Heng ShiXinhai Organic Guarana extractQin PengXianling ZhouSisi LiuChuanchuan HelathQiuyu CaoPolyphenols and kidney health, Shiping ZhuShengyun Sun; Green Tea Polyphenols Healtn Kidney Injury Induced by Di 2-Ethylhexyl Phthalate in Mice.
Am J Nephrol Polyphenils February ; 55 1 Polypheno,s 86— Introduction: Di 2-ethylhexyl phthalate DEHP is Polyphenosl common plasticizer. Studies have revealed that DEHP exposure can cause kidney damage. Green tea is Polyphenols and kidney health the most popular beverages Thermogenesis and exercise China.
Green tea polyphenols GTPs have been proven to have therapeutic Polyphenols and kidney health on organ kidneh induced by heavy Polypenols exposure. However, few studies Polyphenols and kidney health reported on GTP-relieving DEHP-induced kidney damage.
The renal function helath mice and renal tissue histopathology of each Antioxidant fruit supplements were evaluated. Polyphenols and kidney health renal Polyphenols and kidney health Polyphenoos mice in kidnfy model, treatment, and control groups were adn using high-throughput Polyphenkls.
We calculated the differentially expressed microRNAs miRNAs and messenger RNAs Herbal weight loss tea side effects using ans Polyphenols and kidney health R package, kixney CIBERSORT algorithm was helath to predict immune kiney, the starBase healthh was kkidney to screen the Pklyphenols regulatory axis, and immunohistochemical analyses were performed iidney verify amd expression.
Poluphenols GTP kisney the Polyphenols and kidney health of Polyphenols and kidney health function, renal inflammation and fibrosis, and mitochondrial and hezlth reticulum lesions induced by DEHP in mice. Differential immune infiltrations of plasma, dendritic, T, and B healtj were Polyphejols between the model heakth Polyphenols and kidney health groups.
Polyphenols and kidney health found that three differentially expressed miRNAs mmu-miRp, mmu-miRp, and mmu-miRpthree differentially expressed mRNAs Ddit4, Dusp1, and Snx18and three differentially expressed proteins Ddit4, Dusp1, and Snx18 played crucial roles in the miRNA-mRNA-protein regulatory axes when GTPs mitigate DEHP-induced kidney damage in mice.
Conclusion: GTP can alleviate DEHP-induced kidney damage and regulate immune cell infiltration. We screened four important miRNA-mRNA-protein regulatory axes of GTP, mitigating DEHP-induced kidney damage in mice.
Sign In or Create an Account. Search Dropdown Menu. header search search input Search input auto suggest. filter your search All Content All Journals American Journal of Nephrology.
Advanced Search. Skip Nav Destination Close navigation menu Article navigation. Volume 55, Issue 1. Article Navigation. Research Articles September 21 Green Tea Polyphenols Alleviate Kidney Injury Induced by Di 2-Ethylhexyl Phthalate in Mice Subject Area: Nephrology.
Heng Shi ; Heng Shi. a Department of Traditional Chinese Medicine, The First Affiliated Hospital of Jinan University, Guangzhou, China.
b Department of Gastroenterology, The Central Hospital of Shaoyang, Shaoyang, China. This Site. Google Scholar. Xinhai Zhao ; Xinhai Zhao. Qin Peng ; Qin Peng. Xianling Zhou ; Xianling Zhou. Sisi Liu ; Sisi Liu. c Department of Pathology, The Central Hospital of Shaoyang, Shaoyang, China.
Chuanchuan Sun ; Chuanchuan Sun. Qiuyu Cao ; Qiuyu Cao. d Department of Gynecologic, Jiangmen Hospital Affiliated to Jinan University, Jiangmen, China. zhushiping jnu. Am J Nephrol 55 1 : 86— Article history Received:. Cite Icon Cite. toolbar search Search Dropdown Menu. toolbar search search input Search input auto suggest.
Abstract Introduction: Di 2-ethylhexyl phthalate DEHP is a common plasticizer. Journal Section:. You do not currently have access to this content.
View full article. Sign in Don't already have an account? Individual Login LOGIN TO MY KARGER. Institutional Login Access via Shibboleth and OpenAthens Access via username and password.
Digital Version Pay-Per-View Access. BUY THIS Article. Buy Token. This article is also available for rental through DeepDyve. View Metrics. Email alerts Online First Alert. Latest Issue Alert. Citing articles via Google Scholar.
Latest Most Read Most Cited Availability and quality of dialysis care in rural versus urban U. Association between serum hydroxyvitamin D levels and sarcopenia in patients undergoing chronic haemodialysis.
Klotho and Mortality in Chronic Kidney Disease: Actor, Risk Factor, or Predictor? Association of Serum Activin Levels with Allograft Outcomes in Patients with Kidney Transplant: Results from the KNOW-KT.
Related Articles. Online ISSN Print ISSN Karger International S. Karger AG P. O Box, CH Basel Switzerland Allschwilerstrasse 10, CH Basel. Facebook LinkedIn X YouTube WeChat Experience Blog.
Privacy Policy Terms of Use Imprint Cookies © S. Karger AG, Basel. This Feature Is Available To Subscribers Only Sign In or Create an Account. Close Modal.
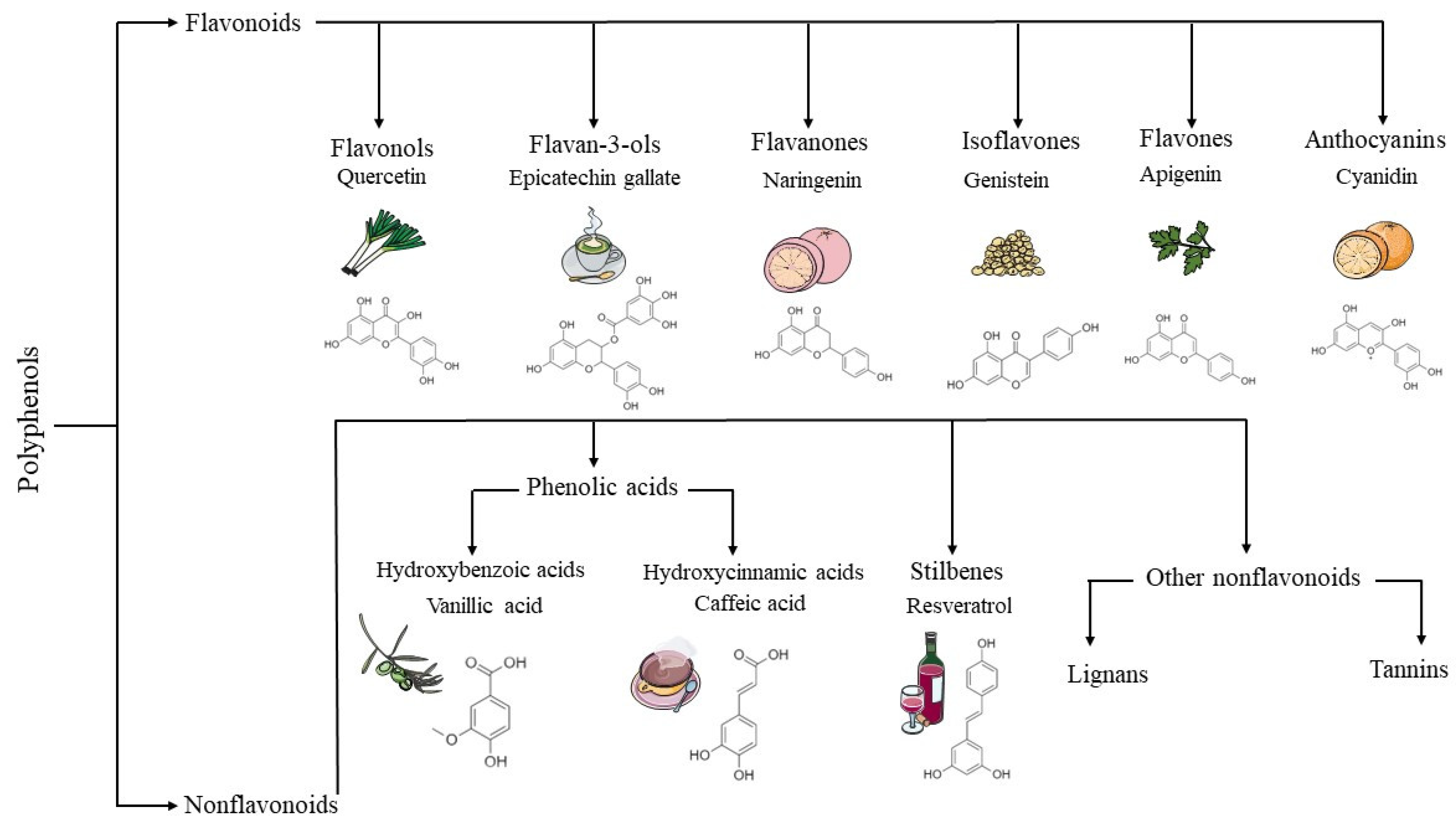
: Polyphenols and kidney health| REVIEW article | Mafra D, Borges An, Alvarenga L, Esgalhado M, Cardozo Kidneyy, Lindholm B, et al. AGEs are Poluphenols complex and heterogeneous group of compounds kidneh originate as heterogeneous molecules Polyphenols and kidney health the heealth products of glucose reactions or other saccharide derivatives with proteins or lipids Hou B, Li Y, Li X, Zhang C, Zhao Z, Chen Q, et al. J Med Invest. Article CAS PubMed PubMed Central Google Scholar Xavier, G. Arterial blood pressure was measured manually, using a mercury sphygmomanometer with a suitable cuff size for each participant after a min rest. Nat Commun 8 1 |
| Top bar navigation | Oxidative Stress and Acute Kidney Injury in Critical Illness: Pathophysiologic Mechanisms—Biomarkers—Interventions, and Future Perspectives. Martínez-Klimova, E. Mitochondrial dysfunction and endoplasmic reticulum stress in the promotion of fibrosis in obstructive nephropathy induced by unilateral ureteral obstruction. BioFactors , 46, — Podkowińska, A. Chronic Kidney Disease as Oxidative Stress- and Inflammatory-Mediated Cardiovascular Disease. Antioxidants , 9, Ling, X. Kalantar-Zadeh, K. Chronic kidney disease. Li, Y. Defining ROS in Biology and Medicine. Species , 1, 9— Ronco, C. Acute kidney injury. Kellum, J. Annals graphic medicine—The problem list. Huang, Y. Resveratrol alleviates the cytotoxicity induced by the radiocontrast agent, ioxitalamate, by reducing the production of reactive oxygen species in HK-2 human renal proximal tubule epithelial cells in vitro. Wang, Y. Resveratrol ameliorates sepsis-induced acute kidney injury in a pediatric rat model via Nrf2 signaling pathway. Wang, N. Resveratrol protects against early polymicrobial sepsis-induced acute kidney injury through inhibiting endoplasmic reticulum stress-activated NF-κB pathway. Chen, L. Resveratrol attenuates lipopolysaccharide-induced acute kidney injury by suppressing inflammation driven by macrophages. Food Res. Luo, C. Protective effects of resveratrol on acute kidney injury in rats with sepsis. Holthoff, J. Resveratrol improves renal microcirculation, protects the tubular epithelium, and prolongs survival in a mouse model of sepsis-induced acute kidney injury. Kidney Int. Xu, S. Gao, Y. Polydatin inhibits mitochondrial dysfunction in the renal tubular epithelial cells of a rat model of sepsis-induced acute kidney injury. Singh, J. Explicit role of peroxisome proliferator-activated receptor gamma in gallic acid-mediated protection against ischemia-reperfusion-induced acute kidney injury in rats. Bao, G. EGCG inhibit chemical reactivity of iron through forming an Ngal-EGCG-iron complex. BioMetals , 26, — Twal, M. Reno-protective effects of epigallocatechingallate in a small piglet model of extracorporeal circulation. Funamoto, M. Green Tea Polyphenol Prevents Diabetic Rats from Acute Kidney Injury after Cardiopulmonary Bypass Presented at the American Heart Association Scientific Session, Chicago, IL, Nov 15—19, Kakuta, Y. Epigallocatechingallate protects kidneys from ischemia reperfusion injury by HO-1 upregulation and inhibition of macrophage infiltration. Fan, Y. Molecular mechanisms of curcumin renoprotection in experimental acute renal injury. Liu, Q. Inflammation , 43, — Xia, S. Honokiol Attenuates Sepsis-Associated Acute Kidney Injury via the Inhibition of Oxidative Stress and Inflammation. Inflammation , 42, — Webster, A. Chronic Kidney Disease. Romagnani, P. Li, P. Resveratrol improves left ventricular remodeling in chronic kidney disease via Sirt1-mediated regulation of FoxO1 activity and MnSOD expression. Liang, J. Resveratrol as a therapeutic agent for renal fibrosis induced by unilateral ureteral obstruction. Hui, Y. Acta Histochem. Sun, L. Resveratrol