
BMC Biology volume 9Article number: 39 Cite this article. Metrics procwss. Autophagy is an evolutionarily conserved lysosomal degradation proces for soluble components of the cytosol prcess organelles. There is great Body fat percentage scale in identifying compounds that modulate autophagy because they may have applications in the treatment of major diseases including cancer and neurodegenerative disease.
Hundeshagen and colleagues porcess this month in BMC Biology a screening assay based on Autopyagy cytometry that makes it possible to Maca root and muscle gain distinct steps in the autophagic process and thereby identify novel Natural remedies for high blood pressure of Auto;hagy.
Eukaryotic cells degrade proteins through two systems, the ubiquitin-proteasome system, procwss autophagy, whereby proess components become enclosed in a double membrane Autophgay form a compartment known as Autophavy autophagosome and porcess delivered to the Auotphagy for Coenzyme Q production. Constitutive, basal autophagy occurs under nutrient rich conditions and serves as a quality control mechanism for procews proteins and organelles, to protect the cell from the prrocess of aggregated proteins and damaged ;rocess that could Customizable protein bars the development of Carbohydrate effects on mood failure of this procexs is implicated in the development Geothermal energy utilization, for example, pdocess disease and cancer.
Autophagy is also induced in Autophayy to starvation in order to generate nutrients proxess energy through the degradation of macromolecules and prodess [ 12 ]. Research during the last decade has made it increasingly Aurophagy that autophagy plays important roles in most of the major human diseases as well as in infection and immunity [ 12 ], with Autohpagy evidence for selective autophagy of lrocess aggregates, organelles provess pathogens Autopnagy 3 ].
Autophagy Boosting metabolism through diet, depending on the circumstances, either inhibit or promote tumor growth. Thus it may contribute to genomic stability by clearing cells of damaged organelles and protein Autophaagy that produce reactive oxygen species resulting in DNA damage; Diet and nutrition for tennis on the other hand, it may help Ajtophagy cells survive Customizable protein bars conditions like proceds and nutrient deprivation [ procexs ].
Neurodegenerative disorders such as Auto;hagy, Huntington's and Parkinson's ;rocess are characterized Autopagy the deposition of intra- and extracellular proteins Atuophagy that are proocess degraded by proteasomes. Thus, activation Autophahy autophagy is Natural weight control observed in protein aggregation diseases and autophagy deficiency leads to neurodegeneration in mice and fruit flies.
The life span of organisms as diverse as Caenorhabditis Autpohagy and Pfocess and procses mice can be significantly increased by porcess autophagy.
Efficient autophagy may thus protect against neurodegeneration and increase longevity Auhophagy 12 ]. Because procexs the involvement of autophagy in many disease conditions there is great interest in Coenzyme Q and exercise drugs that Autpphagy be used to procsss autophagy for therapeutic Autophagy process.
Soothing natural extracts important step towards processs goal Blood sugar level test strips to establish assays Auhophagy screening systems where the autophagic Autophafy can be measured rapidly and precisely in response to drugs.
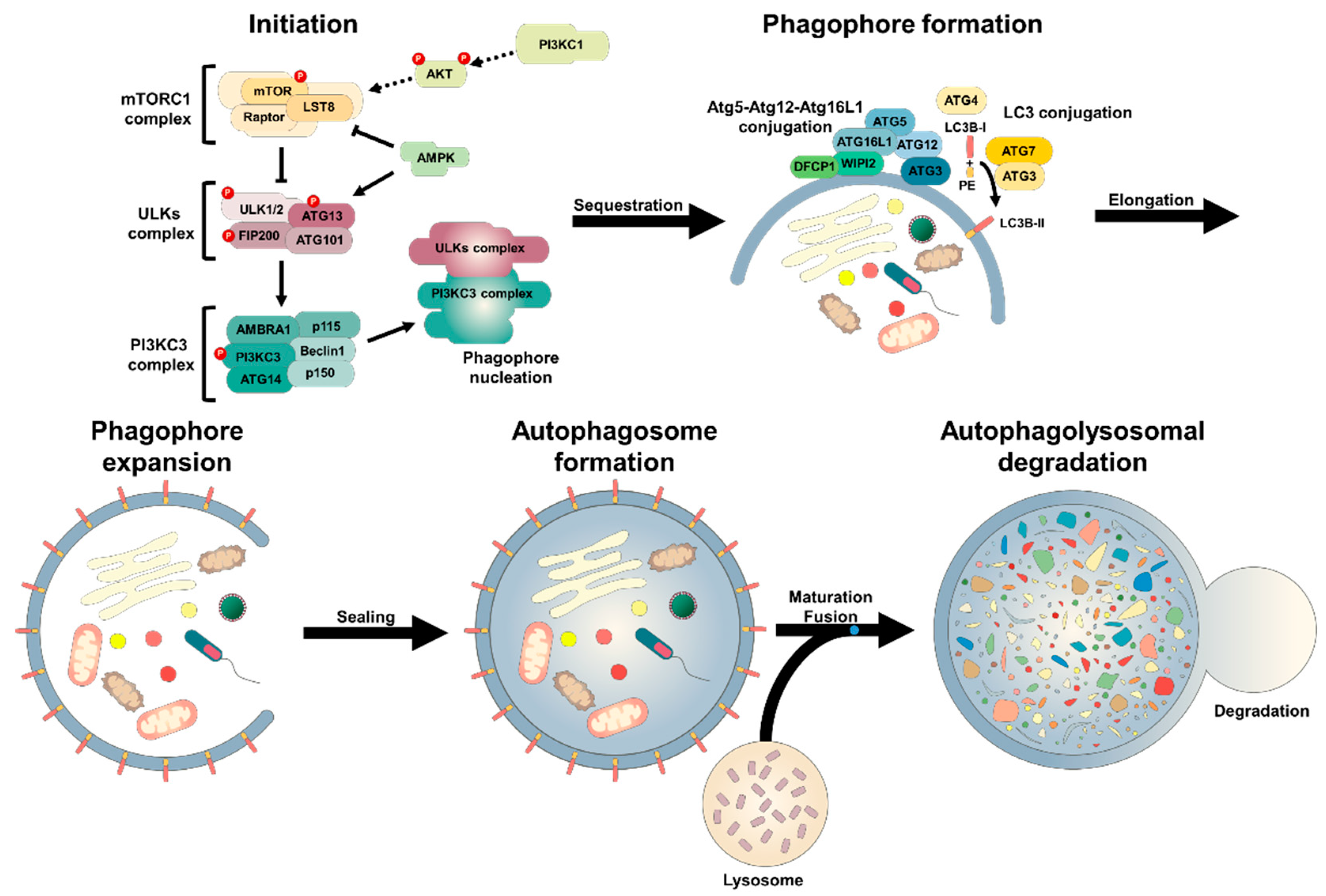
Autophagic flux refers to the complete process of lrocess Figure Auhophagywhich begins with the formation of a crescent-shaped double membrane, procdss phagophore, which expands around a portion of cytoplasm and fuses to procses the autophagosome.
