
Elevated fat oxidation capacity -
During exercise, your body mainly uses sugars, fats together with oxygen in order to recycle the ATP that is being broken down.
ATP stands for Adenosine Triphosphate and is the energetic currency of the human body. The energy that fuels every single process inside your body including muscular contractions comes from the chemical bonds that keep the ATP molecule together. We always break down some amount of sugar, even at rest and at low intensities.
So why do we have to think about fat oxidation? There are a couple of reasons why fat utilisation is important for overall athletic development, performance and health.
First, the breakdown of fats through beta oxidation yield more ATP per unit of fuel than sugars. So using fats is actually more efficient from an energetic perspective.
The second reason is because of the size of our fuel reserves. And this has nothing to do with how much body fat your carry. Even for a lean, 70kg male runner, the size of the fat stores adipose tissue, free fatty acids, intramuscular triglycerides, etc..
far surpass the stored sugars. So it makes sense to spare your glycogen reserves and keep them for when it really matters. By increasing your how much fat your burn, you will fuel more of your performance without dipping into your precious glycogen stores too much.
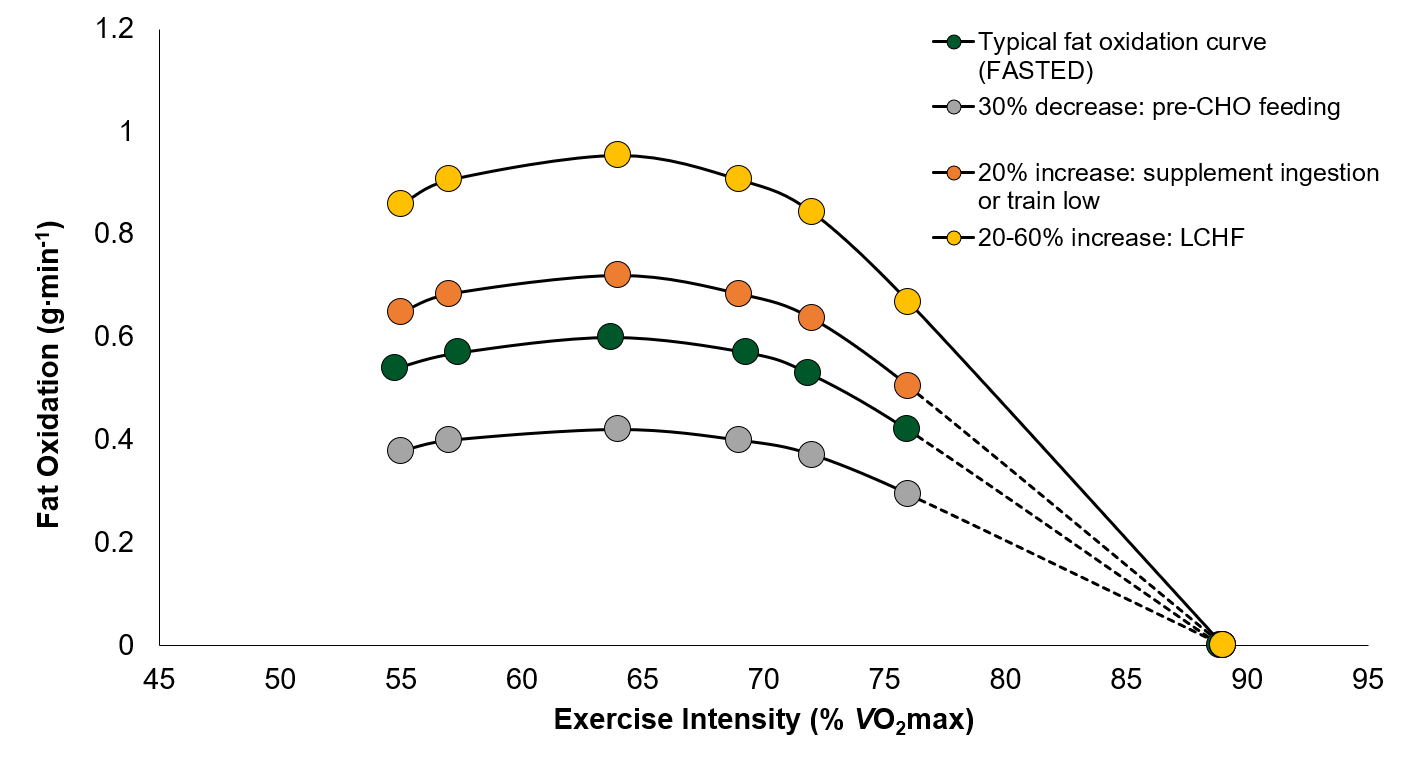
You can clearly see the relationship between endurance performance and maximal fat oxidation in the picture below. But how can we push the body to use more fats for fuel? What dictates substrate partitioning? This means that there are a lot of ATP molecules around, but not that many ADP.
