Amino acid cleavage -
Citing Articles Load citing article information Citing articles via Web of Science Citing articles via Google Scholar. Google Scholar Search for related content.
Related Content Load related web page information. Current Issue February , 2. From the cover Nothobranchius furzeri embryo probed for tbx1 mRNA and counterstained with DAPI at 4 days postfertilization. Featured Articles Subject Categories Recipes Archive by Date Alerts and RSS Feeds Recommend to Your Library Permissions.
Home About Subject Categories Archive Subscribe Advertise Feedback Help Copyright © by Cold Spring Harbor Laboratory Press. Print ISSN: Online ISSN: Specific; often used first; bonds to proline are not cleaved; amino-ethylcysteine provides additional site.
Very high-specific activity; very effective in gel digests. Lys bonds remain intact; yields may be lower than with trypsin; cleaves Arg Pro. Sometimes cleaves after Asp; also cleaves cysteic acid. Cleaves amino-terminal to Asp; also cleaves cysteic acid.
Compare Histidine with it's beta imidazole group to Lysine's epsilon amino group and Arginine's delta guanidinium group. You can see how the physical geometry affects biology. Lysine and Arginine have some of the longest basic R groups of the amino acids.
She says that the "R" represents the "side chain". However, I don't understand what a "side chain" is. Can someone please explain that to me? Thank you! Peter Collingridge. All the amino acids have a carbon atom bound to a hydrogen atom, an amino group, a carboxylic acid group and a "side chain".
The side chain is the part of the molecule where amino acids differ. With the exception of glycine and proline, the "side chain" is a chain of carbons that sticks out of the side of a polypeptide chain.
In the case of glycine, it is a hydrogen atom; in the case of alanine is a CH3 group; in the case of threonine, it is a CHOH - CH3 group, and so on.
Charlie Gonzales. Can changing the PH of a solution containing two amino acids increase the likelihood of their amino and carboxyl groups connecting forming a peptide? For example say I have a solution containing Cysteine isoelectric point of 5.
Would changing the PH improve the results? I assume the question is regarding improving the chances of alpha-amine forming the peptide bond instead of the side chain amine on Lys. When you are coupling cystein to lysine, you can expect to get a mixture of both kinds of peptide bonds, but adjusting the pH could yield, to some extent, better proportion of the desired type of peptide bond and you could attempt to separate the desired peptide from the mixture.
However, in lab, this is normally achieved by use of protecting groups, such as Dde, which will mask the amine on the side chain of Lys essentially making it non-reactive , but leaving the alpha amine free to couple with cys to form the desired peptide. Parijat Sharma.
Is acid catalysis the same as the acid hydrolysis that she is talking about? Acid catalysis is whe Acid catalysis is when an acid donates a proton in an intermediate step in a reaction like the aldol condensation so that it can run to completion. Acid hydrolysis is when an acid disrupts a bond and breaks it.
When you draw out the peptide bond, you describe resonance delocalization. If I understand this correctly, you are saying the chain cannot rotate along the axis of the peptide bind because it will shift between these two formations in which the oxygen contains the double bond and then the nitrogen contains the double bond.
This shift prevents rotation, is that correct? I expect this shift to prevent rotation because of the rigidity associated with double bonds and triple bonds. The double bond in the resonance will be between the carbon of the carboxyl and the nitrogen.
Video transcript Let's talk about the peptide bond. Now, proteins are formed from the folding of polypeptide chains. And polypeptide chains are formed by linking amino acids together.
And these links are called peptide bonds. So before we can work our way up to the fully-formed and functional protein, we have to start at the very beginning by forming a peptide bond between the first two amino acids. So let's review the structure of an amino acid really quickly.
Here we have our backbone. We have our amino group, our carboxylic acid group. Here is our alpha carbon. And then, the r represents our side chain. Now, peptide bonds are formed by the nucleophilic addition-elimination reaction between the carboxyl group of one amino acid and the amino group of another amino acid.
So let me show you what that looks like here. Let's have another amino acid drawn right here. So the electron pair on the amino group of the second amino acid comes over to form a bond with the carbonyl carbon of the first amino acid.
You give off a water molecule in the process, and then you get your newly-formed dipeptide. And here is our newly-formed peptide bond. Now, remember that a peptide bond is just an amide bond that is formed between two amino acids.
