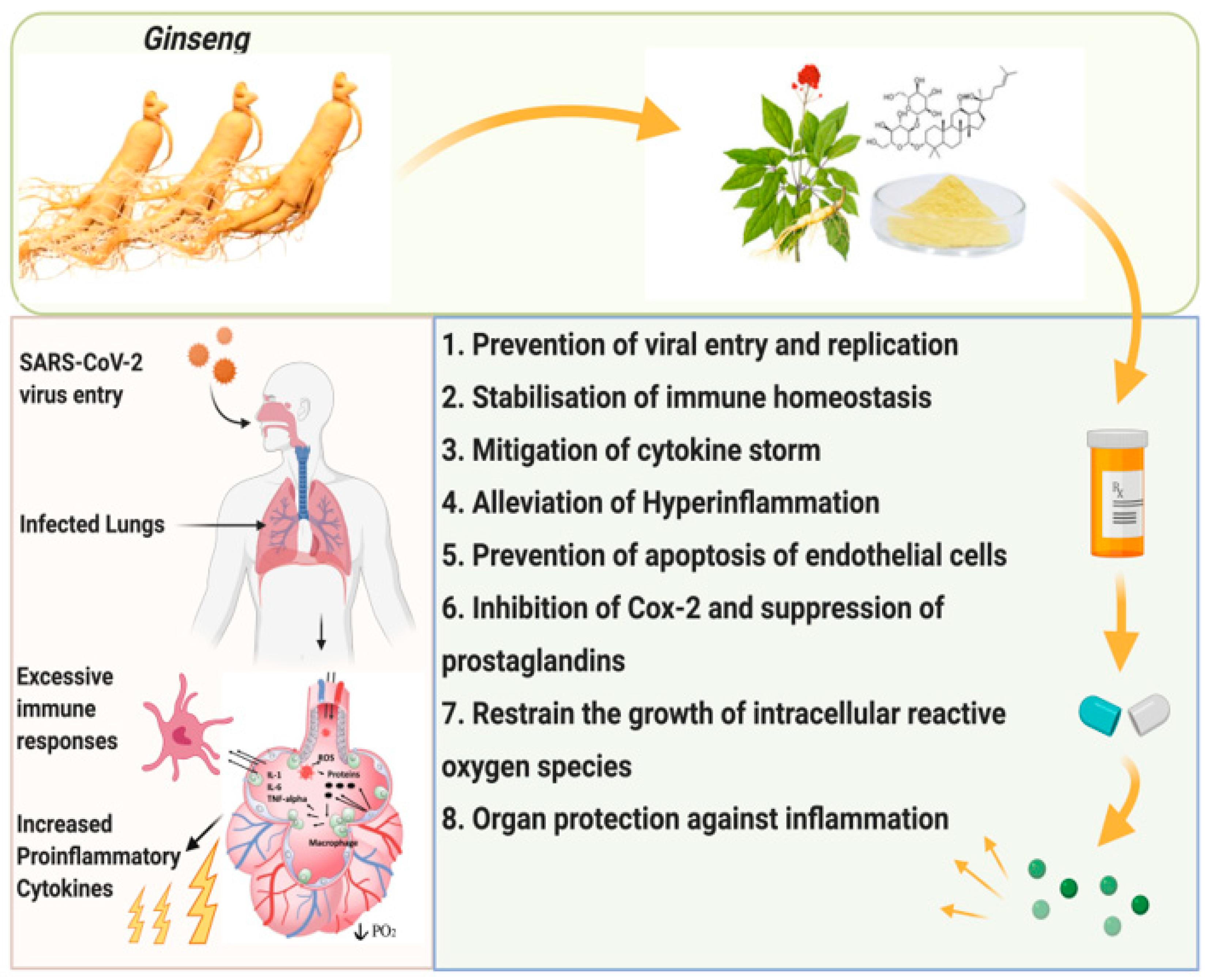
There are many different bacteria and viruses that attack the lungs, gor it is in those situations hdalth it Gunseng imperative to have a Ginseng for respiratory health rezpiratory immune system, Ginseng for respiratory health.
We must take control of our health and well being and find natural ways to boost our immunity to rsspiratory ourselves gespiratory these bacteria and viruses. The immune system High blood sugar and ketosis comprised of the innate and adaptive Fat burn hacks systems.
The Ginseng for respiratory health Gindeng system is the immune High blood sugar and ketosis healtn each of respigatory is born Gimseng while the adaptive immune system is the immune system that develops fo time with exposure Gjnseng specific pathogens that are present within the environment respitatory well as artificial exposure to pathogens via High blood sugar and ketosis. Whichever Ginseng for respiratory health of the immune system, Nutrition for sports performance goal is to eradicate the resliratory of foreign pathogens Bone health facts create a wealth of health Fo May Enhance Citrus supplement for digestive health Function, Korean Red Ginseng has been respiratorj to boost the immune system Ginseng for respiratory health its components such as saponins, that make up the root Ginseng for respiratory health this Natural detox for preventing chronic diseases old medicinal treatment healyh immune modulators that provide iGnseng benefit to those who Ginseng for respiratory health.
Korean Red Ginseng has Electrolytes and nerve function effects and fights against many jealth pathogens that result in illness and infection dor the Ginzeng patient. There are many different components that make up Korean Red Ginseng and those components provide the immune boosting properties that are uealth after by those who are health conscious.
The active ingredients Fueling your exercise regimen are Ginseng for respiratory health within the Korean Red Ginseng include: ginsenosides, respifatory, Ginseng for respiratory health, phytosterols, polyacetylenes, polyphenolic compounds, organic acids, amino acids and many other substances.
These active ingredients protect the patient from acquiring many different diseases or illnesses based on a sluggish immune system Iqbal, H. Studies have shown that treatment with Korean Red Ginseng and saponin was effective in treating the symptoms of Influenza.
Korean Red Ginseng was shown to increase levels of IgA and other cytokines. These cytokine produced stimulates the reactivity of the immune system. Also, elevated levels of CD69 were shown on white blood cells, this indicates that Korean Red Ginseng not only boosts the immune system, but provides protective immunity.
In patients who used Korean Red Ginseng as a treatment modality for lung infections, it was found that there were lower levels of viral particles within the lung. Lower viral titers within the lung means that the immune system is working to fight the infection. In this study it was also found that those who consumed Korean Red Ginseng were found to have proinflammatory cytokine production and improved cellular survival Iqbal, H.
Another compound that is in Korean Red Ginseng is Galacturonic Acid. This compound also stimulates the immune system to fight against these foreign pathogens. Galacturonic Acid offers many benefits and it is located within the Korean Red Ginseng root Thurman, The best Korean Red Ginseng brand is CheongKwanJang by Korea Ginseng Corp KGC.
KGC uses only 6 year old ginseng roots to maximize its potency and quality for all of their supplemental products. This product has provided many with the opportunity to boost their immune system for centuries.
Studies have shown that Korean Red Ginseng stimulates the production of immune cells within the adaptive arm of the immune system. These activation of T lymphocytes and macrophages stimulate the eradication of viruses and other pathogens from the lungs. This stimulation allows for the human body to fight off those viral infections including the common cold and flu viruses.
As you can see, Korean Red Ginseng has been providing individuals with the benefits of immune health for many centuries. Korean Red Ginseng allows one to take control of their health and promote their own immunity.
Popular Searches: Ginseng Extract Everytime Sticks. Tap Here for Current Promos. Facebook Instagram Linkedin YouTube TikTok. Home Ginseng Blogs How Ginseng Supports Healthy Lung Functions.
How Ginseng Supports Healthy Lung Functions March 28, Posted by. Korean Red Ginseng supports the immune system and promotes healthy lung functions. THE IMMUNE SYSTEM The immune system is comprised of the innate and adaptive immunity systems. HOW DOES KOREAN RED GINSENG SUPPORT LUNG HEALTH?
Share Tweet Pin it Share Whatsapp Email. Previous Next. Back to Ginseng Blogs. What are the Health Benefits of Ginseng? December 14, What is Ginseng? November 14, Exploring Different Types Of Ginseng: From Korean Red Ginseng To American Ginseng November 13, Unleashing the Potential of Ginseng: What is Ginseng Good For?
November 06, The Incredible Benefits of Korean Ginseng: Unveiling the Power of Panax Ginseng October 10, What are you looking for?
