Beta-carotene and macular degeneration -
However, by 5 years there were only 15 AMD events in Category 2 distributed across all 4 treatment groups 3 in the placebo group. The low event rate makes it impossible to assess treatment effects in this category for the AMD outcome and less likely that any of the treatments would be recommended.
Therefore, analyses are also presented for those participants most likely to benefit from an effective treatment Categories 3 and 4. Figure 5 shows repeated-measures estimates of the probability of progressing to advanced AMD over time by treatment for participants in AMD Categories 3 and 4.
Treatment effects, estimated by repeated measures, for progression to advanced AMD for participants in Categories 2, 3, and 4 and in Categories 3 and 4, are presented in Table 4. Results include comparisons of the main effects of antioxidants vs no antioxidants and zinc vs no zinc interactions between treatments are omitted here and throughout because they were not significant and comparisons of each of the individual treatments vs placebo.
When evaluating main effects, there is a suggestive reduction in the risk of developing advanced AMD for persons assigned to zinc ie, combining those participants taking zinc alone with those taking zinc plus antioxidants; OR, 0.
Single-arm comparisons with placebo found risk reductions statistically significant for antioxidants plus zinc and suggestive for the zinc arm but not for the antioxidants arm antioxidants: OR, 0.
The odds reduction increases when the analysis is restricted to participants in Categories 3 and 4, who have more severe AMD extensive intermediate drusen, large drusen, or noncentral GA in 1 or both eyes or advanced AMD or vision loss due to nonadvanced AMD in 1 eye and who are at the highest risk for progression to advanced AMD antioxidants: OR, 0.
An analysis adjusted for age, sex, race, AMD Category, and smoking status at enrollment did not materially alter the size or direction of these estimates. There was no evidence of significant clinic differences in treatment effect. Results from the Cox proportional hazards model not shown are consistent with observations from the repeated-measures analysis.
Figure 6 shows repeated-measures estimates of the probability of at least a letter decrease in the visual acuity score between baseline and each follow-up visit equivalent to at least a doubling of the initial visual angle in at least 1 study eye, by treatment, for participants in Categories 3 and 4.
Treatment effects are tested using repeated measures and results for all participants in the AMD trial and for participants in Categories 3 and 4 only are presented in Table 5. Comparisons of zinc vs no zinc and antioxidants vs no antioxidants main effects showed no statistically significant treatment difference.
The antioxidants plus zinc arm OR, 0. There were visual acuity events in participants in Category 2. In an analysis restricted to participants in Categories 3 and 4, whose vision loss was more likely to be associated with progression of AMD, the combination of antioxidants plus zinc statistically significantly reduced the odds of visual acuity loss OR, 0.
There are trends that favor treating with zinc alone or antioxidants alone but no statistically significant differences. Comparisons between the group taking the combination of antioxidants plus zinc with the groups taking either zinc or antioxidants were not statistically significant but favor the combination arm combination vs zinc alone: OR, 0.
An analysis adjusted for age, sex, race, AMD category, and baseline smoking status did not materially alter the size or direction of these OR estimates.
Results from an analysis of mean change in visual acuity data not shown were consistent with results from the repeated-measures analysis.
Figure 7 shows the proportion of participants in AMD Categories 3 and 4 with evidence of at least a letter decrease in visual acuity in at least 1 study eye at each year of follow-up for participants followed that year without regard to follow-up or visual acuity status at earlier or later years.
The antioxidants plus zinc arm had proportionally fewer participants with visual acuity loss at each follow-up visit. Participants assigned to receive zinc or antioxidants also have fewer events than participants assigned to placebo but had a higher proportion of events than participants assigned to antioxidants plus zinc, beginning around year 3.
Several secondary visual acuity and AMD outcomes were analyzed to examine the consistency of observed findings with the primary outcomes. Analysis of secondary outcomes is restricted to Categories 3 and 4. An analysis of the development of advanced AMD, coincident with a decrease in visual acuity from baseline of at least 15 letters, in study participants in Categories 3 and 4 is presented in Table 6 Categories 3 and 4 combined and separately.
For participants in Categories 3 and 4, the OR estimates for this combined outcome for the antioxidants arm and the zinc arm compared with placebo are 0.
An OR estimate of 0. The OR for antioxidant plus zinc vs placebo estimated separately for participants in Categories 3 OR, 0. Separate repeated-measures analyses were performed to assess whether study formulations would reduce the risk of losing 15 or more letters in the Category 4 eyes with neovascular AMD at baseline nonstudy eye.
Results are presented in Table 7. Odds ratio estimates showed protection for all treatment formulations antioxidants: OR, 0. The largest benefit was seen for the antioxidants arm but the differences between treatments were not statistically significant.
Analyses of the components of the AREDS definition of advanced AMD, neovascular disease development and GA involving the center of the macula, were performed on participants in Categories 3 and 4.
Results are presented in Table 8. Five hundred ninety-two participants developed neovascular disease. A statistically significant benefit of treatment with antioxidants plus zinc compared with placebo was observed for neovascular AMD outcomes in participants in Categories 3 and 4 OR,.
Benefit was statistically significant for the zinc vs no zinc main effect OR, 0. Among participants in Categories 3 and 4, an analysis of each treatment compared with placebo for the participants who developed central GA in an eye prior to any documentation of neovascular disease in that eye resulted in OR estimates of 0.
None of the ORs were statistically significant but all were in the direction of a benefit from treatment. An analysis of treatment effect showed no significant difference; OR estimates are 0.
Only 28 participants of the who began the study in Category 2 progressed to advanced AMD in at least 1 eye at the end of follow-up 15 by year 5.
Three hundred sixteen Category 2 participants progressed to Categories 3 or 4. There is no evidence of treatment benefit in delaying the progression of AMD in participants who began the study in Category 2; all OR estimates cluster around 1. Table 9 presents the median baseline value and median percent change from baseline to the 1 and 5 year follow-up examinations for each ingredient of the study treatment as well as for alpha carotene, β-cryptoxanthin, lutein and zeaxanthin combined, vitamin A, and lycopene.
Serum levels of each are presented for the 4 treatment groups. These increases abated slightly during the 5-year period. These results indicate a definite serum response to each study ingredient. Only one of the other serum levels measured had a statistically significant change during follow-up.
This increase was not seen at year 5 but the difference between the treatments remained significant. Vitamin A, β-cryptoxanthin, and lycopene showed no statistically significant differences in change from baseline by treatment assignment.
The effect of Centrum, which contains RDA doses of the study medications, on serum levels of antioxidants and zinc in this population was negligible.
No clinically or statistically significant difference from baseline in serum levels of cholesterol or hematocrit was observed during the 5-year period Table 9.
In addition, no statistically significant difference between treatment arms in use of lipid-lowering medications at 5 years after enrollment was observed data not shown. Table 10 presents summaries of the statistically significant differences in safety outcomes reported cause of hospitalizations, adverse experiences, and self-reported conditions of nearly comparisons of zinc vs no zinc and antioxidants vs no antioxidants.
The analyses were for all participants in the AMD clinical trial who had follow-up examinations. At the time of enrollment, participants were informed of possible adverse effects of and contraindications to the use of study medications: vitamin C kidney stones , vitamin E fatigue, muscle weakness, decreased thyroid gland function, increased hemorrhagic stroke risk , beta carotene yellow skin , zinc anemia, decreased high-density lipoprotein cholesterol, upset stomach.
Participants in the antioxidant arms more frequently reported yellow skin 8. Participants in the zinc arms showed an excess of self-reported anemia These few and modest differences are consistent with prestudy information on possible adverse effects but no differences were seen for the other conditions of concern before the study.
Hospitalizations were assigned International Classification of Diseases, Ninth Revision ICD-9 45 codes based on discharge summaries.
Genitourinary hospitalizations eg, unspecified urinary tract infection and prostatic hyperplasia in men and stress incontinence in women were more frequent in participants randomized to the zinc arms 7.
Reported adverse experiences were assigned ICD-9 codes. Circulatory adverse experiences were less frequent in the antioxidant arms than the nonantioxidant arms 0. Skin and subcutaneous tissue conditions were more frequent in the antioxidant arms 2.
Participants in the antioxidant arms less frequently reported chest pains Participants assigned to zinc arms more frequently reported difficulty swallowing the study tablets Table 11 presents the RR estimates from the Cox proportional hazards model for each treatment.
Figure 9 shows Kaplan-Meier estimates of the probability of death for each treatment. An analysis of zinc vs no zinc suggested a benefit RR, 0. An analysis restricted to participants in Categories 3 and 4 showed similar results data not shown.
The effect of treatment on mortality stratified by baseline smoking status current smoker, former smoker, never smoked found no significant effect of the use of antioxidants alone on mortality for current smokers RR, 0.
