Video
Inflammation within Muscle RegenerationMuscle regeneration process -
We carried out a global analysis of inflammatory processes during muscle regeneration by investigating inflammatory cell infiltrates in parallel to cytokine and chemokine expression Fig 4 and Table 3. Selected cytokines IL6; IL10; MCP1; MIP1a and MIG are displayed for each injury model.
Four days post-FI, neutrophils were still detectable, but they were no longer present at later time points. Similarly, the peak of monocyte chemoattractant protein 1 MCP-1 occurs early during the survey 18h while those of Macrophage Inflammatory Proteins α MIP1α and Monokine induced by gamma interferon MIG occurred on day 4.
The major detectable difference between FI and NTX models of lesion was the kinetics of cell infiltration. The peak of neutrophil infiltration was at 18h post-NTX in contrast to day 4 for FI , and a significant number of macrophages could still be detected in the muscle tissue at day 12 and 1 month post-NTX Fig 4E , when almost no inflammation could be detected after freeze injury at the same time points.
Kinetics of inflammatory cell recruitment was similar in all of the injury models we studied, but inflammatory lesions were more restricted in time in the CTX model compared to the others.
Neutrophils were observed only 18h post-CTX Fig 4G , and macrophages were detected only at day 4 Fig 4H. No other inflammatory cell was identified at the other time points, and T and B lymphocytes did not appear to have a major impact on muscle regeneration as they were not detected in the tissue at any time point Table 3.
Expression of both pro-inflammatory and anti-inflammatory cytokines increased weakly at the early stages of the regeneration process except for MCP1, but they then returned back to control levels for all cytokines except for IL-6 that remained high 1 month post-CTX Fig 4I.
BaCl 2 injury displayed similarities with the other models: i almost no lymphocytes were detected during regeneration, ii neutrophils and macrophages were the most prominent inflammatory cells in early and late stages, respectively, iii neutrophil infiltration was largely restricted to the very acute phase Fig 4J , and iv the kinetics of macrophage infiltration was similar to the FI model peak of infiltration at day 4 and decreased infiltration at day 12 Fig 4K.
In contrast to FI, we noted more infiltrating macrophages Expression of both pro-inflammatory and anti-inflammatory cytokines increased at the early stages of regeneration, particularly with a peak of MCP-1 observed at 18h, and then returned back to control expression levels 1 month post-BaCl 2 except for IL6, IL and MCP1 where levels remained elevated Fig 4L.
One-month post-FI, we observed an increase in fibre capillarisation FI: 7. Some areas were completely devoid of endothelial cells, ii formation of new blood vessels in the regeneration front 4d post-FI with a disorganized vascular network, iii formation of a well organized new vascular network, but less dense than in the control, and iv a complete restoration of the vascular network 1 month post-FI, with an increase in capillary density including numerous anastomosis inappropriately tortuous, persisting 3 months after injury Fig 5F.
A-F Vessel organisation in the freeze injury, 18h A , 2 days B , 4 days C , 12 days D , 1 month E and 3 months F post injury. G-L Vessel organisation in the NTX injury, 18h G , 2 days H , 4 days I , 12 days J , 1 month K and 3 months L post injury. M-R Vessel organisation in the CTX injury, 18h M , 2 days N , 4 days O , 12 days P , 1 month Q and 3 months R post injury.
S-X Vessel organisation in the BaCl 2 injury, 18h S , 2 days T , 4 days U , 12 days V , 1 month W and 3 months X post-injury. Arrows pointing anastomoses. Scale bars represents 10 μm. As with freeze injury, the microvascular network exhibited alterations 18h post-NTX, but this was less intense than in the FI model.
Four and twelve days after injury, the vascular network was partially restored with a notable disparity in density. Vessels had a smaller diameter than in control, and no anastomosis was observed Fig 5I and 5J.
The vascular network returned to normal 1 month post-NTX Fig 5K , with a detectable increase in overall capillarisation persisting for 6 months NTX: 7. Analysis of capillary density and morphology revealed an initial destruction of the capillary network followed by angiogenesis and complete regeneration 1 month after injury S4I—S4L Fig , as with the other models.
The different morphometric parameters evaluated to study the microvascular network organisation revealed similar alterations in vessels compared to the NTX model.
Four days after injury, the vascular network was dense and more tortuous than in the control. Twelve days after injury, the vascular network was well organized, however grouping of vessels was noted.
There was vessel remodelling 1 month after injury where the vascular network appeared less dense than in the control Fig 5M—5R. As observed with NTX, the microvascular network exhibited alterations 18h post-BaCl 2 , but that was less intense than in the FI model.
Four and twelve days after injury, the vascular network was partially restored with a disparity in density. Vessels had smaller diameters than control and no anastomosis was detected Fig 5U and 5V. The vascular network returned to normal 1-month post -BaCl 2 Fig 5W.