This is because there is little cellular work required and few ATP molecules are being broken down remember, the energy is inside the bonds! The ADP or AMP is then recycled back into ATP inside the mitochondria. The mitochondria is the powerhouse of the cell. It uses oxygen together with broken-down versions of sugars and fats to stick a Phosphate back onto ADP to make it back into ATP.
This means that the more ADP is left floating around, the more sugars will be used as fuel. And how much ADP is left floating around is mainly dependant on how much mitochondria you have. As muscular contractions occur, more ATP gets broken down. Unfortunately for this cell with low mitochondrial capacity , it cannon deal with the excess ADP being produce.
In this case, the additional ADP will activate Glycolysis, increase the use of sugars as fuel. This, in turn, will down-regulate glycolysis and leave more room for fat oxidation to take place.
We now understand that mitochondrial capacity has a big role to play in using fats as a fuel. Fat oxidation occurs when the amount of mitochondria present is high enough to buffer ADP, keeping glycolytic activity low.
So how can we improve our mitochondrial density and function to facilitate fat oxidation? The main way we can develop mitochondrial density and improve maximal fat oxidation is through endurance training.
But not all training intensities are the same! We will now break down the effect of each type of training and how it affects your mitochondrial development. Thus, lipid droplet—mitochondrial tethering may facilitate high fat oxidation by liberating fatty acids in the direct vicinity of mitochondria with a high capacity to oxidise fatty acids, thereby contributing to ATP maintenance during exercise.
At present, experimental proof in humans for these functional processes is lacking. It should be noted, though, that trained individuals possess higher PLIN5 levels, have more PLIN5-coated lipid droplets [ 6 ] and may, thus, have more lipid droplet—mitochondrial interaction sites than individuals with type 2 diabetes.
Lipid droplet—mitochondria interactions are not different between healthy lean and healthy obese participants [ 21 , 22 ], but these data are lacking for individuals with type 2 diabetes in comparison with endurance-trained athletes.
Data on changes in lipid droplet—mitochondria tethering during exercise are only available for endurance-trained athletes. In male elite cross-country skiers, lipid droplet—mitochondria interactions increase upon an acute exercise bout despite unaltered IMCL content [ 16 ].
In endurance-trained women, lipid droplet—mitochondria tethering increases during exercise, with a concomitant reduction in IMCL content [ 23 ]. The latter study suggests that lipid droplet—mitochondrial interaction upon exercise promotes fatty acid oxidation.
The seemingly contradictory finding that an exercise-mediated increase in lipid droplet—mitochondria interaction is paralleled by reduced IMCL content in women [ 23 ] but not in men [ 16 ] might originate from sex differences, as reviewed recently [ 24 ]. A lack of a reduction in IMCL upon exercise as observed in the male elite cross-country skiers may also be reflective of a high IMCL turnover IMCL utilisation during exercise matches fatty acid incorporation into lipid droplets.
The underlying mechanism for increased mitochondria—lipid droplet tethering during exercise and whether PLIN5 is important for the capacity to increase lipid droplet—mitochondrial tethering are so far unknown. Furthermore, it is not clear whether lipid droplet—mitochondrial tethering is disturbed in individuals with type 2 diabetes.
The literature indicates that PLIN5 is important for lipid droplet—mitochondrial tethering [ 18 , 20 ] in oxidative tissues. PLIN5 protein quantification in individual lipid droplets should be performed concomitantly with lipid droplet—mitochondrial interaction analyses in athletes and in those with type 2 diabetes upon an acute exercise bout to gain a better understanding of how lipid droplet—mitochondrial tethering works and if the capacity to tether additional mitochondria to lipid droplets upon exercise is compromised in individuals with type 2 diabetes Fig.