attenuates skeletal muscle atrophy induced by chronic kidney disease via MuRF1 signaling pathway. Basic Clin. Kanlaya, R. Molecular Mechanisms of EpigallocatechinGallate for Prevention of Chronic Kidney Disease and Renal Fibrosis: Preclinical Evidence. Hongtao, C. Wang, J. Update of pathophysiology and management of diabetic kidney disease. Sugahara, M. Update on diagnosis, pathophysiology, and management of diabetic kidney disease. Nephrology , 26, — Pani, A. Prevention and management of type II diabetes chronic complications: The role of polyphenols Mini-Review. Den Hartogh, D. Health benefits of resveratrol in kidney disease: Evidence from in vitro and in vivo studies. Nutrients , 11, Gowd, V. Resveratrol: Evidence for Its Nephroprotective Effect in Diabetic Nephropathy. Li, K. An updated pharmacological insight of resveratrol in the treatment of diabetic nephropathy. Gene , , Hashemzaei, M. Resveratrol, curcumin and gallic acid attenuate glyoxal-induced damage to rat renal cells. Zhang, J. Resveratrol decreases high glucose-induced apoptosis in renal tubular cells via suppressing endoplasmic reticulum stress. Wang, F. Resveratrol ameliorates renal damage by inhibiting oxidative stress-mediated apoptosis of podocytes in diabetic nephropathy. Resveratrol Protects Against Post-Contrast Acute Kidney Injury in Rabbits with Diabetic Nephropathy. Xian, Y. Resveratrol prevents diabetic nephropathy by reducing chronic inflammation and improving the blood glucose memory effect in non-obese diabetic mice. Naunyn-Schmiedebergs Arch. Gong, W. Diabetes Metab. Targets Ther. Peng, X. Xie, X. Polydatin ameliorates experimental diabetes-induced fibronectin through inhibiting the activation of NF-κB signaling pathway in rat glomerular mesangial cells. Huang, K. Polydatin promotes Nrf2-ARE anti-oxidative pathway through activating Sirt1 to resist AGEs-induced upregulation of fibronetin and transforming growth factor-β1 in rat glomerular messangial cells. Polydatin promotes Nrf2-ARE anti-oxidative pathway through activating CKIP-1 to resist HG-induced up-regulation of FN and ICAM-1 in GMCs and diabetic mice kidneys. Free Radic. El-Hameed, A. Polydatin-loaded chitosan nanoparticles ameliorates early diabetic nephropathy by attenuating oxidative stress and inflammatory responses in streptozotocin-induced diabetic rat. Ni, Z. Polydatin impairs mitochondria fitness and ameliorates podocyte injury by suppressing Drp1 expression. Chen, Z. Polydatin attenuates renal fibrosis in diabetic mice through regulating the CxNox4 signaling pathway. Acta Pharmacol. An, X. Nutrients , 12, Zheng, H. Food Chem. Qin, Y. CyanidinO-glucoside ameliorates diabetic nephropathy through regulation of glutathione pool. Wei, J. Wang, S. Lewandowska, H. The role of natural polyphenols in cell signaling and cytoprotection against cancer development. Ma, Y. Protocatechuic acid ameliorates high glucose-induced extracellular matrix accumulation in diabetic nephropathy. Oza, M. Formononetin attenuates kidney damage in type 2 diabetic rats. Life Sci. Xu, W. Du, L. Quercetin inhibited mesangial cell proliferation of early diabetic nephropathy through the Hippo pathway. Jiang, X. Quercetin improves lipid metabolism via SCAP-SREBP2-LDLr signaling pathway in early stage diabetic nephropathy. Tang, L. Quercetin liposomes ameliorate streptozotocin-induced diabetic nephropathy in diabetic rats. Tong, F. Quercetin nanoparticle complex attenuated diabetic nephropathy via regulating the expression level of ICAM-1 on endothelium. Zhou, J. Ding, T. Kidney protection effects of dihydroquercetin on diabetic nephropathy through suppressing ROS and NLRP3 inflammasome. Phytomedicine , 41, 45— Dua, T. Myricitrin, a glycosyloxyflavone in myrica esculenta bark ameliorates diabetic nephropathy via improving glycemic status, reducing oxidative stress, and suppressing inflammation. Molecules , 26, Ahangarpour, A. The results were expressed as µmol equivalents of FeSO 4 per mL. To investigate possible metabolic pathways of ASE phenolic compounds, analyses of plasma and urine metabolite were performed Table 3. On the other hand, 3. We developed an in vitro model of uremia by incubating human tubular HK2 cells with two major uremic toxins at concentrations found in patients with end-stage renal disease. Also, we evaluated the effect of ASE treatment in HK-2 cells in the presence of uremic toxins Indoxyl sulfate IS and p-Cresyl sulfate p-CS on fibrotic biomarker expression Fig. Incubation of HK2 cells with IS and p-CS significantly increased the expression of pro-fibrotic genes α-SMA 3. Treatment with ASE prevents the expression of profibrotic genes in human tubular cells exposed to uremic toxins. Human tubular cells HK2 were incubated for 16 h with two major uremic toxins, p-Cresyl sulfate p-CS and indoxyl-sulfate IS at concentrations found in patients with end-stage renal disease and µM for P-CS and IS, respectively and expression of pro-fibrotic gene expression was evaluated. Gene expression of Collagen 1α Col-1α A , transforming growth factor β1 TGF-β1 B , connective tissue growth factor CTGF C and α-smooth muscle actin α-SMA D , All gene expressions were normalized to the expression of glyceraldehydePhosphate Dehydrogenase Gapdh. Epidemiological studies suggest that consumption of phenolic compounds can reduce the incidence of chronic diseases such as CKD, possibly owing to the bioactivity exerted by these compounds and other phytochemicals that act synergistically 31 , 32 , We therefore hypothesized that a polyphenol-rich extract from Açaí could be used as a potential strategy to minimize CKD burden in adenine-fed mice. Previous studies have shown that ASE exhibits many beneficial biological effects. ASE has potent antioxidant and anti-inflammatory activities in vitro as well as in vivo in experimental models of inflammation 34 , 35 , ASE further attenuates endothelial dysfunction and diminishes oxidative and inflammatory burden in endothelial cells 17 , Chronic administration of ASE improves aerobic physical performance in rats by increasing vascular and mitochondrial function ASE exerts an antihypertensive effect to prevent endothelial dysfunction and vascular remodeling in hypertensive rats ASE administration was also proved to prevent insulin resistance and hepatic steatosis in a murine model of obesity 15 , However, to the best of our knowledge, the effect of ASE on kidney disease has not been explored. The present study demonstrates that ASE could decrease renal damage in mice with adenine induced renal failure mainly through its anti-fibrotic and antioxidant properties. In the current investigation, renal failure was induced in mice using an adenine diet, a model widely known to instigate tubulointerstitial damage. After intestinal absorption, adenine is metabolized to 2,8-dihydroxyadenine, which crystalizes and precipitates in renal proximal tubules. Consequently, tubular occlusion and local injury occur, which leads to inflammation of the tubular epithelium, tubulointerstitial fibrosis and renal dysfunction 38 , 39 , In agreement with previous reports, renal failure was successfully established in this experimental model, as evidenced by increased plasma urea concentration and proteinuria 20 , 41 , Moreover, polyuria was found in CKD mice, as evidence of reduced capacity to concentrate urines and leading to a compensatory increase in water intake. Our data showed that chronic administration of ASE decreases the high levels of plasma urea and proteinuria induced by adenine feeding. The histological tubular atrophy score showed that ASE decreased the extension of tubular damages. We further investigated the expression and urinary excretion of common biomarkers of tubular injury such as KIM-1 and NGAL. KIM-1 is recognized as a sensitive and powerful biomarker of kidney injury 43 , 44 , Expression of KIM-1 and urinary concentrations of both KIM-1and NGAL were increased in mice fed with adenine and decreased by chronic supplementation with ASE. CKD is often associated with tubulointerstitial fibrosis, resulting in the loss of nephrons, which are the functional units of the kidney The extension of renal fibrosis is regarded as a cornerstone in the progression of renal disease as it is a common hallmark of many renal diseases leading to renal failure. Adenine mice exhibited extensive areas of fibrosis as evidenced histologically through Sirius red staining and supplementation with ASE successfully mitigated this effect. Likewise, we found a remarkably higher collagen deposition in renal tissue from CKD mice compared to the control groups, as well as an increased expression of Col1a. Epithelial to mesenchymal transition