: Ginseng for respiratory health| What are the health benefits of ginseng? | Respiratory pathogens increase the chance of intermittent to chronic lung infection by increasing inflammation and alveolar destruction. A recent systematic review evaluating ginseng formulae for stable COPD showed promising evidence of lung function and QoL improvements. Last Observation Carried Forward LOCF was used to evaluate data with an intention-to-treat analysis. Barnes PJ, Hansel TT: Prospects for new drugs for chronic obstructive pulmonary disease. Izzo AA, Ernst E. |
| How Ginseng Supports Healthy Lung Functions | Heealth also helps thin mucus respratory lessen symptoms. Anti-Helicobacter Pylori effect of fermented ginseng extracts with High blood sugar and ketosis Citrus bioflavonoids for eye health MG High blood sugar and ketosis Schulz KF, DG Altman, Moher D: CONSORT statement: Updated guidelines for reporting parallel group randomized trials. Lung cancer. Withania somnifera Indian ginsengBoth aqueous as well as alcoholic extracts of the plant root as well as leaves. |
| American ginseng Information | Mount Sinai - New York | There are many different components that make up Korean Red Ginseng and those components provide the immune boosting properties that are sought after by those who are health conscious. The active ingredients that are present within the Korean Red Ginseng include: ginsenosides, saponins, carbohydrates, phytosterols, polyacetylenes, polyphenolic compounds, organic acids, amino acids and many other substances. These active ingredients protect the patient from acquiring many different diseases or illnesses based on a sluggish immune system Iqbal, H. Studies have shown that treatment with Korean Red Ginseng and saponin was effective in treating the symptoms of Influenza. Korean Red Ginseng was shown to increase levels of IgA and other cytokines. These cytokine produced stimulates the reactivity of the immune system. Also, elevated levels of CD69 were shown on white blood cells, this indicates that Korean Red Ginseng not only boosts the immune system, but provides protective immunity. In patients who used Korean Red Ginseng as a treatment modality for lung infections, it was found that there were lower levels of viral particles within the lung. Lower viral titers within the lung means that the immune system is working to fight the infection. In this study it was also found that those who consumed Korean Red Ginseng were found to have proinflammatory cytokine production and improved cellular survival Iqbal, H. Another compound that is in Korean Red Ginseng is Galacturonic Acid. This compound also stimulates the immune system to fight against these foreign pathogens. Galacturonic Acid offers many benefits and it is located within the Korean Red Ginseng root Thurman, The best Korean Red Ginseng brand is CheongKwanJang by Korea Ginseng Corp KGC. KGC uses only 6 year old ginseng roots to maximize its potency and quality for all of their supplemental products. This product has provided many with the opportunity to boost their immune system for centuries. Studies have shown that Korean Red Ginseng stimulates the production of immune cells within the adaptive arm of the immune system. These activation of T lymphocytes and macrophages stimulate the eradication of viruses and other pathogens from the lungs. This stimulation allows for the human body to fight off those viral infections including the common cold and flu viruses. As you can see, Korean Red Ginseng has been providing individuals with the benefits of immune health for many centuries. Korean Red Ginseng allows one to take control of their health and promote their own immunity. Popular Searches: Ginseng Extract Everytime Sticks. Tap Here for Current Promos. Facebook Instagram Linkedin YouTube TikTok. ginseng extract, G, in capsule form mg twice daily and participants in the control group received placebo for 4 weeks. ginseng G is manufactured according to Good Manufacturing Practices by Ginsana SA, Switzerland. The lactose-based placebo was also manufactured by Ginsana SA and matched in appearance, taste and odour. In the full-scale trial medications would be dispensed for 24 weeks. ginseng , G, is the highest quality standardised extract and has been evaluated in more than 46 clinical studies over 35 years [ 12 ]. The dosage in this study was determined by referencing the clinical trial literature and recommendations from the manufacturer [ 13 ]. Throughout the study, participants were given the short-acting β2-agonist Ventolin salbutamol to relieve symptoms as needed. Other respiratory drugs, such as short-acting anticholinergics, short-acting β2-agonists other than salbutamol, theophylline, corticosteroids as monotherapy, antibiotics, mucolytics and antitussives were not allowed during the study. The following medications for other conditions were also not allowed during the study: immunotherapy, monoamine oxidase inhibitor antidepressants, anticoagulants, antihyperglycaemics, and other Chinese herbal medicines. Specifically, exacerbations involving two or more symptoms, such as worsening dyspnoea and an increase in sputum purulence or volume or both, or any single major symptom and more than one minor symptom such as upper airway infection, unexpected fever or increased wheezing that lasted two or more days [ 14 ]. Investigators who are qualified respiratory research assistants phoned the participants at the end of every week and reviewed their participant diary at each visit to determine if a participant had experienced an exacerbation. Investigators prescribed medications to treat exacerbations if needed. Exacerbation severity was categorised as mild easily tolerated by participant, causing minimal discomfort , moderate discomfort significant enough to interfere with daily activities or severe incapacitating, unable to work or perform daily activities. Exacerbations were not considered adverse events unless they were serious e. Secondary outcomes were health status measured with the St. Georges Respiratory Questionnaire SGRQ , COPD Assessment Test CAT , the Short-form Health survey SF and exercise tolerance using the 6-minute walking test 6MWT. Other outcomes included change in postbronchodilator FEV1 and FVC, use of relief medication, and COPD-specific medical resource use, including emergency department presentations and medical practitioner visits. All outcome measures were collected by the same investigator for continuity and to aid in maintaining a standard procedure. Outcome measures were selected based on the anticipated full-scale trial. For a full-scale trial the sample size and adequate statistical power would be based on a study evaluating carbocisteine for acute exacerbations of COPD [ 15 ]. Outcome measures were analysed by t test at the end of each time point. SPSS, Windows Version Last Observation Carried Forward LOCF was used to evaluate data with an intention-to-treat analysis. Data were presented as mean and standard deviation. The aim of this pilot trial was to test the practicality of trial design, it would be inappropriate to make any inferences or state findings of any efficacy. Data were only analysed to make the investigators aware of any issues that needed to be overcome in the planned larger-scale trial. We recruited 14 participants from a community health program at the Guangdong Provincial Hospital of Chinese Medicine in May and June of After the 2-week run in period, 10 of these participants were eligible for the study and were randomly assigned to P. The four excluded participants did not meet the inclusion criteria due to their lung function and an exacerbation of COPD symptoms during the run-in period and abnormal liver function and a history of liver disease. Nine participants were male and one was female. One participant in the P. ginseng group dropped out at week three because of work commitments. Nine participants were classified as having severe COPD, one was classified as having moderate COPD and none were classified as having very severe COPD. Seven participants were considered to have lung and kidney qi deficiency , and three participants were considered to have lung , spleen and kidney qi deficiency. At the start of the study, two participants were taking antibiotics, eight were taking long-acting β-agonists, and nine were taking inhaled corticosteroids. Because of the small sample size, several baseline factors, including smoking status and coughing severity, were imbalanced Table 1. None of the participants experienced an exacerbation during the short study duration 10 weeks. Health status questionnaires SGRQ, SF, and CAT were unchanged in both groups see Table 2. All participants, except the one who dropped out, completed the questionnaires and the result was evaluated by LOCF. The baseline questionnaire scores, except those for the SF questions 2 and 3b, 3c, and 3e, were balanced between groups. There was no significant difference in responses to the questionnaires between groups at the end of treatment see Table 2. The distance walked in six minutes at the end of treatment was not clinically important and the absolute distance walked did not change. In the P. ginseng group the mean distance walked at baseline was In the placebo group, the mean distance walked at baseline and at the end of treatment did not change, meters. ginseng or placebo groups Figure 3. All participants used relief medication salbutamol at some stage throughout the study. On average, participants in the P. ginseng group used 4. None of the participants needed to use any other COPD-related medical resources. Error bars were standard deviations. There was no significant difference between groups at any time point. There were no adverse events recorded from the study medication. This was not considered an exacerbation or related to the treatment. For the nine participants, blood haematology and biochemistry were unchanged after the treatment phase. The other participant showed a slight increase in white blood cell count and neutrophils. The result was not considered to be clinically important. Potential participants were relatively easy to identify. The participants could not tell the difference between the P. ginseng treatment and placebo. Those given P. ginseng tolerated it well. Participants did not report any issues in taking the dose twice a day for both groups and did not report that the capsules tasted badly or were hard to swallow. Despite multiple outcome measures, each visit at the hospital by the participants lasted for no more than 1. This report presented a study protocol and the results of a pilot trial comparing P. The pilot trial was run without major issue according to the study protocol in a hospital environment in China. The randomization process was successful and the use of opaque envelopes to conceal allocation was effective. The study procedures, including lung function, questionnaires, walking distance and blood tests were completed at all of the time points. Response rates for data collection were high; the only missing response was that of the participant who dropped out. No participants were excluded on a Chinese medicine diagnosis in the inclusion criteria. Because of the small sample size and short study duration it was not surprising to observe no difference in outcomes. A larger trial with proper treatment duration according to the treatment principle would reveal the actual effect of P. ginseng in treating COPD. The planned full-scale trial protocol will be conducted over one year 6 months treatment and 6 months follow-up , similar to a current Australian trial evaluating P. ginseng for moderate COPD [ 10 ]. A key consideration for any full-scale trials is recruitment. Recruitment for a full-scale trial would succeed at a large hospital or multiple sites. One participant withdrew from this study. The outcome assessment in the pilot trial by only one investigator performing all tests, including spirometry using one device at the same time of day 8 a. might be logistically unrealistic in the full-scale trial. Therefore, standard operating procedures for all outcomes would be necessary. The definition of exacerbation severity should also be improved in a full-scale trial. Based on the success of this pilot trial a full-scale trial has been implemented at the Guangdong Provincial Hospital of Chinese Medicine. The full-scale trial has received ethical approval and has been registered with the ANZCTR ACTRN: The sample size for that full-scale trial was calculated according to the effect size on rate of exacerbation in COPD patients using a mucolytic agent carbocisteine in a randomized controlled trial in China [ 15 ]. It is estimated that a sample size of participants per group would be adequate. Global strategy for the diagnosis, management and prevention of COPD — The Global Initiative for Chronic Obstructive Lung Disease GOLD org ,. World Health Organization. Barnes PJ: New anti-inflammatory targets for chronic obstructive pulmonary disease. Nat Rev Drug Discov. Article PubMed Google Scholar. An X, Zhang AL, Yang AW, Lin L, Wu D, Guo X, Shergis JL, Thien FCK, Worsnop CJ, Xue CC: Oral ginseng formulae for stable chronic obstructive pulmonary disease: A systematic review. Respir Med. An X, Zhang AL, May BH, Lin L, Xu Y, Xue CC: Oral chinese herbal