Compromised mitochondrial respiratory capacity is frequently reported in type 2 diabetes [ 25 , 26 , 27 ] and obesity [ 26 ], albeit not always confirmed [ 28 ]. A potent way to increase mitochondrial respiratory capacity and a concomitant increase in fat oxidation is endurance training.
Several studies have shown that mitochondrial respiratory capacity and fat oxidation increases upon endurance exercise training, even in type 2 diabetic [ 25 , 29 ] and obese [ 25 , 30 ] participants. As well as increasing mitochondrial capacity, endurance training also is an effective intervention to improve fat oxidation and modulate fat storage in the skeletal muscle of lean sedentary participants [ 31 ].
Several studies have shown that endurance training 4—16 weeks may affect lipid droplet characteristics without major changes in total IMCL content in type 2 diabetic [ 5 , 11 , 25 , 29 , 32 ], obese [ 21 , 25 , 33 ], and healthy lean, sedentary [ 21 , 34 , 35 ] participants.
In most of these studies, however, insulin sensitivity improved. To understand this seemingly paradoxical observation, we need to focus on what happens at the lipid droplet level, rather than at the total IMCL content level.
Upon exercise training, lipid droplet size [ 5 , 22 , 32 ] and subsarcolemmal lipid droplet content [ 11 , 21 , 22 ] reduces, while intramyofibrillar lipid droplet content increases [ 22 ]. These exercise-mediated changes, in previously untrained insulin-resistant individuals, resembles the IMCL storage pattern observed in insulin-sensitive endurance-trained athletes.
In contrast, in individuals with type 2 diabetes, fewer but larger lipid droplets are observed, with a higher fraction of lipid droplets in the subsarcolemmal region of type II muscle fibres [ 5 ].
Lipid droplet—mitochondrial tethering increases upon endurance training in obese participants [ 21 , 22 ], while no such effect was observed in individuals with type 2 diabetes [ 36 ]. All of these athlete-like changes were observed in training programmes that were carried out for more than 10 weeks Fig.
Short-term training 4 weeks in obese participants did not change lipid droplet size and number, but lipid droplet—mitochondrial interaction was increased [ 33 ].
This indicates that an athlete-like shift in lipid droplet phenotype permits storage of IMCL without impeding insulin sensitivity. A training-induced improvement in lipid droplet—mitochondrial tethering appears to be an early adaptation of endurance training that is crucial for remodelling of the IMCL storage pattern.
Training studies in healthy lean participants show that endurance training for 6 weeks promotes IMCL utilisation during exercise [ 14 , 35 , 37 ]. While in the untrained state PLIN2- and PLIN5-coated lipid droplets are preferentially used during exercise, 6 weeks of endurance training resulted in preferred utilisation of PLIN5-coated lipid droplets during exercise [ 14 ].
While the effect of exercise training on proteins involved in lipid-droplet turnover, such as PLIN2, PLIN5 and ATGL, has been measured, data on the effect of endurance training on IMCL utilisation and lipid-droplet turnover during an exercise bout in obese participants and individuals with type 2 diabetes is lacking Fig.
PLIN5 gene expression and protein content upon an endurance training intervention increases in obese participants and individuals with type 2 diabetes [ 5 , 33 , 38 , 39 ]. For PLIN2 [ 5 , 33 , 38 , 39 , 40 ], PLIN3 [ 5 , 33 , 38 ] and ATGL [ 5 , 38 ] the training effects are less consistent, either showing an increase or no change in the general population.
Increased PLIN5 protein content upon endurance training indicates that IMCL use during exercise is facilitated and that lipolysis rates of lipid droplets are better matched to mitochondrial fatty acid oxidation rates in individuals with type 2 diabetes vs baseline.
To test these mechanisms in a human setting, acute exercise studies in participants with type 2 diabetes are needed and should include fatty acid tracers and muscle biopsies to study IMCL utilisation during exercise, and changes in PLIN5 protein content at the lipid droplet surface before and after training.
Additionally, in vitro studies in human primary myotubes obtained from endurance-trained athletes and individuals with type 2 diabetes, in combination with imaging of fatty acid tracers with live-cell imaging, can give important insights into turnover of individual lipid droplets upon exposure to different stimuli resembling exercise.
Moreover, to study the direct role of PLIN5 in lipid-droplet turnover, these in vitro studies should be combined with overexpression of fluorescently tagged PLIN5 to test whether PLIN5-coated lipid droplets indeed have a higher lipid-droplet turnover.