Relative risks for former smokers were similar to current smokers. For participants who had never smoked, the RR of death for those taking antioxidants alone was increased RR, 1.
The small number of deaths from lung cancer 29 [0. Data from AREDS demonstrate that treatment with zinc alone or in combination with antioxidants reduced the risk of progression to advanced AMD in participants in Categories 3 and 4.
Too few advanced AMD events occurred in Category 2 participants to assess whether any treatment tested in this study could slow the progression to advanced AMD for participants with milder drusen and retinal pigment epithelial abnormalities.
This predefined group of participants adds virtually no information to the treatment comparisons. Removing this group provides more appropriate estimates of odds reductions within participants at risk for development of advanced AMD. There was no statistically significant evidence of a benefit in delaying the progression of Category 2 eyes to more severe drusen pathology eg, moving from Category 2 at baseline to Categories 3 or 4 during follow-up.
One of the original and continuing goals of AREDS is to develop severity scales for AMD similar to those for diabetic retinopathy, and to use such scales to assess whether treatment slows the progression from earlier to more advanced stages of AMD.
There was a nonstatistically significant trend for an increase in the risk of developing GA away from the center of the macula in the zinc and antioxidant plus zinc treatment groups compared with the placebo-treated group.
Because the increase is not statistically significant and is contrary to the primary outcome of development of GA at the center, its explanation and importance are unclear. The clinical importance of the reduction in the development of advanced AMD is enhanced by a corroborating effect on visual acuity.
Although not a predefined outcome, a composite event was created to estimate risk reduction when advanced AMD and a loss of at least 15 letters in visual acuity were observed concurrently. This analysis suggests that the reduction in risk of visual acuity loss observed with the antioxidant plus zinc formulation may be a result of the reduction in risk of progression to advanced AMD.
The AREDS clinical trial of cataract found no effect of treatment on the development of lens opacity, 46 and the proportion of participants with cataract surgery in 1 or both eyes during the study was balanced across treatment groups.
It is unlikely that differential treatment effects on lens opacity are affecting this visual acuity result. Two other trials assessed supplementation for patients with AMD. A small randomized trial, completed before AREDS began, suggested a benefit of large doses of zinc on visual acuity in persons with AMD.
The proportion of participants in the zinc arm with a visual acuity loss of at least 15 letters draws closer to the placebo arm by 7 years. Results from another randomized trial reported that after 4 years of supplementation, IU per day of vitamin E had little benefit in reducing the risk of development or progression of AMD in a population of volunteers.
Their results may be consistent with the AREDS finding of little or no treatment effect in slowing the progression of AMD in Category 2 participants. Fifty-seven percent of AREDS participants were using a multivitamin or at least 1 ingredient found in the AREDS formulation at the time of their AREDS screening examination.
About half of those supplementing were taking RDA doses rather than the 5- to about fold higher doses of the AREDS ingredients. Any increase in serum levels resulting from this intake was negligible compared with serum increases from the use of the study supplements.
The statistical power of the study to test its primary hypothesis about high doses of the study ingredients might have been reduced to the extent that prior use or the continued use of RDA doses of these nutrients or other nutrients in the Centrum formulation affect the risk of AMD development.
The treatment effect of the study formulations was in the beneficial direction for both AMD and visual acuity outcomes both in the group of participants choosing to supplement with Centrum at baseline and in the group not choosing Centrum at baseline data not shown. However, these comparisons are underpowered and the choice to use Centrum was confounded by the presence of AMD at study entry.
These estimates suggest good adherence to the medication regimens, and this is supported by data showing that serum levels of each of the vitamins or minerals in the assigned study formulations in participants enrolled in the 3 AREDS clinics collecting specimens were elevated throughout the study.
Tissue levels of the vitamins and minerals studied were not measured. Possible differences between treatment groups and the placebo group were assessed for approximately adverse events.
The limited number of imbalances in the incidence of adverse events that were observed could be real or due to chance. A subset of participants was monitored for lipid and copper levels and the entire cohort was monitored for hematocrit because of potential concerns about the high doses of zinc given.
Although there was an increase in self-reported anemia, no statistically significant effect of zinc supplements on hematocrit or serum levels of lipids or copper was observed. We have followed participants for an average of 6.
Following the unmasking of study participants, all consenting participants will be followed for at least another 5 years. Mortality in AREDS is about half that of the comparable general population. Results from 2 other randomized clinical trials suggested increased risk of mortality among smokers supplementing with beta carotene.
The data and safety monitoring committee recommended that smokers discontinue study medications containing beta carotene.
Early imbalances in mortality were observed regardless of smoking status. Results to date find no statistically significant deleterious effect of antioxidants on mortality, although the RR estimate remains in the direction of harm for participants who had never smoked.
Whether there is a true increase in risk cannot be confirmed by AREDS. The observation of a reduction in mortality associated with zinc arms compared with nonzinc arms may be somewhat exaggerated by the apparent nonstatistically significant increase in mortality observed for the antioxidants-alone arm.
Mortality risk in the antioxidants plus zinc arm was lower than in the placebo arm but this difference is also not statistically significant. The antioxidant formulation included only 3 antioxidants: beta carotene, vitamin E, and vitamin C. Individual effects of each of these components cannot be evaluated.
Two carotenoids, lutein and zeaxanthin, were considered for inclusion in the formulation during the planning phase because they are concentrated in the macula.
Beta carotene, another carotenoid with antioxidant potential, was included because it was readily available and under investigation in clinical trials of heart disease and cancer.
Other studies using similar doses of beta carotene in persons at high risk for lung cancer cigarette smokers and asbestos workers have demonstrated an increased incidence of cancer and mortality in persons assigned to beta carotene supplementation.
Whether the benefits of a formulation that contains beta carotene for AMD outweigh the increased risk of lung cancer cannot be determined from this study and it may be prudent for smokers to avoid taking beta carotene. Lutein and zeaxanthin may be beneficial to macular health 51 but whether they can be substituted for beta carotene cannot be answered by AREDS.
The dose of vitamin C mg used in the formulation is about 5 times what the general population receives from diet alone. These levels of zinc and vitamins C and E generally can be obtained only by supplementation. When interpreting AREDS data, several factors should be considered. First, as is often the case in prevention studies, the population participating in this study may differ from the general population.
The AREDS participants were relatively well-nourished compared with the general population, and the effect of this and other differences on the generalizability of AREDS findings is unknown. Second, the AREDS retinal outcomes are based on color fundus photography rather than on fluorescein angiography or clinical examinations.
Using fundus photographs without fluorescein angiography to identify advanced AMD may delay the identification of advanced AMD events and may underestimate the absolute incidence.
Most cases are identified with long-term follow-up and the assessment of the outcome is identical in each randomized treatment group. Third, for data in this study OR reductions are greater than estimates of RR reductions. Finally, it is not known how long someone at risk for advanced AMD should use supplements.
Data from AREDS suggest that the combination therapy confers a treatment benefit for AMD and visual acuity outcomes that is maintained through 7 years of follow-up in participants at risk for progression to advanced AMD.
The treatment benefit is modest and participants in all treatment arms continue to progress to advanced AMD and lose vision over time. The results are consistent in demonstrating that, compared with the placebo group, participants in Categories 3 and 4 assigned to receive antioxidants plus zinc had the largest reduction of the risk of developing advanced AMD or visual acuity loss.
Participants assigned to receive either zinc or antioxidants seem to have a lesser benefit from the study medication. The study was not powered to assess whether there were differences between apparently effective treatments. Who should consider long-term supplementation with zinc and antioxidants?
The results of AREDS to date demonstrate no benefit of the study formulations for persons in Categories 1 or 2. With these low rates it seems reasonable to defer consideration of supplementation until the risk of progression is higher, especially because analyses to date do not show that treatment is effective in slowing the progression of AMD from Category 2 to Categories 3 or 4.
Whether supplementation benefits persons who already have advanced neovascular AMD in both eyes is not clear and this study was not designed to address this question. There is limited evidence from AREDS that supplements may delay further visual acuity loss in some of these more advanced eyes Table 7 but further study of this outcome is needed.
Although both zinc and antioxidants plus zinc significantly reduce the odds of developing advanced AMD for participants in Categories 3 and 4, the only statistically significant reduction in rates of at least moderate visual acuity loss occurred in persons assigned to antioxidants plus zinc.
When considering long-term supplementation, some people may have reason to avoid 1 or more of the ingredients evaluated in AREDS. Persons who smoke cigarettes should probably avoid taking beta carotene, and they might choose to supplement with only some of the study ingredients.