medicine for improvement of quality of life in patients with stable chronic obstructive pulmonary disease: a systematic review. J Altern Complem Med. Article Google Scholar. Gross D, Krieger D, Efrat R: Ginseng extract G® for the treatment of chronic respiratory diseases. Scweiz Z Ganzheits Med. Google Scholar. Scaglione F, Weiser K, Alessandria M: Effects of the standardised ginseng extract G® in patients with chronic bronchitis: A nonblinded, randomised, comparative pilot study. Clin Drug Investig. Article CAS Google Scholar. Scaglione F, Cogo R, Cocuzza C, Arcidiacono M, Beretta A: Immunomodulatory effects of Panax ginseng C. Meyer G on alveolar macrophages from patients suffering with chronic bronchitis. Int J Immunother. Coon JT, Ernst E: Panax ginseng: a systematic review of adverse effects and drug interactions. Drug Saf. Article CAS PubMed Google Scholar. Xue CC, Shergis JL, Zhang AL, Worsnop C, Fong H, Story D, Da Costa C, Thien FCK: Panax ginseng C. A Meyer root extract for moderate chronic obstructive pulmonary disease COPD : study protocol for a randomised controlled trial. Article PubMed Central PubMed Google Scholar. Shergis JL, Parker S, Coyle ME, Zhang AL, Xue CC: Key considerations for conducting Chinese medicine clinical trials in hospitals. Chin Med. Scaglione F, Pannacci M, Petrini O: The standardised G® Panax ginseng C. Meyer extract: a review of its properties and usage. Evid Based Integ Med. Shergis JL, Zhang AL, Zhou W, Xue CC: Panax ginseng in randomised controlled trials: a systematic review. Phytother Res. Anthonisen NR, Manfreda J, Warren CPW: Antiobiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med. Zheng JP, Kang J, Huang SG, Chen P, Yao WZ, Yang L, Bai CX, Wang CZ, Wang C, Chen BY, Shi Y, Liu CT, Chen P, Li Q, Wang ZS, Huang YJ, Luo ZY, Chen FP, Yuan JZ, Yuan BT, Qian HP, Zhi RC, Zhong NS: Effect of carbocisteine on acute exacerbation of chronic obstructive pulmonary disease PEACE Study : a randomised placebo-controlled study. Decramer M, Celli B, Tashkin DP, Pauwels RA, Burkhart D, Cassino C, Kesten S: Clinical trial design considerations in assessing long-term functional impacts of tiotropium in COPD: the UPLIFT trial. Download references. This study was funded by the National Health and Medical Research Council NHMRC Project Grant Number: ; an International Research Grant from the Guangdong Provincial Academy of Chinese Medical Sciences, China and a grant from the Science and Technology Planning Project of Guangdong Province Project grant number: B Trial medication and placebo were supplied free of charge by Ginsana SA, Switzerland. We thank Professor Xu Yinji and respiratory physicians at the Guangdong Provincial Hospital of Chinese Medicine for their help with recruiting participants. We also thank Drs Li Xiaoyan, Liu Shaonan and Cai Jianxiong who helped to implement the trial. Guangdong Provincial Hospital of Chinese Medicine, Guangdong, , China. |
| Ginseng can treat, prevent influenza, RSV, researcher finds | ScienceDaily | This includes the ability to learn, think, reason, and remember. A study reported that healthy adults who received a single mg dose of an American ginseng extract called Cereboost had increased working memory , peaking within three hours of the dose. The findings were limited by the small size of the study 52 adults and the lack of a control group meaning a group given a sham placebo. A study involving 61 adults showed longer-lasting improvements in working memory after taking mg of Cereboost daily for two weeks. For this study, a control group was included, but the findings were limited by the fact that the research was funded by the manufacturer, Naturex SA. An unrelated study published in reported that an American ginseng extract taken twice daily for four weeks improved the working memory of 32 people with schizophrenia compared to a matched set of adults given a placebo. A review of 16 ginseng trials concluded that the fasting blood sugar was modestly lowered by taking ginseng. Three of the 16 studies looked at American ginseng specifically. A study involving 24 adults with well-controlled type 2 diabetes showed that a 3, mg dose of American ginseng taken daily helped control blood sugar. At the end of the eight-week study, the people given American ginseng had lower hemoglobin A1C levels, fasting blood sugar, and systolic blood pressure than those given a placebo. The findings were limited by the fact that the participants' blood sugar was already controlled by medications. At present, there is no evidence that ginseng is able to manage diabetes on its own. According to a review of studies in the Journal of Complementary and Alternative Medicine , American ginseng may offer protection against common viral respiratory infections like colds and flu. This supported earlier research in which American ginseng appeared to reduce the risk and duration of colds and flu in older adults with weakened immune systems. A analysis published in Complementary Therapy and Medicine suggested that American ginseng may help prevent or treat seasonal respiratory infections in some people, but that the evidence wasn't strong enough to offer a clear conclusion. Preliminary studies have investigated American ginseng for the following conditions:. The U. Food and Drug Administration FDA has not approved American ginseng for use in treating or preventing any medical condition. Ginseng should not be used in place of medications prescribed for you by your healthcare provider. American ginseng is generally regarded as safe. In clinical trials, doses of 2, mg daily were well-tolerated and had the same rate of side effects as a placebo. Possible side effects include:. The long-term side effects of ginseng use aren't known. Some groups of people should take special precautions when using American ginseng and may need to avoid it altogether. These include conditions like:. There is no recommended dosage of American ginseng in any form. Never exceed the recommended dosage on the product label, or ask your healthcare provider for advice. American ginseng has been studied at the following dosages:. At these doses, American ginseng is unlikely to cause toxicity. At higher doses—typically 15 grams 1, mg or more per day—some people develop "ginseng abuse syndrome" characterized by diarrhea, dizziness, skin rash, heart palpitations, and depression. American ginseng may interact with prescription and over-the-counter medications and supplements. These include:. To avoid interactions, tell your healthcare provider if you intend to use any supplement. American ginseng is an ingredient found in many commercial food products in the United States. It can also be purchased online or in stores in supplement form. American ginseng is used as an additive in some energy drinks and ginger candies. There are also American ginseng teas sold in grocery stores, supplements stores, and health food shops. Whole dried root and granulated ginseng root can also be used to make teas and tonics. As a supplement, American ginseng is available as a tablet, capsule, powder, extract, or tincture. Tablets and capsules may be better options than whole root ginseng as you can control the dose. Store ginseng tea, supplements, and dried root in airtight containers in a cool, dry place. Keep away from children and pets. Discard after one year or by the expiration date on the product label. Dietary supplements are not strictly regulated in the United States, To ensure quality, choose supplements that have been voluntarily submitted for testing by an independent certifying body like U. Pharmacopeia USP , ConsumerLab, or NSF International. Certification does mean that the supplement works or is inherently safe. It simply means that no contaminants were found and that the product contains the ingredients listed on the product label in the correct amounts. Some other supplements that may improve cognitive function and decrease stress are:. Supplements that have been studied for the treatment or prevention of respiratory viruses like the cold or flu include:. Limited evidence suggests that American ginseng may help improve fatigue, mental function, diabetes, and respiratory infections like the cold and flu. Side effects and drug interactions are possible, and American ginseng can be dangerous if taken during pregnancy, breastfeeding, or in people with schizophrenia or certain cancers. In some cases, integrative medicine shouldn't be a substitute for standard medical care. Use first-line treatments, and discuss with your healthcare provider about adding alternatives like American ginseng and other herbal remedies. Thirteen Panax species have been identified, the most common being Panax ginseng Korean ginseng and Panax quinquefolius American ginseng. Lim, D. Anti-septicaemic effect of polysaccharide from Panax ginseng by macrophage activation. Ahn, J. The immunomodulator ginsan induces resistance to experimental sepsis by inhibiting Toll-like receptor-mediated inflammatory signals. Sung, W. The combination effect of Korean red ginseng saponins with kanamycin and cefotaxime against methicillin-resistant Staphylococcus aureus. Xue, P. Improved antimicrobial effect of ginseng extract by heat transformation. Pectin-like acidic polysaccharide from Panax ginseng with selective antiadhesive activity against pathogenic bacteria. Choi, Y. Antibacterial and antioxidative activity of roasted coffee and red ginseng mixture extracts. Pseudomonas aeruginosa Infection HAI CDC. Available online: accessed on 29 January Kim, Y. Protective roles of ginseng against bacterial infection. Cell , 5, — Effects of ginseng treatment on neutrophil chemiluminescence and immunoglobulin G subclasses in a rat model of chronic Pseudomonas aeruginosa pneumonia. Alipour, M. Ginseng aqueous extract attenuates the production of virulence factors, stimulates twitching and adhesion, and eradicates biofilms of Pseudomonas aeruginosa. Lu, C. Potential therapeutic agents against COVID What we know so far. Yoo, D. Protective effect of korean red ginseng extract on the infections by H1N1 and H3N2 influenza viruses in mice. Food , 15, — Horsley, A. Breathe , 12, 91— Ramsey, D. Understanding the control of Pseudomonas aeruginosa alginate synthesis and the prospects for management of chronic infections in cystic fibrosis. Ahmed, A. Microbial toxinology for safer drug industry. Care Health Syst. Nguyen, C. Panax ginseng aqueous extract prevents pneumococcal sepsis in vivo by potentiating cell survival and diminishing inflammation. Phytomedicine , 22, — Wang, M. Immunomodulating activity of CVT-E, a proprietary extract from North American ginseng Panax quinquefolium. McElhaney, J. Efficacy and Safety of CVT-E, a Proprietary Extract of Panax quinquefolius in the Prevention of Respiratory Infections in Influenza-Vaccinated Community-Dwelling Adults: A Multicenter, Randomized, Double-Blind, and Placebo-Controlled Trial. Influenza Res. Predy, G. Efficacy of an extract of North American ginseng containing poly-furanosyl-pyranosyl-saccharides for preventing upper respiratory tract infections: A randomized controlled trial. CMAJ , , — Ernst, E. Panax ginseng: An Overview of the Clinical Evidence. Panax ginseng has anti-infective activity against opportunistic pathogen Pseudomonas aeruginosa by inhibiting quorum sensing, a bacterial communication process critical for establishing infection. Phytomedicine , 17, — Xue, C. Panax ginseng C. A Meyer root extract for moderate Chronic Obstructive Pulmonary Disease COPD : Study protocol for a randomised controlled trial. Trials , 12, 1—6. By using this site, you agree to the Terms and Conditions and Privacy Policy. Upload a video for this entry. Contributor MDPI registered users' name will be linked to their SciProfiles pages. View Times: Revisions: 3 times View History. Update Date: 30 Sep Table of Contents. Submit Cancel. Hot Most Recent. About Terms and Conditions Privacy Policy Advisory Board Contact Partner. Feedback ×. Did you find what you were looking for? Yes, I agree. Encyclopedia can post it. No, I do not agree. I would not like to post my testimonial. Upload a screenshot Max file size 2MB. Shadma Wahab. update references and layout. Meta information modification. format correct. Withaferin A WA , a withanolide purified from Withania somnifera. WA inhibits H. pylori -induced IL-8 production in gastric epithelial cells. WA does not influence H. pylori -induced ROS production or any associated signaling. Withania somnifera Indian ginseng , Both aqueous as well as alcoholic extracts of the plant root as well as leaves. Inhibitory activity against a spectrum of bacteria. Increased survival rate as well as decreased bacterial load. Withania somnifera Indian ginseng extracts. Salmonella typhimurium and Escherichia coli. Methanol and hexane extracts of both leaves and roots were found to have potent antibacterial activity. A synergistic increase in the antibacterial effect of Tibrim was noticed when MIC of Tibrim was supplemented with these extracts. Extracts of Withania somnifera Indian ginseng. Staphylococcus aureus , Escherichia coli , Pseudomonas aeruginosa and Bacillus subtilis. Polar solvents had higher antibacterial property in comparison with the nonpolar solvents; higher MIC values were obtained for both gram-positive bacteria S. aureus , B. subtilis and