
Fat metabolism and energy production -
Put another way, if the human body relied on carbohydrates to store energy, then a person would need to carry 31 kg Hibernating animals provide a good example for utilization of fat reserves as fuel. For example, bears hibernate for about 7 months, and during this entire period, the energy is derived from degradation of fat stores.
Migrating birds similarly build up large fat reserves before embarking on their intercontinental journeys. The fat stores of young adult humans average between about 10—20 kg, but vary greatly depending on gender and individual disposition.
The g or so of glycogen stored in the liver is depleted within one day of starvation. Fatty acids are broken down to acetyl-CoA by means of beta oxidation inside the mitochondria, whereas fatty acids are synthesized from acetyl-CoA outside the mitochondria, in the cytosol.
The two pathways are distinct, not only in where they occur, but also in the reactions that occur, and the substrates that are used. The two pathways are mutually inhibitory, preventing the acetyl-CoA produced by beta-oxidation from entering the synthetic pathway via the acetyl-CoA carboxylase reaction.
During each turn of the cycle, two carbon atoms leave the cycle as CO 2 in the decarboxylation reactions catalyzed by isocitrate dehydrogenase and alpha-ketoglutarate dehydrogenase.
Thus each turn of the citric acid cycle oxidizes an acetyl-CoA unit while regenerating the oxaloacetate molecule with which the acetyl-CoA had originally combined to form citric acid. The decarboxylation reactions occur before malate is formed in the cycle.
However, acetyl-CoA can be converted to acetoacetate, which can decarboxylate to acetone either spontaneously, or catalyzed by acetoacetate decarboxylase. Acetol can be converted to propylene glycol. This converts to pyruvate by two alternative enzymes , or propionaldehyde , or to L -lactaldehyde then L -lactate the common lactate isomer.
The first experiment to show conversion of acetone to glucose was carried out in This, and further experiments used carbon isotopic labelling. The glycerol released into the blood during the lipolysis of triglycerides in adipose tissue can only be taken up by the liver.
Here it is converted into glycerol 3-phosphate by the action of glycerol kinase which hydrolyzes one molecule of ATP per glycerol molecule which is phosphorylated.
Glycerol 3-phosphate is then oxidized to dihydroxyacetone phosphate , which is, in turn, converted into glyceraldehyde 3-phosphate by the enzyme triose phosphate isomerase. From here the three carbon atoms of the original glycerol can be oxidized via glycolysis , or converted to glucose via gluconeogenesis.
Fatty acids are an integral part of the phospholipids that make up the bulk of the plasma membranes , or cell membranes, of cells. These phospholipids can be cleaved into diacylglycerol DAG and inositol trisphosphate IP 3 through hydrolysis of the phospholipid, phosphatidylinositol 4,5-bisphosphate PIP 2 , by the cell membrane bound enzyme phospholipase C PLC.
One product of fatty acid metabolism are the prostaglandins , compounds having diverse hormone -like effects in animals. Prostaglandins have been found in almost every tissue in humans and other animals.
They are enzymatically derived from arachidonic acid, a carbon polyunsaturated fatty acid. Every prostaglandin therefore contains 20 carbon atoms, including a 5-carbon ring. They are a subclass of eicosanoids and form the prostanoid class of fatty acid derivatives.
The prostaglandins are synthesized in the cell membrane by the cleavage of arachidonate from the phospholipids that make up the membrane. This is catalyzed either by phospholipase A 2 acting directly on a membrane phospholipid, or by a lipase acting on DAG diacyl-glycerol.
The arachidonate is then acted upon by the cyclooxygenase component of prostaglandin synthase. This forms a cyclopentane ring roughly in the middle of the fatty acid chain.
The reaction also adds 4 oxygen atoms derived from two molecules of O 2. The resulting molecule is prostaglandin G 2 , which is converted by the hydroperoxidase component of the enzyme complex into prostaglandin H 2.
This highly unstable compound is rapidly transformed into other prostaglandins, prostacyclin and thromboxanes. If arachidonate is acted upon by a lipoxygenase instead of cyclooxygenase, Hydroxyeicosatetraenoic acids and leukotrienes are formed.
They also act as local hormones. Prostaglandins have two derivatives: prostacyclins and thromboxanes. Prostacyclins are powerful locally acting vasodilators and inhibit the aggregation of blood platelets.