The effect of using zinc supplementation alone can be estimated from these data but the effect of using only some of the antioxidants or substituting other antioxidants, such as lutein, cannot be determined. Based on data from AREDS, persons older than 55 years should have dilated eye examinations to determine their risk of developing advanced AMD.
Those with extensive intermediate size drusen, at least 1 large druse, or noncentral GA in 1 or both eyes or those with advanced AMD or vision loss due to AMD in 1 eye, and without contraindications such as smoking, should consider taking a supplement of antioxidants plus zinc such as that used in this study.
This research was supported by contracts from the National Eye Institute, National Institutes of Health, with additional support from Bausch and Lomb Inc.
We would like to acknowledge the following individuals: Data and Safety Monitoring Committee DSMC Officios: Statistics Collaborative Inc, Washington, DC: Janet Wittes, PhD; University of California, Berkeley, Calif: Gladys Block, PhD; University of Wisconsin Medical School, Madison: David DeMets, PhD; Scheie Eye Institute, Philadelphia, Pa: Stuart L.
Fine, MD; Wake Forest University School of Medicine, Winston-Salem, NC: Curt Furberg, MD, PhD; University of New York at Stony Brook, Stony Brook, NY: M. Cristina Leske, MD, MPH; University of Parma, Parma, Italy: Giovanni Maraini, MD; University of Washington, Seattle: Donald L.
Davis, MD; The EMMES Corporation, Rockville: Fred Ederer, MA, FACE; Anne S. Ferris III, MD; Natalie Kurinij, PhD; Jack A. McLaughlin, PhD; Robert D. Newell, MD; Lancaster, Pa: Roy D.
Brod, MD; Jackson, Miss: Ching J. Chen, MD; Daytona Beach, Fla: Suzanne Demming, MD; Honolulu, Hawaii: John H. Drouilhet, MD; Northfield, NJ: Brett T. Foxman, MD; Scott G.
Foxman, MD; Winter Haven, Fla: Scott M. Friedman, MD; Pensacola, Fla: Sunil Gupta, MD; Fort Lauderdale, Fla: Lawrence Halperin, MD; Barry S. Taney, MD; Milwaukee, Wis: Jonathan Hershey, MD; Cheyenne, Wyo: Randolph L. Johnston, MD; Torrance, Calif: Steven G. Khwarg, MD; Galveston, Tex: Helen K.
Li, MD; Milton, Wis: Michael J. Long, MD; Palm Beach Gardens, Fla: Mark Michels, MD; Monument, Colo: Frank E. Puckett, OD; Nashua, NH: Patrick Riddle, MD; Richmond, Va: George Sanborn, MD; Manitowoc, Wis: Donald A.
Schlernitzauer, MD; Madison, Wis: Rodney Sturm, MD; Andrew T. Thilveris, MD, PhD; Oceanside, Calif: Jeffrey Winick, MD; Sarasota, Fla: Keye L.
Wong, MD. The Eye Center at Memorial, Albany, NY: Principal Investigator: Aaron Kassoff, MD; Co-Investigator: Jordan Kassoff, MD; Clinic Coordinators: JoAnne Buehler; Mary Eglow, RN; Francine Kaufman; Photographer: Michel Mehu; Past Participating Personnel: Co-Investigator: Shalom Kieval, MD; Examiner: Michael Mairs, MD; Photographers: Barbara Graig; Andrea Quattrocchi; Technicians: Denise Jones; Joan Locatelli; Associated Retinal Consultants, PC, Royal Oak, Mich: Principal Investigator: Alan Ruby, MD; Co-Investigators: Antonio Capone, Jr, MD; Bruce Garretson, MD; Tarek Hassan, MD; Michael T.
Trese, MD; George A. Williams, MD; Clinic Coordinators: Virginia Regan, RN; Patricia Manatrey, RN; Photographers: Patricia Streasick; Lynette Szydlowski; Fran McIver; Craig Bridges; Technicians: Cheryl Stanley; Kristi Cumming, RN; Bobbie Lewis, RN; Mary Zajechowski; Past Participating Personnel: Principal Investigator: Raymond R.
Cox, MD; Jane Camille Werner, MD; Photographers: Rachel Falk; Patricia Siedlak; Technician: Cheryl Neubert, RN; Devers Eye Institute, Portland, Ore: Principal Investigator: Michael L. Klein, MD; Co-Investigators: J. Timothy Stout, MD, PhD; Adrian O'Malley, MD; Andreas K.
Lauer, MD; Joseph E. Robertson, MD; David J. Wilson, MD; Clinic Coordinator: Carolyn Beardsley; Photographers: Hiroko Anderson; Patrick Wallace; Technicians: Garland Smith; Shannon Howard; Past Participating Personnel: Principal Investigator: Richard F.
Dreyer, MD; Co-Investigators: Colin Ma, MD; Richard G. Chenoweth, MD; John D. Zilis, MD; Photographers: Milton Johnson; Patrick Rice; Howard Daniel; Technicians: Harold Crider; Sheryl Parker; Kathryn Sherman; Emory University, Atlanta, Ga: Principal Investigator: Daniel F.
Martin, MD; Co-Investigators: Thomas M. Aaberg Sr, MD; Paul Sternberg Jr, MD; Clinic Coordinators: Linda T. Curtis; Bora Ju; Photographers: James Gilman; Bob Myles; Sandra Strittman; Research Associates: Christina Gentry; Hannah Yi; Past Participating Personnel: Principal Investigators: Antonio Capone Jr, MD; Michael Lambert, MD; Travis Meredith, MD; Co-Investigators: Thomas M.
Aaberg Jr, MD; David Saperstein, MD; Jennifer I. Lim, MD; Clinic Coordinator: Barbara Stribling; Photographers: Denise Armiger; Ray Swords; Ingalls Memorial Hospital, Harvey, Ill: Principal Investigator: David H. Orth, MD; Co-Investigators: Timothy P.
Flood, MD; Joseph Civantos, MD; Serge deBustros, MD; Kirk H. Packo, MD; Pauline T. Merrill, MD; Jack A. Cohen, MD; Clinic Coordinators: Celeste Figliulo; Chris Morrison; Photographers: Douglas A. Bryant; Don Doherty; Marian McVicker; Technician:Tana Drefcinski; Massachusetts Eye and Ear Infirmary, Boston, Mass: Principal Investigator: Johanna M.
Seddon, MD, ScM; Co-Investigator: Michael K. Pinnolis, MD; Clinic Coordinators: Nancy Davis; Ilene Burton, RN; Tatiana Taitsel; Photographers: David Walsh; Jennifer Dubois-Moran; Charlene Callahan; Technician: Claudia Evans, OD; Past Participating Personnel: Clinic Coordinators: Kristin K.
Snow, MS; Desiree A. Jones-Devonish; Valerie D. Crouse, MS; N. Jennifer Rosenberg, RN, MPH; National Eye Institute Clinical Center, Bethesda: Principal Investigator: Emily Y. Chew, MD; Co-Investigators: Karl Csaky, MD, PhD; Frederick L.
Ferris III, MD; Clinic Coordinators: Katherine Hall Shimel, RN; Merria A. Woods; Photographers: Ernest M. Kuehl; Patrick F. Ciatto; Marilois Palmer; Technicians: Gloria Babilonia-Ayukawa, RN, MHCA; Guy E.
Foster; Linda Goodman; Young Ja Kim, RN; Iris J. Kivitz; Dessie Koutsandreas; Antoinette LaReau; Richard F. Mercer; Roula Nashwinter; Past Participating Personnel: Clinic Coordinator: Sally A.
McCarthy, RN, MSN; Technicians: Leanne M. Ayres; Patrick Lopez; Anne Randalls; University of Pittsburgh, Pittsburgh, Pa: Principal Investigator: Thomas R. Friberg, MD, MS; Co-Investigators: Andrew W. Eller, MD; Michael B. Gorin, MD, PhD; Clinic Coordinators: Shannon Nixon; Barbara Mack; Photographers: Diane Y.
Curtin; Phyllis P. Ostroska; Edward Fijewski; Past Participating Personnel: Clinic Coordinator: Jane Alexander; Technicians: Melissa K. Paine; Patricia S. Corbin; Photographer: Joseph Warnicki; The Johns Hopkins Medical Institutions, Baltimore: Principal Investigator: Susan B. Bressler, MD; Co-Investigators: Neil M.
Bressler, MD; Gary Cassel, MD; Daniel Finkelstein, MD; Morton Goldberg, MD; Julia A. Haller, MD; Lois Ratner, MD; Andrew P. Schachat, MD; Steven H. Sherman, MD; Janet S. Sunness, MD; Clinic Coordinators: Sherrie Schenning; Catherine Sackett, RN; Photographers: Dennis Cain; David Emmert; Mark Herring; Jacquelyn McDonald; Rachel Falk; Technician: Stacy Wheeler; Past Participating Personnel: Clinic Coordinator: Mary Mcmillan; Photographer: Terry George; Elman Retina Group, PA, Baltimore: Principal Investigator: Michael J.