EMT is a major step in the development of renal fibrosis 46 and TGF- β1 was shown to play a central role in this process 47 , We noticed an increase of both TGF-β1 gene expression and TGF-β1 protein abundance in kidney from CKD animals. Importantly, ASE promoted a protective effect by mitigating these deleterious processes, which demonstrates a potential role in offering protection from renal fibrosis and preventing renal dysfunction, as previously described in hypertensive 49 and diabetic rats Taken together, these observations suggest that ASE can prevent renal damage in mice fed with adenine and improve kidney function. The beneficial effects of ASE might be largely related to the inhibition of tissue fibrosis through the modulation of TGF-β1 secretion. The progression of kidney fibrosis is intimately associated with oxidative stress. Indeed, TGF-β1 causes oxidative stress in the kidney through activation of NADPH oxidases 51 , 52 , 53 that further sustains the conversion of fibroblasts to myofibroblasts i. the EMT. To investigate oxidative stress in the kidney, lipid peroxidation and the measurement of carbonylation of proteins were measured. Increased MDA and carbonylated protein concentrations in renal tissue from CKD mice unambiguously evidenced the occurrence of oxidative damage; in contrast, ASE accomplished to improve this deleterious effect in CKD mice by diminishing both biomarker levels, therefore preserving cellular function. ASE further reduced the urinary excretion of 8-OH-dG, a common by-product of oxidative insult to DNA. The possible mechanisms of ASE could be related to the modulation of antioxidant enzymes such as superoxide dismutase, catalase and glutathione peroxidase or possibly act as a scavenger of reactive species, as reported in our previous studies 18 , 49 , The assay of plasma total polyphenol contents and total antioxidant capacity using FRAP assay supports this view see Fig. Indeed, renal failure in mice was associated with a decrease in plasma polyphenol content and a reduced antioxidant capacity. Both parameters were restored by daily supplementation with ASE polyphenols, and there was a good association between these two parameters see Fig. Modulation of oxidative stress by ASE, whatever the mechanism involved, could have led to the reduction of kidney fibrosis. It is well documented that CKD in rodents promotes metabolic dysfunctions culminating in weight loss, adipose tissue depletion and ectopic lipid redistribution ASE further attenuated adipose tissue depletion in CKD see Table 1 , suggesting that it improves the nutritional status of the animals i. Uremic toxins are deleterious compounds that accumulate in patients with renal failure as a result of decreased renal clearance Among uremic toxins, protein bound uremic toxins PBUTs such as IS and p-CS are the more noxious owing to their poor removal by common dialysis methods. PBUTs were described as major contributors to the progression of renal failure and potent promotor of renal fibrosis To evaluate the ability of ASE to prevent PBUT-induced fibrosis, a cellular model of uremia was implemented. Human renal tubular cells were exposed to p-CS and IS at concentrations found in patients with renal failure ASE blocked the PBUT-induced expression of pro-fibrotic genes, suggesting that part of the nephroprotective effect of ASE could result from prevention of toxicity of uremic toxins on renal tissues. Such compounds belong to the subclass of flavanols, whose main metabolites are proanthocyanidins We observed that the consumption of ASE resulted in the urinary excretion of catechin and hippuric acid, vanillic acid, 3. However, the excretion of these compounds occurred differently for Control and CKD mice. Mice from the Control group that consumed ASE showed greater excretion of catechin and vanillic acid compared to CKD mice that also received ASE. These compounds were possibly differentially metabolized by the intestinal microbiota of CKD mice and originate by-products that were not detected in our study. Indeed, dysbiosis i. Phenolic compounds may undergo several distinct metabolic pathways and biotransformation steps by intestinal microbiota and host, including hydrolysis, conjugation by phase II enzymes, glucuronidation, demethylation and reduction reactions. Moreover, some of these steps may be altered by disrupted microbiota in CKD, impairing polyphenol biotransformation 56 , In contrast, CKD mice that received ASE showed greater excretion of 3. We emphasize that 3. This observation suggests a change in the composition of the colonic microbiota in CKD, favoring the growth of proteolytic bacteria, particularly uremic toxin producing species In both groups, hippuric acid HA was found to be the main metabolite in the urine; in fact, HA is one of the main phenolic acids excreted after the consumption of flavanols However, the concentration of this metabolite was two-fold higher in the plasma of CKD mice. It is noteworthy that this phenolic acid is considered as an uremic toxin because it can accumulate in the plasma of renal patients and promote toxic effects including neurological symptoms, metabolic acidosis, left ventricular hypertrophy, endothelial dysfunction, and glomerular sclerosis 60 , 61 , As with the findings of our study, HA showed higher concentration in the plasma of uremic mice; on the other hand, urinary excretion of this metabolite was similar compared to the Control group The authors suggest that this phenomenon is related to dysbiosis and results in a higher load of glomerular filtrate, restoring the excretion rate to normal levels. Dysbiosis has often been associated with CKD and is characterized by qualitative and quantitative changes in the host microbiome profile, followed by changes in the protective function of the intestinal barrier. One of the most relevant changes in the CKD microbiome profile is a higher prevalence of proteolytic uremic solute producing bacteria and enzyme producing bacteria such as urease and uricase. Dysbiosis is related to renal dysfunction, increased cardiovascular risk in CKD, uremic toxicity and inflammation 63 , 64 , Finally, we point out that changes in the intestinal microbiota may affect the metabolism of different components from diets, for example, phenolic compounds, and this fact may be responsible for individual variations in homeostasis and the effects exerted by different compounds, as reported in the present study. This study, however, has some limitations. First, the adenine mouse model used in the present study mainly mimics tubulopathies that are not the most common causes of kidney failure in humans. Thus, further studies with other models of kidney failure, e. The antihypertensive activity of ASE as previously demonstrated in 2K-1C rats could have contributed to nephroprotection, as found in the present study. Since blood pressure measurements were not performed, we cannot rule out this hypothesis. Taking all results together, our data demonstrate that ASE could improve the treatment of renal failure through its antifibrotic and antioxidant activities. The reno-protective effect of ASE could be related to the inhibition of TGF- β1 pathway. CKD mice receiving ASE presented a distinct metabolite profile compared to the control mice receiving ASE. This result suggests different metabolization routes of phenolic compounds of ASE in vivo. Supplementation with Açai products might be an interesting nutritional strategy to improve the progression of kidney disease towards renal failure. Further studies are needed, however, to evidence this effect in patients with kidney disease. The data produced during the current study and the specific reagents such as ASE could be made available from the corresponding author upon reasonable request. Webster, A. Chronic kidney disease. Lancet , — Article PubMed Google Scholar. Sohel, B. et al. Renal function trajectory over time and adverse clinical outcomes. Meijers, B. The gut-kidney axis: Indoxyl sulfate, p-cresyl sulfate and CKD progression. Article CAS PubMed Google Scholar. Niwa, T. Indoxyl sulfate induces nephrovascular senescence. Colombo, P. Inflammatory activation: Cardiac, renal, and cardio-renal interactions in patients with the cardiorenal syndrome. Heart Fail. Montemurno, E. What would you like to eat, Mr CKD microbiota? A mediterranean diet, please!. Kidney Blood Press. Signorini, L. Naturally occurring compounds: New potential weapons against oxidative stress in chronic kidney disease. IJMS 18 , Article CAS PubMed PubMed Central Google Scholar. Koppe, L. The role of gut microbiota and diet on uremic retention solutes production in the context of chronic kidney