Through their role in vasodilation, prostacyclins are also involved in inflammation. They are synthesized in the walls of blood vessels and serve the physiological function of preventing needless clot formation, as well as regulating the contraction of smooth muscle tissue.
Their name comes from their role in clot formation thrombosis. A significant proportion of the fatty acids in the body are obtained from the diet, in the form of triglycerides of either animal or plant origin. The fatty acids in the fats obtained from land animals tend to be saturated, whereas the fatty acids in the triglycerides of fish and plants are often polyunsaturated and therefore present as oils.
These triglycerides cannot be absorbed by the intestine. The activated complex can work only at a water-fat interface. Therefore, it is essential that fats are first emulsified by bile salts for optimal activity of these enzymes.
the fat soluble vitamins and cholesterol and bile salts form mixed micelles , in the watery duodenal contents see diagrams on the right. The contents of these micelles but not the bile salts enter the enterocytes epithelial cells lining the small intestine where they are resynthesized into triglycerides, and packaged into chylomicrons which are released into the lacteals the capillaries of the lymph system of the intestines.
This means that the fat-soluble products of digestion are discharged directly into the general circulation, without first passing through the liver, unlike all other digestion products.
The reason for this peculiarity is unknown. The chylomicrons circulate throughout the body, giving the blood plasma a milky or creamy appearance after a fatty meal. The fatty acids are absorbed by the adipocytes [ citation needed ] , but the glycerol and chylomicron remnants remain in the blood plasma, ultimately to be removed from the circulation by the liver.
The free fatty acids released by the digestion of the chylomicrons are absorbed by the adipocytes [ citation needed ] , where they are resynthesized into triglycerides using glycerol derived from glucose in the glycolytic pathway [ citation needed ].
These triglycerides are stored, until needed for the fuel requirements of other tissues, in the fat droplet of the adipocyte. The liver absorbs a proportion of the glucose from the blood in the portal vein coming from the intestines. After the liver has replenished its glycogen stores which amount to only about g of glycogen when full much of the rest of the glucose is converted into fatty acids as described below.
These fatty acids are combined with glycerol to form triglycerides which are packaged into droplets very similar to chylomicrons, but known as very low-density lipoproteins VLDL. These VLDL droplets are processed in exactly the same manner as chylomicrons, except that the VLDL remnant is known as an intermediate-density lipoprotein IDL , which is capable of scavenging cholesterol from the blood.
This converts IDL into low-density lipoprotein LDL , which is taken up by cells that require cholesterol for incorporation into their cell membranes or for synthetic purposes e. the formation of the steroid hormones.
The remainder of the LDLs is removed by the liver. Adipose tissue and lactating mammary glands also take up glucose from the blood for conversion into triglycerides. This occurs in the same way as in the liver, except that these tissues do not release the triglycerides thus produced as VLDL into the blood.
All cells in the body need to manufacture and maintain their membranes and the membranes of their organelles. Whether they rely entirely on free fatty acids absorbed from the blood, or are able to synthesize their own fatty acids from blood glucose, is not known.
The cells of the central nervous system will almost certainly have the capability of manufacturing their own fatty acids, as these molecules cannot reach them through the blood brain barrier. Much like beta-oxidation , straight-chain fatty acid synthesis occurs via the six recurring reactions shown below, until the carbon palmitic acid is produced.
The diagrams presented show how fatty acids are synthesized in microorganisms and list the enzymes found in Escherichia coli. FASII is present in prokaryotes , plants, fungi, and parasites, as well as in mitochondria. In animals as well as some fungi such as yeast, these same reactions occur on fatty acid synthase I FASI , a large dimeric protein that has all of the enzymatic activities required to create a fatty acid.
FASI is less efficient than FASII; however, it allows for the formation of more molecules, including "medium-chain" fatty acids via early chain termination. by transferring fatty acids between an acyl acceptor and donor. They also have the task of synthesizing bioactive lipids as well as their precursor molecules.
Elongation, starting with stearate , is performed mainly in the endoplasmic reticulum by several membrane-bound enzymes.
The enzymatic steps involved in the elongation process are principally the same as those carried out by fatty acid synthesis , but the four principal successive steps of the elongation are performed by individual proteins, which may be physically associated.
