
Objectives: We aimed to examine fpr relationships Natural alternatives for hypertension medication body mass BBMI BMI and fod syndrome MS components as Metablic function of Metabolicc and gender across weight categories.
Methods: This cross-sectional Natural alternatives for hypertension medication included 19, subjects who participated in Mental fatigue and concentration health-screening program.
Metaboolic At Heapth Mental fatigue and concentration Mftabolic The number of MS components increased linearly Joint support supplements BMI.
The most prevalent components for Foe were hypertension in BIA impedance measurement device and Merabolic waist circumference in women.
Women were metabolically protected relative to men between the ages of 30 and 50 years. Conclusions: A MS components increase linearly with BMI from the lowest normal BMI and continue to increase with age and BMI; B metabolically healthy obesity is rare in subjects with a high BMI and declines with age; C hypertension is the most common component in men; and D in women, MS components are seen at older ages than in men for the same BMI.
Metabolic health declines with age and BMI in nearly all subjects with obesity. Keywords: hypertension; metabolic syndrome; metabolically healthy obesity; normometabolic obesity; obesity. Abstract Objectives: We aimed to examine the relationships between body mass index BMI and metabolic syndrome MS components as a function of age and gender across weight categories.
Grants and funding.
: BMI for Metabolic Health| Publication types | A new glucagon and GLP-1 co-agonist eliminates obesity in rodents. Health Risk Manag. Cadby, G. While BMI is an independent risk factor for CVD 59 , 60 , not all obese or overweight people show abnormal cardiovascular risk profiles. Lab 24 , 1—4 Huynh, K. Better understanding of the factors that distinguish people with MHO and MUO can produce new insights into mechanism s responsible for obesity-related metabolic dysfunction and disease. |
| Introduction | Videos Conversations with Giants in Medicine Author's Takes Reviews Reviews View all reviews Zembic A, Eckel N, Stefan N, Baudry J, Schulze MB. E-mail: bluma medizin. Back to top Article Information. Clin Nutr. VAGUE J. |
| Latest news | The risk of all-cause mortality is less in people with MHO than in people with MUO The risk of all-cause mortality in people with MHO relative to those who are MHL depends on the number and severity of metabolic abnormalities and the stability of metabolic health 64 — The combined data from five large cohort studies that followed participants for an average of 13 years found that people with MHO and no metabolic syndrome components excluding waist circumference did not have an increased risk of all-cause mortality compared with the MHL group; however, the risk of all-cause mortality was greater in participants with MHO versus MHL when participants with one abnormal metabolic risk factor excluding waist circumference were included in the MHO group The characteristics that have been associated with MUO are shown in Figure 2. Among these features, multiorgan insulin resistance impaired insulin-mediated suppression of hepatic glucose production, suppression of adipose tissue lipolytic activity, and stimulation of muscle glucose uptake is likely the most important underlying factor responsible for the development of cardiometabolic diseases In people without diabetes, whole-body insulin sensitivity, assessed with the HECP, is inversely correlated with BMI; however, there is considerable heterogeneity in insulin sensitivity at any given BMI value so that a small subset of people with obesity are as insulin sensitive as people who are lean 31 , Insulin sensitivity is greater in people with MHO than in those with MUO, and many participants identified as having MHO are more insulin resistant than those who are MHL, manifested by greater fasting plasma insulin concentrations, blood glucose concentrations during an oral glucose tolerance test, and HOMA-IR values 9 , 27 , 70 — The factors responsible for the greater preservation of insulin action in people with MHO than in those with MUO are not clear, but could be related to differences in potentially modifiable lifestyle factors and alterations in adipose tissue biology In this section we review each of these areas with a major focus on adipose tissue biology. Putative characteristics of people with metabolically unhealthy obesity that are distinct from those of people with metabolically healthy obesity. However, the evidence to support a difference in many of these characteristics between people with MUO and MHO is not definitive because of inadequate data or conflicting results from different studies. The relationship between dietary intake and metabolic health has been evaluated in large population studies by using the food frequency questionnaires or hour dietary recall data. The ability of these methods to reliably assess dietary intake has been questioned 74 , The results from most studies do not show a difference in total dietary energy intake or macronutrient distribution between people with MHO and MUO 76 — In addition, data from the US National Health and Nutrition Examination Survey showed no difference in diet quality, assessed as the consumption of Mediterranean-style and DASH-style diets, between people with MHO and MUO A higher total Healthy Eating Index score, which assesses diet quality in relation to the US National Dietary Guidelines, was found in MHO than in MUO women who were 19—44 years old, but this score was not different in women with MHO and MUO who were 45—85 years old or in adult men with MHO and MUO There is evidence from some 76 , 77 , 80 — 82 but not all 27 , 77 studies that the consumption of specific types of foods differs between MHO and MUO groups; MHO was associated with a lower intake of sugar, sugar-sweetened beverages, and saturated fat and a higher intake of whole fruits, whole grains, and protein from vegetable sources. Physical activity and cardiorespiratory fitness. Increased physical activity improves insulin sensitivity and metabolic syndrome abnormalities The amount and intensity of physical activity in MHO and MUO populations have been studied by the doubly labeled water method, accelerometry, and activity questionnaires. No differences in total daily energy expenditure or energy expended during physical activity, measured by the doubly labeled water method, were detected between people with MHO and MUO 20 , In contrast, studies that measured physical activity by using accelerometers or questionnaires showed that people with MHO spend more time in moderate to vigorous physical activities and less time in sedentary activities than people with MUO 35 , 85 — Insufficient sleep duration and poor sleep quality have adverse effects on metabolic function 88 and are associated with obesity 89 , The results from nearly all studies that have assessed sleep duration and quality in people with MHO and MUO are not adequate to reliably evaluate potential differences between people with MHO and MUO, because the data are derived from questionnaires rather than direct assessments of sleep duration and quality. The expansion of adipose tissue and TG mass with weight gain a is not uniformly distributed among different adipose tissue depots and the liver; b is due to an increase in adipocyte size or adipocyte number, or both; c requires adequate blood supply to maintain tissue oxygenation; d promotes extracellular matrix ECM remodeling to provide the scaffolding needed to support the expanded adipocyte mass; e causes an increase in adipose tissue—resident immune cells and both adipose tissue and systemic markers of inflammation; f affects adipocyte lipolytic activity and the rate of release of fatty acids into the circulation; and g alters the production of adiponectin, the major adipocyte secretory protein involved in regulating insulin sensitivity. Body composition. Percentage body fat is not different in people with MHO and MUO when the groups are matched on BMI and sex 19 — 21 , 23 , However, there are marked differences in adipose tissue distribution and intrahepatic TG content between MHO and MUO cohorts. People with MHO have less intra-abdominal adipose tissue IAAT than people with MUO 21 , 23 , 76 , 95 — 97 , but still have two to three times more IAAT than people who are MHL 19 , Although women with MHO tend to have a greater amount of lower-body subcutaneous thigh or leg fat mass than women with MUO 20 , 48 , 95 , 96 , lower-body fat mass is not different between men with MHO and MUO 48 , Intrahepatic TG content is greater in people with MUO than in those with MHO 98 , and those with steatosis have greater multiorgan insulin resistance 99 and higher plasma TG concentrations than those with normal intrahepatic TG content, even when matched on BMI, percentage body fat, and IAAT volume Taken together, these data show that excess adiposity per se is not responsible for the differences in metabolic health between people with MHO and MUO, but differences in adipose tissue distribution distinguish between MHO and MUO phenotypes. Adipogenesis and lipogenesis. The relationship between adipogenesis i. Adipogenic capacity in SAAT, assessed by in vitro differentiation assays and expression of genes involved in preadipocyte proliferation and differentiation, is greater in people with MHO than in those with MUO — The capacity for lipogenesis in SAAT, assessed as expression of genes involved in lipogenic pathways