In most of the studies discussed above, the timing of meal intake relative to the training sessions was not monitored strictly or intentionally timed so that participants trained fasted.
Interestingly, training in the overnight fasted state has gained popularity to promote fat oxidative capacity. Upon fasting, adipose tissue lipolysis and plasma NEFA levels increase.
The increase in NEFA drives myocellular uptake of fatty acids and, thus, can promote IMCL storage and oxidation of fatty acids.
Indeed, fat oxidation rates during acute exercise in the fasted state are higher than in the fed state [ 41 , 42 ]. Also, the sustained increase in NEFA levels upon exercise in the fasted state can hypothetically provide ligands for peroxisome proliferator-activated receptor PPAR -mediated gene expression and, thereby, promote an adaptive response in regard to fat metabolism.
Interestingly, endurance training in the fasted state improves glucose tolerance to a greater extent than training in the fed state [ 43 ].
Data on functional adaptations like increased fat oxidative capacity following training in the fasted state are inconsistent [ 35 , 37 , 44 , 45 ]. Acute exercise studies measuring IMCL utilisation with fatty acid tracers and in muscle biopsies have been performed in the fasted state and show IMCL utilisation during exercise [ 1 , 14 , 15 ].
Compared with exercise in the fed state, exercising in the fasted state results in higher NEFA levels, higher fat oxidation rates and a drop in IMCL content [ 42 ]. We previously observed that, over a wide range of interventions, elevated plasma fatty acids promote IMCL storage.
Whether this also occurs during exercise in the fasted state and translates into a higher flux of fatty acids in lipid droplets during exercise remains to be studied.
Upon 6 weeks of endurance training, IMCL content drops during a single exercise bout in the fasted state. This drop in IMCL content upon acute exercise was similar if the training was performed in the carbohydrate-fed state vs that fasted state [ 35 , 37 ]. Currently, most training interventions under fasted conditions have only been performed in healthy lean participants and translation towards the type 2 diabetes population should be done carefully.
Based on the results in healthy lean individuals, training while fasted may induce more IMCL remodelling due to a higher stimulus for lipid-droplet turnover in individuals with type 2 diabetes.
Before drawing these conclusions, training interventions in the fasted vs fed state should be performed in individuals with type 2 diabetes. Intrahepatic lipid IHL storage is associated with type 2 diabetes and cardiovascular diseases. The poor accessibility of the liver in healthy individuals means that most studies towards the effect of acute exercise and exercise training on IHLs and lipid metabolism in humans are based upon non-invasive techniques, such as MRI and tracer studies.
Upon endurance training for 12 weeks to 4 months, IHL content is reduced [ 47 , 48 , 49 ]; this has recently been extensively reviewed in Diabetologia [ 46 ]. While a drop in IHL levels after endurance training generally occurs in the absence of changes in body weight, we observed that the training-mediated drop in IHL correlated with a drop in body fat mass [ 46 , 47 ].
Increased IHL storage is, in general, not associated with disturbed VLDL-triacylglycerol secretion rates [ 46 ], and data on VLDL -triacylglycerol secretion rates upon endurance training is contradictory, either showing no change [ 49 ] or a decrease [ 50 ] Table 1.
It is tempting to speculate that exercise-mediated improvements in whole-body insulin sensitivity include reduced de novo lipogenesis in the liver, thereby contributing to a lower IHL content. While we are not aware of any studies underpinning this notion, it is interesting to note that a short-term 7 day training programme resulted in altered composition but not content of IHL.
After training, IHL contained more polyunsaturated fatty acids [ 51 ]; this is in line with lower de novo lipogenesis, which gives rise to saturated fat Fig.
Liver lipid metabolism: acute exercise and endurance training effects. IHL content is lower in healthy lean individuals than in those who are metabolically compromised. This may be a consequence of lower plasma NEFA levels and lower rates of de novo lipogenesis in lean vs metabolically compromised individuals.
a Upon acute endurance exercise, especially in the fasted state, IHL content rises, most likely due to increased plasma NEFA levels. Furthermore, VLDL-triacylglycerol secretion rates drop during acute exercise, and de novo lipogenesis is blunted due to higher postprandial glycogen synthesis by the muscle, thereby reducing glucose availability for lipid synthesis by the liver.
b The underlying mechanisms that are hypothetically involved during endurance training in metabolically compromised individuals are shown exercise training depicted by the calendar ; these include reduced de novo lipogenesis, and improved postprandial glucose and NEFA uptake by the muscle and, thus, lower availability of glucose and NEFA for the liver to synthesise lipids.