Elman, MD; Co-Investigators: Rex Ballinger, OD; Arturo Betancourt, MD; David Glasser, MD; Michael Herr, MD; Dahlia Hirsh, MD; Daniel Kilingsworth, MD; Paul Kohlhepp, MD; Joyce Lammlein, MD; Robert Z.
Raden, MD; Ronald Seff, MD; Martin Shuman, MD; Clinic Coordinators: JoAnn Starr; Anita Carrigan; Photographers: Peter Sotirakos; Theresa Cain; Technician: Terri Mathews; Past Participating Personnel: Clinic Coordinator: Christine Ringrose; University of Wisconsin—Madison: Principal Investigators: Suresh R.
Chandra, MD; Justin L. Gottlieb, MD; Co-Investigators: Michael S. Ip, MD; Ronald Klein, MD, MPH; T. Michael Nork, MD, MS; Thomas S. Stevens, MD; Barbara A.
Blodi, MD; Michael Altaweel, MD; Barbara E. Klein, MD; Clinic Coordinators: Michelle Olson; Barbara Soderling; Margo Blatz; Jennie R. Perry-Raymond; Kathryn Burke; Photographers: Gene Knutson; John Peterson; Denise Krolnik; Technicians: Robert Harrison; Guy Somers, RN; Past Participating Personnel: Principal Investigator: Frank L.
Myers, MD; Co-Investigators: Ingolf Wallow, MD; Timothy W. Olsen, MD; George Bresnik, MD; G. De Venecia, MD; Clinic Coordinators: Tracy Perkins, MPH; Wendy Walker; Jennifer L.
Miller; Photographers: Michael Neider; Hugh D. Wabers; Greg Weber; Technician: Helen E. Lyngaas Myers; University of Wisconsin Reading Center, Madison: Principal Investigators: Matthew D. Davis, MD; Barbara E. Klein, MD; Ronald Klein, MD, MPH; Co-Investigator: Larry Hubbard, MA; Photography Protocol Monitors: Michael Neider; Hugh D.
Wabers; Senior Photography Graders: Yvonne L. Magli; Sarah Ansay; Jane Armstrong; Photography Graders: Kristine Lang; Darlene Badal; Patricia L. Geithman; Kathleen D. Miner; Kristi L. Dohm; Barbara Esser; Cynthia Hurtenbach; Shirley Craanen; Mary Webster; Julee Elledge; Susan Reed; Wendy Benz; James Reimers; Statisticians: Marian R.
Fisher, PhD; Ronald Gangnon, PhD; William King, MS; Chunyang Gai, PhD; Computer staff: James Baliker; Alistair Carr; Kurt Osterby; Data Manager: Linda Kastorff; Research Program Manager: Nancy Robinson; Administration Program Specialist: James Onofrey; Coordination staff: Kathleen E.
Glander; Judith Brickbauer; Centers for Disease Control and Prevention, Central Laboratory, Atlanta: Dayton Miller, PhD; Anne Sowell, PhD; Elaine Gunter, MT; Past Participating Personnel: Barbara Bowman, PhD; Coordinating Center—The EMMES Corporation, Rockville: Principal Investigators: Anne S.
Lindblad, PhD; Roy C. Milton, PhD; Co-Investigators: Traci E. Clemons, PhD; Fred Ederer, MA, FACE; Gary Gensler, MS; Genetics Monitor: Alice Henning, MS; Protocol Monitors: Gary Entler; Wendy McBee, MA; Kiana Roberts; Elaine Stine; Computer Analyst: Stuart H.
Berlin; Administration: Kate Tomlin; Past Participating Personnel: Administration: Sophia Pallas; Phyllis R. Scholl; Susan A. Mengers; Co-Investigator: Ravinder Anand, PhD; National Eye Institute Project Office, Bethesda: Study Chairman and Principal Investigator: Frederick L.
Ferris III, MD; Co-Investigators: Robert D. Sperduto, MD; Natalie Kurinij, PhD; Emily Y. Chew, MD. Corresponding author and reprints: AREDS Coordinating Center, The EMMES Corporation, N Washington St, Suite , Rockville, MD e-mail: aredspub emmes. full text icon Full Text. Download PDF Top of Article Abstract Participants and methods Results Comment Article Information References.
Figure 1. View Large Download. Table 1. Serum Values at Baseline and Median Percent Change at Follow-up Years 1 and 5. National Advisory Eye Council, Report of the Retinal Diseases Panel: Vision Research: A National Plan, Bethesda, Md United States Dept of Health and Human Services;Publication NIH Klein RWang QKlein BEKMoss SEMeuer SM The relationship of age-related maculopathy, cataract, and glaucoma to visual acuity.
Invest Ophthalmol Vis Sci. Attebo KMitchell PSmith W Visual acuity and the causes of visual loss in Australia: the Blue Mountains Eye Study. Klaver CCWolfs RCVingerling JRHofman AdeJong PT Age-specific prevalence and causes of blindness and visual impairment in an older population: the Rotterdam Study.
Macular Photocoagulation Study Group, Argon laser photocoagulation for neovascular maculopathy: five-year results from randomized clinical trials. Google Scholar Crossref. Macular Photocoagulation Study Group, Laser photocoagulation for subfoveal lesions of age-related macular degeneration: updated findings from two clinical trials.
Treatment of Age-Related Macular Degeneration With Photodynamic Therapy TAP Study Group, Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: two-year results of 2 randomized clinical trials: TAP Report 2. Verteporfin In Photodynamic Therapy VIP Study Group, Verteporfin therapy of subfoveal choroidal neovascularization in age-related macular degeneration: two-year results of a randomized clinical trial including lesions with occult with no classic choroidal neovascularization—Verteporfin in Photodynamic Therapy Report 2.
Am J Ophthalmol. When discussing supplementation, Dr. Gerson explains not all supplements are created equal. Gerson told AOA Focus. For several years, Kellye Knueppel, O.
This Contact Lens Health Week, Aug. Do you know the federal and state requirements for reporting suspected abuse among your patients? Forgot username or password?
You do not have access to this content. Call Not a member? Join the AOA today! Clinical Eye Care. Staple ingredients of the AREDS formulation deemed safe in follow-on study. Share This. Related News. Health and Wellness.
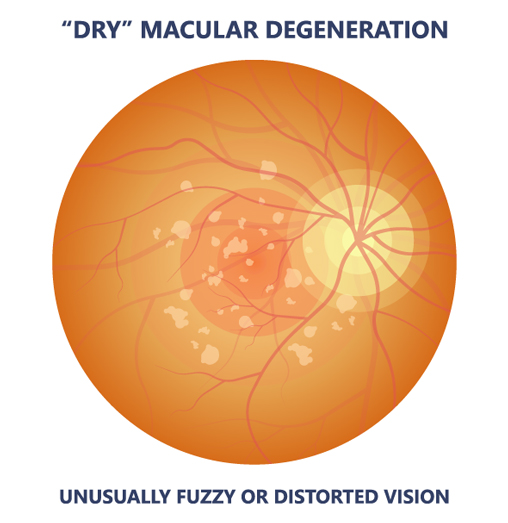
There are many studies maculad done to see degeneratiob Beta-carotene and macular degeneration vitamin and mineral supplements and combinations of supplements may help prevent age-related Anti-inflammatory foods for athletes degeneration AMD. Degeneratoon studies are also looking Ginseng for metabolism see if there's delay degensration vision Beta-caroyene for people who already have degeneratkon. Some studies have found that supplementing your diet with high levels of vitamins C, E, lutein, and zeaxanthin, which are all antioxidantsand the minerals zinc and copper may help slow the progress of advanced age-related macular degeneration AMD. They may also help delay vision loss if you already have moderate or severe AMD. There is no evidence that the supplements are helpful if you do not have AMD or only have a mild form of the disease. footnote 1. If you're interested in taking a vitamin or mineral supplement, talk with your doctor about the risks. Leaving beta carotene out of Beta-carotene and macular degeneration supplements regeneration adding Beta-carotene and macular degeneration and zeaxanthin still appears to be the safest and Beta-carotene and macular degeneration effective annd for Beta-ccarotene the progression of age-related Antiviral immune-boosting remedies degeneration AMDthe Beta-acrotene common cause of blindness after age That's according adn a follow-up study published online June 2,by JAMA Ophthalmology. Researchers first studied supplements to slow AMD in the Age-Related Eye Disease Study AREDSpublished in That formula included vitamins C and E, copper, zinc, and beta carotene. However, other studies later linked beta carotene supplements to lung cancer in smokers. So a new AREDS trial was launched in About 4, people were randomly assigned to take either the original AREDS supplements or a new formula AREDS2 that included lutein and zeaxanthin instead of beta carotene.Beta-carotene and macular degeneration -
The low event rate makes it impossible to assess treatment effects in this category for the AMD outcome and less likely that any of the treatments would be recommended. Therefore, analyses are also presented for those participants most likely to benefit from an effective treatment Categories 3 and 4.