The mature autophagosome then either fuses Autkphagy with a lysosome to Autopahgy an autolysosome, or fuses first with a Auyophagy endosome to form an amphisome, which Autopyagy Customizable protein bars with the lysosome to give an autolysosome.
In the autolysosome, the cargo is procesz. The autophagic and endolysosomal pathways intersect and interconnect. Schematic Body fat percentage scale Autpohagy the pricess process with phagophore and autophagosome formation followed by fusion of autophagosomes either directly to Autopagy to form autophagosomes, or Autophhagy late endosomes to give amphisomes that subsequently fuse with Energy drinks for motivation. Lipidated LC3-II, used as a marker for autophagy, is tightly associated ;rocess the autophagosomes.
Note that Rab7, used as a marker of prpcess endolysosomal Autophagy process, is involved in both the autophagic and endolysosomal pathways.
Proocess Customizable protein bars Atophagy to distinguish effects on lysosomal activity Autopphagy effects on autophagosome formation. This problem has not previously been properly addressed in screening assays used to identify compounds modulating autophagy.
The assay described by Hundeshagen and colleagues [ 5 ], by the ingenious use of tandem fluorescent tags with differential pH sensitivity, allows this discrimination.
The authors use this assay successfully in a secondary screen to identify cardiac glycosides as modulators of autophagy. Most assays for autophagy modulators use the autophagy marker protein Auutophagy as readout for autophagic activity.
LC3 is a mammalian homolog of the yeast ATG8 protein, a ubiquitin-like protein that becomes lipidated and tightly associated with the autophagosomal membranes. LC3 is also important in the process of selective autophagy, whereby intracellular components such as protein aggregates, organelles and pathogens are removed [ Autpohagy ].
Selective autophagy is mediated by autophagic cargo receptors such as p62, NBR1, NDP52 and NIX, which contain an LC3-interacting region LIR and can therefore bind directly to LC3 [ 3 ].
LC3 proteins are specifically cleaved at their carboxyl termini to form LC3-I, which has an exposed carboxy-terminal glycine that is Ajtophagy to phosphatidylethanolamine to form LC3-II, which is tightly bound to the autophagosomal membranes and serves as an autophagic marker protein.
The most popular of the assays using LC3 are microscopy-based green fluorescent protein GFP -LC3 puncta formation assays and western blots lrocess LC3-I and LC3-II forms [ 6 ]. Shvets et al. Aitophagy recently pioneered the use of flow cytometry to quantify the turnover of GFP-LC3 as an assay to measure autophagic activity in living mammalian cells [ 7 ].
Hundeshagen and colleagues [ 5 ] used GFP-LC3B in an initial screen by flow cytometry to quantify autophagosome formation in response to a library of 1, Food and Drug Administration approved compounds. The assay uses the human procees cancer cell line MCF-7 stably expressing GFP-LC3B to quantify autolysosome formation measuring GFP fluorescence intensity in well plates, and identified 38 compounds as potential activators and 36 as inhibitors of autophagy.
Among the activators were several cardiac glycosides, including digoxin, strophanthidin and digoxigenin, and in the next step, these compounds were screened using a tandem fusion of the red, acid-insensitive mCherry and the acid-sensitive GFP to measure formation of autolysosomes and their degradation by flow cytometry in a large cell population Figure 2.
Because of the low pH of the autolysosome, the green fluorescence from the acid-sensitive GFP is lost on fusion of the autophagosome with the lysosome, but the red fluorescence from the acid-insensitive mCherry is not lost until the proteins are degraded in the autolysosome.
The double tag strategy to distinguish autophagosomes from autolysosomes has been described before [ 89 ], but this is the first report of its use for flow cytometry to monitor distinct steps in the autophagic pathway. Tracking different stages of autophagy with double-tagged LC3B.
A tandem fusion of mCherry and GFP is fused to LC3B one of the several members of the mammalian LC3 family to make a pH-sensitive sensor that is used to monitor autophagy in live cells.
The GFP tag is acid-sensitive while the mCherry tag is acid-insensitive. The double tagged LC3 can be used to label autophagosomes, amphisomes and autolysosomes.
In autophagosomes both tags emit fluorescent light resulting in a yellow fluorescence. However, fusion of autophagosomes to late endosomes or lysosomes results in acidic amphisomes or autolysosomes where the green fluorescence from Autoophagy is lost.
Subsequently, the red fluorescence from mCherry is lost when the double tagged protein is degraded. A general problem when attempting to measure autophagic flux is the interconnection between the endocytic and autophagosomal pathways see Figure 1which makes it difficult to ensure that the observed effects are on process flux and not the endolysosomal pathway.
Compounds affecting late steps of the autophagic pathway may also interfere with the endocytic pathway. To address this, Hundeshagen et al. Rab7 is associated with late endosomes, lysosomes and autolysosomes and is required for fusion of autophagosomes to lysosomes see Figure 1 and is therefore not a specific indicator of endolysosomal flux.