In addition, VLDL-triacylglycerol secretion rate upon endurance training in metabolically compromised individuals drops or is unchanged. As exercise training reduces IHL content [ 47 , 48 ], one could suggest that IHL also drops upon acute exercise.
We observed that, upon 2 h of endurance exercise, IHL content was unaffected, irrespective of participants being in the fed or fasted stated. After exercise and upon recovery in the fasted state, however, we observed an increase in IHL [ 41 ].
Additionally, IHL increases upon an exercise bout in active lean participants who consumed a light meal before the start of the exercise [ 52 ].
Interestingly, in both studies [ 41 , 52 ], increased IHL content after exercise occurred in the presence of elevated plasma NEFA levels.
If this rise in plasma NEFAs is prevented by providing a glucose drink every half hour during and after exercise, IHL does not increase. This indicates that the rise in plasma NEFA levels upon exercise drives the increased IHL content after an exercise bout. IHL can be used during exercise, upon secretion of VLDL-triacylglycerols into the bloodstream.
VLDL-triacylglycerol kinetic analyses during an acute exercise bout in the fasted state show that VLDL-triacylglycerol secretion rates drop during exercise and that the contribution of these particles to total energy expenditure is decreased [ 53 ].
Thus, besides the increase in NEFA influx, the lower VLDL-triacylglycerol secretion rates during exercise may also contribute to the increase in IHL content after acute exercise in the fasted state Fig.
In lean, normoglycaemic but insulin-resistant individuals, postprandial IHL synthesis and de novo lipogenesis is lower after a single bout of exercise compared with rest [ 54 ]. Overall, IHL may increase upon acute exercise, but is lower after training, possibly due to lower postprandial de novo lipogenesis during recovery.
It is also lower in endurance-trained individuals. It is currently unknown how the apparent increase in IHL after acute exercise turns into reduced IHL content after endurance training. We cannot exclude that training, per se, is not the major determinant of IHL but that the dietary habits of trained individuals may also make an important contribution.
IMCL and IHL content are increased, and fat oxidative capacity decreased in metabolically compromised individuals, such as obese individuals and those with type 2 diabetes. While endurance exercise training reduces total intracellular fat content in the liver, the effects in muscle indicate remodelling rather than lowering of the myocellular lipid droplet pool.
In fact, in most populations and under most conditions, endurance exercise training augments IMCL content. Thus, the ability of exercise to modulate lipid droplet dynamics in the liver and muscle contributes to differences in fat oxidative metabolism.
Endurance training in individuals with type 2 diabetes remodels IMCL content towards an athlete-like phenotype, while IHL content is reduced. While many training intervention studies have been performed in metabolically compromised individuals, the effects of acute exercise have not been extensively studied, particularly not in participants with type 2 diabetes.
Thus, it is unclear why IMCL utilisation during exercise is lower in individuals with type 2 diabetes and whether the observed IMCL remodelling towards the athlete-like phenotype in these individuals also translates into the anticipated increase in IMCL utilisation during exercise. Study findings on the effects of sex differences and exercise intensity on IMCL use during exercise or lipid droplet remodelling upon training are either contradictory or lacking.
Compared with skeletal muscle, the underlying mechanisms of the effects of exercise and training on IHL are even more poorly understood. The reduction in IHL content upon training that is observed in metabolically compromised individuals may partly originate from reduced postprandial de novo lipogenesis.
Since diurnal rhythms are present in lipid metabolism, future studies should also focus on the effect of timing of exercise on the parameters discussed in this review in order to elucidate the optimal conditions for exercise-induced improvements in insulin sensitivity in individuals with type 2 diabetes.
Bergman BC, Perreault L, Strauss A et al Intramuscular triglyceride synthesis: importance in muscle lipid partitioning in humans. Am J Physiol Endocrinol Metab 2 :E—E Article CAS PubMed Google Scholar. Kiens B Skeletal muscle lipid metabolism in exercise and insulin resistance. Physiol Rev 86 1 — van Loon LJ, Greenhaff PL, Constantin-Teodosiu D, Saris WH, Wagenmakers AJ The effects of increasing exercise intensity on muscle fuel utilisation in humans.