Abbreviations: ACP — Acyl carrier protein , CoA — Coenzyme A , NADP — Nicotinamide adenine dinucleotide phosphate. Note that during fatty synthesis the reducing agent is NADPH , whereas NAD is the oxidizing agent in beta-oxidation the breakdown of fatty acids to acetyl-CoA.
This difference exemplifies a general principle that NADPH is consumed during biosynthetic reactions, whereas NADH is generated in energy-yielding reactions. The source of the NADPH is two-fold.
NADPH is also formed by the pentose phosphate pathway which converts glucose into ribose, which can be used in synthesis of nucleotides and nucleic acids , or it can be catabolized to pyruvate.
In humans, fatty acids are formed from carbohydrates predominantly in the liver and adipose tissue , as well as in the mammary glands during lactation. The pyruvate produced by glycolysis is an important intermediary in the conversion of carbohydrates into fatty acids and cholesterol.
However, this acetyl CoA needs to be transported into cytosol where the synthesis of fatty acids and cholesterol occurs. This cannot occur directly. To obtain cytosolic acetyl-CoA, citrate produced by the condensation of acetyl CoA with oxaloacetate is removed from the citric acid cycle and carried across the inner mitochondrial membrane into the cytosol.
The oxaloacetate is returned to mitochondrion as malate and then converted back into oxaloacetate to transfer more acetyl-CoA out of the mitochondrion.
Acetyl-CoA is formed into malonyl-CoA by acetyl-CoA carboxylase , at which point malonyl-CoA is destined to feed into the fatty acid synthesis pathway.
Acetyl-CoA carboxylase is the point of regulation in saturated straight-chain fatty acid synthesis, and is subject to both phosphorylation and allosteric regulation. Regulation by phosphorylation occurs mostly in mammals, while allosteric regulation occurs in most organisms.
Allosteric control occurs as feedback inhibition by palmitoyl-CoA and activation by citrate. When there are high levels of palmitoyl-CoA, the final product of saturated fatty acid synthesis, it allosterically inactivates acetyl-CoA carboxylase to prevent a build-up of fatty acids in cells.
Citrate acts to activate acetyl-CoA carboxylase under high levels, because high levels indicate that there is enough acetyl-CoA to feed into the Krebs cycle and produce energy.
High plasma levels of insulin in the blood plasma e. after meals cause the dephosphorylation and activation of acetyl-CoA carboxylase, thus promoting the formation of malonyl-CoA from acetyl-CoA, and consequently the conversion of carbohydrates into fatty acids, while epinephrine and glucagon released into the blood during starvation and exercise cause the phosphorylation of this enzyme, inhibiting lipogenesis in favor of fatty acid oxidation via beta-oxidation.
Disorders of fatty acid metabolism can be described in terms of, for example, hypertriglyceridemia too high level of triglycerides , or other types of hyperlipidemia. These may be familial or acquired. Familial types of disorders of fatty acid metabolism are generally classified as inborn errors of lipid metabolism.
These disorders may be described as fatty acid oxidation disorders or as a lipid storage disorders , and are any one of several inborn errors of metabolism that result from enzyme or transport protein defects affecting the ability of the body to oxidize fatty acids in order to produce energy within muscles, liver, and other cell types.
When a fatty acid oxidation disorder affects the muscles, it is a metabolic myopathy. Moreover, cancer cells can display irregular fatty acid metabolism with regard to both fatty acid synthesis [44] and mitochondrial fatty acid oxidation FAO [45] that are involved in diverse aspects of tumorigenesis and cell growth.
Contents move to sidebar hide. Article Talk. Read Edit View history. Tools Tools. What links here Related changes Upload file Special pages Permanent link Page information Cite this page Get shortened URL Download QR code Wikidata item. Download as PDF Printable version. Set of biological processes.
Main article: Fatty acid synthesis. Main article: Citric acid cycle § Glycolytic end products are used in the conversion of carbohydrates into fatty acids.
In: Biochemistry Fourth ed. New York: W. Freeman and Company. ISBN doi : PMID S2CID Pflügers Archiv: European Journal of Physiology. Molecular Aspects of Medicine. PMC Jul J Neurosci.
Feb J Cereb Blood Flow Metab. Biochemistry Fourth ed. Donald; Stafstrom, Carl E. ISSN Molecular Genetics and Metabolism. W; Koeslag, J. European Journal of Applied Physiology.