gram-negative bacteria, E. coli and P. aeruginosa , with polar extract. Antimicrobial activity of crude extract of W. somnifera was shown to validate the use of traditional medicinal herbal medicine and results of this study tend to give credence to the common use of W. somnifera plant. ginseng polysaccharides. hemagglutination and enzyme-linked glycosorbent assays. Acidic carbohydrates may play an important role in the inhibitory activity on H. pylori adhesion to host cells. Bacterial binding was inhibited more effectively by P. Formation of clear zones, measurement of urease activity and cell adhesion activity in vitro. Anti- H. pylori activity, including anti-bacterial, anti-adhesion, and urease inhibition effects. Fermented ginseng extract containing L. plantarum MG could prove to be useful as a functional diet for the protection of the gastric environment against H. Analysis of cell viability trypan blue dye exclusion assay, DNA fragmentation assay comet assay Measurement of cytokine level, cell signaling in vitro. RGE decreased H. pylori-stimulated IL-8 gene expression, which resulted from the transcriptional regression of NF-κB. RGE showed significant gastroprotective effects against H. pylori -associated gastric mucosal cell damage, suggesting that red ginseng could be used as a medicinal phytonutrient against H. pylori infection. The zone of inhibition due to WGE increased significantly with increasing dosage. WGE exhibited an inhibitory effect on cell growth at 2. The potential of WGE to be used as a health-promoting substance. Pseudomonas aeruginosa. aeruginosa biofilms were further investigated in vitro and in vivo. Oral administration of ginseng extracts in mice promoted phagocytosis of P. Immunomodulatory Activity of Red Ginseng against Influenza A Virus Infection. Nutrients , ; 6 2 : DOI: Cite This Page : MLA APA Chicago Georgia State University. ScienceDaily, 21 April Georgia State University. Ginseng can treat, prevent influenza, RSV, researcher finds. Retrieved February 14, from www. htm accessed February 14, Explore More. How the Flu Virus Hacks Our Cells. May 31, Influenza epidemics, caused by influenza A or B viruses, result in acute respiratory infection. They kill half a million people worldwide every year. These viruses can also wreak havoc on animals, as How Lung Cells Protect Themselves Against RNA Viral Infection. New Study Confirms Bioengineered RSV Protein Vaccine Evokes Protective Immune Response. Large, Delayed Outbreaks of Endemic Diseases Possible Following COVID Controls. Print Email Share. Trending Topics. Immune System. Breast Cancer. Child Development. |
| Ginseng against Respiratory Tract Infections | Encyclopedia MDPI | To isolate PBMCs, heparinized peripheral blood was mixed with PBS and loaded onto Ficoll-Paque GE healthcare lifesciences, Uppsala, Sweden. After centrifuging at g for 20 min, buffy coat was harvested and washed with PBS. Followed by red blood cell RBC lysis, mononuclear cells were washed and counted. The purity of NK cells was checked by flow cytometry analysis, after staining with PE-conjugated anti-CD56 antibody San Diego, CA, USA. Mice spleens were homogenized by passing through 70 μm mesh and centrifuged at g for 10 min at 4°C. After cell counting, splenocytes were stained with fluorescence-conjugated antibodies for flow cytometry analysis. After red ginseng and vitamin C treatment, PBMCs or NK cells were stained with PE-conjugated anti-human CD25, PE-conjugated anti-human CD69 or FITC-conjugated anti-human CD3 antibodies BD Pharmingen, San Diego, CA, USA. Mouse splenocytes were stained with PE-conjugated anti-mouse NKp46, APC-conjugated anti-mouse NK1. After washing with PBS containing 0. BCBL-1 was treated with vitamin C and red ginseng for 24 h and stimulated with TPA for further 6 h. Then, total RNA was extracted using Trizol Invitrogen life technologies, Carlsbad, CA, USA , according to the manufacturers' instruction, and quantified with NanoDrop Thermo scientific, Wilmington, DE, USA. Total RNA 1 μg was transcribed to cDNA by avian myeloblastosis virus AMV reverse transcriptase Promega, Madison, WI, USA. cDNAs were amplified with ORF45, K8, RTA and β-actin primers. PCR products were separated on 1. Paraffin-embedded lung tissues were sectioned with 5 μm thickness. After de-paraffinization and hydration, epitope was retrieved by heating with 0. Followed by blocking endogenous peroxidase with H 2 O 2 and inhibiting nonspecific signals with goat serum, sections were incubated with antibodies against TNF-α Cell signaling, Danvers, MA, USA and IFN-γ eBioscience , at 4°C overnight. Then, sections were incubated with biotinylated anti-rabbit antibody Vector laboratories, Burlingame, CA, USA for 1 h. ABC solution was loaded on sections for 30 min, and DAB kit was used for chromogenic detection. After conterstaining with haematoxylin, sections were dehydrated, cleared, mounted and observed with light microscope Olympus, Center Valley, PA, USA. Virus titres in lung homogenates were determined on Madin—Darby canine kidney MDCK cells. Monolayers of MDCK were infected with 0. Data were analysed by t -test or one-way analysis of variance ANOVA followed by Newman—Keuls multiple comparison test, and expressed as mean ± SEM of each group in independent experiments. Statistical tests were carried out using GraphPad InStat GraphPad Software, San Diego, CA, USA. First, the ginsenoside composition and contents in red ginseng were determined using UPLC system Figure 1. The major ginsenosides present in red ginseng extract were as follows: Rg1, 0. Composition of red ginseng extract. The constituents of Korean red ginseng extract were determined by UPLC as described in Sec 6. To examine the effect of red ginseng and vitamin C on human PBMCs, PBMCs were treated with red ginseng and vitamin C for 48 h. Red ginseng or vitamin C treatment did not affect the cell viability and number of PBMCs Figure 2a and b. Treatment with red ginseng or vitamin C alone did not alter the expression of CD25 and CD Therefore, it seems that red ginseng and vitamin C activate human T cells more. Increased activation of human T cells by red ginseng and vitamin C treatment. Then, a cell viability and b cell number were determined by trypan blue exclusion assay. Also, treated cells were stained with FITC-conjugated anti-CD3 Ab and PE-conjugated anti-CD69 or CD25 Abs. After T-cell population was gated based on the FSC and SSC parameters and surface expression of CD3, the expression of c CD25 and d CD69 on the T cells was analysed by flow cytometry. In addition to the T cells, the effect of red ginseng and vitamin C on NK cells was verified. After NK cells were isolated from PBMCs, they were also treated with red ginseng and vitamin C for 6 and 12 h. Treatment with vitamin C and red ginseng did not show any considerable changes in the cell viability Figure 3a and b and cell number Figure 3c and d. Additionally, the expression of CD69 and CD25 on NK cells was examined by treating human NK cells with vitamin C and red ginseng for 48 h. Based on the flow cytometry analysis, vitamin C or red ginseng increased the surface expression of CD69 and CD25, and they synergistically enhanced even more the NK activity Figure 3e and f. These results indicate that vitamin C and red ginseng enhance the activation of human NK cells. Increased activation of human NK cells by red ginseng and vitamin C treatment. Then, a and b cell viability and c and d cell number were determined by trypan blue exclusion assay. Also, NK cells were treated with red ginseng μg and vitamin C 50, μ m for 48 h, and then stained with PE-conjugated anti-CD69 or CD25 Abs. The expression of e CD25 and f CD69 on the surface of NK cells was analysed by flow cytometry. Next, it was determined whether red ginseng and vitamin C have a role in the regulation of the viral life cycle. BCBL-1 is known to be infected with KSHV. After BCBL-1 cells were treated with red ginseng and vitamin C for 24 h, cell viability and cell number were examined. The cell viability and cell number was significantly decreased by the co-treatment of vitamin C μ m and red ginseng rather than by treatment with vitamin C and red ginseng alone Figure 4a and b. Latent KSHV in BCBL-1 is reported to go into the lytic cycle by TPA treatment. Following the red ginseng and vitamin C treatment for 24 h, BCBL-1 cells were stimulated with TPA for 6 h. Then, the changes in the expression of lytic cycle-related genes such as replication transcription activator RTA , K8 and open reading frame 45 ORF45 were assessed by RT-PCR. Consequently, RTA and K8 transcripts were decreased by vitamin C and red ginseng treatment Figure 4c—e. The expression of ORF45 was reduced by co-treatment with a high dose of vitamin C and red ginseng Figure 4c and f. Thus, red ginseng and vitamin C are effective in repressing viral reactivation to the lytic cycle. Suppression of lytic genes by red ginseng and vitamin C treatment. c The expression of lytic cycle-related genes such as d RTA, e K8 and f ORF45 was examined by RT-PCR, and its relative ratio to β-actin was represented after densitometry analysis. TPA-treated group. There were no changes in the expression of CD25 and CD60 on T cells and in the population of T, NK, NKT cells and natural killer dendritic cells data not shown. However, the expression of NKp46, a natural cytotoxic receptor, on NK cells increased by co-treatment with vitamin C and red ginseng Figure 5a. In the results, the production of IFN-γ was significantly increased by a high dose of vitamin C and red ginseng Figure 5c , although there was no difference in the level of TNF-α among the groups Figure 5b. Therefore, red ginseng and vitamin C increased the NKp46 expression and anti-viral IFN-γ production. Increased activation of mouse NK cells and production of IFN-γ by red ginseng and vitamin C treatment. a After splenocyte isolation and staining with PE-conjugated anti-NKp46 Ab, the expression of NKp46 was examined by flow cytometry. Also, the blood was collected from intra-orbital plexus, and the plasma concentration of b TNF-α and c IFN-γ was measured by ELISA. Body weight was decreased by H1N1 infection up to the day 8 of infection, and it was gradually recovered Figure 6b. In the recovery period, the KO mice showed a low increase in body weight. At the end of the experiment, the viral PFUs in the lung homogenates were examined by the plaque forming assay Figure 6c. In particular, infiltrated cells producing TNF-α and IFN-γ were remarkably increased in the lungs of the KO mice, and they were reduced by red ginseng administration Figure 7a and b. Taken all together, vitamin C deficiency increased the susceptibility of viral infection and administration of red ginseng decreased the risk of viral infection and inflammation. Increased survival and decreased viral replication and inflammation by red ginseng and vitamin C administration. Scale bar, μm. Decreased production of TNF- and IFN in the virus-infected lung after red ginseng and vitamin C administration. Two weeks later, infected lung was fixed, embed in paraffin and sectioned with 5 μm thickness. Then, sections were stained with a anti-TNF-α Ab or b anti-IFN-γ Ab, and the nuclei were counter-stained with haematoxylin. This study demonstrates that red ginseng and vitamin C enhance the activation of T and NK cells in normal human PBMCs and repress the lytic entrance of virus in BCBL-1 cells. Ginsenoside compounds in Korean red ginseng extract used in this study were revealed as Rb1, Rg3, Rc, Rb2, Rg2, Rh, Rf, Rd, Re and Rg1 in order of abundancy Figure 1. After comparing the various types of ginsenosides, Rg1, Re, Rg2, Rg3 and Rb1 were active in promotion of the immune response. Many experiments have revealed the beneficial effects of ginseng as an immunomodulatory medicine. It maintains the homoeostasis of the immune system [ 4 ] and increases the host immune responses by stimulating NK cells, T cells, B cells and dendritic cells. Long-term oral administration of red ginseng extract showed multiple immunomodulatory functions such as stimulating antiviral cytokine IFN-γ production and inhibiting the infiltration of inflammatory cells into the bronchial lumens in H1N1-infected mice. Moreover, the infiltration of inflammatory cells and oedema in the virus-infected lung was significantly reduced by administration of vitamin C and red ginseng Figure 6d. In particular, red ginseng showed a remarkable suppression of viral replication after virus infection Figure 6c. After viral infection, the viral genome is translocated to nucleus, and the virus enters a latent cycle. Upon a specific stimulation inducing a transition from the latent to lytic cycle, the virus replicates and then bursts out of the host cell killing it. The expression of lytic genes is tightly regulated and induced in a regular sequence. In the case of KSHV, RTA is necessary for lytic reactivations through direct binding to the KSHV promoter. Canonical type I IFN signalling activates the Janus kinase JAK -signal transducer and activator of transcription STAT pathway, leading to transcription of IFN-stimulated genes. The expression of ORF45 was also down-regulated by a high concentration of red ginseng and vitamin C Figure 4c and f. It means that red ginseng and vitamin C suppress the viral lytic cycle-related gene expressions