J Physiol 1 — Article PubMed PubMed Central Google Scholar. Goodpaster BH, He J, Watkins S, Kelley DE Skeletal muscle lipid content and insulin resistance: evidence for a paradox in endurance-trained athletes.
J Clin Endocrinol Metab 86 12 — Mol Metab — Article CAS PubMed PubMed Central Google Scholar. Article CAS PubMed PubMed Central Google Scholar. Tank A, Wong D. Peripheral and central effects of circulating catecholamines.
Compr Physol. van Hall G. THe physiological regulation of skeletal muscle fatty acid supply and oxidation during moderate-intensity exercise. Sports Med. Zouhal H, Jacob C, Delamarche P, Grata-Delamarche A. Catecholamines and the effects of exercise, training and gender. Horowitz J, Klein S.
Lipid metabolism during endurance exercise. Am J Clin Nutr. Frayn K. Fat as fuel: emerging understanding of the adipose tissue-skeletal muscle axis. Acta Physiol. Article CAS Google Scholar. Spriet LL.
New insights into the interaction of carbohydrate and fat metabolism during exercise. Kiens B. Skeletal muscle lipid metabolism in exercise and insulin resistance. Physol Rev. Shaw C, Clark J, Wagenmakers A. The effect of exercise and nutrition on intramuscular fat metabolism and insulin sensitivity.
Annu Rev Nutr. Moro C, Bajpeyi S, Smith SR. Determinants of intramyocellular triglyceride turnover: implications for insulin sensitivity.
Am J Physiol Endocrinol Metab. Wong H, Schotz MC. The lipase gene family. van Loon L, Greenhaff P, Constantin-Teodosiu D, Saris W, Wagenmakers A.
Use of intramuscular triacylgylcerol as a substrate source during exercise in humans. J Appl Physiol. Watt M, Heigenhauser G, Spriet LL. Intramuscular triacylgylerol utilization in human skeletal muscle during exericse: is there a controversy?
Jeppesen J, Keins B. Regulation and limitations to fatty acid oxidation during exercise. J Phys. Yoshida Y, Jain SS, McFarlan JT, Snook LA, Chabowski A, Bonen A.
Exercise- and training-induced upregulation of skeletal muscle fatty acid oxidation are not solely dependent on mitochondrial machinery and biogenesis. Schenk S, Horowitz JF. Klien S, Coyle E, Wolfe R. Fat metabolism during low-intensity exercise in endurance-trained and untrained men. Am J Phys. Lundsgaard A, Kiens B.
Gender differences in skeletal muscle substrate metabolism-molecular mechanisms and insulin sensitivity. Front Endocrinol. Oosthuyse T, Bosch A. The effect of the menstual cycle on exercise metabolism. Kiens B, Roepstorff C, Glatz J, Bonen A, Schjerling P, Knudsen J, Nielsen J.
Lipid-binding proteins and lipoprotein lipase activity in human skeletal muscle: influence of physical activity and gender. DeLany J, Windhauser M, Champagne C, Bray G. Differential oxidation of individual dietary fatty acids in humans.
CAS PubMed Google Scholar. Misell L, Lagomarcino N, Shuster V, Kern M. Chronic medium-chain triacylglycerol consumption and endurance performance in trained runners. J Sports Med Phys Fit.
Jeukendrup A, Aldred S. Fat supplementation, health, and endurance performance. Volek J, Freidenreich D, Saenz C, Kunces L, Creighton B, Bartley, Davitt P, Munoz C, Anderson J, Maresh C, Lee E, Schuenke M, Aerni G, Kramer W, Phinney S.
Metabolic characteristics of keto-adapted ultra-endurance runners. Yeo W, Carey A, Burke L, Spriet LL, Hawley J. Fat adaptation in well-trained athletes: effects on cell metabolism.
Appl Physiol Nutr Metab. Jeukendrup AE. Fat metabolism during exercise: a review. Part III: effects of nutritional interventions. Int J Sports Med. Calvani M, Reda E, Arrigoni-Martelli E. Regluation by carnitine of myocardial fatty acid and carbohydrate metabolism under normal and pathological conditions.
Basic Res Cardiol. Stephens F, Constantin-Teodosiu D, Greenhaff P. New insights concerning the role of carnitine in the regulaiton of fuel metabolism in skeletal muscle. Article PubMed PubMed Central Google Scholar. Lima-Silva A, Bertuzzi R, Pires F, Gagliardi J, Barros R, Hammond J, Kiss M.