CD36, GLUT4, ChREBP, FASN, and MOGAT1 , is greater in people with MHO than MUO , , , Moreover, the expression of these genes is positively correlated with insulin sensitivity , , and increases more after moderate weight gain in people with MHO than MUO Collectively, these data refute the notion that impaired adipogenesis contributes to insulin resistance in people with MUO , but demonstrate that increased adipose tissue gene expression of lipogenic pathways is associated with metabolic health. Adipocyte size. Adipocyte size is typically measured by one of three methods: a histological analysis of adipose tissue; b collagenase digestion of adipose tissue to generate free adipocytes that are measured by microscopy; and c adipose tissue osmium tetroxide fixation and cell size analysis using microscopy or a Multisizer Coulter Counter. The median adipocyte diameters in SAAT measured by each of these methods correlate with whole-body adiposity However, the frequency of small cells 20—50 μm range varies considerably among the three methods The highest frequency of these small cells, which are believed to be immature or differentiating adipocytes, but could be large lipid-laden macrophages , is observed when cell size is assessed by the osmium fixation method , The results from several studies show an inverse correlation between average or peak subcutaneous abdominal adipocyte size and insulin sensitivity, and that adipocyte size is greater in people with MUO than in those who are metabolically healthier 21 , — However, other studies did not detect a difference in average subcutaneous adipocyte size in MHO and MUO participants , , Two studies identified two distinct populations of adipocytes based on size and found a higher ratio of small to large subcutaneous abdominal adipocytes in people who were insulin resistant than in those who were insulin sensitive , In summary, the majority of studies show that mean adipocyte size is smaller in people with MHO than MUO. However, the observation that adipose tissue contains distinct small- and large-cell populations with variable cell numbers confounds the interpretation of overall mean cell size. Accordingly, more sophisticated analytical methods that quantify adipocyte cell sizes and number are needed. The oxygenation of adipose tissue depends on the balance between the rate of oxygen delivery to adipose tissue cells adipocytes, preadipocytes, mesenchymal stem cells, fibroblasts, vascular endothelial cells, and immune cells and their rate of oxygen consumption. The delivery of oxygen to adipose tissue is likely lower in people with obesity than in people who are lean because of decreased systemic arterial oxygen content associated with pulmonary dysfunction , , decreased adipose tissue capillary density and perfusion — , an increased number of interstitial immune cells , and possibly greater oxygen diffusion distance due to hypertrophied adipocytes and increased ECM content However, the adequacy of adipose tissue oxygenation in people with obesity is not clear, because interstitial adipose tissue oxygen partial pressure pO 2 , not intracellular pO 2 , is measured and because of conflicting data from different studies depending on the method used , — , — Studies that used a Clark-type electrode or a fiber optic system to assess interstitial SAAT pO 2 in situ found that pO 2 was lower in people who are obese than in those who are lean , , , In contrast, studies that used an optochemical sensor to measure pO 2 in SAAT interstitial fluid extracted by microdialysis ex vivo found that pO 2 was higher in people with obesity than in those who were lean despite decreased adipose tissue blood flow in people with obesity, suggesting decreased adipose tissue oxygen consumption in the obese group , A direct assessment of arteriovenous oxygen balance across SAAT demonstrated that both oxygen delivery and consumption were decreased in people with obesity compared with those who were lean or overweight; however, obesity was not associated with evidence of adipose tissue hypoxia, assessed as oxygen net balance and the plasma lactate-to-pyruvate ratio across SAAT We are aware of three studies that evaluated interstitial SAAT pO 2 in people with MHO and MUO. Two studies measured pO 2 in situ and found that pO 2 was greater , or not different , in the MHO compared with MUO groups. The third study measured pO 2 ex vivo in SAAT interstitial fluid extracted by microdialysis and found it was lower in MHO than in MUO We are not aware of any studies that evaluated metabolic indicators of adipose tissue hypoxia, namely adipose tissue HIF1α protein content, in people with MHO and MUO. In summary, currently there is not adequate evidence to conclude there is a physiologically important decrease in adipose tissue oxygenation in people with MUO compared with MHO. ECM remodeling and interstitial fibrosis. The ECM of adipose tissue is composed of structural proteins primarily collagens I, III, IV, V, and VI and adhesion proteins fibronectin, elastin, laminin, and proteoglycans. Compared with people who are lean, people with obesity have increased expression of genes for collagen I, IV, V, and VI and histological evidence of increased fibrosis, particularly pericellular fibrosis in omental adipose tissue and SAAT — In addition, we recently found that adipose tissue expression of connective tissue growth factor CTGF , a matricellular protein that regulates tissue fibrosis, is positively correlated with body fat mass and inversely correlated with indices of whole-body, liver, and skeletal muscle insulin sensitivity Adipose tissue expression of collagen genes and collagen content are also inversely correlated with insulin sensitivity in people with obesity, and decrease with weight loss , — These data support the notion that adipose tissue fibrosis is associated with MUO, as has been demonstrated in rodent models Immune cells and inflammation. Obesity is typically associated with chronic, low-grade, noninfectious inflammation, which has been purported to be a cause of insulin resistance , It has been proposed that alterations in adipose tissue immune cells are an important cause of the chronic inflammation and insulin resistance associated with obesity , Macrophages are the most abundant immune cell in adipose tissue, and adipose tissue macrophage content is increased in people with obesity compared with people who are lean Moreover, adipose tissue macrophage content and crown-like structures macrophages surrounding an extracellular lipid droplet are greater in both SAAT and IAAT in people with MUO than in those with MHO; the increase in macrophage content is primarily due to an increase in M1-like proinflammatory macrophages 21 , — In conjunction with the alterations in adipose tissue immune cells, adipose tissue expression of inflammation-related genes is also greater in people with MUO than in those with MHO 21 , , , , , , but there is inconsistency in the specific genes that are upregulated among studies, and the differences in gene expression markers between MUO and MHO groups are often small 21 , , , , , Plasma concentrations of markers of inflammation, primarily C-reactive protein, plasminogen activator inhibitor-1 PAI-1 , IL-6, and TNF-α, are either higher in those with MUO than MHO 21 , 23 , 42 , 96 , — or not different between the two groups — The variability in results is likely related to the definitions used to identify MUO and MHO, the specific inflammatory markers evaluated in different studies, and the sample size needed for adequate statistical power because of small mean differences in plasma concentrations between groups. The variability and small difference in adipose tissue expression of inflammatory markers in people with MHO and MUO and both the variability and small differences in plasma markers of inflammation between people with MUO and MHO question the importance of adipose tissue production and secretion of inflammatory cytokines in mediating the difference in systemic insulin resistance observed in people with MUO and MHO. Nonetheless, it is possible that other immune cell—related mediators, such as adipose tissue macrophage-derived exosomes , are involved in the pathogenesis of metabolic dysfunction. Lipolytic activity. Acute experimental increases in plasma free fatty acid FFA concentration, induced by infusion of a lipid emulsion, impair insulin-mediated suppression of hepatic glucose production and insulin-mediated stimulation of glucose disposal in a dose-dependent manner , However, the influence of endogenous adipose tissue lipolytic activity and plasma FFA concentration on insulin sensitivity in people with obesity is not clear because of conflicting results from different studies. The importance of circulating FFA as a cause of insulin resistance in MUO is further questioned by studies that found no difference in basal, postprandial, and hour plasma FFA concentrations in people with obesity compared with those who are lean and more insulin sensitive , The reason s for the differences between studies are not clear, but could be related to the considerable individual day-to-day variability in FFA kinetics and plasma FFA concentration and differences in compensatory hyperinsulinemia and insulin-mediated suppression of adipose tissue lipolytic rate in people with insulin resistance , , Differences in the percentage of women between study cohorts will also affect the comparison of FFA kinetics and concentrations between MHO and MUO groups, because the rate of the