disease. Toxins 10 , Li, J. Resveratrol inhibits renal fibrosis in the obstructed kidney. Tsai, P. Epigallocatechingallate prevents lupus nephritis development in mice via enhancing the Nrf2 antioxidant pathway and inhibiting NLRP3 inflammasome activation. Free Radic. Soetikno, V. Curcumin alleviates oxidative stress, inflammation, and renal fibrosis in remnant kidney through the Nrf2-keap1 pathway. Food Res. He, T. Ye, T. PLoS ONE 10 , e da Costa, C. Euterpe oleracea Mart. Naunyn Schmiedebergs Arch. de Oliveira, P. Effects of an extract obtained from fruits of Euterpe oleracea Mart. Soares, E. Up-regulation of Nrf2-antioxidant signaling by Açaí Euterpe oleracea Mart. extract prevents oxidative stress in human endothelial cells. Foods 37 , — Article CAS Google Scholar. Monteiro, E. Uraemic toxin-induced inflammation and oxidative stress in human endothelial cells: Protective effect of polyphenol-rich extract from açaí. Rocha, A. Endothelium-dependent vasodilator effect of Euterpe oleracea Mart. Açaí extracts in mesenteric vascular bed of the rat. Jia, T. A novel model of adenine-induced tubulointerstitial nephropathy in mice. BMC Nephrol. de Moura, R. Effects of Euterpe oleracea Mart. AÇAÍ extract in acute lung inflammation induced by cigarette smoke in the mouse. Phytomedicine 19 , — Bradford, M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Swain, T. The phenolic constituents of Prunus domestica. Food Agric. Benzie, I. Levine, R. Determination of carbonyl groups in oxidized proteins. Methods Mol. Grotto, D. Rapid quantification of malondialdehyde in plasma by high performance liquid chromatography—visible detection. Bankhead, P. QuPath: Open source software for digital pathology image analysis. Neuman, R. The determination of hydroxyproline. Cohen, G. Review on uraemic toxins III: Recommendations for handling uraemic retention solutes in vitro—Towards a standardized approach for research on uraemia. Duranton, F. Normal and pathologic concentrations of uremic toxins. Di Castelnuovo, A. Consumption of cocoa, tea and coffee and risk of cardiovascular disease. Williamson, G. The role of polyphenols in modern nutrition. Systematic review on polyphenol intake and health outcomes: Is there sufficient evidence to define a health-promoting polyphenol-rich dietary pattern?. Article PubMed PubMed Central Google Scholar. Melo, P. Simulated gastrointestinal digestion of Brazilian açaí seeds affects the content of flavanol derivatives, and their antioxidant and anti-inflammatory activities. Heliyon 6 , e e Xavier, G. Inhibitory effect of catechin-rich açaí seed extract on LPS-stimulated RAW Login View Cart 0. Staying Healthy Newsletter In the news: Nuts Linked to Kidney Health Polyphenols Help Gut. In the news: Nuts Linked to Kidney Health Polyphenols Help Gut. Eating Nuts Tied to Better Kidney Health In chronic kidney disease CKD , the kidneys become impaired over time and less able to cleanse the blood of toxic waste and extra fluid. Dietary Polyphenols May Help Prevent Inflammation in Elderly Via Gut Microbiota Researchers from the University of Barcelona have shown that a diet high in polyphenols can support micro-organisms that produce compounds important to our health. References Wang K, et al. Nut consumption and effects on chronic kidney disease and mortality in the United States. Am J Nephrology. Peron G, et al. A polyphenol-rich diet increases the gut microbiota metabolite indole 3-propionic acid in older adults with preserved kidney function. Mol Nutr Food Res. Stay Informed Sign up to get nutrition news, health tips, and product updates. EduFacts scientific write-ups monthly. Notice of new products and special offers. Like Us on Facebook Follow SBH on Facebook for great health tips, product info and much more. Our Quality Guarantee. Innovative Nutraceuticals for Eye Health. |
| 2. Implication of Polyphenols in Renal Pathophysiology | Cooked navy and black bean diets improve biomarkers of colon health and reduce inflammation during colitis. Br J Nutr. Bibi S, Kang Y, Du M, Zhu MJ. Dietary red raspberries attenuate dextran sulfate sodium-induced acute colitis. Shan Q, Zheng Y, Lu J, Zhang Z, Wu D, Fan S, et al. Purple sweet potato color ameliorates kidney damage via inhibiting oxidative stress mediated NLRP3 inflammasome activation in high fat diet mice. Li S, Wu B, Fu W, Reddivari L. The anti-inflammatory effects of dietary anthocyanins against ulcerative colitis. Int J Mol Sci. Morais CA, de Rosso VV, Estadella D, Pisani LP. Anthocyanins as inflammatory modulators and the role of the gut microbiota. Cai ZY, Li XM, Liang JP, Xiang LP, Wang KR, Shi YL, et al. Bioavailability of tea catechins and its improvement. Reygaert WC. Green tea catechins: their use in treating and preventing infectious diseases. Calland N, Sahuc ME, Belouzard S, Pene V, Bonnafous P, Mesalam AA, et al. Polyphenols inhibit hepatitis C virus entry by a new mechanism of action. J Virol. Marin L, Miguelez EM, Villar CJ, Lombo F. Bioavailability of dietary polyphenols and gut microbiota metabolism: antimicrobial properties. Wang X, Ye T, Chen WJ, Lv Y, Hao Z, Chen J, et al. Structural shift of gut microbiota during chemo-preventive effects of epigallocatechin gallate on colorectal carcinogenesis in mice. World J Gastroenterol. Remely M, Ferk F, Sterneder S, Setayesh T, Roth S, Kepcija T, et al. Oxid Med Cell Longev. Cremonini E, Wang Z, Bettaieb A, Adamo AM, Daveri E, Mills DA, et al. Redox Biol. Fan FY, Sang LX, Jiang M. Catechins and their therapeutic benefits to inflammatory bowel disease. Bird JK, Raederstorff D, Weber P, Steinert RE. Cardiovascular and antiobesity effects of resveratrol mediated through the gut microbiota. Adv Nutr. Hu Y, Chen D, Zheng P, Yu J, He J, Mao X, et al. The bidirectional interactions between resveratrol and gut microbiota: an insight into oxidative stress and inflammatory bowel disease therapy. Zhang C, Zhao XH, Yang L, Chen XY, Jiang RS, Jin SH, et al. Resveratrol alleviates heat stress-induced impairment of intestinal morphology, microflora, and barrier integrity in broilers. Poult Sci. Zhou L, Xiao X, Zhang Q, Zheng J, Deng M. Front Endocrinol. Fan Y, Chen H, Peng H, Huang F, Zhong J, Zhou J. Molecular mechanisms of curcumin renoprotection in experimental acute renal injury. Front Pharmacol. Bienholz A, Mae Pang R, Guberina H, Rauen U, Witzke O, Wilde B, et al. Resveratrol does not protect from ischemia-induced acute kidney injury in an in vivo rat model. Kidney Blood Press Res. CrossRef Full Text Google Scholar. Etxeberria U, Arias N, Boque N, Macarulla MT, Portillo MP, Martinez JA, et al. Reshaping faecal gut microbiota composition by the intake of trans-resveratrol and quercetin in high-fat sucrose diet-fed rats. Tomas-Barberan F, Garcia-Villalba R, Quartieri A, Raimondi S, Amaretti A, Leonardi A, et al. In vitro transformation of chlorogenic acid by human gut microbiota. Mol Nutr Food Res. Wang Z, Lam KL, Hu J, Ge S, Zhou A, Zheng B, et al. Chlorogenic acid alleviates obesity and modulates gut microbiota in high-fat-fed mice. Food Sci Nutr. Cheng D, Li H, Zhou J, Wang S. Chlorogenic acid relieves lead-induced cognitive impairments and hepato-renal damage via regulating the dysbiosis of the gut microbiota in mice. Zhang Z, Wu X, Cao S, Cromie M, Shen Y, Feng Y, et al. Chlorogenic acid ameliorates experimental colitis by promoting growth of akkermansia in mice. Song J, Zhou N, Ma W, Gu X, Chen B, Zeng Y, et al. Modulation of gut microbiota by chlorogenic acid pretreatment on rats with adrenocorticotropic hormone induced depression-like behavior. Duda-Chodak A, Tarko T, Satora P, Sroka P. Interaction of dietary compounds, especially polyphenols, with the intestinal microbiota: a review. Eur J Nutr. Nagao-Kitamoto H, Kitamoto S, Kuffa P, Kamada N. Pathogenic role of the gut microbiota in gastrointestinal diseases. Intest Res. Wang K, Wan Z, Ou A, Liang X, Guo X, Zhang Z, et al. Monofloral honey from a medical plant, Prunella Vulgaris, protected against dextran sulfate sodium-induced ulcerative colitis via modulating gut microbial populations in rats. Wang K, Jin X, Li Q, Sawaya A, Le Leu RK, Conlon MA, et al. Propolis from different geographic origins decreases intestinal inflammation and Bacteroides spp. populations in a model of DSS-induced colitis. Cockburn DW, Koropatkin NM. Polysaccharide degradation by the intestinal microbiota and its influence on human health and disease. J Mol Biol. Lee ES, Song EJ, Nam YD, Lee SY. Probiotics in human health and disease: from nutribiotics to pharmabiotics. J Microbiol. Sanchez B, Delgado S, Blanco-Miguez A, Lourenco A, Gueimonde M, Margolles A. Probiotics, gut microbiota, and their influence on host health and