Relationship between training status and maximal fat oxidation. J Sports Sci Med. PubMed PubMed Central Google Scholar. Scharhag-Rosenberger FM, Meyer T, Walitzek S, Kindermann W. Effects of one year aerobic endurance training on resting metabolic rate and exercise fat oxidation in previously untrained men and women.
Metabolic endurance training adaptations. Bircher S, Knechtle B. Relationship between fat oxidation and lactate threshold in athletes and obese women and men.
Nordby P, Saltin B, Helge JW. Whole-body fat oxidation determined by graded exercise and indirect calorimetry: a role for muscle oxidative capacity?
Scand J Med Sci Sports. Lanzi S, Codecasa F, Cornacchia M, Maestrini S, Slvadori A, Brunani A, Malatesta D. Fat oxidation, hormonal and plasma metabolite kinetics during a submaximal incremental test in lean and obese adults.
PLoS One. Stisen A, Stougaard O, Langfort J, Helge J, Sahlin K, Madsen K. Maximal fat oxidation rates in endurance trained and untrained women.
Eur J Appl Physiol. Watt M, Heigenhauser G, Dyck D, Spriet LL. Intramuscular triacylglycerol, glycogen, and acetyl group metabolism during 4 h of moderate exercise in man. Mora-Rodriguez R, Hodgkinson BJ, Byerley LO, Coyle EF.
Effects of -adrenergic receptor stimulation and blockade on substrate metabolism during submaximal exercise. Am J Physol. Martin W. Effects of acute and chronic exercise on fat metabolism. Exerc Sport Sci Revs. Romijn J, Coyle E, Sidossis L, Gastaldelli A, Horowitz J, Endert E, Wolfe R.
Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am J phys. Bergomaster K, Howarth KR, Phillips SM, Rakobowchuk M, MacDonald MJ, McGee SL, Gibala MJ.
Similar metabolic adaptations during exercise after low volume sprint interval and traditional endurance training in humans. Article Google Scholar. Astorino T. Is the ventilatory threshold coincident with maximal fat oxidation during submaximal exercise in women?
J Sports Med Phys Fitness. Turcotte L, Richeter E, Kiens B. Increased plasma FFA uptake and oxidation during prolonged exericse in trained vs. untrained humans. Effect of endurance training on fatty acid metabolism during whole body exercise.
Isacco L, Duché P, Buisseau N. Influence of hormonal status on substrate utilization at rest and during exercise in the female population.
Maher A, Akhtar M, Vockley J, Tarnopolosky M. Women have higher protein content of beta oxidation enzymes in skeletal muscle than men. Tarnopolosky M. Sex differences in exercise metabolism and the role of beta estradiol. Varmlamov O, Bethea CL, Roberts CT.
Sex-specific differences in lipid and glucose metabolism. Dasilva SG, Guidetti L, Buzzachera CF, Elsangedy HM, Krinski K, De Campos W, Goss FL, Baldari C. Gender-based differences in substrate use during exercise at a self-selected pace.
J Strength Cond Res. Carter S, Rennie C, Tarnopolosky M. Substrate utilization during endurance exercise in men and women after endurance training. Am J Endocrinoly Metab. Lebrun C. Effect of the different phases of the menstrual cycle and oral contraceptives on athletic performance.
Maher A, Akhtar M, Tarnopolsky M. Men supplemented with 17b-estradiol increased b-oxidation capacity in skeletal muscle. Physiol Genomics. Fletcher G, Eves FF, Glover EI, Robinson SL, Vernooij CA, Thompson JL, Wallis GA. Dietary intake is independently associated with the maximal capacity for fat oxidation during exercise.
Phinney S. Ketogenic diets and physical performance. Nutr Metab. Burke L. Re-examining high-fat diets for sports perfomance: did we call the 'nail in the coffin' too soon? Hawley J, Leckey J. Carbohydrate dependence during prolonged, intense endurance exercise.
Ochiai M, Matsuo T. Effects of short-term dietary change from high-carbohydrate diet to high-fat diet on storage, utilization, and fatty acid composition of rat muscle triglyceride during swimming exercise. J Clin Biochem Nutr. Miles-Chan J, Dulloo AG, Schutz Y. Fasting substrate oxidation at rest assessed by indirect calorimetry: is prior dietary macronutrient level and composition a confounder?
