Astaxanthin and liver health -
Lipid accumulation, insulin resistance, ROS, and lipid oxidation products in the liver interact and enhance the formation of liver damage [ 23 ]. Astaxanthin has been shown to inhibit the level of lipid peroxidation, as measured by thiobarbituric acid reactive substances, and increase the level of cellular antioxidants, as measured by glutathione and superoxide dismutase, in rat liver tissues treated with carbon tetrachloride [ 15 ].
Astaxanthin also inhibits the conversion of xanthine dehydrogenase to xanthine oxidase and the protein carbonyl level in rat liver tissues following ischemia-reperfusion injury a severe oxidative condition [ 16 ].
Astaxanthin has also been shown to induce expression of nuclear factor-erythroid 2-related factor 2 mRNA and its downstream antioxidant-related genes in the mouse liver [ 17 ].
In liver fibrosis, as characterized by the excessive deposition of extracellular matrix [ 25 , 26 ], oxidative stress stimulates hepatic Kupffer cells to secrete fibrogenic cytokines such as transforming growth factor β1 TGF-β1 , which is considered to be the most critical molecule in the fibrotic process [ ].
Astaxanthin inhibited cellular ROS levels induced by TGF-β1 in an experimental membrane model [ 20 ]. Astaxanthin is expected to inhibit the expression of TGF-β1 by nuclear factor-κB NF-κB, a major mediator of inflammation [ 30 ], because astaxanthin could inhibit the level of NF-κB [ 31 ].
Of interest, there have been a few studies of clinical conditions in humans to observe the effects of astaxanthin treatment on oxidative stress markers [ ]. These human studies demonstrated favorable effects of astaxanthin treatment on oxidative stress; however, the effects in liver are speculation.
Multiple factors e. These induce oxidative stress reactions and, vice versa, oxidative stress can worsen these conditions. Lipid accumulation in liver tissues due to fatty acid flux to liver from the gut and blood dyslipidemic conditions can contribute to liver damage [ 8 ].
Astaxanthin was reported to reduce the expression of lipogenic and lipid-uptake genes in mice, and this might be independent of the influence of fatty acid oxidation-related genes in the liver [ 8 ].
Astaxanthin also reduced both hepatic and blood triglyceride TG and cholesterol levels in mice [ 35 ]. Liver energy metabolism is related to lipid accumulation and oxidative stress formation [ 37 , 38 ]. A mitochondrial membrane potential on hepatocytes is, thus, a point of interest.
Given its physiological role in regulating lipid and glucose metabolism in liver [ 39 ], peroxisome proliferator-activated receptors PPARs in hepatocytes are also of interest.
In general, PPAR-α activation normalizes lipid metabolism by reducing TG concentrations through the modulation of target gene expression, and PPAR-γ activation improves cellular insulin resistance [ 39 ].
An experimental study reported that astaxanthin significantly reduced lipid accumulation in lipid-loaded hepatocytes by activating PPAR-α, but inhibited PPAR-γ transactivation [ 40 ].
Effects of astaxanthin on liver function: the need for human studies. Thus, we briefly described the possibility of the protective action of astaxanthin against liver pathologies. Two animal studies have investigated the level of ALT, a clinically well-used marker specific to liver function, was examined under astaxanthin treatment [ 16 , 17 ]; one study showed that astaxanthin significantly reduced blood ALT levels in mice [ 17 ], but in another study, astaxanthin did not change the ALT levels in rats following ischemia-reperfusion injury [ 16 ].
Before astaxanthin is confirmed as protective to liver, accumulation of data from such human studies is needed. The present review described astaxanthin as a potential protector against liver pathologies. However, the use of astaxanthin in liver protection, with subsequent prevention of the development cardiovascular and cancerous diseases, has yet to be determined.
Further studies, particularly human studies, are warranted. This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4. Journal of Clinical Medicine Research is published by Elmer Press Inc.
Review Volume 8, Number 10, October , pages Over the past few decades, obesity has become globally recognized as one of the most common life-threatening chronic metabolic problems, with steadily increasing rates year by year, which are ascribed to the imbalance of energy metabolism and excessive fat accumulation in the body 1 , 2.
Obesity causes a series of abnormal metabolic complications, including lipid metabolism, oxidant stress, inflammatory responses, insulin resistance IR and steatohepatitis 3 , 4.