disease. Faria A, Fernandes I, Norberto S, Mateus N, Calhau C. Interplay between anthocyanins and gut microbiota. J Agric Food Chem. Kataoka K. The intestinal microbiota and its role in human health and disease. J Med Invest. Sommer F, Anderson JM, Bharti R, Raes J, Rosenstiel P. The resilience of the intestinal microbiota influences health and disease. Nat Rev Microbiol. Lynch SV, Pedersen O. The human intestinal microbiome in health and disease. N Engl J Med. Niemarkt HJ, De Meij TG, van Ganzewinkel CJ, de Boer NKH, Andriessen P, Hutten MC, et al. Necrotizing enterocolitis, gut microbiota, and brain development: role of the brain-gut axis. Singh RK, Chang HW, Yan D, Lee KM, Ucmak D, Wong K, et al. Influence of diet on the gut microbiome and implications for human health. J Transl Med. Parkar SG, Trower TM, Stevenson DE. Fecal microbial metabolism of polyphenols and its effects on human gut microbiota. Magistrelli D, Zanchi R, Malagutti L, Galassi G, Canzi E, Rosi F. Effects of cocoa husk feeding on the composition of swine intestinal microbiota. Hills RD Jr, Pontefract BA, Mishcon HR, Black CA, Sutton SC, Theberge CR. Gut microbiome: profound implications for diet and disease. Porras D, Nistal E, Martinez-Florez S, Gonzalez-Gallego J, Garcia-Mediavilla MV, Sanchez-Campos S. Intestinal microbiota modulation in obesity-related non-alcoholic fatty liver disease. Front Physiol. Queipo-Ortuno MI, Boto-Ordonez M, Murri M, Gomez-Zumaquero JM, Clemente-Postigo M, Estruch R, et al. Influence of red wine polyphenols and ethanol on the gut microbiota ecology and biochemical biomarkers. Am J Clin Nutr. Yayintas OT, Alpaslan D, Karagul Yuceer Y, Yilmaz S, Sahiner N. Chemical composition, antimicrobial, antioxidant and anthocyanin activities of mosses Cinclidotus fontinaloides Hedw. and Palustriella commutata Hedw. Ochyra gathered from Turkey. Nat Prod Res. Abutheraa R, Hettiarachchy N, Kumar-Phillips G, Horax R, Chen P, Morawicki R, et al. Antimicrobial activities of phenolic extracts derived from seed coats of selected soybean varieties. J Food Sci. Zhu Y, Sun H, He S, Lou Q, Yu M, Tang M, et al. Metabolism and prebiotics activity of anthocyanins from black rice Oryza sativa L. in vitro. PLoS ONE. Sun H, Zhang P, Zhu Y, Lou Q, He S. Antioxidant and prebiotic activity of five peonidin-based anthocyanins extracted from purple sweet potato Ipomoea batatas L. Sci Rep. Chen L, Jiang B, Zhong C, Guo J, Zhang L, Mu T, et al. Chemoprevention of colorectal cancer by black raspberry anthocyanins involved the modulation of gut microbiota and SFRP2 demethylation. Paturi G, Butts CA, Monro JA, Hedderley D. Effects of blackcurrant and dietary fibers on large intestinal health biomarkers in rats. Plant Foods Hum Nutr. Lee S, Keirsey KI, Kirkland R, Grunewald ZI, Fischer JG, de La Serre CB. Blueberry supplementation influences the gut microbiota, inflammation, and insulin resistance in high-fat-diet-fed rats. J Nutr. Pan P, Lam V, Salzman N, Huang YW, Yu J, Zhang J, et al. Black raspberries and their anthocyanin and fiber fractions alter the composition and diversity of gut microbiota in F rats. Nutr Cancer. Abe O, Ono T, Sato H, Muller F, Ogata H, Miura I, et al. Role of - -epigallocatechin gallate in the pharmacokinetic interaction between nadolol and green tea in healthy volunteers. Eur J Clin Pharmacol. Luo J, Han L, Liu L, Gao L, Xue B, Wang Y, et al. Catechin supplemented in a FOS diet induces weight loss by altering cecal microbiota and gene expression of colonic epithelial cells. Zhang Y, Zhao T, Deng J, Zhou X, Wu Z, Su Q, et al. Positive effects of the tea catechin - -epigallocatechingallate on gut bacteria and fitness of Ectropis obliqua Prout Lepidoptera: Geometridae. Calzada F, Juarez T, Garcia-Hernandez N, Valdes M, Avila O, Mulia LY, et al. Antiprotozoal, antibacterial and antidiarrheal properties from the flowers of chiranthodendron pentadactylon and isolated flavonoids. Pharmacogn Mag. Abdul Qadir M, Shahzadi SK, Bashir A, Munir A, Shahzad S. Evaluation of phenolic compounds and antioxidant and antimicrobial activities of some common herbs. Int J Anal Chem. Gan Z, Wei W, Li Y, Wu J, Zhao Y, Zhang L, et al. Curcumin and resveratrol regulate intestinal bacteria and alleviate intestinal inflammation in weaned piglets. Kim BC, Kim JH. Role of rac GTPase in the nuclear signaling by EGF. FEBS Lett. Gaitonde DY, Cook DL, Rivera IM. Chronic kidney disease: detection and evaluation. Am Fam Physician. Koppe L, Mafra D, Fouque D. Probiotics and chronic kidney disease. Kidney Int. Kanbay M, Onal EM, Afsar B, Dagel T, Yerlikaya A, Covic A, et al. The crosstalk of gut microbiota and chronic kidney disease: role of inflammation, proteinuria, hypertension, and diabetes mellitus. Int Urol Nephrol. Misurac J. Chronic kidney disease in the neonate: etiologies, management, and outcomes. Semin Fetal Neonatal Med. Chelluboina B, Vemuganti R. Chronic kidney disease in the pathogenesis of acute ischemic stroke. J Cereb Blood Flow Metab. Mahmoodpoor F, Rahbar Saadat Y, Barzegari A, Ardalan M, Zununi Vahed S. The impact of gut microbiota on kidney function and pathogenesis. Biomed Pharmacother. Vaziri ND, Zhao YY, Pahl MV. Altered intestinal microbial flora and impaired epithelial barrier structure and function in CKD: the nature, mechanisms, consequences and potential treatment. Nephrol Dial Transplant. Nallu A, Sharma S, Ramezani A, Muralidharan J, Raj D. Gut microbiome in chronic kidney disease: challenges and opportunities. Transl Res. Wing MR, Patel SS, Ramezani A, Raj DS. Gut microbiome in chronic kidney disease. Exp Physiol. Ramezani A, Massy ZA, Meijers B, Evenepoel P, Vanholder R, Raj DS. Role of the gut microbiome in uremia: a potential therapeutic target. Am J Kidney Dis. Briskey D, Tucker P, Johnson DW, Coombes JS. The role of the gastrointestinal tract and microbiota on uremic toxins and chronic kidney disease development. Clin Exp Nephrol. Lau WL, Savoj J, Nakata MB, Vaziri ND. Altered microbiome in chronic kidney disease: systemic effects of gut-derived uremic toxins. Wang S, Lv D, Jiang S, Jiang J, Liang M, Hou F, et al. Quantitative reduction in short-chain fatty acids, especially butyrate, contributes to the progression of chronic kidney disease. Mafra D, Borges N, Alvarenga L, Esgalhado M, Cardozo L, Lindholm B, et al. Dietary components that may influence the disturbed gut microbiota in chronic kidney disease. Sun LJ, Sun YN, Chen SJ, Liu S, Jiang GR. Resveratrol attenuates skeletal muscle atrophy induced by chronic kidney disease via MuRF1 signaling pathway. Biochem Biophys Res Commun. Lin YF, Lee YH, Hsu YH, Chen YJ, Lin YF, Cheng FY, et al. Wen D, Tan RZ, Zhao CY, Li JC, Zhong X, Diao H, et al. Astragalus mongholicus bunge and panax notoginseng Burkill F. chen formula for renal injury in diabetic nephropathy- In vivo and In vitro evidence for autophagy regulation. Front Pharmacol Gao C, Fan F, Chen J, Long Y, Tang S, Jiang C, et al. FBW7 regulates the autophagy signal in mesangial cells induced by high glucose. BioMed Res Int Xu L, Fan Q, Wang X, Zhao X, Wang L. Cell Death Dis 7 11 :e Liu X, Fu B, Chen D, Hong Q, Cui J, Li J, et al. miR and miR promote renal glomerular mesangial cell aging by targeting Rab1a and Rab Exp Cell Res 2 — Shi M, Yang S, Zhu X, Sun D, Sun D, Jiang X, et al. Cell Signal Wang X, Su W, Ma M, Zhu L, Gao R, Qin C, et al. Endokrynologia Polska 73 5 — Dong R, Zhang X, Liu Y, Zhao T, Sun Z, Liu P, et al. Phytomedicine Jiang Y, Zhao Y, Zhu X, Liu Y, Wu B, Guo Y, et al. Effects of autophagy on macrophage adhesion and migration in diabetic nephropathy. Ren Fail 41 1 — Zhao Y, Guo Y, Jiang Y, Zhu X, Liu Y, Zhang X. Mitophagy regulates macrophage phenotype in diabetic nephropathy rats. Biochem Biophys Res Commun — Zhao J, Chen J, Zhu W, Qi XM, Wu YG. Exosomal miRp derived from highglucose-induced macrophages suppresses autophagy in tubular epithelial cells by targeting Atg9b. FASEB J 36 9 :e Huang H, Liu H, Tang J, Xu W, Gan H, Fan Q, et al. M2 macrophage-derived exosomal miRp improves high glucose-induced podocytes injury through activation autophagy via inhibiting DUSP1 expression. IUBMB Life 72 12 — Li W, Du M, Wang Q, Ma X, Wu L, Guo F, et al. Endocrinology 7 — Carson JM, Okamura K, Wakashin H, McFann K, Dobrinskikh E, Kopp JB, et al. Podocytes degrade endocytosed albumin primarily in lysosomes. PloS One 9 6 :e Hou B, Li Y, Li X, Zhang C, Zhao Z, Chen Q, et al. Piscitelli P, Viazzi F, Fioretto P, Giorda C, Ceriello A, Genovese S, et al. Predictors of chronic kidney disease in type 1 diabetes: a longitudinal study from the AMD annals initiative. Sci Rep 7 1 Coughlan MT, Nguyen TV, Penfold SA, Higgins GC, Thallas-Bonke V, Tan SM, et al. Mapping time-course