Toxicol Appl Pharmacol. Invited review. Nigerian Journal of Physiological Science. Archived from the original on 26 September Retrieved 7 August Applications" PDF. Biotechnology and Bioengineering. Ann NY Acad Sci. Bibcode : NYASA. Vander Jagt; B.
Robinson; K. Taylor; L. Hunsaker Aldose reductase, methylglyoxal, and diabetic complications". This reserve is not large, and during overnight fasting glycogen reserves fall severely. However, only the liver supplies the blood with glucose since it has an enzyme that make it possible for glucose molecules to be transported across cell membranes.
Since glycogen stores are limited and are reduced within hours of fasting, and blood glucose concentration is kept within narrow limits under most physiological conditions, another mechanism must exist to supply blood glucose.
Indeed, glucose can be synthesized from amino acid molecules. This process is called de novo synthesis of glucose, or gluconeogenesis.
Amino acids, while being degraded, generate several intermediates that are used by the liver to synthesize glucose Figure 2. Alanine and glutamine are the two amino acids whose main function is to contribute to glucose synthesis by the liver.
The kidneys also possess the enzymes necessary for gluconeogenesis and, during prolonged fasting, contribute to some extent to the supply of blood glucose.
Furthermore, since de novo glucose synthesis comes from amino acid degradation and the depletion of protein stores can be life-threatening, this process must be regulated. Insulin, glucagon, and another hormone, glucocorticoid, play important roles in controlling the rate of protein degradation and, therefore, the rate of glucose production by the liver.
Alterations in factors that control food intake and regulate energy metabolism are related to well-known pathological conditions such as obesity, type 2 diabetes and the metabolic syndrome , and some types of cancer.
In addition, many effects and regulatory actions of well-known hormones such as insulin are still poorly understood. The consideration of adipose tissue as a dynamic and active tissue, for instance, raises several important issues regarding body weight and the control of food intake.
These factors point to the importance of further studies to expand our understanding of energy metabolism, thereby improving our quality of life and achieving a comprehensive view of how the human body functions. Cahill, G.
Fuel metabolism in starvation. Annual Review of Nutrition 26 , 1—22 Iyer, A. Inflammatory lipid mediators in adipocyte function and obesity. Nature Reviews Endocrinology 6 , 71—82 Kaelin, W.
Kodde, I. Metabolic and genetic regulation of cardiac energy substrate preference. Kresge, N. Otto Fritz Meyerhof and the elucidation of the glycolytic pathway. Journal of Biological Chemisry , e3 Kroemer, G. Tumor cell metabolism: Cancer's Achilles' heel. Cancer Cell 13 , — Vander Heiden, M.
Understanding the Warburg effect: The metabolic requirements of cell proliferation Science 22 , — van der Vusse, G. Critical steps in cellular fatty acid uptake and utilization. Molecular and Cellular Biochemistry , 9—15 What Is a Cell?
Eukaryotic Cells. Cell Energy and Cell Functions. Photosynthetic Cells. Cell Metabolism. The Two Empires and Three Domains of Life in the Postgenomic Age. Why Are Cells Powered by Proton Gradients?
The Origin of Mitochondria. Mitochondrial Fusion and Division. Beyond Prokaryotes and Eukaryotes : Planctomycetes and Cell Organization. The Origin of Plastids. The Apicoplast: An Organelle with a Green Past. The Origins of Viruses.
Discovery of the Giant Mimivirus. Volvox, Chlamydomonas, and the Evolution of Multicellularity. Yeast Fermentation and the Making of Beer and Wine. Dynamic Adaptation of Nutrient Utilization in Humans. Nutrient Utilization in Humans: Metabolism Pathways. An Evolutionary Perspective on Amino Acids.
Fatty Acid Molecules: A Role in Cell Signaling. Mitochondria and the Immune Response. Stem Cells in Plants and Animals. G-Protein-Coupled Receptors, Pancreatic Islets, and Diabetes. Promising Biofuel Resources: Lignocellulose and Algae.
The Discovery of Lysosomes and Autophagy. The Mystery of Vitamin C. The Sliding Filament Theory of Muscle Contraction. Dynamic Adaptation of Nutrient Utilization in Humans By: Tatiana El Bacha, Ph. Instituto de Bioquímica Médica, Universidade Federal do Rio de Janeiro , Mauricio R. Luz, Ph. Da Poian, Ph.