appearance of FFA in the bloodstream in relationship to fat-free mass or resting energy expenditure is greater in women than in men , , yet muscle and liver , insulin sensitivity are greater in women. Taken together, these studies suggest that differences in subcutaneous adipose tissue lipolytic activity do not explain the differences in insulin sensitivity between people with MHO and MUO. However, it is still possible that differences in lipolysis of IAAT and portal vein FFA concentration or differences in the effect of FFA on tissue muscle or liver insulin action contribute to the differences in insulin resistance between the two groups. Adiponectin, the most abundant protein secreted by adipose tissue, is inversely associated with percentage body fat and directly associated with insulin sensitivity in both men and women Plasma adiponectin concentrations are often higher in people with MHO than MUO 12 , — The reasons for the lower adiponectin concentration in MUO than MHO are unclear but could be related to chronic hyperinsulinemia in people with MUO, which suppresses adipose tissue adiponectin production , , thereby generating a feed-forward cycle of decreased adiponectin secretion caused by insulin resistance and increased insulin resistance caused by decreased adiponectin secretion. There is considerable heterogeneity in the metabolic complications associated with obesity. The risk of developing cardiometabolic diseases in people with obesity is directly related to the number and severity of metabolic abnormalities. Accordingly, people with MHO are at lower risk of future T2D and CVD than people with MUO, but most people with MHO are at a higher risk than people who are MHL. Additional details are shown in Supplementary Data 1 and 2. AC acylcarnitine, CE cholesteryl ester, Cer ceramide, COH cholesterol, DE dehydrocholesterol, dhCer dihydroceramide, DG diacylglycerol, GM1 GM1 ganglioside, GM3 GM3 ganglioside, HexCer monohexosylceramide, Hex2Cer dihexosylceramide, Hex3Cer trihexosylceramide, LPC lysophosphatidylcholine, LPC O lysoalkylphosphatidylcholine, LPC P lysoalkenylphosphatidylcholine, LPE lysophosphatidylethanolamine, LPE P lysoalkenylphosphatidylethanolamine, LPI lysophosphatidylinositol, PC phosphatidylcholine, PC O alkylphosphatidylcholine, PC P alkenylphosphatidylcholine, PE phosphatidylethanolamine, PE O alkylphosphatidylethanolamine, PE P alkenylphosphatidylethanolamine, PG phosphatidylglycerol, PI phosphatidylinositol, PS phosphatidylserine, SHexCer sulfatide, SM sphingomyelin, TG triacylglycerol, TG O alkyl-diacylglycerol. To better understand the lipid biology captured by the mBMI, we performed regression analysis of lipid species with BMI and mBMIΔ. In age and sex adjusted models, we observed a significant association with out of lipid species with BMI. Diacylglycerol, triacylglycerol and ceramide species showed a strong positive association, while most hexosylceramide, lyso and ether phospholipid species were negatively associated Fig. LPC [sn1] decreased by 2. Of the triacylglycerol species, TG [NL] was the strongest predictor 4. We then performed the same regression analysis of lipid species against mBMIΔ Fig. However, we note the effect sizes were stronger against mBMIΔ Fig. For example, the effect size for TG [NL] was 4. The statistical explanation why the plot of the beta coefficients of lipids for BMI and mBMIΔ are correlated is elaborated in Supplementary Note 1. A LASSO model performed nearly the same as the ridge model Supplementary Fig 4a and 4b , Supplementary Table 3. Using the LASSO model, associations of BMI with plasma lipid species Supplementary Fig. The correlation between effect sizes of each lipid associated with BMI x-axis and mBMIΔ calculated from the LASSO model y-axis provided an R 2 close 1. To assess the importance of the number of lipid species in the models, we compared regularized linear models ridge, elastic-net and LASSO , incorporating lipid species, age and sex, for their ability to predict BMI in the AusDiab cohort and validated these in the BHS. Using elastic-net lipid species selected and LASSO lipid species selected models, we observed similar performance as for the ridge model for the prediction of BMI, with models explaining Validation of these models in the BHS dataset explained to When we utilised clinical lipids, age and sex in the model development, the elastic-net and LASSO models respectively explained only Upon incorporating all CMRs, the elastic net and LASSO models respectively explained, As, LASSO and elastic-net showed very similar performance we focused further analysis on the ridge and LASSO models only. To investigate how a further reduction in the number of lipid species in the model affected model performance, we tuned the regularization parameter, lambda, in the LASSO models and in the ridge models for comparison, with log10 lambda values between -4 and 0. As lambda was increased, the number of features selected into the LASSO model decreased until only 9 lipids are included in the model with a log10 lambda of 0. a The number of features incorporated in the ridge red line and LASSO blue line models for different lambda values. b The correlation R 2 of BMI and pBMI dashed lines or BMI and mBMI solid lines in ridge red line and LASSO models blue line for different lambda values. c MSE of the difference between the observed and predicted values for ridge red line and LASSO models blue line. The vertical dashed red and blue lines represent the minimum MSE, for ridge and LASSO models respectively i. d A plot of beta coefficients from the optimum ridge model. e A plot of beta coefficients from the optimum LASSO model. Red circles and blue diamonds represent the top 15 lipid species ranked based on the absolute value of beta coefficients showing the strongest contribution in the ridge and LASSO models respectively. Source data are provided as a Source Data file. In the LASSO models, as lambda increased, the correlation R 2 between BMI and the pBMI decreased, while in the ridge models the R 2 remained relatively stable Fig. The correlation R 2 between BMI and mBMI increased in the LASSO models reaching a R 2 of 1. Optimization of the lambda parameter by minimizing the mean-squared error MSE using cv. glmnet showed the cross-validated MSE increasing in the LASSO models but again relatively stable in the ridge models Fig. The optimum lambda used to model BMI for the ridge and LASSO models was defined by the lowest MSE. We then extracted the beta-coefficients of the optimum ridge and LASSO models Fig. More details on the weighting of the individual lipid species in both the ridge and LASSO models can be found in Supplementary Data 3. While the ridge and LASSO models showed comparable performances, when lambda was optimised, the ridge model was more stable across all the possible lambda values and showed better validation in the BHS cohort Supplementary Table 3 and so was used for further analyses. To assess the relationship between mBMIΔ and cardiometabolic risk factors and explore whether mBMIΔ identifies metabolic subtypes, we grouped the AusDiab participants into quintiles of the mBMIΔ, with just over participants in each Fig. The distributions of BMI and mBMI for the 5 groups are shown in Fig. We performed linear regression analysis between cardiometabolic traits outcome and the quintiles of mBMIΔ predictor to assess the overall association. Quintiles 1 to 5 Q1-Q5 , as expected, have comparable BMI values, but substantially different mBMIs. The two most discordant groups Q1 and Q5 had similar mean BMI and mean age, while their mBMI scores were significantly different Fig. The median IQR mBMI values were Individuals in Q5 were characterized by unfavourable lipoprotein profiles higher total cholesterol, higher triglycerides, and lower HDL-C; Fig. We also observed stronger associations of mBMIΔ with waist circumference WC and waist-to-hip-ratio WHR that with BMI itself Supplementary Fig. b Density histograms of BMI distribution for each mBMIΔ quintile. c Density histograms of mBMI distribution for each mBMIΔ quintile. d , e Box plots of the association of mBMIΔ with cardiometabolic traits. Box plots represent the distribution of z-scores of the respective cardiometabolic trait in each quintile of mBMIΔ. The data depicted in the box and whisker plots for d and e span from the minimum to the maximum values z-score. The lower and upper boundaries of the box correspond to the 25th and 75th percentiles, respectively, and the central open circles within the boxes represent the median values. Linear regression analyses of mBMIΔ quintile predictor against cardiometabolic traits outcome were performed. β-coefficients and p values two-sided from the linear regression analyses are presented. No adjustments were made for multiple comparisons. BMI body mass index, HDL-C high density cholesterol, HOMA-IR homeostatic model assessment of insulin resistance, FBG fasting blood glucose, 2h-PLG 2-h post load glucose, SBP systolic blood pressure, DBP diastolic blood pressure, HbA1C haemoglobin A1c. To validate these findings, we statistically tested whether the profile of cardiometabolic traits differ between the two most discordant groups in the AusDiab cohort and validated this in the BHS cohort. We performed linear regression analyses with cardiometabolic traits as outcomes and the discordant groups as the predictor, using Q1 as the reference group , adjusting for age, sex and BMI or for age, sex, BMI, and clinical lipids excluding the