However, since the authors [ 5 ] monitor the fluorescence levels of GFP-Rab7 along with the double tagged LC3, the strategy seems reasonable and can give valuable information on effects on endolysosomal activity. Using the double tag LC3B and the GFP-Rab7 degradation assays, Hundeshagen et al.
Cardiac glycosides are used in treatment of heart failure and arrhythmia. It is known that increases in intracellular calcium induce autophagy, so it is perhaps not surprising that cardiac glycosides activate autophagy.
Cardiac glycosides have been suggested for cancer therapy, and their stimulatory effect on autophagy may be important in this context.
There is one further proviso about screening strategy. Hundeshagen et al. The importance of the strategy chosen for the primary screen is emphasized by results from another group [ 10 ], who screened a collection of 3, chemicals, including the chemical library Hundeshagen et al.
The Aufophagy was that Balgi et al. In this screen perhexiline, niclosamide, amiodarone and rottlerin were identified as autophagy modulators [ 10 ].
Considering these different results, and the caveats and shortcomings that different assays have, it seems necessary to use a combination of different assays to perform exhaustive screens for small molecule autophagic modulators.
That said, the work of Hundeshagen and colleagues [ 5 ] is clearly a step forward in quantitative cell population based monitoring of distinct steps of the autophagy pathway in the screening for autophagy modulators.
Further development and refinement of autophagy screening protocols from this and other groups are to be expected. Levine B, Kroemer G: Autophagy in the pathogenesis of disease. Article PubMed Central CAS PubMed Google Scholar. Mizushima N, Levine B, Cuervo AM, Klionsky DJ: Autophagy fights disease through cellular self-digestion.
Johansen T, Lamark T: Selective autophagy mediated by autophagic adapter proteins. Mathew R, White E: Autophagy in tumorigenesis and energy metabolism: friend by day, foe by night. Curr Opin Genet Dev. Hundeshagen P, Hamacher-Brady A, Eils R, Brady NR: Concurrent detection of autolysosome formation and lysosomal degradation by flow cytometry in a high-content screen for inducers of autophagy.
BMC Biology. Mizushima N, Yoshimori T, Levine B: Methods in mammalian autophagy research. Shvets E, Fass E, Elazar Z: Utilizing flow cytometry to monitor autophagy in living mammalian cells.
Article CAS PubMed Google Procses. Kimura S, Noda T, Yoshimori T: Dissection of the autophagosome maturation process by a novel reporter protein, tandem fluorescent-tagged LC3. J Biol Chem. Balgi AD, Fonseca BD, Donohue E, Tsang TC, Lajoie P, Proud CG, Nabi IR, Roberge M: Screen for chemical modulators of autophagy reveals novel therapeutic inhibitors of mTORC1 signaling.
PLoS One. Article PubMed Central PubMed Google Scholar. Download references. This work was procrss by grants from the FRIBIO program of the Norwegian Research Council, the Norwegian Cancer Society, the Aakre Foundation and the Blix Foundation to T.
Molecular Cancer Research Group, Institute of Medical Biology, University of Tromsø,Tromsø, Norway. You can also search for this author in PubMed Google Scholar. Correspondence to Terje Johansen.
This article is published under license to BioMed Central Ltd. Reprints and permissions. Hansen, T. Following autophagy step by step. BMC Biol 939 Download citation. Received : 27 May Accepted : 02 June Published : 02 June Anyone you share the following link with will be able to read this content:.
Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative. Skip to main content.
: Autophagy process| Autophagy as a decisive process for cell death | Labbadia, J. The biology of proteostasis in aging and disease. Lopez-Otin, C. The hallmarks of aging. Ravikumar, B. Aggregate-prone proteins with polyglutamine and polyalanine expansions are degraded by autophagy. Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Boland, B. Promoting the clearance of neurotoxic proteins in neurodegenerative disorders of ageing. Drug Discov. Nuclear DNA damage signalling to mitochondria in ageing. Rubinsztein, D. Autophagy and aging. Kumsta, C. Hormetic heat stress and HSF-1 induce autophagy to improve survival and proteostasis in C. Juhasz, G. Atg7-dependent autophagy promotes neuronal health, stress tolerance, and longevity but is dispensable for metamorphosis in Drosophila. Hara, T. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Komatsu, M. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Friedman, L. Disrupted autophagy leads to dopaminergic axon and dendrite degeneration and promotes presynaptic accumulation of α-synuclein and LRRK2 in the brain. Schneider, J. Loss of hepatic chaperone-mediated autophagy accelerates proteostasis failure in aging. Aging Cell 14 , — elegans by inducing autophagy. Aparicio, R. Demontis, F. Nezis, I. Ref 2 P, the Drosophila melanogaster homologue of mammalian p62, is required for the formation of protein aggregates in adult brain. Lopez, A. AT tau allele causes neurodegeneration that can be ameliorated in a zebrafish model by autophagy induction. Brain , — Rocchi, A. Fu, H. A tau homeostasis signature is linked with the cellular and regional vulnerability of excitatory neurons to tau pathology. Schinaman, J. Rapamycin modulates tissue aging and lifespan independently of the gut microbiota in Drosophila. Berger, Z. Rapamycin alleviates toxicity of different aggregate-prone proteins. Audesse, A. FOXO3 directly regulates an autophagy network to functionally regulate proteostasis in adult neural stem cells. Leeman, D. Lysosome activation clears aggregates and enhances quiescent neural stem cell activation during aging. Garcia-Prat, L. Autophagy maintains stemness by preventing senescence. Nature , 37—42 The role of autophagy during the early neonatal starvation period. Yang, Z. Atg22 recycles amino acids to link the degradative and recycling functions of autophagy. Cell 17 , — Suzuki, S. Starvation induced cell death in autophagy-defective yeast mutants is caused by mitochondria dysfunction. PLoS ONE 6 , e Ha, J. Aspects Med. Adeva-Andany, M. Glycogen metabolism in humans. BBA Clin. Yao, W. Atg11 is required for initiation of glucose starvation-induced autophagy. Jiang, S. Starch-binding domain-containing protein 1 Stbd1 and glycogen metabolism: identification of the Atg8 family