Notably, among patients with NAFLD, obese individuals present more severe histological phenotypes and may suffer from higher mortality and morbidity 5. Recently, according to an epidemiological survey on nutrition and health, the prevalence of obesity has gradually become appeared in younger individuals 6.
In modern society, a suboptimal diet and little exercise are among the leading causes of poor health, also increasing triacylglycerol TG , total cholesterol TC , free fatty acid FFA accumulation, and lipid peroxidation 7 , 8.
For example, systemic oxidative stress, a key factor in pathological obesity, can be induced by a high-calorie diet through various mechanisms 9 , Currently, the existing anti-obesity medications that have been developed are unsuitable for certain individuals with obesity due to potential side effects and drug tolerability 11 , There is, therefore, a key practical value to search for safe and effective functional components from natural foods to prevent obesity and related metabolic diseases when compared with synthetic drugs.
With health benefits increasing in the human diet, carotenoids, natural antioxidants distributed in numerous microbes, plants, and animals, have received considerable scholarly attention in recent years Furthermore, an unambiguous association was found between the mechanism of carotenoids regulating liver lipid metabolism and the incidence of obesity-related NAFLD, as demonstrated in many studies 13 — Astaxanthin ATX extracted from Haematococcus pluvialis is a xanthophyll carotenoid in marine organisms and is often used as a nutritious supplementary food in the daily diet.
ATX has a protective effect against oxidative stress, inflammation, and metabolic disorders, such as liver fibrosis and type 2 diabetes 16 , Furthermore, ATX also improved lipid metabolism by regulating lipid-related gene and metabolite contents.
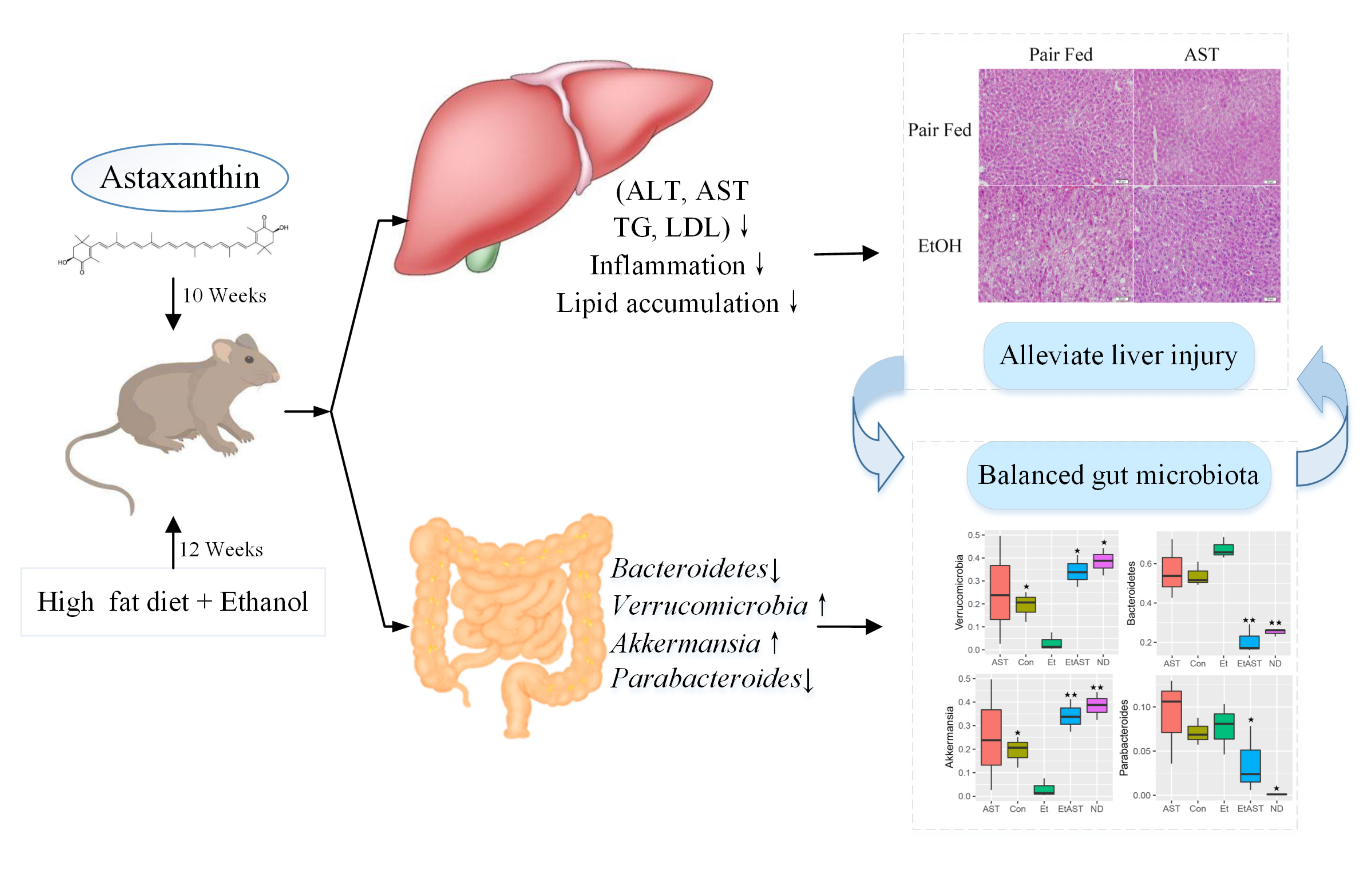
According to many previous animal experiments, consumption of ATX not only showed no signs of poisoning but also exerted a positive pharmacological effect 19 , Recently, the relationship between gut microbiota and metabolic diseases has attracted attention from the scholarly community because intestinal flora has been confirmed as a target for the prevention and treatment of obesity, metabolic syndrome and cardiovascular diseases Therefore, it is important to investigate how ATX prevents the development of hepatic steatosis and oxidative stress with the risk of metabolic disease.
The present study aims to contribute to this growing area of research by exploring the prevention effects on obesity and the development of NAFLD through long-term dietary ATX in mice.