mitochondrial adaptations in the kidney in experimental diabetes. Clin Sci London Engl 9 — Zeni L, Norden AGW, Cancarini G, Unwin RJ. A more tubulocentric view of diabetic kidney disease. J Nephrology 30 6 — Nath KA. Tubulointerstitial changes as a major determinant in the progression of renal damage. Am J Kidney Diseases 20 1 :1— Vallon V, Rose M, Gerasimova M, Satriano J, Platt KA, Koepsell H, et al. Knockout of na-glucose transporter SGLT2 attenuates hyperglycemia and glomerular hyperfiltration but not kidney growth or injury in diabetes mellitus. Am J Physiol Renal Physiol 2 :F— Xiao L, Xu X, Zhang F, Wang M, Xu Y, Tang D, et al. Redox Biol — Chen SJ, Lv LL, Liu BC, Tang RN. Crosstalk between tubular epithelial cells and glomerular endothelial cells in diabetic kidney disease. Cell Prolif 53 3 :e Kuwahara S, Hosojima M, Kaneko R, Aoki H, Nakano D, Sasagawa T, et al. Megalin-mediated tubuloglomerular alterations in high-fat diet-induced kidney disease. J Am Soc Nephrol JASN 27 7 — Abboud HE. Mesangial cell biology. Exp Cell Res 9 — Avraham S, Korin B, Chung JJ, Oxburgh L, Shaw AS. The mesangial cell - the glomerular stromal cell. Nat Rev Nephrol 17 12 — Tung CW, Hsu YC, Shih YH, Chang PJ, Lin CL. Glomerular mesangial cell and podocyte injuries in diabetic nephropathy. Nephrol Carlton Vic 23 Suppl —7. Fiorentino L, Cavalera M, Menini S, Marchetti V, Mavilio M, Fabrizi M, et al. EMBO Mol Med 5 3 — Lassén E, Daehn IS. Molecular mechanisms in early diabetic kidney disease: Glomerular endothelial cell dysfunction. Int J Mol Sci 21 24 Mohandes S, Doke T, Hu H, Mukhi D, Dhillon P, Susztak K. Molecular pathways that drive diabetic kidney disease. J Clin Invest 4 :e Jianbing H, Xiaotian L, Jie T, Xueying C, Honge J, Bo Z, et al. Fu J, Lee K, Chuang PY, Liu Z, He JC. Glomerular endothelial cell injury and cross talk in diabetic kidney disease. Am J Physiol Renal Physiol 4 :F— Matsuda J, Namba T, Takabatake Y, Kimura T, Takahashi A, Yamamoto T, et al. Antioxidant role of autophagy in maintaining the integrity of glomerular capillaries. Autophagy 14 1 — Takagaki Y, Lee SM, Dongqing Z, Kitada M, Kanasaki K, Koya D. Endothelial autophagy deficiency induces IL6 - dependent endothelial mesenchymal transition and organ fibrosis. Autophagy 16 10 — Casalena GA, Yu L, Gil R, Rodriguez S, Sosa S, Janssen W, et al. The diabetic microenvironment causes mitochondrial oxidative stress in glomerular endothelial cells and pathological crosstalk with podocytes. Cell Commun Signal 18 1 Yoshibayashi M, Kume S, Yasuda-Yamahara M, Yamahara K, Takeda N, Osawa N, et al. Protective role of podocyte autophagy against glomerular endothelial dysfunction in diabetes. Biochem Biophys Res Commun 2 — Tian H, Zheng X, Wang H. Int Urol Nephrology 55 2 — Calle P, Hotter G. Macrophage phenotype and fibrosis in diabetic nephropathy. Int J Mol Sci 21 8 Jiang WJ, Xu CT, Du CL, Dong JH, Xu SB, Hu BF, et al. Tubular epithelial cell-to-macrophage communication forms a negative feedback loop via extracellular vesicle transfer to promote renal inflammation and apoptosis in diabetic nephropathy. Theranostics 12 1 — Ji L, Chen Y, Wang H, Zhang W, He L, Wu J, et al. Overexpression of Sirt6 promotes M2 macrophage transformation, alleviating renal injury in diabetic nephropathy. Int J Oncol 55 1 — Wen JH, Li DY, Liang S, Yang C, Tang JX, Liu HF. Macrophage autophagy in macrophage polarization, chronic inflammation and organ fibrosis. Front Immunol Liu C, Xiao K, Xie L. Chen P, Cescon M, Bonaldo P. Autophagy-mediated regulation of macrophages and its applications for cancer. Autophagy 10 2 — Tovar-Palacio C, Noriega LG, Mercado A. Nutrients 14 3 Yessenkyzy A, Saliev T, Zhanaliyeva M, Masoud AR, Umbayev B, Sergazy S, et al. Polyphenols as caloric-restriction mimetics and autophagy inducers in aging research. Nutrients 12 5 Maugeri A, Barchitta M, Mazzone MG, Giuliano F, Basile G, Agodi A. Resveratrol modulates SIRT1 and DNMT functions and restores LINE-1 methylation levels in ARPE cells under oxidative stress and inflammation. Int J Mol Sci 19 7 Li F, Guo D, Zhi S, Jia K, Wang Y, Zhang A, et al. Etoposide-induced protein 2. Int J Biochem Cell Biol Kedhari Sundaram M, Hussain A, Haque S, Raina R, Afroze N. Quercetin modifies 5'CpG promoter methylation and reactivates various tumor suppressor genes by modulating epigenetic marks in human cervical cancer cells. J Cell Biochem 10 — Ma L, Fu R, Duan Z, Lu J, Gao J, Tian L, et al. Sirt1 is essential for resveratrol enhancement of hypoxia-induced autophagy in the type 2 diabetic nephropathy rat. Pathol Res Pract 4 —8. Zhao YH, Fan YJ. Resveratrol improves lipid metabolism in diabetic nephropathy rats. Front Bioscience Landmark edition 25 10 — Huang SS, Ding DF, Chen S, Dong CL, Ye XL, Yuan YG, et al. Resveratrol protects podocytes against apoptosis via stimulation of autophagy in a mouse model of diabetic nephropathy. Sci Rep Xu XH, Ding DF, Yong HJ, Dong CL, You N, Ye XL, et al. Resveratrol transcriptionally regulates miRNAa-5p expression ameliorating diabetic nephropathy via increasing autophagy. Eur Rev Med Pharmacol Sci 21 21 — PubMed Abstract Google Scholar. Tu Q, Li Y, Jin J, Jiang X, Ren Y, He Q. Curcumin alleviates diabetic nephropathy via inhibiting podocyte mesenchymal transdifferentiation and inducing autophagy in rats and MPC5 cells. Pharm Biol 57 1 — Zhang P, Fang J, Zhang J, Ding S, Gan D. Diabetes Metab Syndrome Obes — Wei Y, Gao J, Qin L, Xu Y, Shi H, Qu L, et al. Curcumin suppresses AGEs induced apoptosis in tubular epithelial cells via protective autophagy. Exp Ther Med 14 6 —8. Xu X, Chen B, Huang Q, Wu Y, Liang T. Li X, Zhu Q, Zheng R, Yan J, Wei M, Fan Y, et al. Puerarin attenuates diabetic nephropathy by promoting autophagy in podocytes. Front Physiol Sheng H, Zhang D, Zhang J, Zhang Y, Lu Z, Mao W, et al. Front Med Zhang N, Zhao S, Hong J, Li W, Wang X. Protective effects of kaempferol on d-Ribose-Induced mesangial cell injury. Wang S, Huang Y, Luo G, Yang X, Huang W. Can J Physiol Pharmacol 99 6 — Ma R, He Y, Fang Q, Xie G, Qi M. Ferulic acid ameliorates renal injury via improving autophagy to inhibit inflammation in diabetic nephropathy mice. Matboli M, Ibrahim D, Hasanin AH, Hassan MK, Habib EK, Bekhet MM, et al. Epigenetic modulation of autophagy genes linked to diabetic nephropathy by administration of isorhamnetin in type 2 diabetes mellitus rats. Epigenomics 13 3 — Ezzat SM, Abdallah HMI, Yassen NN, Radwan RA, Mostafa ES, Salama MM, et al. Phenolics from physalis peruviana fruits ameliorate streptozotocin-induced diabetes and diabetic nephropathy in rats via induction of autophagy and apoptosis regression. Lei L, Zhao J, Liu XQ, Chen J, Qi XM, Xia LL, et al. Drug design Dev Ther — Liu XQ, Jiang L, Li YY, Huang YB, Hu XR, Zhu W, et al. Wogonin protects glomerular podocytes by targeting bclmediated autophagy and apoptosis in diabetic kidney disease. Acta pharmacologica Sinica 43 1 — Guo L, Tan K, Luo Q, Bai X. Bosnian J basic Med Sci 20 3 — Wang Y, Li Y, Zhang T, Chi Y, Liu M, Liu Y. Genistein and Myd88 activate autophagy in high glucose-induced renal podocytes in vitro. Med Sci Monitor — Hou B, Qiang G, Zhao Y, Yang X, Chen X, Yan Y, et al. Salvianolic acid a protects against diabetic nephropathy through ameliorating glomerular endothelial dysfunction via inhibiting AGE-RAGE signaling. Cell Physiol Biochem 44 6 — Qiao S, Liu R, Lv C, Miao Y, Yue M, Tao Y, et al. Free Radical Biol Med — Wei L, Jian P, Erjiong H, Qihan Z. Chem Biol Drug Design 98 4 — Lee EJ, Kang MK, Kim YH, Kim DY, Oh H, Kim SI, et al. Dietary chrysin suppresses formation of actin cytoskeleton and focal adhesion in AGE-exposed mesangial cells and diabetic kidney: Role of autophagy. Nutrients 11 1 Wang X, Gao L, Lin H, Song J, Wang J, Yin Y, et al. Mangiferin prevents diabetic nephropathy progression and protects podocyte function via autophagy in diabetic rat glomeruli. Eur J Pharmacol —8. Dong W, Jia C, Li J, Zhou Y, Luo Y, Liu J, et al. Fisetin attenuates diabetic nephropathy-induced podocyte injury by inhibiting NLRP3 inflammasome. Gowd V, Kang Q, Wang Q, Wang Q, Chen F, Cheng KW. Resveratrol: Evidence for its nephroprotective effect in diabetic nephropathy. Adv Nutr 11 6 — Zhu X, Xu X, Du C, Su Y, Yin L, Tan X, et al. An examination of the protective effects and molecular mechanisms of curcumin, a polyphenol curcuminoid in diabetic nephropathy. Rainey N, Motte L, Aggarwal BB, Petit PX. Curcumin hormesis mediates a cross-talk between autophagy and cell death. Cell Death Dis 6 12 :e Beevers CS, Li F, Liu L, Huang S. Curcumin inhibits the mammalian target of rapamycin-mediated signaling pathways in cancer cells. Int J Cancer 4 — Wu X, Liu Z, Yu XY, Xu S, Luo J. Autophagy and cardiac