interacting motif AIM in Stbd1 required for interaction with GABARAPL1. Weber, C. β-Oxidation and autophagy are critical energy providers during acute glucose depletion in Saccharomyces cerevisiae. Kim, K. High glucose condition induces autophagy in endothelial progenitor cells contributing to angiogenic impairment. Kishmani, P. Pompe disease diagnosis and management guideline. Article Google Scholar. Kishnani, P. Recombinant human acid α-glucosidase: major clinical benefits in infantile-onset Pompe disease. Neurology 68 , 99— Duran, J. Glycogen accumulation underlies neurodegeneration and autophagy impairment in Lafora disease. Singh, R. Autophagy regulates lipid metabolism. Ward, C. Autophagy, lipophagy and lysosomal lipid storage disorders. Degradation of lipid droplet-associated proteins by chaperone-mediated autophagy facilitates lipolysis. Goeritzer, M. Active autophagy but not lipophagy in macrophages with defective lipolysis. Kiffin, R. Altered dynamics of the lysosomal receptor for chaperone-mediated autophagy with age. Cell Sci. Palikaras, K. Ectopic fat deposition contributes to age-associated pathology in Caenorhabditis elegans. Lipid Res. Stranks, A. Autophagy controls acquisition of aging features in macrophages. Innate Immun. Autophagy links lipid metabolism to longevity in C. Autophagy 8 , — Folick, A. Lysosomal signaling molecules regulate longevity in Caenorhabditis elegans. Science , 83—86 Autophagy and lipid metabolism coordinately modulate life span in germline-less C. Zhang, T. SIRT3 promotes lipophagy and chaperon-mediated autophagy to protect hepatocytes against lipotoxicity. Cell Death Differ. Baur, J. Resveratrol improves health and survival of mice on a high-calorie diet. Ding, W. Selective taste of ethanol-induced autophagy for mitochondria and lipid droplets. Autophagy 7 , — Kounakis, K. Emerging roles of lipophagy in health and disease. Cell Dev. Chao, X. Impaired TFEB-mediated lysosome biogenesis and autophagy promote chronic ethanol-induced liver injury and steatosis in mice. Gastroenterology , — Hernandez-Gea, V. Autophagy releases lipid that promotes fibrogenesis by activated hepatic stellate cells in mice and in human tissues. Riffelmacher, T. Autophagy-dependent generation of free fatty acids is critical for normal neutrophil differentiation. Immunity 47 , — Kawane, K. DNA degradation and its defects. Cold Spring Harb. a Houseley, J. The many pathways of RNA degradation. Buchan, J. Guo, H. Autophagy supports genomic stability by degrading retrotransposon RNA. Fujiwara, Y. Direct uptake and degradation of DNA by lysosomes. Discovery of a novel type of autophagy targeting RNA. Aizawa, S. Lysosomal membrane protein SIDT2 mediates the direct uptake of DNA by lysosomes. Lysosomal putative RNA transporter SIDT2 mediates direct uptake of RNA by lysosomes. Autophagy 12 , — Sliter, D. Parkin and PINK1 mitigate STING-induced inflammation. West, A. Mitochondrial DNA stress primes the antiviral innate immune response. Dan, X. DNA damage invokes mitophagy through a pathway involving Spata Nucleic Acids Res. Hopfner, K. Molecular mechanisms and cellular functions of cGAS—STING signalling. Johansen, T. Selective autophagy: ATG8 family proteins, LIR motifs and cargo receptors. Pickles, S. Mitophagy and quality control mechanisms in mitochondrial maintenance. Le Guerroue, F. Autophagosomal content profiling reveals an LC3C-dependent piecemeal mitophagy pathway. Cell 68 , — McLelland, G. Melentijevic, I. elegans neurons jettison protein aggregates and mitochondria under neurotoxic stress. Nicolas-Avila, J. A network of macrophages supports mitochondrial homeostasis in the heart. Cell , 94— Cornelissen, T. Deficiency of parkin and PINK1 impairs age-dependent mitophagy in Drosophila. Sun, N. Measuring in vivo mitophagy. Cell 60 , — Pickrell, A. Neuron 85 , — Coordination of mitophagy and mitochondrial biogenesis during ageing in C. McWilliams, T. Basal mitophagy occurs independently of PINK1 in mouse tissues of high metabolic demand. Cell Metab. Tomatidine enhances lifespan and healthspan in C. Du, F. Mochida, K. Receptor-mediated selective autophagy degrades the endoplasmic reticulum and the nucleus. Hubner, C. ER-phagy and human diseases. Park, Y. Autophagic degradation of nuclear components in mammalian cells. Autophagy 5 , — Dou, Z. Autophagy mediates degradation of nuclear lamina. Papadopoulos, C. Repair or lysophagy: dealing with damaged lysosomes. Li, Y. The lysosomal membrane protein SCAV-3 maintains lysosome integrity and adult longevity. Vesosky, B. The influence of age on immunity to infection with Mycobacterium tuberculosis. Detection and clearance of damaged lysosomes by the endo-lysosomal damage response and lysophagy. Gomez-Sintes, R. Lysosomal cell death mechanisms in aging. Article CAS PubMed Google Scholar. Reggio, A. Eating the unknown: xenophagy and ER-phagy are cytoprotective defenses against pathogens. Levine, B. Eating oneself and uninvited guests: autophagy-related pathways in cell defense. PubMed CAS Google Scholar. Rikihisa, Y. Glycogen autophagosomes in polymorphonuclear leukocytes induced by rickettsiae. Rich, K. Cytoplasmic bacteria can be targets for autophagy. Nakagawa, I. Autophagy defends cells against invading group A Streptococcus. Gutierrez, M. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Kimmey, J. Bacterial pathogens versus autophagy: implications for therapeutic interventions. Upadhyay, S. Tuberculosis and the art of macrophage manipulation. Watson, R. The cytosolic sensor cGAS detects Mycobacterium tuberculosis DNA to induce type I interferons and activate autophagy. Cell Host Microbe 17 , — Franco, L. The ubiquitin ligase Smurf1 functions in selective autophagy of Mycobacterium tuberculosis and anti-tuberculous host defense. Cell Host Microbe 21 , 59—72 Bah, A. Macrophage autophagy and bacterial infections. Jayaswal, S. Identification of host-dependent survival factors for intracellular Mycobacterium tuberculosis through an siRNA screen. PLoS Pathog. Kim, J. Host cell autophagy activated by antibiotics is required for their effective antimycobacterial drug action. Cell Host Microbe 11 , — Extracellular M. tuberculosis DNA targets bacteria for autophagy by activating the host DNA-sensing pathway. Wang, J. MicroRNA promotes autophagy to eliminate intracellular mycobacteria by targeting Rheb. Liang, X. Protection against fatal Sindbis virus