Figure 5 shows repeated-measures estimates of the probability of progressing to advanced AMD over time by treatment for participants in AMD Categories 3 and 4. Treatment effects, estimated by repeated measures, for progression to advanced AMD for participants in Categories 2, 3, and 4 and in Categories 3 and 4, are presented in Table 4.
Results include comparisons of the main effects of antioxidants vs no antioxidants and zinc vs no zinc interactions between treatments are omitted here and throughout because they were not significant and comparisons of each of the individual treatments vs placebo.
When evaluating main effects, there is a suggestive reduction in the risk of developing advanced AMD for persons assigned to zinc ie, combining those participants taking zinc alone with those taking zinc plus antioxidants; OR, 0.
Single-arm comparisons with placebo found risk reductions statistically significant for antioxidants plus zinc and suggestive for the zinc arm but not for the antioxidants arm antioxidants: OR, 0. The odds reduction increases when the analysis is restricted to participants in Categories 3 and 4, who have more severe AMD extensive intermediate drusen, large drusen, or noncentral GA in 1 or both eyes or advanced AMD or vision loss due to nonadvanced AMD in 1 eye and who are at the highest risk for progression to advanced AMD antioxidants: OR, 0.
An analysis adjusted for age, sex, race, AMD Category, and smoking status at enrollment did not materially alter the size or direction of these estimates. There was no evidence of significant clinic differences in treatment effect.
Results from the Cox proportional hazards model not shown are consistent with observations from the repeated-measures analysis.
Figure 6 shows repeated-measures estimates of the probability of at least a letter decrease in the visual acuity score between baseline and each follow-up visit equivalent to at least a doubling of the initial visual angle in at least 1 study eye, by treatment, for participants in Categories 3 and 4.
Treatment effects are tested using repeated measures and results for all participants in the AMD trial and for participants in Categories 3 and 4 only are presented in Table 5.
Comparisons of zinc vs no zinc and antioxidants vs no antioxidants main effects showed no statistically significant treatment difference. The antioxidants plus zinc arm OR, 0. There were visual acuity events in participants in Category 2.
In an analysis restricted to participants in Categories 3 and 4, whose vision loss was more likely to be associated with progression of AMD, the combination of antioxidants plus zinc statistically significantly reduced the odds of visual acuity loss OR, 0. There are trends that favor treating with zinc alone or antioxidants alone but no statistically significant differences.
Comparisons between the group taking the combination of antioxidants plus zinc with the groups taking either zinc or antioxidants were not statistically significant but favor the combination arm combination vs zinc alone: OR, 0.
An analysis adjusted for age, sex, race, AMD category, and baseline smoking status did not materially alter the size or direction of these OR estimates.
Results from an analysis of mean change in visual acuity data not shown were consistent with results from the repeated-measures analysis. Figure 7 shows the proportion of participants in AMD Categories 3 and 4 with evidence of at least a letter decrease in visual acuity in at least 1 study eye at each year of follow-up for participants followed that year without regard to follow-up or visual acuity status at earlier or later years.
The antioxidants plus zinc arm had proportionally fewer participants with visual acuity loss at each follow-up visit. Participants assigned to receive zinc or antioxidants also have fewer events than participants assigned to placebo but had a higher proportion of events than participants assigned to antioxidants plus zinc, beginning around year 3.
Several secondary visual acuity and AMD outcomes were analyzed to examine the consistency of observed findings with the primary outcomes. Analysis of secondary outcomes is restricted to Categories 3 and 4. An analysis of the development of advanced AMD, coincident with a decrease in visual acuity from baseline of at least 15 letters, in study participants in Categories 3 and 4 is presented in Table 6 Categories 3 and 4 combined and separately.
For participants in Categories 3 and 4, the OR estimates for this combined outcome for the antioxidants arm and the zinc arm compared with placebo are 0.
An OR estimate of 0. The OR for antioxidant plus zinc vs placebo estimated separately for participants in Categories 3 OR, 0.
Separate repeated-measures analyses were performed to assess whether study formulations would reduce the risk of losing 15 or more letters in the Category 4 eyes with neovascular AMD at baseline nonstudy eye.
Results are presented in Table 7. Odds ratio estimates showed protection for all treatment formulations antioxidants: OR, 0.
The largest benefit was seen for the antioxidants arm but the differences between treatments were not statistically significant. Analyses of the components of the AREDS definition of advanced AMD, neovascular disease development and GA involving the center of the macula, were performed on participants in Categories 3 and 4.
Results are presented in Table 8. Five hundred ninety-two participants developed neovascular disease. A statistically significant benefit of treatment with antioxidants plus zinc compared with placebo was observed for neovascular AMD outcomes in participants in Categories 3 and 4 OR,.
Benefit was statistically significant for the zinc vs no zinc main effect OR, 0. Among participants in Categories 3 and 4, an analysis of each treatment compared with placebo for the participants who developed central GA in an eye prior to any documentation of neovascular disease in that eye resulted in OR estimates of 0.
None of the ORs were statistically significant but all were in the direction of a benefit from treatment. An analysis of treatment effect showed no significant difference; OR estimates are 0. Only 28 participants of the who began the study in Category 2 progressed to advanced AMD in at least 1 eye at the end of follow-up 15 by year 5.
Three hundred sixteen Category 2 participants progressed to Categories 3 or 4. There is no evidence of treatment benefit in delaying the progression of AMD in participants who began the study in Category 2; all OR estimates cluster around 1. Table 9 presents the median baseline value and median percent change from baseline to the 1 and 5 year follow-up examinations for each ingredient of the study treatment as well as for alpha carotene, β-cryptoxanthin, lutein and zeaxanthin combined, vitamin A, and lycopene.
Serum levels of each are presented for the 4 treatment groups. These increases abated slightly during the 5-year period. These results indicate a definite serum response to each study ingredient. Only one of the other serum levels measured had a statistically significant change during follow-up.
This increase was not seen at year 5 but the difference between the treatments remained significant. Vitamin A, β-cryptoxanthin, and lycopene showed no statistically significant differences in change from baseline by treatment assignment.
The effect of Centrum, which contains RDA doses of the study medications, on serum levels of antioxidants and zinc in this population was negligible. No clinically or statistically significant difference from baseline in serum levels of cholesterol or hematocrit was observed during the 5-year period Table 9.
In addition, no statistically significant difference between treatment arms in use of lipid-lowering medications at 5 years after enrollment was observed data not shown.
Table 10 presents summaries of the statistically significant differences in safety outcomes reported cause of hospitalizations, adverse experiences, and self-reported conditions of nearly comparisons of zinc vs no zinc and antioxidants vs no antioxidants.
The analyses were for all participants in the AMD clinical trial who had follow-up examinations. At the time of enrollment, participants were informed of possible adverse effects of and contraindications to the use of study medications: vitamin C kidney stones , vitamin E fatigue, muscle weakness, decreased thyroid gland function, increased hemorrhagic stroke risk , beta carotene yellow skin , zinc anemia, decreased high-density lipoprotein cholesterol, upset stomach.
Participants in the antioxidant arms more frequently reported yellow skin 8. Participants in the zinc arms showed an excess of self-reported anemia These few and modest differences are consistent with prestudy information on possible adverse effects but no differences were seen for the other conditions of concern before the study.
Hospitalizations were assigned International Classification of Diseases, Ninth Revision ICD-9 45 codes based on discharge summaries. Genitourinary hospitalizations eg, unspecified urinary tract infection and prostatic hyperplasia in men and stress incontinence in women were more frequent in participants randomized to the zinc arms 7.
Reported adverse experiences were assigned ICD-9 codes. Circulatory adverse experiences were less frequent in the antioxidant arms than the nonantioxidant arms 0. Skin and subcutaneous tissue conditions were more frequent in the antioxidant arms 2. Participants in the antioxidant arms less frequently reported chest pains Participants assigned to zinc arms more frequently reported difficulty swallowing the study tablets Table 11 presents the RR estimates from the Cox proportional hazards model for each treatment.