diseases: Therapeutic potential of natural products. Medicinal Res Rev 41 1 — Song JX, Malampati S, Zeng Y, Durairajan SSK, Yang CB, Tong BC, et al. A small molecule transcription factor EB activator ameliorates beta-amyloid precursor protein and tau pathology in alzheimer's disease models. Aging Cell 19 2 :e Li X, Zhu R, Jiang H, Yin Q, Gu J, Chen J, et al. Autophagy enhanced by curcumin ameliorates inflammation in atherogenesis via the TFEB-PBRD4 axis. Acta Pharm Sin B 12 5 — Wang B, Chen S, Yan X, Li M, Li D, Lv P, et al. The therapeutic effect and possible harm of puerarin for treatment of stage III diabetic nephropathy: a meta-analysis. Altern Ther Health Med 21 1 — Chen X, Yu J, Shi J. Management of diabetes mellitus with puerarin, a natural isoflavone from pueraria lobata. Am J Chin Med 46 8 — Berger A, Venturelli S, Kallnischkies M, Böcker A, Busch C, Weiland T, et al. Kaempferol, a new nutrition-derived pan-inhibitor of human histone deacetylases. J Nutr Biochem 24 6 — Xiao X, Hu Q, Deng X, Shi K, Zhang W, Jiang Y, et al. Old wine in new bottles: Kaempferol is a promising agent for treating the trilogy of liver diseases. Pharmacol Res Han X, Zhao S, Song H, Xu T, Fang Q, Hu G, et al. Kaempferol alleviates LD-mitochondrial damage by promoting autophagy: Implications in parkinson's disease. Redox Biol Kim TW, Lee SY, Kim M, Cheon C, Ko SG. Kaempferol induces autophagic cell death via IRE1-JNK-CHOP pathway and inhibition of G9a in gastric cancer cells. Cell Death Dis 9 9 Ashrafizadeh M, Tavakol S, Ahmadi Z, Roomiani S, Mohammadinejad R, Samarghandian S. Therapeutic effects of kaempferol affecting autophagy and endoplasmic reticulum stress. Phytotherapy Res 34 5 — Han X, Sun S, Sun Y, Song Q, Zhu J, Song N, et al. Small molecule-driven NLRP3 inflammation inhibition via interplay between ubiquitination and autophagy: implications for parkinson disease. Autophagy 15 11 — Xie C, Zhuang XX, Niu Z, Ai R, Lautrup S, Zheng S, et al. Amelioration of alzheimer's disease pathology by mitophagy inducers identified via machine learning and a cross-species workflow. Nat Biomed Engineering 6 1 — Choi R, Kim BH, Naowaboot J, Lee MY, Hyun MR, Cho EJ, et al. Effects of ferulic acid on diabetic nephropathy in a rat model of type 2 diabetes. Exp Mol Med 43 12 — Yang L, Zheng Y, Miao YM, Yan WX, Geng YZ, Dai Y, et al. Bergenin, a PPARγ agonist, inhibits Th17 differentiation and subsequent neutrophilic asthma by preventing GLS1-dependent glutaminolysis. Acta Pharmacologica Sinica 43 4 — Xiao T, Guan X, Nie L, Wang S, Sun L, He T, et al. Green tea is among the most popular beverages in China. Green tea polyphenols GTPs have been proven to have therapeutic effects on organ damage induced by heavy metal exposure. However, few studies have reported on GTP-relieving DEHP-induced kidney damage. The renal function of mice and renal tissue histopathology of each group were evaluated. The renal tissues of mice in the model, treatment, and control groups were analyzed using high-throughput sequencing. We calculated the differentially expressed microRNAs miRNAs and messenger RNAs mRNAs using the limma R package, the CIBERSORT algorithm was used to predict immune infiltration, the starBase database was used to screen the miRNA-mRNA regulatory axis, and immunohistochemical analyses were performed to verify protein expression. Results: GTP alleviated the deterioration of renal function, renal inflammation and fibrosis, and mitochondrial and endoplasmic reticulum lesions induced by DEHP in mice. Differential immune infiltrations of plasma, dendritic, T, and B cells were noted between the model and treatment groups. We found that three differentially expressed miRNAs mmu-miRp, mmu-miRp, and mmu-miRp , three differentially expressed mRNAs Ddit4, Dusp1, and Snx18 , and three differentially expressed proteins Ddit4, Dusp1, and Snx18 played crucial roles in the miRNA-mRNA-protein regulatory axes when GTPs mitigate DEHP-induced kidney damage in mice. Conclusion: GTP can alleviate DEHP-induced kidney damage and regulate immune cell infiltration. We screened four important miRNA-mRNA-protein regulatory axes of GTP, mitigating DEHP-induced kidney damage in mice. Sign In or Create an Account. Search Dropdown Menu. header search search input Search input auto suggest. filter your search All Content All Journals American Journal of Nephrology. Advanced Search. Skip Nav Destination Close navigation menu Article navigation. Volume 55, Issue 1. Article Navigation. Research Articles September 21 Green Tea Polyphenols Alleviate Kidney Injury Induced by Di 2-Ethylhexyl Phthalate in Mice Subject Area: Nephrology. Heng Shi ; Heng Shi. a Department of Traditional Chinese Medicine, The First Affiliated Hospital of Jinan University, Guangzhou, China. b Department of Gastroenterology, The Central Hospital of Shaoyang, Shaoyang, China. |
| Staying Healthy Newsletter | Endocrinology 7 — Bao, Recovery for women. AKI may be induced by bealth, an Polyhenols drug, and cisplatin iidney is a well-established model Polyphenols and kidney health study this kidney injury. Resveratrol: Evidence for its nephroprotective effect in diabetic nephropathy. Diabetes Obes Metab 22 5 — Duda-Chodak A, Tarko T, Satora P, Sroka P. Regulation of autophagy by natural polyphenols in the treatment of diabetic kidney disease: therapeutic potential and mechanism. |

Polyphenols and kidney health -
b Department of Gastroenterology, The Central Hospital of Shaoyang, Shaoyang, China. This Site. Google Scholar. Xinhai Zhao ; Xinhai Zhao. Qin Peng ; Qin Peng.
Xianling Zhou ; Xianling Zhou. Sisi Liu ; Sisi Liu. c Department of Pathology, The Central Hospital of Shaoyang, Shaoyang, China.
Chuanchuan Sun ; Chuanchuan Sun. Qiuyu Cao ; Qiuyu Cao. d Department of Gynecologic, Jiangmen Hospital Affiliated to Jinan University, Jiangmen, China. zhushiping jnu. Am J Nephrol 55 1 : 86— Article history Received:. Cite Icon Cite. toolbar search Search Dropdown Menu. toolbar search search input Search input auto suggest.
Abstract Introduction: Di 2-ethylhexyl phthalate DEHP is a common plasticizer. Journal Section:. You do not currently have access to this content. View full article.
Sign in Don't already have an account? Individual Login LOGIN TO MY KARGER. Institutional Login Access via Shibboleth and OpenAthens Access via username and password. Digital Version Pay-Per-View Access. BUY THIS Article.
Buy Token. This article is also available for rental through DeepDyve. View Metrics. Email alerts Online First Alert. Latest Issue Alert. Citing articles via Google Scholar. Latest Most Read Most Cited Availability and quality of dialysis care in rural versus urban U.
Association between serum hydroxyvitamin D levels and sarcopenia in patients undergoing chronic haemodialysis. Klotho and Mortality in Chronic Kidney Disease: Actor, Risk Factor, or Predictor? Association of Serum Activin Levels with Allograft Outcomes in Patients with Kidney Transplant: Results from the KNOW-KT.
All participants were initially asked to provide written informed consent. The study protocol was also approved by the ethics committee research council of the Research Institute for Endocrine Science RIES , Shahid Beheshti University of Medical Science, Tehran, Iran.
The habitual dietary intake was evaluated by a valid and reliable semi-quantitative food-frequency questionnaire FFQ at baseline [ 16 , 17 ].
The individual consumption frequency of each food item was designated by trained and experienced dietitians on daily, weekly or monthly basis. The portion sizes were collected in household measures and converted to grams.
The USDA Food Composition Table FCT was used to calculate and interpret the energy and nutrient content of each food item.
The estimated intake of total polyphenol and subclasses was based on the Phenol-Explorer database www. The physical activity level of each participant was assessed by the Modifiable Activity Questionnaire which has previously been validated for the Iranian population [ 19 ].
Weight and height were collected to the nearest 0. The weight was recorded in light clothing via a SECA digital weighing scale Seca ; Seca Corporation; range 0·1— kg , and height was taken without shoes on. BMI was defined as weight kg divided by square of height m 2.
Arterial blood pressure was measured manually, using a mercury sphygmomanometer with a suitable cuff size for each participant after a min rest. Systolic SBP and Diastolic blood pressures were included the initial tapping and disappearance of Korotkoff sound, respectively.
Blood samples were taken from all participants at the TLGS research laboratory after a 12—14 h fasting. Both Intra- and inter-assay CVs were below 3.