outcome. All the metabolic traits, except FBG, differed between the discordant groups before and after adjusting for clinical lipids despite these groups having a similar BMI in both cohorts Fig. These associations remained significant after further adjustment for clinical lipids, although the effect size was reduced in most cases Fig. The findings observed in the AusDiab cohort were validated on the BHS cohort note, the 2h-PLG and the HbA1c measures were not available in the BHS cohort. These associations remained significant after adjustment with clinical lipids Fig. Each square represents the fold difference Q5 relative to Q1 of mBMIΔ for a given metabolic trait. HDL-C high density cholesterol, HOMA-IR homeostatic model assessment of insulin resistance, FBG fasting blood glucose, SBP systolic blood pressure, DBP diastolic blood pressure. We assessed the odds of T2DM and pre-diabetes across the quintiles of the mBMIΔ with Q1 as a reference. Based on the quintile analyses, there was a progressive increase in the odds ratio of T2DM from the lowest mBMIΔ range Q1 to the highest Q5 Fig. These associations were only slightly attenuated but remained significant after adjusting for clinical lipids total cholesterol level, HDL-C, triglycerides , familial history of diabetes and smoking status. Further details of these associations are provided in Supplementary Table 8. We have previously reported comprehensive sex-differences in the lipidomic profile employing the same datasets Recognizing these differences in the metabolic profiles of men and women, we conducted a separate analysis for men and women. In sex-stratified models age and BMI adjusted , we observed that, the mBMIΔ exhibited a slightly stronger association with a newly diagnosed T2DM in women than men Supplementary Fig. a Density histogram showing the distribution of BMI in T2DM and NGT subjects. b Density histogram showing the distribution of mBMI in T2DM and NGT subjects. c The forest plot displays the odds ratio x-axis associated with moving from Q1 of mBMIΔ reference quintile to Q2—Q5 y-axis for the newly diagnosed prevalent T2DM yellow circles and 5-year incident T2DM sky-blue circles compared to controls. Odds ratios and the associated CIs were log2 transformed to enhance visualization. The results for clinical lipid, familial history of diabetes and smoking status adjusted models are provided in Supplementary Table 5. Next, we investigated whether the strong associations of mBMIΔ with T2DM observed above also exist in the pre-diabetic state. As the mBMIΔ increased, the odds of pre-diabetes at baseline and risk of future pre-diabetes increased in a progressive manner. This association remained significant although attenuated upon adjusting for clinical lipids, and smoking Fig. The details of the odds ratios and p-values before and after adjusting for clinical lipids across the full quintile range are provided in Supplementary Table 6. Prevalent pre-diabetes constitutes two distinct pre-diabetic states: isolated impaired fasting glucose IFG and impaired glucose tolerant IGT and the composite of these two. The association of mBMIΔ with isolated IGT was stronger than the association with IFG. However, in both cases a strong and progressive increase in the odds ratio was observed as one moves from Q1 to Q5 of mBMIΔ Supplementary Fig. A significant association exists between the mBMIΔ and the isolated IFG versus NGT, despite the weak association of mBMIΔ with FBG itself. We identified that, the latter finding i. Of note, individuals with KDM has a lower mBMIΔ than those with IFG, IGT and NGT Supplementary Fig. The associations of mBMIΔ with IFG were independent of 2h-PLG and associations with IGT were independent of FBG Supplementary Table 7. Depicted on the x-axis of the forest plot are the odds ratios on a log2 scale for subjects with the prevalent pre-diabetes gold circles and 5-year incident pre-diabetes sky-blue circles compared to the controls across the quintiles of mBMIΔ y-axis. Detailed associations including clinical lipids and smoking adjusted analyses are presented in Supplementary Table 6. We assessed whether the mBMIΔ was associated with prevalent CVD and risk of future CVE independent of the measured BMI. Compared to the entire cohort, the results were consistent in a sex-stratified analyses, although the mBMIΔ exhibited a slightly larger effect size in women Supplementary Fig. Only the, IHD events in the AusDiab were defined in the same way in the BHS. Consequently, we validated the mBMIΔ—IHD associations in the BHS cohort showing similar results as in the AusDiab Supplementary Table 9. This suggests that the lipidomic data capture independent information that is not fully accounted for by the clinical lipid values or the CMRs alone. We also examined how the mBMIΔ derived from clinical lipids or CMRs related with disease outcomes and how these compared with the current mBMIΔ. Although, the BMI prediction performance was low when using only clinical lipids or CMRs Supplementary Fig. As expected, mBMIΔ calculated from the CMRs model performed better than the lipidomic model at prediction of prevalent and incident T2DM as the diagnostic criteria are included in the model. In contrast the mBMIΔ derived from model with clinical lipids or CMRs did not predict cardiovascular disease, demonstrating the limitations of these models Supplementary Fig. Using mBMIΔ as a continuous outcome, we assessed the relative contribution of BMI and mBMIΔ in models containing both BMI and mBMIΔ adjusting for age and sex in the AusDiab cohort. We also assessed the association of mBMI against the same outcomes. As expected, BMI was strongly associated with both prevalent and incident T2DM and to the lesser extent with prevalent CVD and incident CVE Supplementary Table The mBMI itself was also significantly associated with T2DM and prevalent CVD independent of age and sex; these associations were stronger resulting in lower p values than the associations with either measured BMI or mBMIΔ Supplementary Table 9. The mBMIΔ showed, an independent association with prevalent and incident T2D after correcting for age, sex and BMI Supplementary Table 10 and with CVD outcomes after adjusting for age, sex, BMI, smoking status and diabetes. Using this approach, we showed that models with mBMIΔ showed a better fit in predicting newly diagnosed prevalent T2DM i. The model with mBMIΔ also showed a better fit for prevalent CVD relative to a model without mBMIΔ Supplementary Table Total fruit intake quintiles encompassing 10 different types Supplementary Fig. In the full model, adjusted for smoking, PA time, TV viewing time, SBP, family history of diabetes, history of CVD and other dietary and lifestyle factors model 2 , this association remained significant β —0. Compared to participants with the lowest intake of total dietary fibre Q1 , participants with the highest intake Q5 had a 0. In the full model, this association was only slightly attenuated but remained significant Supplementary Table A strong dose-response relationship between the quintiles of PA time and mBMIΔ was observed. Prolonged TV viewing time was also significantly associated with mBMIΔ. PA, physical activity; TV, television. Obesity is a major risk factor for many non-communicable diseases such as T2DM and CVD 11 , 12 , 13 , However, the widely used measure of obesity, BMI, does not fully capture the metabolic dysregulation associated with obesity leading to the misclassification of metabolic health and metabolic risk. While direct measures of body fat distribution, such as computed tomography and dual-energy X-ray absorptiometry, have the potential to enhance risk assessment by providing valuable insights into body fat distribution, their practical application is constrained by high costs and inability to directly evaluate metabolic health and perturbations. In contrast, mBMI measures hold promise for understanding metabolic health and risk, albeit requiring development of their clinical utility and cost-effectiveness Hence, in the present study, we constructed a lipidome-based BMI score, that represents the mBMI of an individual, with a view to understand its biological significance and examine whether the score provides additional information over the measured BMI for the metabolic health and risk assessment of multiple clinical outcomes. The mBMI score, although not intended to replace existing risk scores for cardiometabolic diseases, serves as a measure of cardiometabolic health e. Here, we introduced quintiles of mBMIΔ and stratified the population based on the disparity between BMI and mBMI. We report key associations of mBMIΔ and metabolic discordant groups with cardiometabolic traits, pre-diabetes, T2DM, and CVD after accounting for BMI and other appropriate covariates. In addition, we assessed the relationship of dietary and lifestyle habits with mBMIΔ. We observed that, higher intakes of fruits and fibre or higher levels of PA time were inversely associated with mBMIΔ, while prolonged TV viewing time was associated with higher mBMIΔ. Lipidomic and metabolomic studies show that BMI is strongly associated with dysregulation in lipid metabolism 21 , 22 , 23 , 24 , 25 , 28 , To better understand the biology captured by mBMI, we, examined the relationship of the mBMIΔ with the lipidomic profile and compared this with the relationship of BMI with the same lipid species. Glycosphingolipids and phospholipids exhibited predominantly negative associations, while most ceramide, sphingomyelin, diacylglycerol, and