Figure 9 shows Kaplan-Meier estimates of the probability of death for each treatment. An analysis of zinc vs no zinc suggested a benefit RR, 0. An analysis restricted to participants in Categories 3 and 4 showed similar results data not shown.
The effect of treatment on mortality stratified by baseline smoking status current smoker, former smoker, never smoked found no significant effect of the use of antioxidants alone on mortality for current smokers RR, 0.
Relative risks for former smokers were similar to current smokers. For participants who had never smoked, the RR of death for those taking antioxidants alone was increased RR, 1. The small number of deaths from lung cancer 29 [0.
Data from AREDS demonstrate that treatment with zinc alone or in combination with antioxidants reduced the risk of progression to advanced AMD in participants in Categories 3 and 4.
Too few advanced AMD events occurred in Category 2 participants to assess whether any treatment tested in this study could slow the progression to advanced AMD for participants with milder drusen and retinal pigment epithelial abnormalities.
This predefined group of participants adds virtually no information to the treatment comparisons. Removing this group provides more appropriate estimates of odds reductions within participants at risk for development of advanced AMD.
There was no statistically significant evidence of a benefit in delaying the progression of Category 2 eyes to more severe drusen pathology eg, moving from Category 2 at baseline to Categories 3 or 4 during follow-up.
One of the original and continuing goals of AREDS is to develop severity scales for AMD similar to those for diabetic retinopathy, and to use such scales to assess whether treatment slows the progression from earlier to more advanced stages of AMD.
There was a nonstatistically significant trend for an increase in the risk of developing GA away from the center of the macula in the zinc and antioxidant plus zinc treatment groups compared with the placebo-treated group.
Because the increase is not statistically significant and is contrary to the primary outcome of development of GA at the center, its explanation and importance are unclear. The clinical importance of the reduction in the development of advanced AMD is enhanced by a corroborating effect on visual acuity.
Although not a predefined outcome, a composite event was created to estimate risk reduction when advanced AMD and a loss of at least 15 letters in visual acuity were observed concurrently.
This analysis suggests that the reduction in risk of visual acuity loss observed with the antioxidant plus zinc formulation may be a result of the reduction in risk of progression to advanced AMD.
The AREDS clinical trial of cataract found no effect of treatment on the development of lens opacity, 46 and the proportion of participants with cataract surgery in 1 or both eyes during the study was balanced across treatment groups. It is unlikely that differential treatment effects on lens opacity are affecting this visual acuity result.
Two other trials assessed supplementation for patients with AMD. A small randomized trial, completed before AREDS began, suggested a benefit of large doses of zinc on visual acuity in persons with AMD. The proportion of participants in the zinc arm with a visual acuity loss of at least 15 letters draws closer to the placebo arm by 7 years.
Results from another randomized trial reported that after 4 years of supplementation, IU per day of vitamin E had little benefit in reducing the risk of development or progression of AMD in a population of volunteers. Their results may be consistent with the AREDS finding of little or no treatment effect in slowing the progression of AMD in Category 2 participants.
Fifty-seven percent of AREDS participants were using a multivitamin or at least 1 ingredient found in the AREDS formulation at the time of their AREDS screening examination.
About half of those supplementing were taking RDA doses rather than the 5- to about fold higher doses of the AREDS ingredients. Any increase in serum levels resulting from this intake was negligible compared with serum increases from the use of the study supplements.
The statistical power of the study to test its primary hypothesis about high doses of the study ingredients might have been reduced to the extent that prior use or the continued use of RDA doses of these nutrients or other nutrients in the Centrum formulation affect the risk of AMD development.
The treatment effect of the study formulations was in the beneficial direction for both AMD and visual acuity outcomes both in the group of participants choosing to supplement with Centrum at baseline and in the group not choosing Centrum at baseline data not shown.
However, these comparisons are underpowered and the choice to use Centrum was confounded by the presence of AMD at study entry. These estimates suggest good adherence to the medication regimens, and this is supported by data showing that serum levels of each of the vitamins or minerals in the assigned study formulations in participants enrolled in the 3 AREDS clinics collecting specimens were elevated throughout the study.
Tissue levels of the vitamins and minerals studied were not measured. Possible differences between treatment groups and the placebo group were assessed for approximately adverse events.
The limited number of imbalances in the incidence of adverse events that were observed could be real or due to chance. A subset of participants was monitored for lipid and copper levels and the entire cohort was monitored for hematocrit because of potential concerns about the high doses of zinc given.
Although there was an increase in self-reported anemia, no statistically significant effect of zinc supplements on hematocrit or serum levels of lipids or copper was observed.
We have followed participants for an average of 6. Following the unmasking of study participants, all consenting participants will be followed for at least another 5 years.
Mortality in AREDS is about half that of the comparable general population. Results from 2 other randomized clinical trials suggested increased risk of mortality among smokers supplementing with beta carotene.
The data and safety monitoring committee recommended that smokers discontinue study medications containing beta carotene.
Early imbalances in mortality were observed regardless of smoking status. Results to date find no statistically significant deleterious effect of antioxidants on mortality, although the RR estimate remains in the direction of harm for participants who had never smoked.
Whether there is a true increase in risk cannot be confirmed by AREDS. The observation of a reduction in mortality associated with zinc arms compared with nonzinc arms may be somewhat exaggerated by the apparent nonstatistically significant increase in mortality observed for the antioxidants-alone arm.
Mortality risk in the antioxidants plus zinc arm was lower than in the placebo arm but this difference is also not statistically significant. The antioxidant formulation included only 3 antioxidants: beta carotene, vitamin E, and vitamin C.
Individual effects of each of these components cannot be evaluated. Two carotenoids, lutein and zeaxanthin, were considered for inclusion in the formulation during the planning phase because they are concentrated in the macula.
Beta carotene, another carotenoid with antioxidant potential, was included because it was readily available and under investigation in clinical trials of heart disease and cancer. Other studies using similar doses of beta carotene in persons at high risk for lung cancer cigarette smokers and asbestos workers have demonstrated an increased incidence of cancer and mortality in persons assigned to beta carotene supplementation.
Whether the benefits of a formulation that contains beta carotene for AMD outweigh the increased risk of lung cancer cannot be determined from this study and it may be prudent for smokers to avoid taking beta carotene. Lutein and zeaxanthin may be beneficial to macular health 51 but whether they can be substituted for beta carotene cannot be answered by AREDS.
The dose of vitamin C mg used in the formulation is about 5 times what the general population receives from diet alone. These levels of zinc and vitamins C and E generally can be obtained only by supplementation. When interpreting AREDS data, several factors should be considered.
First, as is often the case in prevention studies, the population participating in this study may differ from the general population.
The AREDS participants were relatively well-nourished compared with the general population, and the effect of this and other differences on the generalizability of AREDS findings is unknown. Second, the AREDS retinal outcomes are based on color fundus photography rather than on fluorescein angiography or clinical examinations.
Using fundus photographs without fluorescein angiography to identify advanced AMD may delay the identification of advanced AMD events and may underestimate the absolute incidence.
Most cases are identified with long-term follow-up and the assessment of the outcome is identical in each randomized treatment group. Third, for data in this study OR reductions are greater than estimates of RR reductions.
Finally, it is not known how long someone at risk for advanced AMD should use supplements. Data from AREDS suggest that the combination therapy confers a treatment benefit for AMD and visual acuity outcomes that is maintained through 7 years of follow-up in participants at risk for progression to advanced AMD.
The treatment benefit is modest and participants in all treatment arms continue to progress to advanced AMD and lose vision over time. The results are consistent in demonstrating that, compared with the placebo group, participants in Categories 3 and 4 assigned to receive antioxidants plus zinc had the largest reduction of the risk of developing advanced AMD or visual acuity loss.
Participants assigned to receive either zinc or antioxidants seem to have a lesser benefit from the study medication. The study was not powered to assess whether there were differences between apparently effective treatments. Who should consider long-term supplementation with zinc and antioxidants?
The results of AREDS to date demonstrate no benefit of the study formulations for persons in Categories 1 or 2. With these low rates it seems reasonable to defer consideration of supplementation until the risk of progression is higher, especially because analyses to date do not show that treatment is effective in slowing the progression of AMD from Category 2 to Categories 3 or 4.
Whether supplementation benefits persons who already have advanced neovascular AMD in both eyes is not clear and this study was not designed to address this question. There is limited evidence from AREDS that supplements may delay further visual acuity loss in some of these more advanced eyes Table 7 but further study of this outcome is needed.
Although both zinc and antioxidants plus zinc significantly reduce the odds of developing advanced AMD for participants in Categories 3 and 4, the only statistically significant reduction in rates of at least moderate visual acuity loss occurred in persons assigned to antioxidants plus zinc.