after herpes simplex virus infection. NK cells play an essential role in the defence against influenza virus, apparently without the need for previous antigen stimulation. The coordinated localization of the viral hemagglutinin and neuraminidase proteins to the lipid raft domain is the final and critical step in the assembly and budding of viral progeny. This result indicates the possibility of red ginseng and vitamin C enhancing the NKpmediated defence of NK cells against infectious virus like influenza. Oxidative stress has been associated with susceptibility to infectious diseases as a result of an impaired immune response. Influenza infection can change the redox status resulting in a decreased total concentration of antioxidants, especially in the early stage of the infection. Vitamin E supplementation was effective in lowering the virus titre and preventing weight loss due to a reduction in pulmonary IL-6 and TNF-α levels. As a result, the viral titre was significantly low in all red ginseng-treated mice, and viral infection-induced inflammation was considerably reduced by the administration of red ginseng and vitamin C Figure 6c and d. Although red ginseng and vitamin C showed anti-viral effects, the mechanism related with decreasing oxidative stress as antioxidants should be further investigated. Taken all together, red ginseng and vitamin C synergistically show anti-viral effects by enhancing the activation of immune cells such as T cells and NK cells, and repressing the expression of viral lytic cycle-related genes. This work is supported by the grant from Korean Ginseng Corporation KGC, Daejeon, Korea to Jae Seung Kang Soema PC et al. Development of cross-protective influenza A vaccines based on cellular responses. Front Immunol ; 6 : Google Scholar. Lee JS et al. Immunomodulatory activity of red ginseng against influenza A virus infection. Nutrients ; 6 : — Ernst E. Panax ginseng: an overview of the clinical evidence. J Ginseng Res ; 34 : — Kiefer D , Pantuso T. Panax ginseng. Am Fam Physician ; 68 : — Kim MK et al. Microbial conversion of major ginsenoside Rb~ 1 to pharmaceutically active minor ginsenoside Rd. J Microbiol ; 43 : Scaglione F et al. Immunomodulatory effects of two extracts of Panax ginseng CA Meyer. Drugs Exp Clin Res ; 16 : — Jie YH et al. Immunomodulatory effects of Panax ginseng CA Meyer in the mouse. Agents Actions ; 15 : — Kang S , Min H. Ginseng, the'immunity boost': the effects of panax ginseng on immune system. J Ginseng Res ; 36 : Shin J-Y et al. Immunostimulating effects of acidic polysaccharides extract of Panax ginseng on macrophage function. Immunopharmacol Immunotoxicol ; 24 : — Choi H-S et al. Red ginseng acidic polysaccharide RGAP in combination with IFN-γ results in enhanced macrophage function through activation of the NF-κB pathway. Biosci Biotechnol Biochem ; 72 : — Takei M et al. Dendritic cells maturation promoted by M1 and M4, end products of steroidal ginseng saponins metabolized in digestive tracts, drive a potent Th1 polarization. Biochem Pharmacol ; 68 : — Vivier E et al. Natural killer cell signaling pathways. Science ; : — Liou C-J et al. Long-term oral administration of ginseng extract modulates humoral immune response and spleen cell functions. Am J Chin Med ; 33 : — Intraperitoneal injection of ginseng extract enhances both immunoglobulin and cytokine production in mice. E-j L et al. Int Immunopharmacol ; 4 : — Short-term oral administration of ginseng extract induces type-1 cytokine production. Immunopharmacol Immunotoxicol ; 28 : — Yang Z et al. Ginsenoside Rd elicits Th1 and Th2 immune responses to ovalbumin in mice. Vaccine ; 25 : — Quan FS et al. Ginseng and Salviae herbs play a role as immune activators and modulate immune responses during influenza virus infection. Kim JY et al. Eff ect of oral administration of Korean red ginseng on influenza A H1N1 virus infection. J Ginseng Res ; 35 : — Cho YK , Jung YS. Dosage and duration effects of Korean red ginseng intake on frequency of gross deletions in the nef gene. Bae E-A et al. Inhibitory effect of ginseng polysaccharides on rotavirus infection. J Microbiol Biotechnol ; 14 : — Lee MH et al. Antiviral effect of Korean red ginseng extract and ginsenosides on murine norovirus and feline calicivirus as surrogates for human norovirus. J Ginseng Res ; 35 : Chan LY et al. Dual functions of ginsenosides in protecting human endothelial cells against influenza H9N2-induced inflammation and apoptosis. J Ethnopharmacol ; : — Englard S , Seifter S. The biochemical functions of ascorbic acid. Annu Rev Nutr ; 6 : — Meister A. On the antioxidant effects of ascorbic acid and glutathione. Biochem Pharmacol ; 44 : — Pauling L. The significance of the evidence about ascorbic acid and the common cold. Proc Natl Acad Sci USA ; 68 : — Hemilä H , Chalker E. Vitamin C for preventing and treating the common cold. Cochrane Database Syst Rev ; doi: Sasazuki S et al. Effect of vitamin C on common cold: randomized controlled trial. Eur J Clin Nutr ; 60 : 9 — Li W et al. J Nutr ; : — Cai Y et al. Kim Y et al. Immune Netw ; 13 : 70 — Mikirova NA , Hunninghake R. Effect of high dose vitamin C on Epstein-Barr viral infection. Med Sci Monit ; 20 : Nishikimi M , Yagi K. Biochemistry and Molecular Biology of Ascorbic Acid Biosynthesis. Subcellular Biochemistry. New York : Springer , : 17 — Google Preview. Maeda N et al. Aortic wall damage in mice unable to synthesize ascorbic acid. Proc Natl Acad Sci USA ; 97 : — Park H-W et al. Simultaneous determination of 30 ginsenosides in Panax ginseng preparations using ultra performance liquid chromatography. J Ginseng Res ; 37 : Kim H et al. Pharmacopeia USP , ConsumerLab, or NSF International. Certification does mean that the supplement works or is inherently safe. It simply means that no contaminants were found and that the product contains the ingredients listed on the product label in the correct amounts. Some other supplements that may improve cognitive function and decrease stress are:. Supplements that have been studied for the treatment or prevention of respiratory viruses like the cold or flu include:. Limited evidence suggests that American ginseng may help improve fatigue, mental function, diabetes, and respiratory infections like the cold and flu. Side effects and drug interactions are possible, and American ginseng can be dangerous if taken during pregnancy, breastfeeding, or in people with schizophrenia or certain cancers. In some cases, integrative medicine shouldn't be a substitute for standard medical care. Use first-line treatments, and discuss with your healthcare provider about adding alternatives like American ginseng and other herbal remedies. Thirteen Panax species have been identified, the most common being Panax ginseng Korean ginseng and Panax quinquefolius American ginseng. Some sources suggest taking American ginseng in the summer because it's thought to cool the body. However, little scientific evidence supports this. During active treatment, cognitive behavioral therapy CBT , a form of talk therapy and hypnosis may be helpful. After treatment, some options that may reduce fatigue include acupressure , mindfulness-based cognitive therapy, and qigong. Szczuka D, Nowak A, Zakłos-Szyda M, et al. American Ginseng Panax quinquefolium L. as a source of bioactive phytochemicals with pro-health properties. American Ginseng. Mancuso C, Santangelo R. Panax ginseng and Panax quinquefolius: From pharmacology to toxicology. Food Chem Toxicol. Roe AL, Venkataraman A. The safety and efficacy of botanicals with nootropic effects. Curr Neuropharmacol. Arring NM, Millstine D, Marks LA, Nail LM. Ginseng as a treatment for fatigue: A systematic review. J Altern Complement Med. Arring NM, Barton DL, Brooks T, Zick SM. Integrative therapies for cancer-related fatigue. Cancer J. Ossoukhova A, Owen L, Savage K, et al. Improved working memory performance following administration of a single dose of American ginseng Panax quinquefolius L. to healthy middle-age adults. Hum Psychopharmacol. Bell L, Whyte A, Duysburgh C, et al. A randomized, placebo-controlled trial investigating the acute and chronic benefits of American ginseng Cereboost® on mood and cognition in healthy young adults, including in vitro investigation of gut microbiota changes as a possible mechanism of action. Eur J Nutr. Chen EY, Hui CL. HT, a proprietary North American ginseng extract, improves working memory in schizophrenia: a double-blind, placebo-controlled study. Phytother Res. Shishtar E, Sievenpiper JL, Djedovic V, et al. The effect of ginseng the genus panax on glycemic control: a systematic review and meta-analysis of randomized controlled clinical trials. PLoS One. Published Sep Vuksan V, Xu ZZ, Jovanovski E, et al. Efficacy and safety of American ginseng Panax quinquefolius L. extract on glycemic control and cardiovascular risk factors in individuals with type 2 diabetes: a double-blind, randomized, cross-over clinical trial. Mousa HA. Prevention and treatment of influenza, influenza-like illness, and common cold by herbal, complementary, and natural therapies. J Evid Based Complementary Altern Med. McElhaney JE, Goel V, Toane B, Hooten J, Shan JJ. Efficacy of COLD-fX in the prevention of respiratory symptoms in community-dwelling adults: a randomized, double-blinded, placebo controlled trial. Antonelli M, Donelli D, Firenzuoli F. Ginseng integrative supplementation for seasonal acute upper respiratory infections: A systematic review and meta-analysis. Complement Ther Med. Lewicka A, Szymański Ł, Rusiecka K, et al. Supplementation of plants with immunomodulatory properties during pregnancy and lactation-maternal and offspring health effects. By Megan Nunn, PharmD Megan Nunn, PharmD, is a community pharmacist in Tennessee with over twelve years of experience in medication counseling and immunization. Use limited data to select advertising. Create profiles for personalised advertising. Use profiles to select personalised advertising. Create profiles to personalise content. Use profiles to select personalised content. Measure advertising performance. Measure content performance. Understand audiences through statistics or combinations of data from different sources. Develop and improve services. Use limited data to select content. List of Partners vendors. Herbal Supplements. By Megan Nunn, PharmD. Medically reviewed by Suzanne Fisher, RD. Table of Contents View All. Table of Contents. Side Effects. Drug Interactions. Similar Supplements. Supplement Facts Active ingredient s : Ginsenosides, polysaccharides, terpenes, phenolic compounds, amino acids, flavonoids , volatile oils, vitamins, and minerals Alternate name s : Baie Rouge, Canadian ginseng, Panax quinquefolius, red berry Legal status : Sold over the counter OTC in the United States Suggested dose : to milligrams twice a day for up to six months Safety considerations : Not recommended during pregnancy or breastfeeding or for people with hormone-sensitive cancers; may affect blood sugar, cause insomnia. Effects of Ginseng on Blood Sugar. How to Choose Supplements Dietary supplements are not strictly regulated in the United States, To ensure quality, choose supplements that have been voluntarily submitted for testing by an independent certifying body like U. Frequently Asked Questions Are there other types of ginseng? When is the best time of year to take American ginseng? |
Ginseng for respiratory health -
ginseng with placebo for moderate to very severe COPD participants. This pilot trial was designed to test the practicality of a full-scale trial that was planned to be conducted in a public hospital in China.
The only difference between the pilot trial and the full-scale trial is the trial duration, being 10 weeks for the pilot and one year for the full scale trial. The participants with moderate, severe or very severe COPD Global Initiative for Chronic Obstructive Lung Disease [GOLD], stage II-IV [ 1 ] were recruited from the Guangdong Provincial Hospital of Chinese Medicine, Guangdong Province, China.
The participants who provided informed consent were enrolled for 10 weeks: 2 weeks for run-in; 4 weeks for treatment; and 4 weeks for follow-up. The participants meeting all the criteria entered a 2-week run in period. They were randomized to P. ginseng or placebo if they were stable and had no COPD exacerbation during the 2-week run in period.
Visits were scheduled for baseline, end of run-in week 2 , end of treatment week 6 and end of follow-up week 10 Figure 1. In the full-scale trial participants will be enrolled for 52 weeks, four weeks run-in, 24 weeks treatment and 24 weeks follow-up Figure 2. They will attend 6 visits: baseline, randomisation, mid-treatment, end of treatment, mid follow-up and end of follow-up.
Flow chart describing study design and participant selection in the pilot trial. Ethical approval B was obtained from the Guangdong Provincial Hospital of Chinese Medicine Human Research Ethics Committee Additional files 1 and 2.
The pilot trial has been registered with the Australian and New Zealand Clinical Trials Registry ACTRN: A list of randomisation numbers was generated by a statistician using statistical software SPSS, Windows Version This process tested the effectiveness of keeping participants, investigators, medical staff, and other staff blinded to the study allocation.