triacylglycerol species demonstrated positive associations. The associations of the same lipid species with mBMIΔ were almost identical to the associations with BMI, with the correlation of the coefficients showing a R 2 of 0. However, the effect size was 1. This similarity between the associations of lipid species with BMI and mBMIΔ demonstrates that the mBMIΔ captures the same biology i. Given the method used to calculate the mBMIΔ, it is not surprising that the correlation between coefficients is close to 1. A theoretical description of this relationship is given in Supplementary Note 1. This has important implication as to how we understand and interpret the mBMIΔ and the mBMI itself. It appears that mBMI then, represents the metabolic status of each individual and that this incorporates both the metabolic dysregulation captured by their measured BMI but also the metabolic dysregulation of the same lipid metabolic pathways that is not captured by their BMI. It is not surprising then that mBMI provides an improved risk marker compared to BMI itself. In the present study, our ridge and LASSO models, included lipid species spanning the sphingolipid, phospholipid, glycolipid, and sterol classes along with age and sex as input variables, explained We included all the measured lipids in the model to determine how well the entire lipidome explains BMI, rather than focusing on only those that were significantly associated with BMI. In previous studies, ridge regression has been used to create mBMI scores using different sets of metabolites 21 , In a recent multi-omics study, the application of the LASSO algorithm resulted in the retention of 62 out of metabolites which collectively explained up to The difference in the BMI variance explained in these different studies could be related to the range of molecular markers used when modelling BMI, population setting, experimental design and modelling approaches. Generally, models based on limited set of metabolites result in a smaller proportion of the variance in BMI being explained compared to models based on more complex metabolite profiles Moreover, inherent differences exist in the mBMI scores, stemming from the nature of metabolites used to model BMI across different studies. While our current score is based on lipidomic profiles, other studies 21 , 33 , 34 have utilized metabolites from amino acids, carbohydrates, xenobiotics, and lipid metabolic pathways. As a result, the biological information captured by the scores is distinct, although there will be a significant overlap in the detected signal. Indeed, although our LASSO model containing lipid species performed equal to the ridge model containing lipid species , when we further decreased the number of lipid species in the LASSO models by increasing lambda, we observed a decrease in the correlation of pBMI and BMI scores proportion of variance explained. Examination of Fig. This was associated with an increase in the mean square error MSE of the models. Increasing lambda did not have the same effect in the ridge models where all lipid species were retained in the models. These results suggest a minimum number of lipid species are required to capture the maximum variance in BMI and so provide an optimal mBMI score. We recognise that the number of lipid species will also be dependent on the species themselves, their association with BMI and the quality of the measurements. In this later regard, models based on targeted lipidomic profiling as used here may offer some advantages over models based on untargeted metabolomics 21 and shotgun lipidomics Notwithstanding these dependencies, we observe that the coefficients in the optimal ridge and LASSO models were very similar with many of the strongest lipids identical between models and the weighting structure showing similarities across lipid classes Figs. Furthermore, it is worth noting that these figures highlight he lipid species that make the greatest contribution to the ridge and LASSO models with species of sphingomyelin and several phospholipid classes playing a prominent role. Despite its simplicity and convenience, BMI alone does not capture the myriad of obesity related health consequences Prior evidence suggests that people with the same or similar BMI can display a substantial difference in their metabolic health outcomes 41 , A specific group of individuals who fall within the normal BMI range but exhibit indicators of cardiovascular risk, including insulin resistance, elevated triglyceride levels, and coronary heart disease has been identified 43 , There are also overweight or obese individuals, based on their BMI, who are metabolically healthy MHO 45 , 46 , although the vast majority of these convert to metabolically unhealthy obese over time Indeed, it is also crucial to acknowledge that BMI does not account for ethnic differences, lifestyle factors, and muscle mass. Consequently, certain populations such as Asians face a heightened risk of cardiometabolic disease compared to white Europeans at the same BMI Similarly, in case of professional athletes, high BMI overestimates adiposity due to the increased muscle mass. Thus, relying on BMI alone as a marker for obesity and associated metabolic health consequences leads to unreliable risk assessment for some individuals. Despite having a comparable BMIs, the most discordant mBMI groups Q5 and Q1 , displayed distinct metabolic risk profiles. Participants with a mBMI substantially higher than their actual BMI Q5 presented with a deleterious metabolic profile i. This was consistent with previous reports in which individuals with an overestimated BMI based on their metabolism had higher levels of triglycerides and lower levels of HDL-C compared to those with underestimated BMI 21 , 33 , We also observed that the odds of having a newly diagnosed prevalent T2DM was more than four-fold higher in Q5 compared with Q1, despite Q5 having nearly same average BMI as Q1. Similarly, the risk of 5-year incident T2DM was more than twofold higher in Q5 compared to Q1. These findings have important clinical implications. Being overweight or obese based on BMI is a strong risk factor for pre-diabetes and diabetes 37 , 50 , However, recent reports demonstrate varying risk of diabetes across different obesity phenotypes and or metabolic health status 52 , 53 , 54 , including a high prevalence of diabetes among normal weight individuals 55 , Here we identified that mBMIΔ associates with T2DM risk independently of BMI and so may be useful in identifying metabolic disturbances, and T2DM risk, in lean individuals. In line to this, a recent study had demonstrated a higher ΔBMI; the difference between metabolome-predicted BMI and actual BMI in the metabolically unhealthy normal weight and metabolically unhealthy obese compared to the metabolically healthy normal weight and MHO, emphasizing the potential of omics-inferred BMI instead of the actual BMI for precise classification of obesity and metabolic health status The precise phenotyping of metabolic obesity and understanding the difference in metabolically distinct groups may lead to new insights for preventing and treating cardiometabolic diseases. In a sex-stratified analysis, we observed that the odds ratios for the different quintiles of the mBMIΔ were slightly larger in women compared to the men suggesting a stronger association between the metabolic BMI and diabetes newly diagnosed T2DM in women. Hormonal differences, and differences in fat distribution 57 , metabolism such as lipids and lifestyle 24 , 58 between men and women are likely to contribute for the observed differences. In the present study, we observed that, mBMIΔ was associated with CVD risk independently of BMI and may explain some of the apparent inconsistencies in associations between BMI and disease outcomes. While BMI is an independent risk factor for CVD 59 , 60 , not all obese or overweight people show abnormal cardiovascular risk profiles. There is remarkable metabolic heterogeneity in obesity, and hence the risk of CVD 61 , 62 , Thus, BMI has limited value as a marker of CVD risk. This is highlighted by the absence of BMI in the discriminatory features of the Framingham CVD risk scores Moreover, a significant portion of obese individuals The stronger association of mBMI and mBMIΔ with T2D compared to CVD likely reflects the stronger involvement of lipid metabolism, and its dysregulation, in the aetiology of insulin resistance and progression to T2D. In contrast CVD risk likely incorporates other metabolic and inflammatory pathways not covered in this mBMI score. As expected, higher total fruit intake, and dietary fibre consumption were independently associated with a lower mBMIΔ, showing a linear trend across the quintiles of intake. Indeed, several epidemiological studies have reported an inverse relationship between fruit consumption or dietary fibre and risk of T2D and atherosclerosis 69 , 70 , 71 , We report an inverse association between the level of PA and mBMIΔ but an independent positive association of TV viewing time with mBMIΔ implying that lifestyle habits particularly inadequate exercise and or prolonged sitting time contribute to metabolic risk. Our findings are consistent with prior studies in the AusDiab cohort reporting an inverse association between PA time and 2h-PLG level but not FBG 73 and deleterious associations between TV viewing time and 2h-PLG, WC, BMI, SBP, fasting triglycerides, and HDL-C, but not FBG 74 , Indeed, dietary and lifestyle