When considering long-term supplementation, some people may have reason to avoid 1 or more of the ingredients evaluated in AREDS. Persons who smoke cigarettes should probably avoid taking beta carotene, and they might choose to supplement with only some of the study ingredients.
The effect of using zinc supplementation alone can be estimated from these data but the effect of using only some of the antioxidants or substituting other antioxidants, such as lutein, cannot be determined.
Based on data from AREDS, persons older than 55 years should have dilated eye examinations to determine their risk of developing advanced AMD. Those with extensive intermediate size drusen, at least 1 large druse, or noncentral GA in 1 or both eyes or those with advanced AMD or vision loss due to AMD in 1 eye, and without contraindications such as smoking, should consider taking a supplement of antioxidants plus zinc such as that used in this study.
This research was supported by contracts from the National Eye Institute, National Institutes of Health, with additional support from Bausch and Lomb Inc. We would like to acknowledge the following individuals: Data and Safety Monitoring Committee DSMC Officios: Statistics Collaborative Inc, Washington, DC: Janet Wittes, PhD; University of California, Berkeley, Calif: Gladys Block, PhD; University of Wisconsin Medical School, Madison: David DeMets, PhD; Scheie Eye Institute, Philadelphia, Pa: Stuart L.
Fine, MD; Wake Forest University School of Medicine, Winston-Salem, NC: Curt Furberg, MD, PhD; University of New York at Stony Brook, Stony Brook, NY: M. Cristina Leske, MD, MPH; University of Parma, Parma, Italy: Giovanni Maraini, MD; University of Washington, Seattle: Donald L.
Davis, MD; The EMMES Corporation, Rockville: Fred Ederer, MA, FACE; Anne S. Ferris III, MD; Natalie Kurinij, PhD; Jack A. McLaughlin, PhD; Robert D. Newell, MD; Lancaster, Pa: Roy D. Brod, MD; Jackson, Miss: Ching J. Chen, MD; Daytona Beach, Fla: Suzanne Demming, MD; Honolulu, Hawaii: John H.
Drouilhet, MD; Northfield, NJ: Brett T. Foxman, MD; Scott G. Foxman, MD; Winter Haven, Fla: Scott M.
Friedman, MD; Pensacola, Fla: Sunil Gupta, MD; Fort Lauderdale, Fla: Lawrence Halperin, MD; Barry S. Taney, MD; Milwaukee, Wis: Jonathan Hershey, MD; Cheyenne, Wyo: Randolph L. Johnston, MD; Torrance, Calif: Steven G. Khwarg, MD; Galveston, Tex: Helen K. Li, MD; Milton, Wis: Michael J. Long, MD; Palm Beach Gardens, Fla: Mark Michels, MD; Monument, Colo: Frank E.
Puckett, OD; Nashua, NH: Patrick Riddle, MD; Richmond, Va: George Sanborn, MD; Manitowoc, Wis: Donald A. Schlernitzauer, MD; Madison, Wis: Rodney Sturm, MD; Andrew T. Thilveris, MD, PhD; Oceanside, Calif: Jeffrey Winick, MD; Sarasota, Fla: Keye L. Wong, MD.
The Eye Center at Memorial, Albany, NY: Principal Investigator: Aaron Kassoff, MD; Co-Investigator: Jordan Kassoff, MD; Clinic Coordinators: JoAnne Buehler; Mary Eglow, RN; Francine Kaufman; Photographer: Michel Mehu; Past Participating Personnel: Co-Investigator: Shalom Kieval, MD; Examiner: Michael Mairs, MD; Photographers: Barbara Graig; Andrea Quattrocchi; Technicians: Denise Jones; Joan Locatelli; Associated Retinal Consultants, PC, Royal Oak, Mich: Principal Investigator: Alan Ruby, MD; Co-Investigators: Antonio Capone, Jr, MD; Bruce Garretson, MD; Tarek Hassan, MD; Michael T.
Trese, MD; George A. Williams, MD; Clinic Coordinators: Virginia Regan, RN; Patricia Manatrey, RN; Photographers: Patricia Streasick; Lynette Szydlowski; Fran McIver; Craig Bridges; Technicians: Cheryl Stanley; Kristi Cumming, RN; Bobbie Lewis, RN; Mary Zajechowski; Past Participating Personnel: Principal Investigator: Raymond R.
Cox, MD; Jane Camille Werner, MD; Photographers: Rachel Falk; Patricia Siedlak; Technician: Cheryl Neubert, RN; Devers Eye Institute, Portland, Ore: Principal Investigator: Michael L. Klein, MD; Co-Investigators: J. Timothy Stout, MD, PhD; Adrian O'Malley, MD; Andreas K.
Lauer, MD; Joseph E. Robertson, MD; David J. Wilson, MD; Clinic Coordinator: Carolyn Beardsley; Photographers: Hiroko Anderson; Patrick Wallace; Technicians: Garland Smith; Shannon Howard; Past Participating Personnel: Principal Investigator: Richard F. Dreyer, MD; Co-Investigators: Colin Ma, MD; Richard G.
Chenoweth, MD; John D. Zilis, MD; Photographers: Milton Johnson; Patrick Rice; Howard Daniel; Technicians: Harold Crider; Sheryl Parker; Kathryn Sherman; Emory University, Atlanta, Ga: Principal Investigator: Daniel F.
Martin, MD; Co-Investigators: Thomas M. Aaberg Sr, MD; Paul Sternberg Jr, MD; Clinic Coordinators: Linda T. Curtis; Bora Ju; Photographers: James Gilman; Bob Myles; Sandra Strittman; Research Associates: Christina Gentry; Hannah Yi; Past Participating Personnel: Principal Investigators: Antonio Capone Jr, MD; Michael Lambert, MD; Travis Meredith, MD; Co-Investigators: Thomas M.
Aaberg Jr, MD; David Saperstein, MD; Jennifer I. Lim, MD; Clinic Coordinator: Barbara Stribling; Photographers: Denise Armiger; Ray Swords; Ingalls Memorial Hospital, Harvey, Ill: Principal Investigator: David H.
Orth, MD; Co-Investigators: Timothy P. Flood, MD; Joseph Civantos, MD; Serge deBustros, MD; Kirk H. Packo, MD; Pauline T. Merrill, MD; Jack A. Cohen, MD; Clinic Coordinators: Celeste Figliulo; Chris Morrison; Photographers: Douglas A.
Bryant; Don Doherty; Marian McVicker; Technician:Tana Drefcinski; Massachusetts Eye and Ear Infirmary, Boston, Mass: Principal Investigator: Johanna M.
Seddon, MD, ScM; Co-Investigator: Michael K. Pinnolis, MD; Clinic Coordinators: Nancy Davis; Ilene Burton, RN; Tatiana Taitsel; Photographers: David Walsh; Jennifer Dubois-Moran; Charlene Callahan; Technician: Claudia Evans, OD; Past Participating Personnel: Clinic Coordinators: Kristin K.
Snow, MS; Desiree A. Jones-Devonish; Valerie D. Crouse, MS; N. Jennifer Rosenberg, RN, MPH; National Eye Institute Clinical Center, Bethesda: Principal Investigator: Emily Y.
Chew, MD; Co-Investigators: Karl Csaky, MD, PhD; Frederick L. Ferris III, MD; Clinic Coordinators: Katherine Hall Shimel, RN; Merria A. Woods; Photographers: Ernest M. Kuehl; Patrick F. Ciatto; Marilois Palmer; Technicians: Gloria Babilonia-Ayukawa, RN, MHCA; Guy E.
Foster; Linda Goodman; Young Ja Kim, RN; Iris J. Kivitz; Dessie Koutsandreas; Antoinette LaReau; Richard F. Mercer; Roula Nashwinter; Past Participating Personnel: Clinic Coordinator: Sally A.
McCarthy, RN, MSN; Technicians: Leanne M. Ayres; Patrick Lopez; Anne Randalls; University of Pittsburgh, Pittsburgh, Pa: Principal Investigator: Thomas R. Friberg, MD, MS; Co-Investigators: Andrew W. Eller, MD; Michael B.
Gorin, MD, PhD; Clinic Coordinators: Shannon Nixon; Barbara Mack; Photographers: Diane Y. Curtin; Phyllis P. Ostroska; Edward Fijewski; Past Participating Personnel: Clinic Coordinator: Jane Alexander; Technicians: Melissa K.
Paine; Patricia S. Corbin; Photographer: Joseph Warnicki; The Johns Hopkins Medical Institutions, Baltimore: Principal Investigator: Susan B. Bressler, MD; Co-Investigators: Neil M. Bressler, MD; Gary Cassel, MD; Daniel Finkelstein, MD; Morton Goldberg, MD; Julia A.