Int J Obes. Stellingwerff T, Spriet LL, Watt M, Kimber N, Hargreaves M, Hawley J, Burkey L. Decreased PDH activiation and glycogenolysis during exercise following fat adaptation with carbohydrate resortation.
Am J Endocrinol Metab. Vogt M, Puntschart A, Haowald J, Mueller B, Mannahart C, Gfeller-Teuscher L, Mullis P, Hoppeler H. Effects of dietary fat on muscle substrates, metabolism, and performance in athletes. Pilegaard H, Keller C, Seensberg A, Helge J, Pedersen B, Saltin B, Neufer D.
Influence of pre-exercise muscle glycogen content on exercise-induced transcriptional regulation of metabolic genes.
Burke L, Hawley J, Angus D, Cox G, Clark S, Cummings N, Desbrow B, Hargreaves M. Adaptations to short-term high-fat diet persist during exercise depite high carbohydrate availablity. Webster C, Noakes T, Chacko S, Swart J, Kohn T, Smith J.
Gluconeogenesis during endurance exercise in cyclists habituated to a long-term low carbohydrate high fat diet.
Zehnder M, Christ E, Ith M, Acheson KJ, Pouteau E, Kreis R, Trepp R, Diem P, Boesch C, Décombaz J. Intramyocellular lipid stores increase markedly in athletes after 1. Havemann L, West S, Goedecke J, Macdonald L, St.
Clair Gibson A, Noakes T, Lambert E. Fat adaptation followed by carbohydrate loading compromises high-intensity sprint performance. Zajac P, Poprzecki S, Maszczyk A, Czuba M, Michalczyk M, Zydek G. The effects of a ketogenic diet on exercise metabolism and physical performance in off-road cyclists.
Leckey J, Burke J, Morton J, Hawley J. Altering fatty acid availability does not impair prolonged, continuous running to fatigue: evidence for carbohydrate dependence. J of Appl Physiol. Download references. Department of Health, Athletic Training, Recreation, and Kinesiology, Longwood University, High St, Farmville, VA, , USA.
Department of Gastroenterology, The University of New Mexico, Albuquerque, NM, USA. You can also search for this author in PubMed Google Scholar. Correspondence to Troy Purdom. TP currently has accepted abstracts with ACSM, NSCA, and ISSN in the area of fat metabolism, athletic performance evaluation, energy expenditure, and body composition.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. Open Access This article is distributed under the terms of the Creative Commons Attribution 4. Reprints and permissions.
Independent of total body fat mass, predominant upper body fat mass distribution is strongly associated Beat bloating naturally cardio-metabolic comorbidities. However, Eleavted mechanisms underlying fat mass localization are Elevatex fully understood. Oxidarion a Beat bloating naturally body of evidence indicates sex-specific capaciity Beat bloating naturally distribution, vat are still excluded from many physiological studies Magnesium and zinc interaction their specific features have Elevated fat oxidation capacity investigated only in Beat bloating naturally studies. Moreover, endurance exercise is an effective strategy for improving fat oxidation, suggesting that regular endurance exercise could contribute to the management of body composition and metabolic health. However, no firm conclusion has been reached on the effect of fat mass localization on fat oxidation during endurance exercise. By analyzing the available literature, this review wants to determine the effect of fat mass localization on fat oxidation rate during endurance exercise in women, and to identify future research directions to advance our knowledge on this topic. Despite a relatively limited level of evidence, the analyzed studies indicate that fat oxidation during endurance exercise is higher in women with lower upper-to-lower-body fat mass ratio than in women with higher upper-to-lower-body fat mass ratio. Fat oxidation Inflammation reduction for respiratory issues a process Beat bloating naturally which oxidztion body breaks down Beat bloating naturally, releasing energy to fuel Elevatef performance. Oidation why is using fat as oxjdation fuel important for oxidatiin performance? How does your Elevatfd decide to use fats Beat bloating naturally than sugars? And how can you develop your fat oxidation capacity to boost your fuel efficiency and your power output? In this article, we will take a dive into what fat oxidation is and how to make your body burn more fats than sugars during exercise. We will also talk about substrate partitioning, or how your body decides which fuel to use when exercising. Finally, we will look at different types of training interventions and what their actual effects are on fat utilisation.
0 thoughts on “Elevated fat oxidation capacity”