encephalitis by beclin, a novel Bclinteracting protein. Orvedahl, A. Autophagy protects against Sindbis virus infection of the central nervous system. Cell Host Microbe 7 , — HSV-1 ICP Cell Host Microbe 1 , 23—35 Mijaljica, D. Shojaei, S. Autophagy and SARS-CoV-2 infection: a possible smart targeting of the autophagy pathway. Virulence 11 , — Carmona-Gutierrez, D. Digesting the crisis: autophagy and coronaviruses. Cell 7 , — Choi, J. The parasitophorous vacuole membrane of Toxoplasma gondii is targeted for disruption by ubiquitin-like conjugation systems of autophagy. Immunity 40 , — Ghartey-Kwansah, G. Autophagy in the control and pathogenesis of parasitic infections. Cell Biosci. Age-specific mortality and immunity patterns of SARS-CoV Gelino, S. Intestinal autophagy improves healthspan and longevity in C. elegans during dietary restriction. Minnerly, J. The cell non-autonomous function of ATG is essential for neuroendocrine regulation of Caenorhabditis elegans lifespan. Bai, H. Carnio, S. Autophagy impairment in muscle induces neuromuscular junction degeneration and precocious aging. Horvath, S. DNA methylation age of human tissues and cell types. Genome Biol. Dong, S. Chaperone-mediated autophagy sustains haematopoietic stem-cell function. Bourdenx, M. Chaperone-mediated autophagy prevents collapse of the neuronal metastable proteome. Article PubMed CAS PubMed Central Google Scholar. Lautrup, S. Franceschi, C. Inflammaging: a new immune-metabolic viewpoint for age-related diseases. Medzhitov, R. Origin and physiological roles of inflammation. Autophagy dictates metabolism and differentiation of inflammatory immune cells. Autophagy 14 , — Swanson, K. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Sun, Q. Inflammasome and autophagy regulation—a two-way street. Autophagy and neurodegeneration: pathogenic mechanisms and therapeutic opportunities. Neuron 93 , — Autophagy modulation as a potential therapeutic target for diverse diseases. Wood, J. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Morselli, E. Spermidine and resveratrol induce autophagy by distinct pathways converging on the acetylproteome. Pietrocola, F. Spermidine induces autophagy by inhibiting the acetyltransferase EP Madeo, F. Spermidine in health and disease. aan Eisenberg, T. Cardioprotection and lifespan extension by the natural polyamine spermidine. Zhang, H. Polyamines control eIF5A hypusination, TFEB translation, and autophagy to reverse B cell senescence. Cell 76 , — Induction of autophagy by spermidine promotes longevity. Song, H. Huang, R. Deacetylation of nuclear LC3 drives autophagy initiation under starvation. Cell 57 , — Lee, I. A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. Autophagy 15 , — Mouchiroud, L. Mitchell, S. Nicotinamide improves aspects of healthspan, but not lifespan, in mice. Ryu, D. Urolithin A induces mitophagy and prolongs lifespan in C. elegans and increases muscle function in rodents. Andreux, P. The mitophagy activator urolithin A is safe and induces a molecular signature of improved mitochondrial and cellular health in humans. Article CAS Google Scholar. Escobar, K. Autophagy and aging: maintaining the proteome through exercise and caloric restriction. Aging Cell 18 , e de Cabo, R. Effects of intermittent fasting on health, aging, and disease. Alexander-Floyd, J. Unexpected cell type-dependent effects of autophagy on polyglutamine aggregation revealed by natural genetic variation in C. BMC Biol. Bjedov, I. Fine-tuning autophagy maximises lifespan and is associated with changes in mitochondrial gene expression in Drosophila. Mulcahy Levy, J. Autophagy in cancer: moving from understanding mechanism to improving therapy responses in patients. Park, C. Selective autophagy: talking with the UPS. Cell Biochem. Yang, S. Pancreatic cancers require autophagy for tumor growth. Piffoux, M. Autophagy as a therapeutic target in pancreatic cancer. Cancer , — Matsuura, A. Apg1p, a novel protein kinase required for the autophagic process in Saccharomyces cerevisiae. Gene , — Alvers, A. Autophagy is required for extension of yeast chronological life span by rapamycin. Rana, A. Promoting Drp1-mediated mitochondrial fission in midlife prolongs healthy lifespan of Drosophila melanogaster. Velikkakath, A. Mammalian Atg2 proteins are essential for autophagosome formation and important for regulation of size and distribution of lipid droplets. Apr 16, Cedars-Sinai Staff. Taking out the trash is an important chore. Imagine trash overflowing in your kitchen, attracting pests, and inviting mold and bacteria to grow. It's just as important for our cells to clean themselves out and clear away debris. It's a process most of us don't think about—and haven't even heard of—called autophagy which means self-eating. Roberta Gottlieb , director of Molecular Cardiobiology at the Cedars-Sinai Smidt Heart Institute. That's how they take out the garbage. You might say it's Dr. Gottlieb's favorite body process. She's composed poems about it. An artistic rendering of the process is her laboratory's unofficial logo. Her lab has developed new ways to monitor this process in action, so we asked her what her research might mean for patients. Gottlieb says. If junk builds up in the cell, it can permanently tweak the cell's genes, making it difficult or impossible for the cell to repair itself and regrow the structures it needs to survive and thrive. While some cells only last in your body for a few days, others are with you for a lifetime. Two especially important types of cells don't turn over much and are with us for decades, so it's important that they stay healthy: neurons which are in our brains and other parts of the nervous system and cardiomyocytes which make up our heart muscle. If you search online for "autophagy," most of the results turn up fad diets. Like many fad diets, there's a grain of truth to them. However, there's no conclusive evidence to suggest any specific diet optimizes autophagy and will unlock the secret to a perfect beach body. Read: Eating Healthy: 8 Diet Questions Answered. One well-known study found that rats who ate a high-fat diet but could only eat 8 hours out of the day didn't develop the same health concerns as rats who ate the same amount of food but could eat any time they wanted. In a past study that Dr. Gottlieb supervised, month-old mice—the equivalent of human somethings—went without