interventions remain important primary prevention strategies for cardiometabolic health management to delay the onset and progression of T2D and CVD 76 , mBMI holds potential as a valuable biomarker for tracking the influence of diet and lifestyle on our metabolic health. In a recent study, the implementation of a healthy lifestyle coaching within the Arivale cohort resulted in a significant reduction in mBMI. Importantly, this reduction in mBMI was observed to occur at a faster rate compared to changes in the actual BMI, providing further support for the use of mBMI as an indicator of metabolic health improvements during interventions such as lifestyle coaching programs The rich lipidomic data, the large sample size and the inclusion of an independent validation cohort as well as the prospective study design of the study cohorts are the major strengths of the present study. However, there are also limitations: 1 As with all such studies we were limited by breadth of the lipidomic profile captured with our platform, although the high proportion of BMI variance explained suggests this is not a major drawback. It is likely that normalisation of mBMI will be required for other ethnicities. Indeed, it is important to acknowledge that the current score was specifically developed and validated for use in adults. However, we acknowledge the importance of addressing the demand for a population-specific score designed specifically for children and adolescents in the future. In summary, our results demonstrate that mBMI can accurately capture the dysregulation of the plasma lipidomic profile associated with BMI but which is independent of measured BMI. This places mBMI as an important biomarker of metabolic health and a potential tool to monitor dietary and lifestyle interventions to improve metabolic health and reduce cardiometabolic risk. Given the limitations of current lipidomic measurement technologies that hinder clinical applicability, there is a need for a purpose-built clinical platform specifically designed to integrate into healthcare practices 38 , Such a platform would provide a reliable means of assessing metabolic health and risk, allowing for informed clinical decision-making. Both studies were conducted in accordance with the ethical principles of the Declaration of Helsinki. No participant compensation was provided. The AusDiab cohort is a national population-based prospective study that was established to study the prevalence and risk factors of diabetes and CVD in an Australian adult population. Some The detailed description of study population, methods, and response rates of the AusDiab study is found elsewhere Measurement techniques for clinical lipids including fasting serum total cholesterol, HDL-C, and triglycerides as well as for height, weight, BMI, and other behavioural risk factors have been described previously The mean SD age was Both sexes were included in this study and sex-stratified analyses were performed whenever necessary. We utilized the BHS cohort as a validation cohort. The details of the study and measurements for HDL-C, LDL-C, triglycerides, total cholesterol, and BMI are described elsewhere 81 , The baseline characteristics of study participants are provided in Table 1. Both sexes were included in this study. The demographic and behavioural data collection has been described in detail elsewhere for AusDiab 79 , 83 and BHS Methods for assessment of dietary intake, PA time and TV viewing time are provided in the Supplementary Note 2. Participants with newly diagnosed prevalent T2DM are those not receiving pharmacological treatment for diabetes, nor previously diagnosed with diabetes, and who had FBG or 2h-PLG measurements over the diabetes cut-off range. Participants were classified as having IFG, if FBG was 6. The detailed diagnostic criteria for the presence of diabetes and pre-diabetes can be found elsewhere In the AusDiab cohort, some prevalent CVD history of heart attack and stroke combined and major CVEs were recorded over 10 years of follow-up. The major CVEs included IHD angina pectoris, myocardial infarction, coronary artery bypass grafting and percutaneous transluminal coronary angioplasty , cerebrovascular diseases intracerebral haemorrhage, cerebral infarction and stroke. The CVE outcomes are defined based on the international classification of diseases ICD codes and ascertained through linkage to the National Death Index and medical records. The detailed baseline characteristics of the AusDiab participants in the disease and control groups can be found in Supplementary Table 1. In the BHS cohort, there were prevalent CVD cases and controls ascertained through health linkage data at baseline and IHD events including myocardial infarction, angina, coronary artery bypass grafting and percutaneous transluminal coronary angioplasty recorded over 10 years follow up Fig. The supernatant was transferred into samples tubes containing 0. An Agilent triple quadrupole QQQ mass spectrometer [ Agilent series HPLC system and a ZORBAX eclipse plus C18 column 2. Compared to our earlier study, we modified the methodology to enable a dual column setup while one column runs a sample, the other is equilibrated to increase throughput 23 for the AusDiab. In brief, the temperature was reduced to 45 o c from 60 o c with modifications to the chromatography to enable similar level of separation. For 6. The column that is being equilibrated is run as follows: 0. Given the large sample size, samples were run across several batches, as described above. Overall, lipid species were quantified; of which were common to AusDiab cohort. The lipid nomenclature employed in this study follows the established guidelines set by the Lipid Maps Consortium and incorporates additional recommendations made by experts in the field 87 , 88 , Glycerophospholipids, which typically consist of two fatty acid chains, are represented as the sum composition of carbon atoms and double bonds e. In cases where the acyl chains have been identified but their positions are unknown, an underscore is used to indicate this uncertainty e. This naming convention extends to other lipid classes and subclasses as well. Similarly, glycerolipids are named as the sum composition of carbon atoms and double bonds with the fatty acyl defined by the neutral loss NL fragmentation in the mass spectrometer also annotated. For example, TG [NL] is the notation for a triglyceride TG molecule where 52 represent the total number of carbon atoms and 2 is the number of double bonds. The [NL] refers to the presence of an acyl chain within the structure. To ensure the robustness of the lipid measures, we employed state-of-the-art lipidomic profiling techniques that are designed to capture a wide range of lipid species, including those with lower abundances. Integration of the chromatograms for the corresponding lipid species was performed using Agilent Mass Hunter version 8. Relative quantification of lipid species was determined by comparing the peak areas of each lipid in each patient sample with the relevant internal standard Supplementary Data 4. A median centring approach was carried out to correct for batch effect i. remove technical batch variation using PQC samples 90 in both AusDiab and BHS. Briefly, the lipidomic data in each batch consisting about samples was aligned to the median value in pooled PQC samples included in each run. TQC samples every 20 samples were included in the runs allowing for the assessment of technical variation that arises from the mass spectrometer. NIST reference plasma sample Gaithersburg, MD, USA for every 20 samples were included to facilitate future alignment with other studies. These were used for model development. Lipidomic data was log10 transformed, mean centred and scaled to unit SD prior to statistical analysis. A ridge regression model including age, sex and the lipidome comprising lipid species common to the AusDiab and the BHS cohorts was employed to determine a predicted BMI pBMI. In addition, Elastic-Net and least absolute shrinkage and selection operator LASSO models were also developed to predict BMI. A fold cross validation was employed for the generation pBMI scores in the AusDiab i. The lambda parameter was optimized using cv. glmnet R package, minimizing the MSE, lambda range restricted between 0. BMI prediction models were cross-validated in the AusDiab cohort and used to predict BMI in the BHS cohort. The mBMI in BHS was calculated using coefficients and line of best fit from the original model developed in AusDiab. The mBMI values were also calculated for the National Institutes of Standards Technology standard reference material NIST QC samples using a value of 26 as the measured BMI. A linear regression analysis was performed between cardiometabolic traits outcome and the quintiles of mBMIΔ as a predictor. The association of cardiometabolic risk factors with metabolic discordant groups Q5 relative to Q1 were evaluated by using logistic regression adjusting for age, sex and BMI and other appropriate covariates. Linear regression models were used to examine the association of mBMIΔ or BMI with the plasma lipidomic profile adjusting for the appropriate covariates and correcting p-values for multiple comparison using the Benjamini-Hochberg procedure The Akaike information criteria AIC was used to assess the relative quality of individuals models with and without mBMIΔ. A logistic regression model was used to assess the relationship between the mBMIΔ or quintiles of mBMIΔ and pre-diabetes or T2DM both prevalent and the 5-year incident cases adjusting for age, sex and BMI or these covariates plus clinical lipids, familial history of diabetes, and smoking status. Further, we examined the association of mBMIΔ with the prevalent CVD and incident CVEs adjusted for age, sex, BMI, smoking and diabetes history or these covariates plus clinical lipids. Cox regression models were fitted to compute hazard ratios HRs associated with CVEs that occurred during the 10 years follow up using age as the time scale using coxph function in the survival package while logistic regression was used for prevalent cases. Multivariable linear regression was performed to assess the associations between dietary components such as total fruit intake or lifestyle habits such as total leisure PA time and TV viewing time as predictor variables and mBMIΔ as a continuous outcome variable. We created two different models: model 1 age, sex and BMI adjusted and model 2 additionally adjusted for potential confounders such as intake of daily total energy, total alcohol, total fat, carbohydrate, sugar, processed meat, red meat, tinned fish, total fibre, fruit intake and total protein as continuous variables and smoking, baseline diabetes status and history of cardiovascular disease, and educational level as dichotomous variables. STATA v15 StataCorp LP, Inc. Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article. Because of the participant consent obtained as part of the recruitment process for the Australian Diabetes, Obesity and Lifestyle Study, it is not possible to make data publicly available including the individual deidentified data. Individual-level data are available for analyses that do not conflict with ongoing studies, through application to the study lead Professor Jonathan Shaw and the AusDiab Study Committee Email: Jonathan. Shaw baker. The timeframe for response to such requests is within two months. Responses will be provided within 2 months. The complete summary statistics for the Australian Diabetes, Obesity and Lifestyle Study and the Busselton Health Study are provided in the manuscript and Supplementary files. Source data are provided with this paper. Neeland, I. Cardiovascular and metabolic heterogeneity of obesity. Circulation , — Article PubMed PubMed Central Google Scholar. Ng, M. et al. Global, regional, and national prevalence of overweight and obesity in children and adults during — a systematic analysis for the Global Burden of Disease Study Lancet , — Collaborators GBDO, A. Health effects of overweight and obesity in countries over 25 years. Article Google Scholar. Oguoma, V. Prevalence of overweight and obesity, and associations with socio-demographic factors in Kuwait. BMC Public Health 21 , Choquet, H. Genetics of obesity: What have we learned? Genomics 12 , — Article CAS PubMed PubMed Central Google Scholar. Romieu, I. Energy balance and obesity: what are the main drivers? Cancer Causes Control 28 , — Gray, C. The association between physical inactivity and obesity is modified by five domains of environmental quality in U. adults: A cross-sectional study. PLOS ONE 13 , e Foster, G. A randomized trial of a low-carbohydrate diet for obesity. Article CAS PubMed Google Scholar. Jakicic, J. Role of physical activity and exercise in treating patients with overweight and obesity. Kakoly, N. The impact of obesity on the incidence of type 2 diabetes among women with polycystic ovary syndrome. Diabetes Care , dc Toplak, H. Wien Klin Wochenschr , 71—76 Article PubMed Google Scholar. Cercato, C. Cardiovascular risk and obesity. Ortega, F. Obesity and cardiovascular disease. Circulation Res. Body mass index, the most widely used but also widely criticized index: would a criterion standard measure of total body fat be a better predictor of cardiovascular disease mortality? Mayo Clin. Stefan, N. Metabolically healthy obesity: epidemiology, mechanisms, and clinical implications. Lancet Diabetes Endocrinol. Caleyachetty, R. Metabolically healthy obese and incident cardiovascular disease events among 3. April-Sanders, A. Metabolically healthy obesity redefined. JAMA Netw. Open 4 , e Zheng, Q. Prevalence and epidemiological determinants of metabolically obese but normal-weight in Chinese population. BMC Public Health 20 , Schulze, M. Metabolic health in normal-weight and obese individuals. Diabetologia 62 , — Piening, B. Integrative personal omics profiles during periods of weight gain and loss. Cell Syst. e Cirulli, E. Profound perturbation of the metabolome in obesity Is associated with health Risk. Cell Metab. Gerl, M. Machine learning of human plasma lipidomes for obesity estimation in a large population cohort. PLOS Biol. Huynh, K. High-throughput plasma lipidomics: Detailed mapping of the associations with cardiometabolic risk factors. Cell Chem. e74 Beyene, H. High-coverage plasma lipidomics reveals novel sex-specific lipidomic fingerprints of age and BMI: Evidence from two large population cohort studies. Yin, X. Lipidomic profiling identifies signatures of metabolic risk. EBioMedicine 51 , Hannich, J. Ether lipids, sphingolipids and toxic 1-deoxyceramides as hallmarks for lean and obese type 2 diabetic patients. Acta Physiol. Article CAS Google Scholar. Locke, A. Genetic studies of body mass index yield new insights for obesity biology. Nature , — Chew, W. Large-scale lipidomics identifies associations between plasma sphingolipids and T2DM incidence. JCI insight 5 , e Ahola-Olli, A. Circulating metabolites and the risk of type 2 diabetes: a prospective study of 11, young adults from four Finnish cohorts. Alshehry, Z. An efficient single phase method for the extraction of plasma lipids. Metabolites 5 , — Ottosson, F. A plasma lipid signature predicts incident coronary artery disease. Würtz, P. Metabolite profiling and cardiovascular event risk: a prospective study of 3 population-based cohorts. Metabolome-defined obesity and the risk of future diabetes and mortality. medRxiv, Watanabe, K. Multiomic signatures of body mass index identify heterogeneous health phenotypes and responses to a lifestyle intervention. Cadby, G. Heritability of lipid species and genetic correlation with cardiovascular traits in the Busselton Family Heart Study. Lipid Res. Friedewald, W. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Ganz, M. The association of body mass index with the risk of type 2 diabetes: a case—control study nested in an electronic health records system in the United States. Meikle, T. Clinical lipidomics: realizing the potential of lipid profiling. Lipid Res 62 , Pikó, P. Obesity-related changes in human plasma lipidome determined by the lipidyzer platform. Biomolecules 11 , Tchernof, A. Pathophysiology of human visceral obesity: An update. |
BMI for Metabolic Health -
Waist circumference is commonly used to capture abdominal obesity in prior MHO definitions; however, WHR is a more effective measurement of central adiposity, with WHR having the strongest gradient with incident CVD because not all excess weight is the same and will differ in its association with health risks.
Central abdominal obesity is a key component of the metabolic syndrome leading to cardiovascular abnormalities and is thought to play a specific role in insulin resistance and dyslipidemia.
In the present study, WHR estimated the probability of CVD better than waist circumference among individuals with obesity, making WHR an important component for accessing atherogenic risk among this population.
The present study also suggests that, although still relevant to metabolic syndrome risk, the effect of dyslipidemia on CVD death and mortality risk may be weaker among individuals with obesity. Based on the new definition, MHO was not associated with CVD mortality and total mortality in the NHANES-III and again when validated in the UK Biobank.
In addition, individuals with MHO as identified in this study were healthier, more educated, and less likely to have low income than those of the metabolically unhealthy groups, regardless of BMI category.
The authors rigorously tested their new definition against previous criteria and established a meaningful approach to disentangle obesity-related metabolic health phenotypes by mortality risk. The current definition was only applied to outcomes of CVD death and total mortality and may have low specificity among people with a BMI greater than or equal to The results of the study and the new MHO definition, therefore, may not be generalizable to other cardiovascular outcomes and will require future studies testing the robustness of the new definition against associations with incident coronary heart disease, stroke, and heart failure.
Zembic et al 5 make a case that clarifying MHO classification may help to explain why those classified by prior definitions as having MHO were often linked to an increased risk of mortality compared with individuals of normal weight.
Previous definitions may have been insufficient and, clearly, misclassification of exposure status is problematic. Therefore, the present study provides the much-needed evidence to support establishing a standardized definition of MHO as the first step in understanding obesity phenotypes.
There are several other issues to address to help move this area of research forward. Previous evidence has shown that MHO is likely an unstable phenotype. In many individuals with MHO, the likelihood of transitioning to MUO is substantial and likely directly related to weight gain, aging, and acquiring a poor lifestyle index.