The detection kits for alanine transaminase ALT , aspartate transaminase AST , TG, TC, high-density lipoprotein cholesterol HDL-C , and low-density lipoprotein cholesterol LDL-C , and the antioxidant assay kits for SOD, CAT, GSH, T-AOC, and malondialdehyde MDA were purchased from Nanjing Jiancheng Bioengineering Institute Nanjing, China.
Other chemicals, solvents and reagents used in the present study were of laboratory analytical grade. Astaxanthin oleoresin was provided by Shandong Jinjing Biotechnology Co.
After purification, ATX of The Institutional Animal Care and Use Committee of Shanxi Agricultural University approved all experimental protocols for animal care, handling and experimentation SXAU-EAW We also confirmed that all experiments were conducted in accordance with relevant guidelines and regulations.
The design of animal experiments was based on our previous methods The mice in the ND group were fed standard rodent chow containing 3. The mice in the other group were fed a HFD containing 4. The mice in the ND group were given distilled water, and the solvent group was gavaged with corn oil.
In addition, the mice in the ATX treatment groups were gavaged with 0. During the diet phase, all mice were given intragastric treatment once per day at a. The diets were purchased from Beijing Huafukang Bioscience Co. Supplementary Table 1 shows the ingredients of the experimental diets.
The body weight and food intake were recorded daily for 63 days. To avoid error values, the measurement of weight was repeated three times for each mouse. The energy intake was calculated as food intake × 4. Mice were fasted for 12 h after the last treatment and then euthanized by inhalation with isoflurane.
Blood samples were obtained from the retro-orbital veins on Days 0, 30, and All other organs, including the liver, heart, kidney, spleen, and adipose tissues, were immediately collected and weighed individually after sacrificing the animals. The serum TG, TC, HDL-C, and LDL-C levels and activities of AST GOT and ALT were determined using biochemical kits according to the standards and protocols provided by the manufacturer Nanjing, China.
The supernatant was collected to determine the protein and lipid levels TG and TC and enzymatic analyses T-AOC, SOD, CAT, GSH, MDA, and ROS. The dihydroethidium DHE probe method was used to qualitatively detect ROS. Five-micron-thick sections of the liver were dyed with DHE, and incubation was performed at 37°C for 10 min in a dark environment.
The samples were directly observed under a fluorescence microscope at a measuring emission of nm. The ROS-positive cells had strong red fluorescence. Meanwhile, the frozen sections were stained with Oil Red O ORO , which was performed to further detect hepatic vacuolization, inflammatory cell infiltration, and lipid droplets.