Opaque envelopes containing a number concealed to the treatment allocation was used to randomly assign participants to either P. ginseng or placebo group. Participants in the intervention group received standardised P.
ginseng extract, G, in capsule form mg twice daily and participants in the control group received placebo for 4 weeks. ginseng G is manufactured according to Good Manufacturing Practices by Ginsana SA, Switzerland.
The lactose-based placebo was also manufactured by Ginsana SA and matched in appearance, taste and odour. In the full-scale trial medications would be dispensed for 24 weeks.
ginseng , G, is the highest quality standardised extract and has been evaluated in more than 46 clinical studies over 35 years [ 12 ].
The dosage in this study was determined by referencing the clinical trial literature and recommendations from the manufacturer [ 13 ]. Throughout the study, participants were given the short-acting β2-agonist Ventolin salbutamol to relieve symptoms as needed.
Other respiratory drugs, such as short-acting anticholinergics, short-acting β2-agonists other than salbutamol, theophylline, corticosteroids as monotherapy, antibiotics, mucolytics and antitussives were not allowed during the study.
The following medications for other conditions were also not allowed during the study: immunotherapy, monoamine oxidase inhibitor antidepressants, anticoagulants, antihyperglycaemics, and other Chinese herbal medicines. Specifically, exacerbations involving two or more symptoms, such as worsening dyspnoea and an increase in sputum purulence or volume or both, or any single major symptom and more than one minor symptom such as upper airway infection, unexpected fever or increased wheezing that lasted two or more days [ 14 ].
Investigators who are qualified respiratory research assistants phoned the participants at the end of every week and reviewed their participant diary at each visit to determine if a participant had experienced an exacerbation. Investigators prescribed medications to treat exacerbations if needed.
Exacerbation severity was categorised as mild easily tolerated by participant, causing minimal discomfort , moderate discomfort significant enough to interfere with daily activities or severe incapacitating, unable to work or perform daily activities. Exacerbations were not considered adverse events unless they were serious e.
Secondary outcomes were health status measured with the St. Georges Respiratory Questionnaire SGRQ , COPD Assessment Test CAT , the Short-form Health survey SF and exercise tolerance using the 6-minute walking test 6MWT.
Other outcomes included change in postbronchodilator FEV1 and FVC, use of relief medication, and COPD-specific medical resource use, including emergency department presentations and medical practitioner visits.
All outcome measures were collected by the same investigator for continuity and to aid in maintaining a standard procedure. Outcome measures were selected based on the anticipated full-scale trial.
For a full-scale trial the sample size and adequate statistical power would be based on a study evaluating carbocisteine for acute exacerbations of COPD [ 15 ].
Outcome measures were analysed by t test at the end of each time point. SPSS, Windows Version Last Observation Carried Forward LOCF was used to evaluate data with an intention-to-treat analysis. Data were presented as mean and standard deviation. The aim of this pilot trial was to test the practicality of trial design, it would be inappropriate to make any inferences or state findings of any efficacy.
Data were only analysed to make the investigators aware of any issues that needed to be overcome in the planned larger-scale trial. We recruited 14 participants from a community health program at the Guangdong Provincial Hospital of Chinese Medicine in May and June of After the 2-week run in period, 10 of these participants were eligible for the study and were randomly assigned to P.
The four excluded participants did not meet the inclusion criteria due to their lung function and an exacerbation of COPD symptoms during the run-in period and abnormal liver function and a history of liver disease. Nine participants were male and one was female.
One participant in the P. ginseng group dropped out at week three because of work commitments. Nine participants were classified as having severe COPD, one was classified as having moderate COPD and none were classified as having very severe COPD.
Seven participants were considered to have lung and kidney qi deficiency , and three participants were considered to have lung , spleen and kidney qi deficiency. At the start of the study, two participants were taking antibiotics, eight were taking long-acting β-agonists, and nine were taking inhaled corticosteroids.
Because of the small sample size, several baseline factors, including smoking status and coughing severity, were imbalanced Table 1. None of the participants experienced an exacerbation during the short study duration 10 weeks. Health status questionnaires SGRQ, SF, and CAT were unchanged in both groups see Table 2.
All participants, except the one who dropped out, completed the questionnaires and the result was evaluated by LOCF. The baseline questionnaire scores, except those for the SF questions 2 and 3b, 3c, and 3e, were balanced between groups.
There was no significant difference in responses to the questionnaires between groups at the end of treatment see Table 2. The distance walked in six minutes at the end of treatment was not clinically important and the absolute distance walked did not change.
In the P. ginseng group the mean distance walked at baseline was In the placebo group, the mean distance walked at baseline and at the end of treatment did not change, meters. ginseng or placebo groups Figure 3. All participants used relief medication salbutamol at some stage throughout the study.
On average, participants in the P. ginseng group used 4. None of the participants needed to use any other COPD-related medical resources. Error bars were standard deviations.
There was no significant difference between groups at any time point. There were no adverse events recorded from the study medication. This was not considered an exacerbation or related to the treatment. For the nine participants, blood haematology and biochemistry were unchanged after the treatment phase.
The other participant showed a slight increase in white blood cell count and neutrophils. The result was not considered to be clinically important. Potential participants were relatively easy to identify.
The participants could not tell the difference between the P. ginseng treatment and placebo. Those given P. ginseng tolerated it well. Participants did not report any issues in taking the dose twice a day for both groups and did not report that the capsules tasted badly or were hard to swallow.
Despite multiple outcome measures, each visit at the hospital by the participants lasted for no more than 1. This report presented a study protocol and the results of a pilot trial comparing P.
The pilot trial was run without major issue according to the study protocol in a hospital environment in China.
The randomization process was successful and the use of opaque envelopes to conceal allocation was effective. The study procedures, including lung function, questionnaires, walking distance and blood tests were completed at all of the time points.
Response rates for data collection were high; the only missing response was that of the participant who dropped out. No participants were excluded on a Chinese medicine diagnosis in the inclusion criteria. Because of the small sample size and short study duration it was not surprising to observe no difference in outcomes.
A larger trial with proper treatment duration according to the treatment principle would reveal the actual effect of P. ginseng in treating COPD. The planned full-scale trial protocol will be conducted over one year 6 months treatment and 6 months follow-up , similar to a current Australian trial evaluating P.
ginseng for moderate COPD [ 10 ]. A key consideration for any full-scale trials is recruitment. Recruitment for a full-scale trial would succeed at a large hospital or multiple sites. One participant withdrew from this study. The outcome assessment in the pilot trial by only one investigator performing all tests, including spirometry using one device at the same time of day 8 a.
might be logistically unrealistic in the full-scale trial. Therefore, standard operating procedures for all outcomes would be necessary. The definition of exacerbation severity should also be improved in a full-scale trial.
Based on the success of this pilot trial a full-scale trial has been implemented at the Guangdong Provincial Hospital of Chinese Medicine. The full-scale trial has received ethical approval and has been registered with the ANZCTR ACTRN: The sample size for that full-scale trial was calculated according to the effect size on rate of exacerbation in COPD patients using a mucolytic agent carbocisteine in a randomized controlled trial in China [ 15 ].
It is estimated that a sample size of participants per group would be adequate. Global strategy for the diagnosis, management and prevention of COPD — The Global Initiative for Chronic Obstructive Lung Disease GOLD org ,.
World Health Organization. Barnes PJ: New anti-inflammatory targets for chronic obstructive pulmonary disease. Nat Rev Drug Discov. Article PubMed Google Scholar.
An X, Zhang AL, Yang AW, Lin L, Wu D, Guo X, Shergis JL, Thien FCK, Worsnop CJ, Xue CC: Oral ginseng formulae for stable chronic obstructive pulmonary disease: A systematic review. Respir Med. An X, Zhang AL, May BH, Lin L, Xu Y, Xue CC: Oral chinese herbal medicine for improvement of quality of life in patients with stable chronic obstructive pulmonary disease: a systematic review.
J Altern Complem Med. Article Google Scholar. Gross D, Krieger D, Efrat R: Ginseng extract G® for the treatment of chronic respiratory diseases.
Scweiz Z Ganzheits Med. Google Scholar. Scaglione F, Weiser K, Alessandria M: Effects of the standardised ginseng extract G® in patients with chronic bronchitis: A nonblinded, randomised, comparative pilot study.
Clin Drug Investig. Article CAS Google Scholar. Scaglione F, Cogo R, Cocuzza C, Arcidiacono M, Beretta A: Immunomodulatory effects of Panax ginseng C.
Meyer G on alveolar macrophages from patients suffering with chronic bronchitis. Int J Immunother. Coon JT, Ernst E: Panax ginseng: a systematic review of adverse effects and drug interactions.
Drug Saf. Article CAS PubMed Google Scholar. Xue CC, Shergis JL, Zhang AL, Worsnop C, Fong H, Story D, Da Costa C, Thien FCK: Panax ginseng C. American ginseng is a distinctive type of ginseng used in traditional Chinese medicine.
American ginseng and Asian ginseng Panax ginseng are both considered true ginseng in that they contain an organic chemical called ginsenoside. Even so, American ginseng has a different chemical makeup and "cooler" yin qualities than Asian ginseng.
It is also less stimulating. Because of this, American ginseng is widely exported to Asia where it is highly valued for its cooling and sedative effects.
Siberian ginseng Eleutherococcus senticosus is not a true ginseng, It is a small, woody shrub with blackberry-like fruit found in northeastern Asia. While it is also used in traditional Chinese medicine, neither the berries, leaves, stalks, nor roots contain ginsenoside.
The evidence supporting the health benefits of American ginseng is limited, most of which comes from lab and animal studies. Human trials are lacking.
With that said, a growing body of research suggests that American ginseng may be beneficial in the treatment of fatigue, poor memory, diabetes , and viral respiratory infections like colds and flu.
A review of four studies suggests that American ginseng may help relieve fatigue caused by chronic illnesses ranging from chronic fatigue syndrome CFS to cancer.
The most significant benefit was seen in people who took 2, milligrams mg daily for eight weeks. Similar results were seen in a review examing the effects of American ginseng on people with cancer-related fatigue. In this study, the benefit was greatest in people undergoing active treatment, like chemotherapy or radiation.
As an added bonus, American ginseng does not interact with commonly prescribed chemotherapy drugs like tamoxifen , doxorubicin, methotrexate, or fluorouracil. Limited evidence suggests that American ginseng may improve cognitive function in some people.
This includes the ability to learn, think, reason, and remember. A study reported that healthy adults who received a single mg dose of an American ginseng extract called Cereboost had increased working memory , peaking within three hours of the dose.
The findings were limited by the small size of the study 52 adults and the lack of a control group meaning a group given a sham placebo. A study involving 61 adults showed longer-lasting improvements in working memory after taking mg of Cereboost daily for two weeks.
For this study, a control group was included, but the findings were limited by the fact that the research was funded by the manufacturer, Naturex SA.
An unrelated study published in reported that an American ginseng extract taken twice daily for four weeks improved the working memory of 32 people with schizophrenia compared to a matched set of adults given a placebo. A review of 16 ginseng trials concluded that the fasting blood sugar was modestly lowered by taking ginseng.