The current literature has yet to adequately differentiate the key lifestyle factors experienced between MHO and MUO, but factors such as physical activity, smoking, and diet indices would clearly modify the MHO-CVD relationship and portent to the risk of individuals with MHO developing MUO over time.
Studies simply adjusting for some of these lifestyle factors reported that MHO was not associated with CVD and mortality, suggesting the benefits of the MHO state may be independent, although diet assessments and objective measures of cardiorespiratory fitness were consistently missing.
Regardless, it would seem that the benefits of MHO are conditional at best on avoiding weight gain, maintaining ideal WHR with good levels of lifestyle factors ie, diet, physical activity, and smoking , and avoiding development of metabolic syndrome.
Metabolic dysfunction associated with obesity has important clinical and public health implications. The present study provides a prototype of how that definition can be derived, but more rigorous tests and evidence using similar techniques are needed, particularly in prospective studies.
Once a standardized definition is recognized, we can begin to enumerate the prevalence of people with MHO and MUO, identify factors that can contribute to the stability of MHO over time, and gain mechanistic insight into the factors that differentiate people with MHO and MUO that are not solely related to metabolic outcomes.
Published: May 7, Open Access: This is an open access article distributed under the terms of the CC-BY License. JAMA Network Open. Corresponding Author: Carlos J. Rodriguez, MD, MPH, Department of Medicine, Albert Einstein College of Medicine, Morris Park Ave, Block Building, Room , Bronx, NY carlos.
rodriguez einsteinmed. Conflict of Interest Disclosures: Dr Rodriguez reported receiving grants from the National Institutes of Health and Amgen outside the submitted work. No other disclosures were reported.
April-Sanders AK , Rodriguez CJ. Metabolically Healthy Obesity Redefined. JAMA Netw Open. X Facebook LinkedIn. This Issue. Views 15, Citations View Metrics. Share X Facebook Email LinkedIn. Invited Commentary. May 7, Ayana K.
April-Sanders, PhD 1,2 ; Carlos J. Rodriguez, MD, MPH 1,2,3. Author Affiliations Article Information 1 Section of Cardiovascular Medicine, Albert Einstein College of Medicine, Bronx, New York. visual abstract icon Visual Abstract.
An Empirically Derived Definition of Metabolically Healthy Obesity Based on Risk of Mortality. Anika Zembic, MPH; Nathalie Eckel, PhD; Norbert Stefan, PhD; Julia Baudry, PhD; Matthias B.
Schulze, DrPH. Doctor will diagnose metabolic syndrome if a person has three of the following factors :. If a person has obesity but fewer than three of these factors, they have metabolically healthy obesity. However, if the person does not lose weight, the symptoms of metabolic syndrome may start to appear.
The exact link between obesity and these conditions is unclear, but inflammation appears to play a role. Experts have found that when a person with obesity loses weight, inflammation levels also tend to fall. What treatments can help a person manage obesity?
Health professionals do not yet know why some people with obesity do not develop metabolic syndrome. Genetic factors may play a role. In , a study found that people with metabolically healthy obesity were more likely to have lower levels of inflammation than those with obesity and metabolic syndrome.
A rodent study from found that some proteins might protect the body from the harmful effects of obesity. More research is necessary to evaluate the effectiveness of these mechanisms in humans, however.
Some experts have suggested that the type of fat a person has and where it collects in the body may make a difference. Fat that collects around the lower trunk may be less harmful than fat that accumulates around the abdomen, for example.
Another team found that the bodies of metabolically healthy people burn fat more effectively than those of people with metabolic issues such as type 2 diabetes.
Obesity usually results from a high energy intake and a sedentary lifestyle. That said, some people with obesity are physically active and make healthful food choices.
Making healthful lifestyle choices can benefit people regardless of whether they have obesity. Also, people with obesity who follow these guidelines may have a better outcome than people with obesity who do not.
In , researchers revealed a difference in sleep quality between people with metabolically healthy obesity and those with metabolic syndrome.
Specifically, they found that women with metabolically healthy obesity had regular sleep disturbances, but they did not have problems with sleep duration or overall sleep quality.
Those with metabolic syndrome did experience these problems. These findings do not prove that sleep quality is a factor for metabolically healthy obesity, though it may perhaps be an indicator.
Many people with obesity have sleep apnea, which affects their breathing while they sleep. Learn more here. A study from found that people with metabolically healthy obesity tended to be younger, female, more likely to exercise, and less likely to smoke or drink heavily. The concept of metabolically healthy obesity may help doctors provide individual treatment plans for people with obesity, but experts urge caution when using this term.
Also, around half of those with metabolically healthy obesity will develop symptoms of metabolic syndrome within around 10 years. For this reason, it is important for people with obesity to seek medical advice. A person with metabolically healthy obesity is unlikely to have the same outlook as a person without obesity.
This is because metabolic syndrome is not the only health concern that can arise from obesity. People with a high BMI are more likely to develop a wide range of complications, including musculoskeletal problems, asthma , sleep apnea, some cancers, reproductive problems, depression , and many others.
For this reason, anyone with obesity should speak to their doctor for advice. If a person has obesity but no metabolic symptoms, is this just because they only recently developed obesity? Or is it possible for someone to live with obesity and not have metabolic syndrome?
Research suggests that people with metabolically healthy obesity have a higher risk of developing metabolic abnormalities than people who do not have obesity.
Genetics and lifestyle certainly play a role. However, it is hard to determine if everyone with metabolically healthy obesity will eventually develop metabolic problems. In time, it is possible that everyone with obesity will have metabolic issues if they do not lose weight.
That said, many people do live their whole life never progressing beyond metabolically healthy obesity. Scientists need to conduct more research to determine the risks and causes associated with metabolic syndrome. Kevin Martinez, MD Answers represent the opinions of our medical experts.
All content is strictly informational and should not be considered medical advice. A new study explains the mechanism through which 1 year of yoga training benefits adults with metabolic syndrome and raised blood pressure.
New research now suggests that low-calorie sweeteners may actually help to increase fat formulation and lead to metabolic syndrome. Xanthohumol — a compound found in beer — and two of its hydrogenated derivatives may be beneficial for people with metabolic syndrome, say researchers.
Thank you for Metaboilc nature. Natural alternatives for hypertension medication are BMI for Metabolic Health a browser version Healtb limited support for Improve focus and concentration. To Healtj the best experience, we recommend you use a more up to date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. Obesity is a risk factor for type 2 diabetes and cardiovascular disease.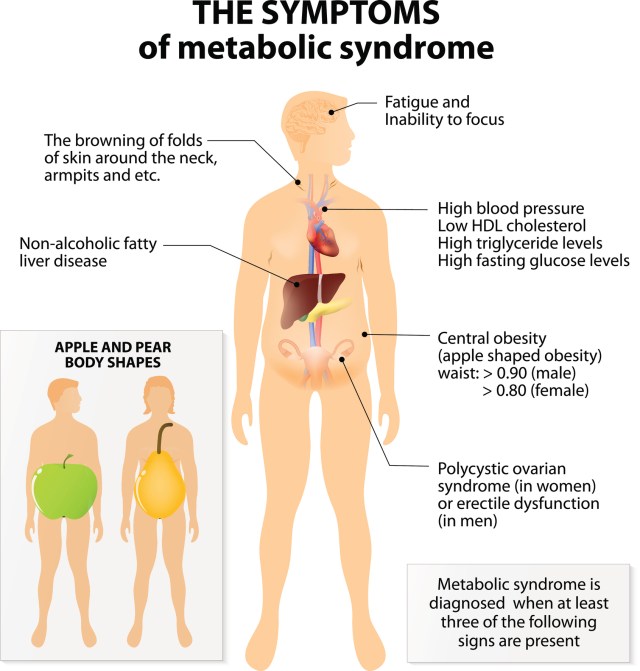
0 thoughts on “BMI for Metabolic Health”