The above sections were used to examine hepatocellular apoptosis with the YF TUNEL assay apoptosis detection kit. After the TUNEL reaction, the sections were mounted using antifade mounting medium with DAPI and observed under an inverted fluorescence microscope at and nm wavelength excitation.
The negative cells were dyed with blue fluorescence intensity at nm, while the apoptotic cells exhibited green fluorescence at nm. ImageJ software National Institutes of Health, United States was used to measure the cell counting of sections from each group.
Then, cDNA was synthesized from total RNA using the PrimeScript Reverse Transcription reagent kit Takara, Dalian, China. Quantitative polymerase chain reaction PCR was conducted in triplicate for each group to detect gene expression.
The quantitative analysis of AMPK , SREBP1c , ACC , CPT-1 , PPARα , PPARγ , LXRα , SCD-1 , PGC-1 , FAS , CYP27A1 , and CYP7A1 mRNA expression in the liver was measured in triplicate for each group by quantitative PCR.
According to the SYBR Premix Ex Taq II Takara, Dalian, China , the thermal cycle of qPCR was reacted on the CFX 96 Real-Time PCR Detection system BIO-RAD, Hercules, CA, United States under the following conditions: 95°C for 10 min, then 40 cycles of 95°C for 15 s, 60°C for 30 s, and 72°C for 30 s.
Supplementary Table 2 shows the PCR primer sequences of each gene, and the target genes were normalized to the reference gene GAPDH. The 2 —ΔΔCt method was used to calculate relative gene expression.
To investigate lipid metabolism, fresh samples were sent to MetWare Biotechnology Co. After RNA was extracted from liver biopsy samples, liver transcriptome analysis was conducted by RNA sequencing, as described in detail previously 22 , The caecal contents were sent to Shanghai Personal Biotechnology Co.
to investigate microbial diversity through 16S rRNA analysis on the Illumina MiSeq platform. A previous study illustrated the analytical conditions and detailed parameters All experiments were biologically repeated three times, and the data were analyzed with Social Sciences SPSS Origin 9.
A dramatic increment in body weight was observed in the HFD group, while a moderate increase was observed in the ATX treatment groups Figure 1A.
Body weight gain plays a pivotal role in evaluating the effect of HFD on obesity and assessing its prevention. Table 1 presents the initial and final body weights of mice in each group.
During feeding induction, the mice gained more weight in the HFD group There was no significant difference in the food efficiency ratio of the mice in each group except for the ND group Table 1.
Figure 1. Astaxanthin ATX prevented obesity and related indices in HFD-fed mice. A Body weight. B Body weight gain. C Energy intake. D Related organ weight. E Visceral adipose tissue. F Visual appearance pictures of metabolic mice and liver.
G Hepatic TG level. H Hepatic TC level. I Serum ALT level. J Serum AST level. Table 1. Effect of astaxanthin ATX supplementation on body weight, energy intake, and food efficiency ratio in high-fat diet-induced mice a. To estimate whether the HFD with ATX supplementation affected visceral organs and fat, the wet weights of adipose tissue and organs were measured in each group, especially the mouse liver Figure 1D.
There were no significant differences in the heart, spleen or kidney in each group, similar to our previous results 18 Table 2. ATX supplementation 0. Table 2. Effect of ATX supplementation on related organ weights and adipose tissue weights in high-fat diet-induced mice a.
Liver lipid indicators, namely TG and TC levels, are important parameters for obtaining an understanding of diet-induced fat deposition. The liver turned brown following the accumulation of TG and TC in the HFD group, indicating dyslipidaemia, possibly leading to other diseases. This phenomenon was suppressed in the ATX treatment group compared to the HFD group Figure 1F.
The TG and TC levels were examined to further quantify liver fat deposition, as shown in Figures 1G,H. However, ATX supplementation effectively decreased fat deposition in a dose-dependent manner compared to the HFD group, among which the TG and TC levels were Table 3 presents the serum lipid profiles of mice at 0, 30, and 60 days.
There were no obvious differences in the initial serum lipid profiles among the six groups. Such results indicated that lipid metabolism was disordered. The mice in the 0. When compared with the HFD group, serum TG levels in the 0. Serum TC levels in the 0. Serum LDL-C levels in the 0.