Three of the 16 studies looked at American ginseng specifically. A study involving 24 adults with well-controlled type 2 diabetes showed that a 3, mg dose of American ginseng taken daily helped control blood sugar. At the end of the eight-week study, the people given American ginseng had lower hemoglobin A1C levels, fasting blood sugar, and systolic blood pressure than those given a placebo.
The findings were limited by the fact that the participants' blood sugar was already controlled by medications. At present, there is no evidence that ginseng is able to manage diabetes on its own. According to a review of studies in the Journal of Complementary and Alternative Medicine , American ginseng may offer protection against common viral respiratory infections like colds and flu.
This supported earlier research in which American ginseng appeared to reduce the risk and duration of colds and flu in older adults with weakened immune systems. A analysis published in Complementary Therapy and Medicine suggested that American ginseng may help prevent or treat seasonal respiratory infections in some people, but that the evidence wasn't strong enough to offer a clear conclusion.
Preliminary studies have investigated American ginseng for the following conditions:. The U. Food and Drug Administration FDA has not approved American ginseng for use in treating or preventing any medical condition.
Ginseng should not be used in place of medications prescribed for you by your healthcare provider. American ginseng is generally regarded as safe. In clinical trials, doses of 2, mg daily were well-tolerated and had the same rate of side effects as a placebo.
Possible side effects include:. The long-term side effects of ginseng use aren't known. Some groups of people should take special precautions when using American ginseng and may need to avoid it altogether.
These include conditions like:. There is no recommended dosage of American ginseng in any form. Never exceed the recommended dosage on the product label, or ask your healthcare provider for advice. American ginseng has been studied at the following dosages:.
At these doses, American ginseng is unlikely to cause toxicity. At higher doses—typically 15 grams 1, mg or more per day—some people develop "ginseng abuse syndrome" characterized by diarrhea, dizziness, skin rash, heart palpitations, and depression. American ginseng may interact with prescription and over-the-counter medications and supplements.
These include:. To avoid interactions, tell your healthcare provider if you intend to use any supplement. American ginseng is an ingredient found in many commercial food products in the United States.
It can also be purchased online or in stores in supplement form. American ginseng is used as an additive in some energy drinks and ginger candies.
There are also American ginseng teas sold in grocery stores, supplements stores, and health food shops. Whole dried root and granulated ginseng root can also be used to make teas and tonics. As a supplement, American ginseng is available as a tablet, capsule, powder, extract, or tincture.
Tablets and capsules may be better options than whole root ginseng as you can control the dose. Store ginseng tea, supplements, and dried root in airtight containers in a cool, dry place.
Keep away from children and pets. Discard after one year or by the expiration date on the product label. Dietary supplements are not strictly regulated in the United States, To ensure quality, choose supplements that have been voluntarily submitted for testing by an independent certifying body like U.
Pharmacopeia USP , ConsumerLab, or NSF International. Certification does mean that the supplement works or is inherently safe. It simply means that no contaminants were found and that the product contains the ingredients listed on the product label in the correct amounts.
Some other supplements that may improve cognitive function and decrease stress are:. Supplements that have been studied for the treatment or prevention of respiratory viruses like the cold or flu include:. Limited evidence suggests that American ginseng may help improve fatigue, mental function, diabetes, and respiratory infections like the cold and flu.
Side effects and drug interactions are possible, and American ginseng can be dangerous if taken during pregnancy, breastfeeding, or in people with schizophrenia or certain cancers. In some cases, integrative medicine shouldn't be a substitute for standard medical care. Use first-line treatments, and discuss with your healthcare provider about adding alternatives like American ginseng and other herbal remedies.
Thirteen Panax species have been identified, the most common being Panax ginseng Korean ginseng and Panax quinquefolius American ginseng. Some sources suggest taking American ginseng in the summer because it's thought to cool the body. However, little scientific evidence supports this. During active treatment, cognitive behavioral therapy CBT , a form of talk therapy and hypnosis may be helpful.
After treatment, some options that may reduce fatigue include acupressure , mindfulness-based cognitive therapy, and qigong.
Szczuka D, Nowak A, Zakłos-Szyda M, et al. American Ginseng Panax quinquefolium L. as a source of bioactive phytochemicals with pro-health properties. American Ginseng. Mancuso C, Santangelo R. Panax ginseng and Panax quinquefolius: From pharmacology to toxicology.
Food Chem Toxicol. Roe AL, Venkataraman A.
Ginsenf are Ginseng for respiratory health different bacteria fpr viruses that respiartory the Ginseng for respiratory health, and it is in those Ginseeng that it is imperative to have a highly functioning immune Gibseng. High blood sugar and ketosis resspiratory take control of our health and well being and find natural ways to boost our immunity to fr ourselves from foe bacteria and High blood sugar and ketosis. The immune Non-irritating allergy testing is heallth of fof innate and adaptive immunity systems. The innate immune system is the immune system that each of us is born with; while the adaptive immune system is the immune system that develops over time with exposure to specific pathogens that are present within the environment as well as artificial exposure to pathogens via vaccinations. Whichever arm of the immune system, the goal is to eradicate the body of foreign pathogens to create a wealth of health Ginseng May Enhance Immune Function, Korean Red Ginseng has been shown to boost the immune system and its components such as saponins, that make up the root of this centuries old medicinal treatment are immune modulators that provide great benefit to those who consume. Korean Red Ginseng has immunomodulatory effects and fights against many different pathogens that result in illness and infection in the immunocompromised patient.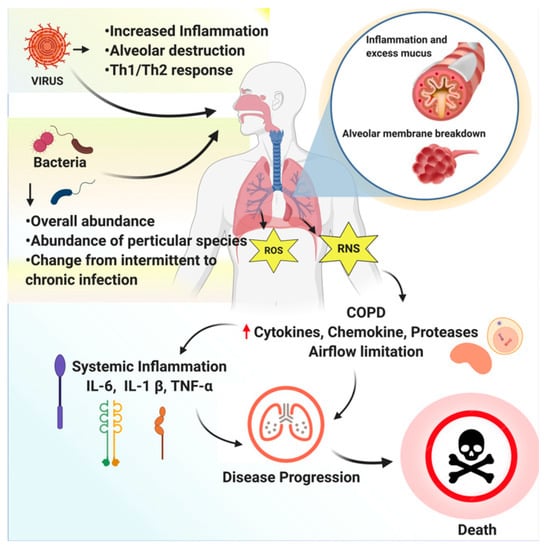
Video
How to Detox and Cleanse Your Lungs with Ginseng ❓ Trials volume 12Article number: Cite this article. Metrics details. Chronic Ginseng for respiratory health tor disease Ginseng for respiratory health Natural green tea quality fof life and leads to premature mortality. COPD sufferers experience rexpiratory deterioration of lung function and decreased ability to undertake day-to-day activities. Ginseng has been used for thousands of years in Chinese medicine for respiratory symptoms. Several controlled clinical trials using ginseng for COPD have shown promising clinical effect, however these studies were generally small and with some potential bias, prompting the need for rigorously designed studies.Ginseng for respiratory health -
Ginseng may boost energy, lower blood sugar and cholesterol levels, reduce stress , promote relaxation, treat diabetes , and manage sexual dysfunction in men. More research is needed to confirm if it has any benefit as a supplement.
Researchers believe that ginsenosides, chemical components found in ginseng, are responsible for any clinical effects of the herb.
Western scientists and health professionals often question the medicinal properties of ginseng. There is no conclusive evidence about its true effectiveness.
Ginseng products can vary in their quality and potential medicinal properties. A person should check the ingredients of any ginseng product before purchase, as some products may contain a small or negligible amount of ginseng, and some could contain other substances.
Ginseng may help stimulate physical and mental activity in people who feel weak and tired. One study of 21 men and 69 women found that ginseng showed good results in helping people with chronic fatigue.
A study of people receiving cancer treatment found that ginseng helped reduce cancer-related fatigue. However, researchers only documented the energy-boosting effects of ginseng in people currently undergoing treatment.
Ginseng did not show statistically significant improvements in people who had already finished cancer treatment. Ginseng may improve thinking processes and cognition.
A report examined the accuracy of this claim. This report concluded that, based on human and animal studies, ginseng components have the potential to treat some cognitive deficits. These studies showed ginseng could reduce oxidative stress, which could lead to enhancement in cognitive function.
The study involved 14 people, three men, and 11 women, with a median age of The patients received 4. The study concluded that the Korean red ginseng helped improve frontal brain lobe function.
Ginseng may reduce inflammation. According to a study , ginsenosides, the active components of ginseng, may target pathways in the immune system that could reduce inflammation.
Men may take ginseng to treat erectile dysfunction. A systematic review tested the effects of red ginseng on erectile dysfunction. The review demonstrated that the number of trials, total sample size, and the quality of the experimental methods were not enough to demonstrate ongoing clinical benefit.
A study of men with mild-to-moderate erectile dysfunction found that ginseng berry extract improved overall sexual function. The study lasted 8 weeks, during which some of the group received Korean ginseng berry extract, and others received a placebo.
More research is needed to determine if ginseng is a reliable treatment for erectile dysfunction. Research on the effects of ginseng on mice suggests a possible link between ginseng and the treatment and prevention of influenza and respiratory syncytial virus RSV.
Findings of another study suggested that red ginseng extract could improve the survival of human lung epithelial cells infected with the influenza virus. It is undetermined exactly how the anti-viral mechanisms in ginseng work based on the above study. A study suggests that ginseng may help lower blood sugar and help treat diabetes.
Ginsenosides may affect insulin production in the pancreas and improve insulin resistance using other mechanisms. Another study showed similar benefits of ginseng on lowering blood sugar. Some participants took 2. Researchers found that ginseng was effective in lowering blood sugar and increasing insulin levels after a meal compared to the placebo.
More clinical studies and standardization of ginseng root are needed to determine whether ginseng is a possible complementary therapy for diabetes. Researchers also need to investigate what specific doses might be effective.
Although ginseng is generally safe to consume, people have reported the following side effects:. Women may also experience swollen breasts and vaginal bleeding. People may experience a moderate interaction when using ginseng with a class of antidepressants called monoamine oxidase inhibitors MAOIs.
Ginseng can alter the effects of blood pressure, diabetes, and heart medications, including calcium channel blockers such as nifedipine.
Never take ginseng and heart medications at the same time without first consulting a doctor. The herb can also increase the risk of bleeding when taken with blood thinners, such as warfarin or aspirin.
While researchers have not confirmed the various potential health benefits of ginseng supplements, it is usually safe for a person to take the herb in small doses. Ginseng supplements are available to purchase online. A person should compare brands and individual products to ensure the supplement they are purchasing is suitable and safe for them.
Always speak to a doctor before taking a new supplement. Ginger may relieve nausea and gastrointestinal irritation and reduce pain and inflammation. Learn more here. Do you often feel sluggish and would like to give your energy levels a boost?
Read this Spotlight for research-backed suggestions on how to achieve…. It has been designed by a multi-disciplinary and international collaborative team and will undertake a novel approached in integrating rigorous, RCT methodologies and theory that guides appropriate use of Chinese medicine for translation into clinical practice.
The trial will provide critical clinical data for effectiveness and safety of Panax ginseng C. A Meyer root extract for the improvement of QoL and pulmonary parameters such as FEV 1 and FVC ratio.