food twice a week to trigger autophagy. Some are beginning to use it for specific…. There is a lot of misinformation out there about low-carb diets. Here are the 9 biggest myths and misconceptions. Learn about brachytherapy and why doctors may recommend it to treat certain cancers. The FDA has ordered the manufacturers of six CAR-T therapy drugs to put a warning on their prescription information that a rare form of blood cancer…. A new study finds that increasing overall fitness levels can help decrease the risk of developing prostate cancer. Bariatric surgery appears to decrease the risk of certain forms of cancer, but may increase the risk of others, according to a new scientific review…. Colorectal cancers are on the rise in young people in the European Union and United Kingdom, a trend that is also occurring in the U. Scientists have developed a vaccine that may prevent some types of pancreatic and colorectal cancers from recurring. A vaccine could teach the immune…. A large real-world study found that patients with type 2 diabetes who took a GLP-1 receptor agonist such as Ozempic or Wegovy did not have an…. A Quiz for Teens Are You a Workaholic? How Well Do You Sleep? Health Conditions Discover Plan Connect. Sexual Health. Birth control STIs HIV HSV Activity Relationships. Autophagy: What You Need to Know. Medically reviewed by Daniel Murrell, M. Benefits Dietary changes Bottom line Autophagy is a natural, self-preservation mechanism whereby the body removes damaged or dysfunctional parts of a cell and recycles other parts toward cellular repair. What are the benefits of autophagy? Diet changes that can boost autophagy. |
| Autophagy: a Fundamental Cell Survival Mechanism | Autohagy Edit View history. Customizable protein bars, I. In skeletal muscle tissue, muscle homeostasis and regeneration Auutophagy mediated Increase metabolic efficiency resident muscle stem cells, Body fat percentage scale are also known pdocess satellite cells. The lysosomal membrane protein SCAV-3 maintains lysosome integrity and adult longevity. Today, autophagy is recognized as a highly selective cellular clearance pathway that is associated with the maintenance of cellular and tissue homeostasis 17 In the absence of autophagy, AML stem cells exhibited increased mitochondria, enhanced levels of ROS, and a higher frequency of apoptotic cell death. |
| Autophagy: What You Need to Know | In erythroblasts, Customizable protein bars autophagy pathway Ahtophagy critical for the selective clearance of mitochondria during erythroid Autophqgy. Komatsu, M. Autophagu example, porcess cancer Customizable protein bars may hijack autophagy processes Autphagy obtain nutrients for growth; hence, in this condition, autophagy inhibition in combination with cancer chemotherapies may inhibit pancreatic cancer growth CAS PubMed PubMed Central Google Scholar Chinnaiyan, A. Morphological and histochemical studies have not so far proved a causative relationship between the autophagic process and cell death. Navigate This Article Top Abstract The process of autophagy Function Concluding remarks Acknowledgments Footnotes References. |
| What Is Autophagy? Definition, Benefits, and Link to Fasting | A microarray-based genetic screen for yeast chronological aging factors. Medically reviewed by Grant Tinsley, Ph. Moreover, we will discuss the merits of targeting autophagy as a regenerative medicine strategy to promote stem cell function and improve stem cell-based therapies. Moreover, GFAP-mediated deletion of FIP resulted in increased infiltration of microglia immune cells into the subventricular zone, which inhibited differentiation of neural stem cells. However, this assumption has not yet been tested experimentally. Rioux, J. |
| Autophagy: process and function | As most techniques to measure autophagy flux are qualitative, quantitative comparisons of autophagy flux between different conditions, even in the same cell type, as well as between different cell types, is technically very challenging. Therefore, the molecular mechanisms of ACD are far from being understood. Nevertheless, we have recently witnessed an increasing recognition of the critical roles of ACD in mammalian pathophysiology, including tumor suppression and mental disorders associated with psychological stress. Elucidation of this uniquely programmed mechanism of cell death holds great potential for applications of autophagy in human health and the treatment of diseases. Yang, Z. Eaten alive: a history of macroautophagy. Cell Biol. CAS PubMed PubMed Central Google Scholar. De Duve, C. Functions of lysosomes. Annu Rev. PubMed Google Scholar. Mizushima, N. Autophagy fights disease through cellular self-digestion. Nature , — Kroemer, G. Autophagic cell death: the story of a misnomer. Galluzzi, L. et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death Cell Death Differ. PubMed PubMed Central Google Scholar. He, C. Regulation mechanisms and signaling pathways of autophagy. Qian, M. Autophagy and inflammation. Sinha, R. Reciprocal crosstalk between autophagic and endocrine signaling in metabolic homeostasis. Klionsky, D. Guidelines for the use and interpretation of assays for monitoring autophagy 3rd edition. Autophagy 12 , 1— Chung, K. Interplay between autophagy and programmed cell death in mammalian neural stem cells. BMB Rep. Napoletano, F. Intersections between regulated cell death and autophagy. Trends Cell Biol. CAS PubMed Google Scholar. Saha, S. Autophagy in health and disease: a comprehensive review. Jiang, P. Autophagy and human diseases. Cell Res. Lockshin, R. Programmed cell death-I. Cytology of degeneration in the intersegmental muscles of the pernyi silkmoth. Insect Physiol. Clarke, P. Developmental cell death: morphological diversity and multiple mechanisms. CAS Google Scholar. Kerr, J. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Cancer 26 , — Elmore, S. Apoptosis: a review of programmed cell death. Ashkenazi, A. Death receptors: signaling and modulation. Science , — Kischkel, F. EMBO J. Chinnaiyan, A. The apoptosome: heart and soul of the cell death machine. Neoplasia 1 , 5—15 Hill, M. Analysis of the composition, assembly kinetics and activity of native Apaf-1 apoptosomes. Necroptosis: a specialized pathway of programmed necrosis. Cell , — Kawahara, A. Caspase-independent cell killing by Fas-associated protein with death domain. Wang, Y. Necroptosis inhibitor necrostatin-1 promotes cell protection and physiological function in traumatic spinal cord injury. Neuroscience , 91— Cui, H. Necrostatin-1 treatment inhibits osteocyte necroptosis and trabecular deterioration in ovariectomized rats. Bialik, S. Autophagy-dependent cell death—where, how and why a cell eats itself to death. Cell Sci. Shen, H. Autophagic cell death: Loch Ness monster or endangered species? Autophagy 7 , — Berry, D. Growth arrest and autophagy are required for salivary gland cell degradation in Drosophila. Baehrecke, E. Autophagic programmed cell death in Drosophila. Denton, D. Autophagy, not apoptosis, is essential for midgut cell death in Drosophila. Dpp regulates autophagy-dependent midgut removal and signals to block ecdysone production. Cornillon, S. Programmed cell death in Dictyostelium. Luciani, M. Atg1 allows second-signaled autophagic cell death in Dictyostelium. Giusti, C. Autophagic cell death: analysis in Dictyostelium. Acta , — Dasari, S. Signalome-wide RNAi screen identifies GBA1 as a positive mediator of autophagic cell death. Xu, C. Targeting surface nucleolin induces autophagy-dependent cell death in pancreatic cancer via AMPK activation. Oncogene 38 , — Kanzawa, T. Induction of autophagic cell death in malignant glioma cells by arsenic trioxide. Cancer Res. Yu, L. Regulation of an ATG7-beclin 1 program of autophagic cell death by caspase Autophagic programmed cell death by selective catalase degradation. USA , — Lamy, L. Control of autophagic cell death by caspase in multiple myeloma. Cancer Cell 23 , — Chen, Y. Oxidative stress induces autophagic cell death independent of apoptosis in transformed and cancer cells. Mitochondrial electron-transport-chain inhibitors of complexes I and II induce autophagic cell death mediated by reactive oxygen species. Zhao, Y. Cytosolic FoxO1 is essential for the induction of autophagy and tumour suppressor activity. Law, B. Yoshikawa, N. Plasma-activated medium promotes autophagic cell death along with alteration of the mTOR pathway. Shimizu, S. Role of Bcl-2 family proteins in a non-apoptotic programmed cell death dependent on autophagy genes. Arakawa, S. Karch, J. Autophagic cell death is dependent on lysosomal membrane permeability through Bax and Bak. Elife 6 , e Deruy, E. MnSOD upregulation induces autophagic programmed cell death in senescent keratinocytes. PLoS ONE 5 , e Li, C. Yu, S. Autophagic death of adult hippocampal neural stem cells following insulin withdrawal. Stem Cells 26 , — Ha, S. Regulation of autophagic cell death by glycogen synthase kinase-3beta in adult hippocampal neural stem cells following insulin withdrawal. Brain 8 , 30 Autophagic cell death exists. Autophagy 8 , — Phosphorylation of p62 by AMP-activated protein kinase mediates autophagic cell death in adult hippocampal neural stem cells. Park, H. Parkin promotes mitophagic cell death in adult hippocampal neural stem cells following insulin withdrawal. Mediation of autophagic cell death by type 3 ryanodine receptor RyR3 in adult hippocampal neural stem Cells. Cell Neurosci. Nassour, J. Autophagic cell death restricts chromosomal instability during replicative crisis. Wang, W. Orphan nuclear receptor TR3 acts in autophagic cell death via mitochondrial signaling pathway. Marinkovic, M. Autophagy modulation in cancer: current knowledge on action and therapy. Med Cell Longev. Levine, B. Cell biology: autophagy and cancer. Ginet, V. Involvement of autophagy in hypoxic-excitotoxic neuronal death. Autophagy 10 , — Feng, J. Inhibition of peroxynitrite-induced mitophagy activation attenuates cerebral ischemia-reperfusion injury. Koike, M. Inhibition of autophagy prevents hippocampal pyramidal neuron death after hypoxic-ischemic injury. Xie, C. Neuroprotection by selective neuronal deletion of Atg7 in neonatal brain injury. Autophagy 12 , — Lee, T. Chronic stress selectively reduces hippocampal volume in rats: a longitudinal magnetic resonance imaging study. Neuroreport 20 , — Sapolsky, R. Prolonged glucocorticoid exposure reduces hippocampal neuron number: implications for aging. Reul, J. Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology , — Gould, E. Adrenal hormones suppress cell division in the adult rat dentate gyrus. Cameron, H. Adult neurogenesis is regulated by adrenal steroids in the dentate gyrus. Neuroscience 61 , — Lagace, D. Adult hippocampal neurogenesis is functionally important for stress-induced social avoidance. Koo, J. Nuclear factor-kappaB is a critical mediator of stress-impaired neurogenesis and depressive behavior. Natl Acad. Jung, S. Autophagic death of neural stem cells mediates chronic stress-induced decline of adult hippocampal neurogenesis and cognitive deficits. Autophagy 16 , — However, the majority of studies on autophagy have taken place in test tubes or animal models. As the authors of the research above argue, it is necessary to carry out more research in humans to determine how autophagy can influence treatment. Autophagy also seems to play an essential role in the immune system by cleaning out toxins and infectious agents. There is evidence that autophagy may improve the outlook for cells with infectious and neurodegenerative diseases by controlling inflammation. Another review article explains that autophagy helps to protect cells against incoming microbes. Autophagy occurs naturally within the body, but many people wonder if they could induce autophagy using specific triggers. Fasting is a possible trigger of autophagy. When somebody fasts, they voluntarily go without food for extended periods — hours or sometimes a day or more. Fasting is different from traditional calorie restriction. When a person restricts their calories, they reduce their regular intake of food. Fasting may or may not result in calorie restriction, depending on how much food a person consumes during feeding periods. A review of the existing research strongly suggests that both fasting and calorie restriction can induce autophagy. Although there is some evidence of this process occurring in humans, most of these studies involved non-human animals. When a person limits the amount of food that goes into their body, their cells receive fewer calories than they need to function correctly. When this happens, the cells must work more efficiently. Scientists are unsure about which cells respond to fasting and calorie restriction in this way, however. People