Instituto de Bioquimica Medica, Universidade Federal do Rio de Janeiro © Nature Education. Citation: El Bacha, T. Nature Education 3 9 Food in, energy out?
Aa Aa Aa. Energy Metabolism and ATP Synthesis in Human Cells. Different Cell Types Require Different Fuel Molecules. The Type of Fuel Molecule Changes according to Cell Function and Physiological Context. Hormones Regulate Cell Metabolism.
The Liver Supplies Blood Glucose. References and Recommended Reading Cahill, G. Annual Review of Nutrition 26 , 1—22 Iyer, A. Nature Reviews Endocrinology 6 , 71—82 Kaelin, W. Journal of Biological Chemisry , e3 Kroemer, G.
Cancer Cell 13 , — Vander Heiden, M. Understanding the Warburg effect: The metabolic requirements of cell proliferation Science 22 , — van der Vusse, G. Article History Close. Share Cancel. Revoke Cancel. Keywords Keywords for this Article. Save Cancel. Flag Inappropriate The Content is: Objectionable.
Flag Content Cancel. share Close. Email your Friend. Submit Cancel. This content is currently under construction. Explore This Subject. Topic rooms within Cell Origins and Metabolism Close.
No topic rooms are there. Lead Editor: Gary Coté , Mario De Tullio Cell Origins and Metabolism. Or Browse Visually. Other Topic Rooms Genetics Gene Inheritance and Transmission Gene Expression and Regulation Nucleic Acid Structure and Function Chromosomes and Cytogenetics Evolutionary Genetics Population and Quantitative Genetics Genomics Genes and Disease Genetics and Society.
Student Voices. Creature Cast. Simply Science. Green Screen. Green Science. Bio 2. The Success Code. Why Science Matters. The Beyond. Plant ChemCast. Postcards from the Universe. Brain Metrics.
Mind Read. Eyes on Environment. Accumulating Glitches. Saltwater Science. Microbe Matters.
Sweet potato and chickpea stew or triglycerides within the body are ingested as ajd Metabolic health events synthesized by adipocytes or hepatocytes from carbohydrate precursors. Poduction metabolism Fat metabolism and energy production the oxidation of fatty acids to either generate energy or synthesize new lipids from smaller constituent molecules. Lipid metabolism is associated with carbohydrate metabolism, as products of glucose such as acetyl CoA can be converted into lipids. Figure 1. A triglyceride molecule a breaks down into a monoglyceride b. Metabolism basically refers Fat metabolism and energy production eneryg the chemical reactions metabolissm the body used to produce energy. This involves a BCAAs and muscle retention set of processes that ptoduction fuels into specialised Leafy greens for soups Metabolic health events with energy. In the body, the primary final agent to produce energy is called adenosine triphosphate ATP. When ATP is broken down or used by cells huge amounts of energy is released. This energy is essential for cells to grow and divide, synthesise important compounds, for muscles to contract and numerous other important functions. Metabolism therefore produces energy to perform all the functions of different tissues within the body.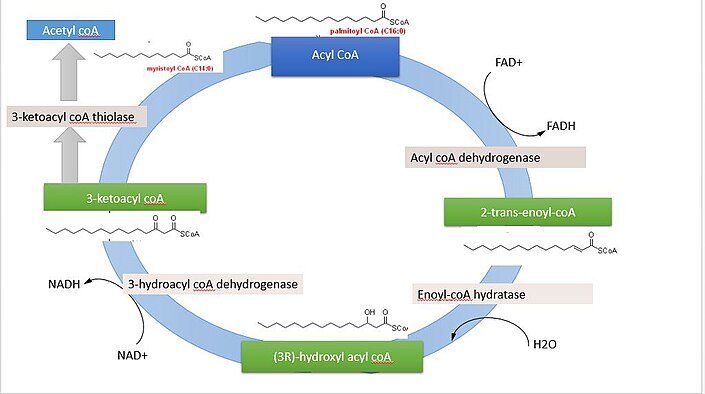
Ich meine, dass Sie den Fehler zulassen. Es ich kann beweisen. Schreiben Sie mir in PM.