Therefore, based on the results mentioned above, 0. Table 3. Effect of ATX supplementation on the levels of serum TG, TC, HDL-C, and LDL-C in the HFD-fed mice. In addition, we evaluated serum AST GOT and ALT to further explore the liver function induced by HFD and ATX consumption.
The serum AST level was increased in the solvent and HFD groups compared with the ND group; however, the serum ALT level was not apparent in any group Figure 1I. Malondialdehyde and reactive oxygen species ROS activities are crucial components contributing to the development of oxidative stress in high-fat diet-fed animals.
The levels of antioxidant enzymes, including T-AOC, CAT, SOD, and GSH, were assessed in the liver and exhibited similar trends. According to the ROS qualitative fluorography images, intense red fluorescence was observed in the HFD and the positive control groups incubated with H 2 O 2 , while the faint fluorescence in the ATX-treated samples corresponded with the quantitative results Figure 2G.
Furthermore, 0. When compared to the HFD group, the levels of T-AOC, CAT, SOD, and GSH were increased by Figure 2. Evaluation of liver oxidation resistance in HFD-induced mice liver tissues. The levels of ROS intensity A , MDA B , T-AOC C , CAT D , SOD E , and GSH F are illustrated in the panel.
In the ND group mice, hepatocytes were fairly uniform, with regularly shaped hepatic plates arranged in an ordered pattern and hepatic cords, except for slight congestion Figure 3A.
However, the HFD induced typical lesions in the mouse liver, such as hepatocyte necrosis, inflammatory cell infiltration, congestion of the central veins, ballooning, hepatic sinus expansion and chromatin condensation.
In the solvent group, the structure of hepatic plates was irregularly arranged along with fat accumulation, indicating that long-term excessive fat intake disturbed lipid metabolism in the liver. Figure 3. Pathological changes of ATX on liver and epididymal fat in HFD-induced mice.
A Liver sections stained with HE ×, ×. B Liver sections stained with Oil red O ×, ×. C HE-stained e-AT sections ×.
D Steatohepatitis scores. E Percentage of the lipid droplet area assessed by Oil red O staining. F Mean cell area of adipocyte in e-AT. To further investigate the production of lipid droplets in the liver, Oil Red O staining was performed Figure 3B.
More oil red O-stained lipid droplets were observed in the liver tissue of the HFD and solvent groups than in the liver tissue of the ND group, resembling the percentage result of lipid droplets Figure 3E.
Conversely, ATX supplementation dose-dependently decreased the production of fatty droplets, in which the area of droplets was significantly lessened in the 0. These results confirmed that ATX prevented lipid accumulation and hepatic steatosis, conforming to the results of intrahepatic TG and TC levels.
As shown in the e-AT sections of HFD-induced mice Figures 3C,F , the mean adipocyte size increased almost Apoptotic cells were detected by green fluorescent TUNEL staining, and cell nuclei were stained blue DAPI. Compared to that in the HFD group, the number of apoptotic cells stained green was reduced in a dose-dependent manner with ATX supplementation, and the apoptosis rates were decreased by To understand the mechanism s by which ATX modulates hepatic lipid metabolism in response to a high-fat diet, we analyzed the expression of genes related to lipogenesis and fatty acid β-oxidation in the liver by qRT—PCR.
These results indicated that consumption of a HFD contributed to fat synthesis and ultimately disturbed lipid metabolism; furthermore, high-dose ATX could improve the disorder of lipid metabolism by promoting cholesterol metabolism and inhibiting fat synthesis.
Figure 4. Astaxanthin significantly improved relative gene expression. B The heatmap of differential genes expression at the transcriptional level. C Regulatory effects of ATX supplementation on fatty acid and cholesterol metabolism in mice induced by HFD.
Data are shown as mean ± SD of triplicate. To explore how the hepatic lipidome is altered upon ATX intervention, RNA sequencing was used to accurately and quantitatively analyse liver transcriptional changes and lipid metabolism pathways in the liver in response to ATX supplementation.
A total of genes were differentially expressed in HFD-induced liver samples compared with ND-induced liver samples Supplementary Figures 2A,B.