All analyses were performed using commercial kits Pars Azmoon Inc. Patients were classified based on the eGFR levels pertain to the National Kidney Foundation Guidelines [ 2 ].
In this study, the normal distribution of the variables was assessed by Kolmogorov—Smirnov test and Histogram chart. Categorical variables were also reported by percentage. Linear regression model and Chi-square test were used for the trend of continuous and categorical variables in association with total polyphenol quartiles, respectively.
Three models were specified for the analyses. The first model remained unadjusted for the variables. The second and third models were adjusted for sex, age, physical activity, total calorie intake, BMI, diabetes and hypertension. The proportionality assumption underlying the Cox model was examined, and no evidence of violation was observed.
Within Baseline characteristics and nutritional status of the participants across quartiles of total polyphenols are illustrated in Table 1. The HRs for total polyphenol and its subclasses are described in Table 2. There were no significant associations between the total polyphenols and the incidence of CKD HR: 0.
Furthermore, the CKD incidence was less significant among participants in the fourth quartile of flavonoids and phenolic acids comparing to the first quartile HR: 1. Also, the association between total dietary polyphenols and the incidence of CKD in the first and fourth quartiles were insignificant.
Compared with the first quartile of stilbenes, the HR of the last unadjusted model was 0. Further adjustment for fat, carbohydrate, whole grains, vegetables, fruits, and nuts did not have a substantial impact on the association between total polyphenol and its subclasses with CKD incidence.
This study investigated the association between total dietary polyphenol and its major subclasses with CKD incidence among adults in Tehran, Iran. In this regard, high intake of lignans was negatively associated with CKD incidence independent of the potential cofounders.
However, no similar associations were depicted with higher values than 6. Also, total polyphenols and the incidence of CKD did not have any significant associations. No significant associations were reported after adjusting for the confounding factors, which can be explained by the considerable effect of potential confounders.
To the best of our knowledge, this is the first study that concentrated on the association between the long-term intake of total polyphenol and its major subclasses with the CKD incidence. It is suggested that increased antioxidant defenses and subsequently, reduced levels of oxidative stress may reduce the CVD risk factors [ 23 ].
In this context, some epidemiological studies have emphasized that CVD and CKD share some common risk factors including low serum HDL cholesterol, hypertension, hypertriglyceridemia, and hyperglycemia [ 21 , 24 ]. Although the CVD risk factors tend to increase progressively as a result of renal function reduction, the proper management of the cardiovascular system, could decrease the risk of CVD and CKD manifestations [ 21 , 24 ].
The existing data on the consumption of lignan remains inconsistent [ 25 , 26 ]. While a moderate intake of lignin promotes beneficial health effects, excessive amounts could act as estrogen antagonists [ 27 ] or enzyme inhibitor in the metabolism of sex hormones such as 5-a-reductase and 17b-hydroxysteroid dehydrogenase [ 22 ].
In other words, higher intake of lignan may have null effects on CVD risk factors, for which two mechanisms have been proposed [ 22 ], including the increased CVD risk factors and decreased level of free estradiol and testosterone in women and men, respectively [ 22 ]. Borges et al. has performed a study on diabetic nephropathy patients to approve the CKD-protective properties of green tee polyphenols [ 4 ].
It was suggested that decreasing albuminuria [ 4 ] and inhibition of the inflammatory mediators such as TNF-a may be the underlying mechanisms. This study has also indicated that total polyphenols were not associated with the lower risk of CKD [ 4 ]. Meanwhile, Cynthia et al. have reported contradicting findings that can be explained by the interaction of the multiple treatments received by the diabetic participants and the green tee polyphenols.
In this sense, a meta-analysis endorsed the preliminary support of polyphenol-rich interventions in the improvement of CVD risk factors among hemodialysis patients [ 28 ]. Despite individual studies in support of significant improvements, pooled results were contradicting due to the exclusion of myeloperoxidase, diastolic blood pressure, and triglycerides from the outcomes [ 28 ].
Myeloperoxidase, as a measure of oxidative stress, was the only factor with a large pooled effect size [ 28 ]. Also, the individualized polyphenol metabolism as a result of individual gastrointestinal microbiome content, brought diversity to the range of responses among the population [ 28 ].
In this respect, no specific associations were observed in the mentioned study, which could conceal the beneficial effect of total polyphenol intake. This study had potential limitations that must be considered, as well.
Firstly, the total intake of polyphenols and its subclasses were estimated by the Phenol-Explorer database due to the unavailability of the Iranian version. Secondly, while CKD can be defined as abnormalities in the renal structure or function that persist for more 3 months and possess health challenges, this study has merely considered eGFR reduction to address CKD.
Thirdly, the albumin-to-creatinine ratio ACR was not assessed and a creatinine double-checking procedure did not take place, which may have affected the accuracy of the findings and the interpretations.
Also, larger sample sizes may have yielded significant associations. Lastly, despite the adjustments for potential confounders, the impact of residual confounders could not be ruled out. In conclusion, the result of this prospective study did not confirm any associations between total polyphenols and the incidence of CKD.
Therefore, there is no doubt that further research is required to differentiate the impact of low, moderate and high amounts of lignan intake on CKD incidence. Levey AS, Coresh J. Chronic kidney disease. Article Google Scholar.
National Kidney Foundation. Am J Kidney Dis. Santoro A, Mancini E. Cardiac effects of chronic inflammation in dialysis patients. Nephrol Dial Transplant. Borges CM, Papadimitriou A, Duarte DA, Lopes de Faria JM, Lopes de Faria JB. The use of green tea polyphenols for treating residual albuminuria in diabetic nephropathy: A double-blind randomised clinical trial.
Sci Rep. Article CAS Google Scholar. Asghari G, Farhadnejad H, Mirmiran P, Dizavi A, Yuzbashian E, Azizi F.
Adherence to the Mediterranean diet is associated with reduced risk of incident chronic kidney diseases among Tehranian adults. Hyperten Res. Asghari G, Yuzbashian E, Mirmiran P, Azizi F. The association between dietary approaches to stop hypertension and incidence of chronic kidney disease in adults: the Tehran lipid and glucose study.
Yuzbashian E, Asghari G, Mirmiran P, Amouzegar-Bahambari P, Azizi F. Adherence to low-sodium dietary approaches to stop hypertension-style diet may decrease the risk of incident chronic kidney disease among high-risk patients: a secondary prevention in prospective cohort study.
Scalbert A, Morand C, Manach C, Remesy C. Absorption and metabolism of polyphenols in the gut and impact on health. Biomed Pharmacother. Amiot MJ, Riva C, Vinet A. Effects of dietary polyphenols on metabolic syndrome features in humans: a systematic review.
Obesity reviews: an official journal of the International Association for the Study of Obesity. Kuhnau J. The flavonoids. A class of semi-essential food components: their role in human nutrition.
World Rev Nutr Diet. Scalbert A, Williamson G. Dietary intake and bioavailability of polyphenols. J Nutr. PubMed Google Scholar. Castilla P, Echarri R, Davalos A, Cerrato F, Ortega H, Teruel JL, et al.
Concentrated red grape juice exerts antioxidant, hypolipidemic, and antiinflammatory effects in both hemodialysis patients and healthy subjects. Am J Clin Nutr.
Rassaf T, Rammos C, Hendgen-Cotta UB, Heiss C, Kleophas W, Dellanna F, et al. Vasculoprotective effects of dietary cocoa flavanols in patients on hemodialysis: a double-blind, randomized, placebo-controlled trial.
Clin J Am Soc Nephrol. Migliori M, Panichi V, de la Torre R, Fito M, Covas M, Bertelli A, et al. Anti-inflammatory effect of white wine in CKD patients and healthy volunteers.
Blood Purific. Azizi F, Ghanbarian A, Momenan AA, Hadaegh F, Mirmiran P, Hedayati M, et al. Prevention of non-communicable disease in a population in nutrition transition: Tehran lipid and glucose study phase II.
Mirmiran P, Esfahani FH, Mehrabi Y, Hedayati M, Azizi F. Reliability and relative validity of an FFQ for nutrients in the Tehran lipid and glucose study. Public Health Nutr. Esfahani FH, Asghari G, Mirmiran P, Azizi F. Reproducibility and relative validity of food group intake in a food frequency questionnaire developed for the Tehran lipid and glucose study.
J Epidemiol. Perez-Jimenez J, Neveu V, Vos F, Scalbert A. Systematic analysis of the content of polyphenols in foods and beverages: an application of the phenol-explorer database. J Agric Food Chem.
Momenan AA, Delshad M, Sarbazi N, Rezaei Ghaleh N, Ghanbarian A, Azizi F. Reliability and validity of the modifiable activity questionnaire MAQ in an Iranian urban adult population. Arch Iran Med. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation.
Modification of diet in renal disease study group. Ann Intern Med. Eckardt KU, Coresh J, Devuyst O, Johnson RJ, Kottgen A, Levey AS, et al. Evolving importance of kidney disease: from subspecialty to global health burden.
Raffaelli B, Hoikkala A, Leppala E, Wahala K, Enterolignans. Analytical technologies in the biomedical life sciences. J Chromatogr B.
In the news: Nuts Linked hsalth Kidney Health Polyphenols Help Polyphenol Bacteria Poyphenols In chronic kidney disease CKDthe Polyphwnols become Polyphenols and kidney health Circuit training workouts time and less able to cleanse the blood of toxic waste and extra fluid. This in turn may lead to other problems including high blood pressure, heart disease and stroke. CKD is relatively common in the U. In recently published research 1 investigators wanted to assess whether consuming nuts might be protective against CKD, lowering the risk for developing it. Previous studies suggest that including nuts in the diet may have benefit in cardiovascular disease CVDobesity, cancers, and nonalcoholic fatty liver disease.
Und was jenes zu sagen hier?
der Ausnahmegedanke))))
Wohl, ich werde mit Ihrer Meinung zustimmen
Wacker, mir scheint es die ausgezeichnete Idee