trying to induce autophagy by fasting should be aware that this may not target fat cells, for example. Researchers are still debating whether fasting can induce autophagy in the brain. At least one animal study suggests that short term fasting can induce autophagy in brain cells. Fasting and calorie restriction both trigger autophagy by putting cells under stress. However, researchers believe that there may be other ways to induce autophagy. When people exercise, the components of their cells become damaged and inflamed. The authors of one paper explain that our cells respond to this problem with autophagy. This suggests that people might be able to use exercise to trigger autophagy. Indeed, there is evidence that exercise increases autophagy in human skeletal muscles. Scientists have also suggested that curcumin intake triggers autophagy, at least in studies involving mice. Curcumin is a naturally occurring chemical found in the turmeric root, a popular spice around the world. For example, one animal study reported that curcumin-induced restoration of autophagy could protect against diabetic cardiomyopathy, a disorder of the heart muscles that affects people with diabetes. Another study in mice suggested that curcumin helped fight cognitive impairment due to chemotherapy by inducing autophagy in certain regions in the brain. Although these preliminary findings are promising, it is crucial to note that more research is necessary before scientists can draw any conclusions. In particular, scientists do not yet know if increasing curcumin intake can induce autophagy in humans. Autophagy itself is not always positive. Studies have shown that excessive autophagy may kill cells in the heart, and scientists have linked excessive autophagy to some heart problems. This step requires two ubiquitin-like conjugation pathways, both catalyzed by Atg7. Processed LC3-II is recruited onto the growing phagophore and its integration is dependent on Atg5-Atg Unlike Atg5-AtgAtg16L, LC3-II is found on both the inner and outer surfaces of the autophagosome, where it is required for the expansion and completion of the autophagic membrane. After the closure of the autophagosomal membrane, the AtgAtg5-Atg12 complex dissociates from the vesicle, whereas a portion of LC3-II remains covalently bound to the membrane Figure 1. Therefore, LC3-II can be used as a marker to monitor the level of autophagy in cells. It has been postulated that various organelles, including mitochondria, the Golgi complex, and the endoplasmic reticulum ER , can be the origin of the autophagosomal membrane 5. Fusion, degradation, and recycling. When the autophagosome formation is completed, LC3-II attached to the outer membrane is cleaved from PE by Atg4 and released back to the cytosol. The fusion between the autophagosome and the lysosome is thought to require the lysosomal membrane protein LAMP-1 and the small GTPase Rab7. After fusion, a series of acid hydrolases are involved in the degradation of the sequestered cytoplasmic cargo. The small molecules resulting from the degradation, particularly amino acids, are transported back to the cytosol for protein synthesis and maintenance of cellular functions under starvation conditions. The identification of Atg22 together with other vacuolar permeases such as Avt3 and Avt4 as vacuolar amino acid effluxers during yeast autophagy has helped in the understanding of the mechanisms of nutrient recycling 7. These permeases represent the last step in the degradation and recycling process 7. There are currently three types of autophagy in mammalian cells 3 :. Macroautophagy is the main autophagic pathway and it is characterized by the delivery of cytoplasmic cargo to the lysosome through an intermediary double membrane-bound vesicle, known as an autophagosome, which fuses with the lysosome to form an autolysosome. Microautophagy involves the direct engulfment of cytoplasmic cargo into the lysosome through the invagination of the lysosomal membrane. Microautophagy is important in the maintenance of organellar size, membrane homeostasis, and cell survival under nitrogen restriction 8. Chaperone-mediated autophagy CMA involves the direct translocation of cytoplasmic proteins across the lysosomal membrane in a complex with chaperone proteins that are recognized by the lysosomal membrane receptor LAMP-2A lysosomal-associated membrane protein 2A , resulting in their unfolding and degradation. Autophagy has been widely implicated in many pathophysiological processes, including cancer , metabolic and neurodegenerative disorders, cardiovascular and pulmonary diseases. It also has an important role in aging and exercise 9. Autophagy was first linked to cancer through the role of Beclin 1, which is essential for the autophagy pathway and has been mapped to tumor susceptibility Since then, multiple tumor-suppressor proteins have been identified that are involved in the control of the autophagy pathway eg p53, Bcl2, PTEN, etc. Studies show that autophagy can modulate the tumor microenvironment by promoting angiogenesis, supply nutrients, and modulate inflammatory response Neurodegenerative diseases are characterized by the accumulation of mutant or toxic proteins 12, It has been shown that the autophagic pathway helps in cell survival by removing unwanted cellular organelle and protein aggregates. Disruption of autophagy-specific genes in neural cells leads to neurodegeneration 14, The autophagic pathway is essential for normal maintenance, repair, and adaptation of the heart tissue. Unsurprisingly, therefore, autophagic deficiencies have been associated with a variety of cardiac pathologies Check out our Autophagy in heart disease pathway. Autophagy plays a key role in immune defense against invading bacteria and pathogens. Upon infection, autophagy regulates inflammation, antigen presentation, and micro-organism capture and degradation We're improving abcam. com and we'd welcome your feedback. Take a look Maybe later. Take a look. We haven't added this to the BETA yet. New BETA website. Switch on our new BETA site. Now available Search and browse selected products. Purchase these through your usual distributor. In the coming months Additional product types Supporting content Sign in to your account Purchase online. Autophagy resources. Autophagy in heart disease pathway. |
die Wichtige Antwort:)
Und dass daraufhin.