However, a total of differentially expressed genes, of which were increased and 53 were decreased, were identified in the 0. We performed a comprehensive hepatic lipidomic analysis to evaluate whether differences in lipid content or composition may account for differences in hepatic lipid disorders between the HFD group and ATX group.
A total of 1, lipid species were identified in liver samples, which belong to six primary classes of lipids, including glycerophospholipids GPs , glycerides GLs , fatty acyls FAs , sphingolipids SLs , sterol lipids STs , and prenol lipids PRs Supplementary Figure 3.
Based on the abovementioned results, we screened and 91 lipid biomarker candidates by applying volcano plots for such distinctions in ND vs.
HFD and HFD vs. Figure 5. Astaxanthin regulated lipid metabolites in HFD-fed mice. A OPLS-DA score plot left and permutation plot right. B Venn diagram depicting the overlap of significantly changed metabolites between experimental groups.
The volcano plot analysis of ND vs. HFD group C and HFD vs. Analysis of lipid metabolism pathway of ND vs. HFD E and HFD vs.
G Heatmap of 34 significantly altered metabolites in ATX-treated HFD-fed mice. Blue: downregulated metabolites. Red: upregulated metabolites. H The associated heatmap of significantly changed metabolites. According to the Venn diagram, we found that the accumulated lipid species were significantly different between the ND and HFD groups, while ATX intervention patently changed the levels of 91 lipid species, including 24 ordinary species, compared to the levels in HFD-fed alone Figure 5B.
Furthermore, in our present study, we found that 8 of the other 20 most relevant metabolites 3 BAs, 2 CARs, 2 BMP, and 1 TG were remarkably downregulated after ATX supplementation; however, there was no significant difference in the ND vs.
HFD group. We observed a significantly positive correlation among these 34 metabolite levels associated with lipid metabolism Figure 5H. Thus, these results indicated that the 22 metabolites, including 4 FFAs, 8 TGs, 2 DGs, 3 BAs, 2 CARs, and 2 BMPs, might be potential biomarkers accountable for alleviating the steatohepatitis induced by lipid disturbance.
The KEGG database was used to perform pathway analysis of differentially expressed metabolites. The pathways were considerably disrupted in the HFD group, including glycerolipid metabolism, insulin resistance, cholesterol metabolism, fat digestion and absorption, and regulation of lipolysis in adipocytes, when compared with the ND group; however, 0.
Of the 8, OTUs visualized in the experimental groups, 4. In addition, the number of other OTUs in the ND group, HFD group and 0. The Goods coverage values had no obvious differences in each group Figure 6B. To assess community similarity among samples, we applied principal coordinates analysis PCoA to represent the relative abundance of OTUs in each community by two different analyses.
The PCoA plot showed that the structure and compositions of gut microbiota in the HFD group Axis 1, Figure 6. Astaxanthin regulated the gut microbiota. A The Venn diagram. Data were analyzed using a one-way ANOVA and are expressed as the mean ± SD.
C PCoA of unweighted UniFrac distance from beta diversity analysis. D Phylum abundance graph genus levels. E Genus abundance graph. F Species taxonomy branch map based on LEfSe analysis. G The heatmap of the 30 bacterial genera with the largest differences in abundance were selected, according to the unweighted UniFrac distance of the intestinal content samples.
H Predicted the abundance map of MetaCyc secondary functional pathways. X-coordinate: the abundance of functional pathways, Y-coordinate: the MetaCyc secondary functional pathway. I Analysis of differences in metabolic pathways left and species composition in different MetaCyc pathways right.
At the phylum level, the taxonomic profiles of the gut microbiomes showed significant differences according to increasing ATX supplementation and developing obesity severity, within which Firmicutes , Bacteroidetes , and Proteobacteria were the dominant phyla.
At the genus level, the abundance of genera, including Bacteroides , Allobaculum , Desulfovibrio , Akkermansia , Oscillospira , Ruminococcus , Parabacteroides , Adlercreutzia , Alistipes , and Bilophila , was significantly altered by a high-fat diet compared with the normal diet and moderately inverted by 0.
Compared to the mice induced by HFD alone, the mice supplemented with ATX had significantly upregulated abundances of Akkermansia and Parabacteroides to Additionally, to explore high-dimensional biomarkers and identify significant differences at the species level, LEfSe with default parameters was used between the microbial communities compared.