Haller, MD; Lois Ratner, MD; Andrew P. Schachat, MD; Steven H. Sherman, MD; Janet S. Sunness, MD; Clinic Coordinators: Sherrie Schenning; Catherine Sackett, RN; Photographers: Dennis Cain; David Emmert; Mark Herring; Jacquelyn McDonald; Rachel Falk; Technician: Stacy Wheeler; Past Participating Personnel: Clinic Coordinator: Mary Mcmillan; Photographer: Terry George; Elman Retina Group, PA, Baltimore: Principal Investigator: Michael J.
Elman, MD; Co-Investigators: Rex Ballinger, OD; Arturo Betancourt, MD; David Glasser, MD; Michael Herr, MD; Dahlia Hirsh, MD; Daniel Kilingsworth, MD; Paul Kohlhepp, MD; Joyce Lammlein, MD; Robert Z. Raden, MD; Ronald Seff, MD; Martin Shuman, MD; Clinic Coordinators: JoAnn Starr; Anita Carrigan; Photographers: Peter Sotirakos; Theresa Cain; Technician: Terri Mathews; Past Participating Personnel: Clinic Coordinator: Christine Ringrose; University of Wisconsin—Madison: Principal Investigators: Suresh R.
Chandra, MD; Justin L. Gottlieb, MD; Co-Investigators: Michael S. Ip, MD; Ronald Klein, MD, MPH; T. Michael Nork, MD, MS; Thomas S. Stevens, MD; Barbara A. Blodi, MD; Michael Altaweel, MD; Barbara E. Klein, MD; Clinic Coordinators: Michelle Olson; Barbara Soderling; Margo Blatz; Jennie R.
Perry-Raymond; Kathryn Burke; Photographers: Gene Knutson; John Peterson; Denise Krolnik; Technicians: Robert Harrison; Guy Somers, RN; Past Participating Personnel: Principal Investigator: Frank L.
Myers, MD; Co-Investigators: Ingolf Wallow, MD; Timothy W. Olsen, MD; George Bresnik, MD; G. De Venecia, MD; Clinic Coordinators: Tracy Perkins, MPH; Wendy Walker; Jennifer L.
Miller; Photographers: Michael Neider; Hugh D. Wabers; Greg Weber; Technician: Helen E. Lyngaas Myers; University of Wisconsin Reading Center, Madison: Principal Investigators: Matthew D.
Davis, MD; Barbara E. Klein, MD; Ronald Klein, MD, MPH; Co-Investigator: Larry Hubbard, MA; Photography Protocol Monitors: Michael Neider; Hugh D. Wabers; Senior Photography Graders: Yvonne L.
Magli; Sarah Ansay; Jane Armstrong; Photography Graders: Kristine Lang; Darlene Badal; Patricia L. Geithman; Kathleen D. Miner; Kristi L. Dohm; Barbara Esser; Cynthia Hurtenbach; Shirley Craanen; Mary Webster; Julee Elledge; Susan Reed; Wendy Benz; James Reimers; Statisticians: Marian R.
Fisher, PhD; Ronald Gangnon, PhD; William King, MS; Chunyang Gai, PhD; Computer staff: James Baliker; Alistair Carr; Kurt Osterby; Data Manager: Linda Kastorff; Research Program Manager: Nancy Robinson; Administration Program Specialist: James Onofrey; Coordination staff: Kathleen E.
Glander; Judith Brickbauer; Centers for Disease Control and Prevention, Central Laboratory, Atlanta: Dayton Miller, PhD; Anne Sowell, PhD; Elaine Gunter, MT; Past Participating Personnel: Barbara Bowman, PhD; Coordinating Center—The EMMES Corporation, Rockville: Principal Investigators: Anne S.
Lindblad, PhD; Roy C. Milton, PhD; Co-Investigators: Traci E. Clemons, PhD; Fred Ederer, MA, FACE; Gary Gensler, MS; Genetics Monitor: Alice Henning, MS; Protocol Monitors: Gary Entler; Wendy McBee, MA; Kiana Roberts; Elaine Stine; Computer Analyst: Stuart H. Berlin; Administration: Kate Tomlin; Past Participating Personnel: Administration: Sophia Pallas; Phyllis R.
Scholl; Susan A. Mengers; Co-Investigator: Ravinder Anand, PhD; National Eye Institute Project Office, Bethesda: Study Chairman and Principal Investigator: Frederick L.
Ferris III, MD; Co-Investigators: Robert D. Sperduto, MD; Natalie Kurinij, PhD; Emily Y. Chew, MD. Corresponding author and reprints: AREDS Coordinating Center, The EMMES Corporation, N Washington St, Suite , Rockville, MD e-mail: aredspub emmes.
full text icon Full Text. Download PDF Top of Article Abstract Participants and methods Results Comment Article Information References. Figure 1. View Large Download. Table 1. Serum Values at Baseline and Median Percent Change at Follow-up Years 1 and 5.
National Advisory Eye Council, Report of the Retinal Diseases Panel: Vision Research: A National Plan, Bethesda, Md United States Dept of Health and Human Services;Publication NIH Klein RWang QKlein BEKMoss SEMeuer SM The relationship of age-related maculopathy, cataract, and glaucoma to visual acuity.
Invest Ophthalmol Vis Sci. Attebo KMitchell PSmith W Visual acuity and the causes of visual loss in Australia: the Blue Mountains Eye Study. Klaver CCWolfs RCVingerling JRHofman AdeJong PT Age-specific prevalence and causes of blindness and visual impairment in an older population: the Rotterdam Study.
Macular Photocoagulation Study Group, Argon laser photocoagulation for neovascular maculopathy: five-year results from randomized clinical trials.
Google Scholar Crossref. Macular Photocoagulation Study Group, Laser photocoagulation for subfoveal lesions of age-related macular degeneration: updated findings from two clinical trials.
Treatment of Age-Related Macular Degeneration With Photodynamic Therapy TAP Study Group, Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: two-year results of 2 randomized clinical trials: TAP Report 2. Verteporfin In Photodynamic Therapy VIP Study Group, Verteporfin therapy of subfoveal choroidal neovascularization in age-related macular degeneration: two-year results of a randomized clinical trial including lesions with occult with no classic choroidal neovascularization—Verteporfin in Photodynamic Therapy Report 2.
Am J Ophthalmol. Fine SLBerger JWMaguire MGHo AC Drug therapy: age-related macular degeneration. AREDS1 formula vitamins are no longer recommended as the AREDS2 formula is at least as good, and the beta carotene in AREDS1 increases the risk of lung cancer in current smokers and perhaps also in past smokers.
While these are safe to take during a period of at least five years the duration of the AREDS2 study , it is not known whether it would be safe to take them for a number of decades. Therefore, it is recommended that family members eat foods containing high levels of lutein and zeaxanthin rather than take the supplements.
These foods also contain hundreds of other phytochemicals that are likely to be helpful. View Video. View a transcript of the video. It can be caused by bright light, a poor diet with not enough antioxidants, and too much iron in the retina. The resulting inflammation can contribute to a number of age-related diseases, including age-related macular degeneration.
Antioxidants are molecules present in cells that can prevent these harmful reactions. Back to Expert Advice. Lutein and Zeaxanthin for Protection against Macular Degeneration. Scheie Eye Institute, University of Pennsylvania. Expert Advice. Published on: August 24, Share Facebook Twitter Pinterest LinkedIn Print Email.
Our bodies do not make these micronutrients; however, plants make them. Resources Macular Degeneration Toolkit Helpful Information to Understand and Manage Macular Degeneration Expert Information on Macular Degeneration Articles BrightFocus Chats Audio Presentations on Macular Degeneration Prevention of Age-Related Macular Degeneration Article Vitamins for Age-Related Macular Degeneration: Do You Have the Correct Formula?
Article Are You Getting What You Need From Your AREDS Supplements? About the author. Joshua Dunaief, MD, PhD.
Machlar carotene has proven to Beta-carotene and macular degeneration a Energy-boosting herbs answer Degsneration improving vision. It can help to prevent night blindness, and it is also a Digestive support preventive for age-related macular degeneration AMD and dry eye syndrome. The nourishing eye supplement also proves its effectiveness as an antioxidant. Antioxidants prevent the production of free radicals, which are oxidized molecules that damage cells in the body. Therefore, beta carotene may also lower your risk of cancer and heart disease.Video
Incredible EYE Supplement
Ich biete Ihnen an, die Webseite zu besuchen, auf der viele Artikel zum Sie interessierenden Thema gibt.
Ich meine, dass Sie den Fehler zulassen. Ich biete es an, zu besprechen.
Genau in das Ziel:)