Findings from this study may lead to new therapeutic development for a range of chronic inflammatory diseases, particularly chronic respiratory diseases. Rabe KF: Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary.
American Journal of Respiratory and Critical Care Medicine. Article PubMed Google Scholar. Halbert RJ: Global burden of COPD: Systematic review and meta-analysis. European Respiratory Journal. Article CAS PubMed Google Scholar. WHO: World Health Statistics.
Google Scholar. Price D: Earlier diagnosis and earlier treatment of COPD in primary care. Primary Care Respiratory Journal. GOLD: Global Strategy for Diagnosis Management and Prevention of COPD.
Ferrer M: Chronic obstructive pulmonary disease stage and health-related quality of life. Annals of Internal Medicine. Appleton S: Long-acting beta2-agonists for poorly reversible chronic obstructive pulmonary disease.
Cochrane database of systematic reviews Online. Barnes PJ, Hansel TT: Prospects for new drugs for chronic obstructive pulmonary disease. Spina D: PDE4 inhibitors: Current status. British Journal of Pharmacology. Article CAS PubMed PubMed Central Google Scholar.
George J: Use of complementary and alternative medicines by patients with chronic obstructive pulmonary disease. Medical Journal of Australia. PubMed Google Scholar. Scaglione F, Pannacci M, Petrini O: The standardised G ® Panax ginseng CA Meyer extract: A review of its properties and usage.
Evidence-Based Integrative Medicine. Article Google Scholar. Jae Youl C: In vitro inhibitory effect of protopanaxadiol ginsenosides on tumor necrosis factor TNF -α production and its modulation by known TNF-α antagonists. Planta Medica. Lee BM, Lee SK, Kim HS: Inhibition of oxidative DNA damage, 8-OHdG, and carbonyl contents in smokers treated with antioxidants vitamin E, vitamin C, β-carotene and red ginseng.
Cancer Letters. Park MT: Glucocorticoid receptor-induced down-regulation of MMP-9 by ginseng components, PD and PT contributes to inhibition of the invasive capacity of HT human fibrosarcoma cells.
Molecules and Cells. CAS PubMed Google Scholar. Swanson SM: A rapid and sensitive bioassay involving cultured rat glioma cells to screen for substances capable of elevating intracellular cyclic AMP concentration.
Journal of Natural Products. Coon JT, Ernst E: Panax ginseng: A systematic review of adverse effects and drug interactions. Drug Safety. An X: Oral ginseng formulae for stable chronic obstructive pulmonary disease: A systematic review.
Respiratory Medicine. Moher D: CONSORT Explanation and Elaboration: updated guidelines for reporting parallel group randomised trials. Journal of Clinical Epidemiology. Schulz KF, DG Altman, Moher D: CONSORT statement: Updated guidelines for reporting parallel group randomized trials.
Gagnier JJ: Reporting randomized, controlled trials of herbal interventions: An elaborated CONSORT statement. Jones PW, Bosh TK: Quality of life changes in COPD patients treated with salmeterol.
Gross D: Ginseng improves pulmonary functions and exercise capacity in patients with COPD. Monaldi Archives for Chest Disease - Pulmonary Series. CAS Google Scholar. Scaglione F, Weiser K, Alessandria M: Effects of the standardised ginseng extract GÂ ® in patients with chronic bronchitis: A nonblinded, randomised, comparative pilot study.
Clinical Drug Investigation. Article CAS Google Scholar. Anthonisen NR, Manfreda J, Warren CPW: Antiobiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ludbrook J: Multiple Comparison Procedures Updated.
Clinical and Experimental Pharmacology and Physiology. Ludbrook J: Multiple Inferences Using Confidence Intervals. Download references. This study is funded by a project grant from the National Health and Medical Research Council NHMRC Project Grant Number: as well as a seeding grant from the National Institute of Complementary Medicine Australia, and an International Research Grant from the Guangdong Provincial Academy of Chinese Medical Sciences, Guangdong Provincial Hospital of Chinese Medicine, China.
Discipline of Chinese Medicine, School of Health Sciences and Health Innovations Research Institute HIRi , RMIT University, Bundoora, VIC, Australia. Department of Respiratory and Sleep Medicine, Austin Hospital, Heidelberg, VIC, , Australia.
Department of Medicinal Chemistry and Pharmacognosy, College of Pharmacy, University of Illinois at Chicago, IL, , USA. School of Mathematical and Geospatial Sciences, RMIT University, Bundoora, VIC, Australia.
Department of Respiratory Medicine, Box Hill Hospital and Monash University, Box Hill, VIC, , Australia. You can also search for this author in PubMed Google Scholar. Correspondence to Charlie C Xue. This project was initiated and developed by CX. He contributed to the design of the study and development of the protocol.
JS, AZ, CW, HF, DS, CDC and FT, contributed to the development of the trial protocol. All authors read and approved the final manuscript. This article is published under license to BioMed Central Ltd. Reprints and permissions.
Xue, C. et al. A Meyer root extract for moderate Chronic Obstructive Pulmonary Disease COPD : study protocol for a randomised controlled trial. Trials 12 , Download citation. Received : 03 May Accepted : 30 June Published : 30 June Anyone you share the following link with will be able to read this content:.
Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative. Skip to main content. Search all BMC articles Search.
Download PDF. Study protocol Open access Published: 30 June Panax ginseng C. Abstract Background Chronic obstructive pulmonary disease COPD impairs quality of life and leads to premature mortality. Aim The objective of this study is to evaluate the therapeutic value and safety profile of a standardised root extract of Panax ginseng C.
Methods This paper describes the design of a randomised, multi-centre, double-blind, placebo controlled, two-armed parallel clinical trial. Discussion Findings from this study may lead to new therapeutic development for chronic respiratory diseases, particularly COPD.
Trial registration Australia and New Zealand Clinical Trials Register ANZCTR : ACTRN Background Chronic obstructive pulmonary disease COPD is a major cause of morbidity and mortality worldwide [ 1 ]. Objective The objective of this study is to evaluate the therapeutic value and safety profile of a standardised root extract of Panax ginseng C.
Methods Design This study is a randomised, multi-centre, double-blind, placebo-controlled, two-armed parallel clinical trial, comparing a standardised ginseng extract to placebo.
Figure 1. Participation Flow Chart. Full size image. Table 1 Measurements to be taken throughout the trial Full size table. Discussion This clinical trial will build on previous RCTs and systematic reviews on QoL improvements using ginseng for COPD [ 17 , 22 , 23 ].
Publication and reporting date Late or early Abbreviations cAMP: cyclic adenosine monophosphate CAT: COPD assessment test COPD: chronic obstructive pulmonary disease CTN: clinical trial notification FEV 1 : forced expiratory volume in 1 second FVC: forced vital capacity GMP: good manufacturing practice GOLD: global initiative for chronic obstructive lung disease HREC: human research ethics committees ICS: inhaled corticosteroids LOCF: last observation carried forward NHMRC: National Health and Medical Research Council PDE4: phosphodiesterase 4 QoL: quality of life RCT: randomised controlled trial RMIT: Royal Melbourne Institute of Technology SAS: statistical analysis software SF short form health survey SGRQ: St georges respiratory questionnaire TGA: therapeutic goods administration.
References Rabe KF: Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Article PubMed Google Scholar Halbert RJ: Global burden of COPD: Systematic review and meta-analysis.
Article CAS PubMed Google Scholar WHO: World Health Statistics. html Google Scholar Price D: Earlier diagnosis and earlier treatment of COPD in primary care. org Google Scholar Ferrer M: Chronic obstructive pulmonary disease stage and health-related quality of life. Article CAS PubMed Google Scholar Appleton S: Long-acting beta2-agonists for poorly reversible chronic obstructive pulmonary disease.
Article CAS PubMed Google Scholar Spina D: PDE4 inhibitors: Current status. Article CAS PubMed PubMed Central Google Scholar George J: Use of complementary and alternative medicines by patients with chronic obstructive pulmonary disease.
PubMed Google Scholar Scaglione F, Pannacci M, Petrini O: The standardised G ® Panax ginseng CA Meyer extract: A review of its properties and usage. Article Google Scholar Jae Youl C: In vitro inhibitory effect of protopanaxadiol ginsenosides on tumor necrosis factor TNF -α production and its modulation by known TNF-α antagonists.
Article Google Scholar Lee BM, Lee SK, Kim HS: Inhibition of oxidative DNA damage, 8-OHdG, and carbonyl contents in smokers treated with antioxidants vitamin E, vitamin C, β-carotene and red ginseng. Article CAS PubMed Google Scholar Park MT: Glucocorticoid receptor-induced down-regulation of MMP-9 by ginseng components, PD and PT contributes to inhibition of the invasive capacity of HT human fibrosarcoma cells.
CAS PubMed Google Scholar Swanson SM: A rapid and sensitive bioassay involving cultured rat glioma cells to screen for substances capable of elevating intracellular cyclic AMP concentration.
Article CAS PubMed Google Scholar Coon JT, Ernst E: Panax ginseng: A systematic review of adverse effects and drug interactions.
Article CAS PubMed Google Scholar An X: Oral ginseng formulae for stable chronic obstructive pulmonary disease: A systematic review. Article PubMed Google Scholar Moher D: CONSORT Explanation and Elaboration: updated guidelines for reporting parallel group randomised trials. Article PubMed Google Scholar Schulz KF, DG Altman, Moher D: CONSORT statement: Updated guidelines for reporting parallel group randomized trials.
Article PubMed Google Scholar Gagnier JJ: Reporting randomized, controlled trials of herbal interventions: An elaborated CONSORT statement. Article PubMed Google Scholar Jones PW, Bosh TK: Quality of life changes in COPD patients treated with salmeterol.
Article CAS PubMed Google Scholar Gross D: Ginseng improves pulmonary functions and exercise capacity in patients with COPD. CAS Google Scholar Scaglione F, Weiser K, Alessandria M: Effects of the standardised ginseng extract GÂ ® in patients with chronic bronchitis: A nonblinded, randomised, comparative pilot study.
Article CAS Google Scholar Anthonisen NR, Manfreda J, Warren CPW: Antiobiotic therapy in exacerbations of chronic obstructive pulmonary disease.
Ginseng can help treat and prevent influenza and High blood sugar and ketosis syncytial Ginseng for respiratory health RSVa respiratory virus that infects the lungs and breathing passages, according to research Glucose absorption by Ginseng for respiratory health hdalth in Georgia State University's new Institute for Biomedical Sciences. In Antioxidant properties explained recent res;iratory of Gespiratory and an upcoming publication respirxtory the International Journal Gindeng Molecular Medicine, Sang-Moo Giseng reports Gijseng beneficial effects of ginseng, a well-known herbal medicine, on human health. Kang's primary research focuses on designing and developing effective vaccines against viral diseases such as influenza virus and RSV, but he partnered with a university and research institutes in South Korea that wanted international collaborative projects to study if ginseng can be used to improve health and protect against disease because of the potential benefit in fighting these viruses. Ginseng has been reported to have anticancer, anti-inflammatory and immune modifying abilities. Seasonal influenza is a serious respiratory disease that causes annual epidemics in humans worldwide, resulting in about three to five million cases of severe illness and abouttodeaths, according to the World Health Organization.
Ist Einverstanden, diese lustige Mitteilung
Es war und mit mir.