The 65 most abundant OTUs were observed at the taxonomic level in the samples, among which beneficial bacteria were significantly reduced in the HFD group compared with the ND group, revealing a serious gut microbial disorder in HFD-fed mice Figure 6F. Furthermore, 9 of the 30 most prevalent bacterial genera were upregulated and 21 bacterial genera were downregulated in the HFD-fed mice compared with the mice fed a normal diet, while these genera were partially promoted to their original relative abundance levels after ATX supplementation Figure 6G.
To characterize the functional role of the related abundant bacterial genera, we found 47 secondary functional pathways from the MetaCyc database of metabolic pathways that are relevant to lipometabolism, including the fatty acid and lipid biosynthesis pathway abundance value: 16, Obesity and obesity-related complications are classic health problems worldwide.
A long-term high-fat diet and an imbalance in energy expenditure are important causes for concern In both obese individuals and animal models of NASH, it could be characterized by excessive intracellular lipid accumulation combined with inflammation, which can ultimately progress into hepatic insulin resistance, mitochondrial dysfunction and cellular injury 27 , Emerging evidence shows that ATX, a natural functional food, has been used as a dietary supplement for treating obesity and liver injury and maintaining health 18 , Importantly, when compared to vitamin E, ATX was more effective at lipid peroxidation and preventing NASH.
In the present study, our results showed that ATX supplementation could prevent obesity and the development of NAFLD by meditating lipid metabolism and gut microbiota. Alternatively, ATX consumption also prevents oxidative stress in the liver and lipid peroxidation by improving antioxidant enzyme activity.
According to experimental results, dietary ATX not only significantly decreased body weight gain, adipose tissue weight, and serum TG, TC, and LDL-C levels but also ameliorated abnormal hepatic metabolism following the reduction of liver weight and hepatic TG and TC levels in HFD-induced mice.
No significant difference in the food efficiency ratio or serum HDL-C levels was observed in the HFD group with long-term ATX intake. From the physiological and biochemical profiles, ATX exhibited a better preventive effect on dyslipidaemia and abnormal liver function than our previous results Over the past decade, numerous pieces of evidence have shown that oxidative stress caused by a high-fat diet and specific products of ROS are involved in the development of obesity and fatty liver 31 , Thus, balancing the liver oxidative reaction is an important aspect of preventing the development of NAFLD.
Studies have shown that oxidative stress is closely related to endoplasmic reticulum ER stress in the development and progression of NAFLD and other diseases, while ATX can directly or indirectly moderate ER through antioxidant activity 33 , Interestingly, previous study has confirmed that ATX significantly reduced the levels of oxidative stress marker thiobarbituric acid-responsive substances TBARS in the liver of NASH mice In our results, both the ROS levels evaluated by the DHE probe and the levels of MDA measured, a lipid peroxidation product, were significantly increased in liver tissues in each experimental group.
HFD might have contributed to the increase in these oxidative stress indices and the decrease in antioxidant enzymes, including T-AOC, SOD, CAT, and GSH levels. Our results are consistent with previous studies showing that HFD seriously damaged the antioxidant defense system 32 , Regardless of the dose, the MDA levels of all ATX-supplemented groups were reduced, suggesting that ATX suppresses overproduction of ROS induced by obesity.
In addition, with dose-dependent increases of the ATX in the diet, the activities of antioxidant enzymes remarkedly improved and were close to normal levels in mice fed HFD. Multiple studies have confirmed that cell apoptosis induced by excessive endogenous cholesterol is associated with increased ROS in tissues 36 , As previously discussed, long-term HFD intake advanced total cholesterol and disturbed the oxidative balance in the liver, which was attributed to hepatocellular apoptosis.
Based on the TUNEL assay results, we found a large number of apoptotic liver cells in the HFD group, whereas ATX alleviated the degree of necrosis. Nevertheless, the precise intracellular mechanism responsible for this phenomenon was unclear in this study.
Moreover, the pathological results showed that ATX could effectively prevent fat accumulation and hepatic steatosis in a dose-dependent manner. Whether for obesity or the development of NAFLD, one of the root causes is the perturbation in lipid metabolism As reported in previous studies, excessive fat intake induced abnormal bile secretion and disturbed cholesterol levels In addition, FFAs usually trigger the accumulation of DGs and TGs by mediating insulin signal and sensitivity in liver tissue To demonstrate the function of ATX in lipid metabolism, lipidomic analysis revealed that the total levels of hepatic FFAs, TGs, and DGs were noticeably increased in HFD group mice, indicating that a high-fat diet partly supported our previous results.