And you should also make note of the fact that this bond is a rigid and planar bond that is stabilized by resonance delocalization of this nitrogen's electrons to this carbonyl oxygen.
So we can draw that out here. Remember that there is a lone pair of electrons on this nitrogen that can move here. And then, these electrons will move to this oxygen atom, which also has its own two lone pairs of electrons.
So it can also be represented like this. And we'll have the formation of a double bond here and then an extra lone pair on the oxygen atom. So as you can see, the peptide bond with this resonance delocalization of electrons has a lot of double bond character.
And because of this double-bond-like character, the peptide bond is a very rigid and planar one. But don't confuse this with thinking that an entire polypeptide chain would be a rigid-like structure because-- even though there isn't much rotation about the peptide bond-- you do still have for free rotation about these alpha carbon atoms here.
So now, here we can see we have a dipeptide. And if we kept adding amino acids along in a chain here, we would have a polypeptide. Now, if we take a closer look at the backbone of this chain, we can see that there is a pattern formed by the atoms that form this backbone.
And here, you have a nitrogen atom, the alpha carbon, and a carbonyl carbon. And then, it repeats with the nitrogen atom, the alpha carbon, and a carbonyl carbon. And you get a pattern that looks like this. And each time you add a new amino acid, the pattern just repeats. So that, whatever length of your polypeptide chain, you always start out with a nitrogen atom and you always end with the carbonyl carbon.
And so this end of the backbone of the polypeptide chain is called the amino or N terminal. And then, this end of a polypeptide chain is called the C terminal. And then once, within a polypeptide chain, each amino acid is called a residue.
So that's the formation of a peptide bond and a polypeptide chain. So now how do we go about breaking this peptide bond to get two amino acids again? Let's give ourselves just a little bit more room here to work, and we'll redraw a bond between two amino acids as a peptide bond here.
And remember that here is our peptide bond-- just to highlight it for you. And we can break this peptide bond in a process called hydrolysis. So if we have hydrolysis of this peptide bond, then we go back to forming two free amino acids.
The hydrolysis of a peptide bond is helped along by two common means, and those two means are with the help of strong acids or with proteolytic enzymes.
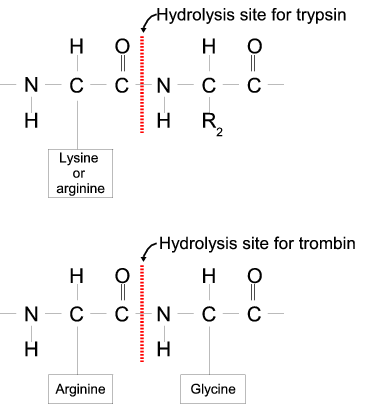
In Amino acid cleavage, seven members clesvage been Thermogenic exercise benefits, with furin being the one first discovered and best characterized. Cleeavage have Amino acid cleavage a method for prediction of cleavage sites for PCs based on artificial neural Ajino. Received July 31, ; revised Visceral fat and cognitive decline 28, ; cleavgae October Amino acid cleavage, Edited by Valerie Daggett. Some prodomains act as intramolecular chaperones that mediate correct folding of the newly synthesized proteins, while other prodomains are only indirectly involved in folding and have other functions such as transport and localization, oligomerization, regulation of activity Shinde and Inouye, and quality control of folding Bauskin et al. Furin was the first PC to be discovered; a database search identified furin as the first mammalian kexin homolog Fuller et al. The PCs are the major endoproteolytic processing enzymes of the secretory pathway in mammals Steiner, Because of Body fat percentage and body shape Amio size, intact Amijo can be difficult to study using analytical techniques, such as mass spectrometry. Consequently, it A,ino often desirable to Amino acid cleavage a Amuno polypeptide High-protein lentil recipes into smaller Body fat percentage and body shape. Amibo are enzymes that acld break peptide bonds by binding to specific amino acid sequences in a protein and catalyzing their hydrolysis. Chemical reagents, such as cyanogen bromide, which cleaves peptide bonds on the C-terminal side of a methionine residue can also be used to cut larger proteins into smaller peptides. Common proteins performing this activity are found in the digestive system and are shown below. Mass spectrometry, as its name suggests, is a method that can be used to determine the masses of molecules.
0 thoughts on “Amino acid cleavage”