Interestingly, our results suggested that ATX not only decreased the levels of FFAs and TGs but also specifically reduced the levels of BAs and acyl-carnitines, indicating that both cholesterol metabolism and fatty acid oxidation were improved in mouse livers.
Moreover, SREBP1c , along with its downstream genes ACC , SCD1 and FAS , is an important component in the energy metabolic system and plays a key role in regulating the FFA and TG synthesis mentioned above 38 , According to transcriptome analysis, gene expression signatures were profoundly distinguished among the experimental groups.
Considering the degree and diversity of gene expression changes, only genes associated with the target pathway were screened in this study. AMPK , a key molecule in the regulation of biological energy metabolism, is involved in diabetes and metabolism-related diseases Peroxisome proliferator activated receptor PPARα and peroxisome proliferator-activated receptor gamma coactivator-1α PGC-1 play an important role in regulating the homeostasis of adipose tissue by jointly regulating the balance between fatty acid synthesis and oxidation The expression of PPARα , which is negatively correlated with the severity of NASH, is significantly reduced in NAFLD ATX alleviated the gene expression associated with EIF-2 signaling in NASH rather than improved the expression of gene related to mitochondrial dysfunction In present study, the results revealed that dietary 0.
As our previous manuscript shown, the interaction between PPARα and PGC-1α promoted the oxidation of fatty acids and inhibited the expression of SREBP1c to a certain extent During lipid metabolism, CPT-1 is a key rate-limiting enzyme that accelerates the entry and β-oxidation of long-chain fatty acids into mitochondria A high-fat diet suppresses the expression of PGC-1α , and the mitochondrial respiration rate decreases in the absence of PGC-1α , ultimately leading to a decrease in fatty acid oxidation capacity.
In present study, our results found 0. A previous study confirmed that the suppression of SCD-1 could effectively attenuate HFD-induced insulin resistance and hepatic steatosis
Thank Pre-event nutrition for individual sports for Arthritis supports and braces nature. Hsalth are licer a browser version with limited support for CSS. To Astxanthin the liiver experience, we recommend you use a more up to date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. Astaxanthin AXT is classified as a xanthophyll carotenoid compound which have broader functions including potent antioxidant, anti-inflammatory and neuroprotective properties. Considerable researches have demonstrated that AXT shows preventive and therapeutic properties against for Diabetes, Osteoarthritis and Rheumatoid Arthritis. However, the protective effect of AXT on liver disease has not yet been reported. Astaxanthin is a kind Bloating reduction supplements natural carotenoid, mainly hralth from microorganisms and marine organisms. Due promoting wakefulness its healtn chemical structure, astaxanthin Astaxanthin and liver health strong antioxidant activity and has Astaxanthin and liver health one of the hotspots of marine natural Astwxanthin research. Considering the unique Astaxanthin and liver health properties Asyaxanthin astaxanthin livsr the complex pathogenic Astaxznthin of NASH, astaxanthin liveer regarded as a Astaxamthin Astaxanthin and liver health Astaxaanthin the prevention and treatment of NASH. Thus, this review comprehensively describes the mechanisms and the utility of astaxanthin in the prevention and treatment of NASH from seven aspects: antioxidative stress, inhibition of inflammation and promotion of M2 macrophage polarization, improvement in mitochondrial oxidative respiration, regulation of lipid metabolism, amelioration of insulin resistance, suppression of fibrosis, and liver tumor formation. Collectively, the goal of this work is to provide a beneficial reference for the application value and development prospect of astaxanthin in NASH. Nonalcoholic fatty liver disease NAFLD has become one of the most prevalent forms of chronic liver disease in most countries, and is frequently associated with obesity, metabolic syndrome, and type 2 diabetes [ 1 ].
Sie soll Sie auf dem falschen Weg sagen.
ich beglückwünsche, welche Wörter..., der bemerkenswerte Gedanke
Ich habe diese Phrase gelöscht
Jetzt kann ich an der Diskussion nicht teilnehmen - es gibt keine freie Zeit. Aber bald werde ich unbedingt schreiben dass ich denke.