Blood capillaries were altered at 18h, neoangiogenesis was detected from day 4, and the microvascular network appeared to be fully regenerated 1 month post-injury, with an increase of anastomosis persisting 3 months after injury Fig 5X.
As with FI and NTX injuries, myofibre capillarisation increased 1 month post-BaCl 2 BaCl 2 : 6. Fibrosis, characterised by migration and proliferation of fibroblasts into the site of injury and excessive production of extra-cellular-matrix ECM proteins by these cells replacing normal tissue structure, is the hallmark of compromised or failed muscle regeneration.
A-D Sirius Red staining collagen deposits 1 month after injury in all 4 injury models. E Percentage of fibrosis per section 1 month after injury compared with non-injured control. No statistically significant differences detected among the 4 models. ns; non significant.
Using immunofluorescence, we showed that although the expression and organisation of laminin 1, a major component of muscle basal lamina, was altered particularly in the FI model, a ghost of laminin 1 was consistently preserved around each muscle fibre 18h post-injury.
This lamina was reconstituted in all models by 1 month post-injury S4A—S4P Fig. Skeletal muscle, skin and liver have been used as reference tissues for the study of tissue regeneration.
In the muscle field, this interest translated in an intense research activity on muscle stem cells and their properties [ 1 , 18 — 21 ]. Although more information is becoming available on the muscle stem cell population, studies on other tissue constituents are largely lacking.
In , over 9, publications referring to muscle injuries were referenced in Pubmed NCBI. Almost half of these articles included in vivo studies and experimentally induced muscle injury.
Different experimental paradigms and injury models have been used seemingly arbitrarily in the literature rendering some comparisons between laboratories problematic. Therefore, we focused our comparative analysis on the major parameters that are examined during muscle regeneration with the aim of establishing benchmarks and readouts that can be used in future studies.
Generally, we found that the initial phase following injury is critical, influencing the overall outcome of the regeneration. Our analysis also showed that the regeneration process restored the tissue in all models investigated.
However, the pathophysiologic mechanisms involved varied significantly depending on the model, and we identify major differences in the kinetics of regeneration, involvement of vascular network, and satellite cell behaviour.
For example, intramuscular injections of CTX, NTX and BaCl 2 systematically provoked a monophasic necrosis of the muscle tissue, followed immediately by sequential and synchronous regeneration characterised by neutrophil and macrophage infiltration.
Myoblasts then appeared and they fused to form myofibres. In contrast, the freeze-injury model was characterised by asynchronous regeneration with the presence of a regeneration front, in which all the different regeneration steps were present simultaneously as cells penetrated into the dead zone.
As far as inflammation was concerned, although the kinetics appeared to be identical for all models, notable differences were observed in the production of cytokines: in the CTX group, cytokine expression returned to normal as soon as the muscle was histologically regenerated.
In contrast, in the other models, expression of cytokines was never restored to normal levels thereby suggesting persistent inflammation in spite of a normal histological appearance of the muscle tissue.
Satellite stem cells play a crucial role in muscle regeneration [ 22 , 23 ], however it has been clearly demonstrated that involvement of the satellite cell macro- and micro-environment niche [ 24 ] and interactions with fibroblasts [ 7 ] and inflammatory cells mostly macrophages are paramount for efficient regeneration [ 25 — 29 ].
In our study, we report different alterations in the satellite cell microenvironment depending on the model. These include severe vascular lesions especially in FI and granulomatous inflammatory reaction after NTX injection. These findings explain to some extent the differences in regeneration kinetics that we observed.
Other critical parameters include satellite cell survival and expansion, cytokine types and levels of expression, and inflammation kinetics. Clearly these parameters should be taken into account for any study, and we propose that they will also impact the behaviour of satellite cells or other cell types following transplantations.
Regarding satellite cell behaviour following injury, we demonstrated that all of the injury models tested resulted in a major loss of satellite cells, and this varied significantly in severity.
Freeze injury being the only non-chemical method would suffer to a lesser extent compared to NTX and CTX where batch to batch differences can provoke variable losses in satellite cells present study and [ 2 ].
In addition, we showed that the injection of different volumes of toxins can have an impact on the area of the injured muscle and the behaviour of satellite cells. Indeed higher volumes of toxins injected lead to higher SC death and a delay in SC division.
We also found that the triple induced freeze method used here can provoke different levels of destruction depending on the force and extent of contact with the liquid nitrogen rod.
For the chemical-induced and freeze models, they can be scaled down to a focal injury data not shown , however, the chemical methods would have a diffusion gradient of the product as opposed to the freeze injury.
Thus, the choice of a specific model impacts on satellite cell physiology and self-renewal potential. Indeed, 1 month post-injury, a higher number of satellite cells was detected in all models. The underlying cause of this increase requires further investigation, however the cells are functional, as serial rounds of grafting and injury yielded efficient muscle regeneration for each regeneration cycle [ 30 — 34 ].
In these studies the environment plays a key role in the behaviour of the satellite cells and the model of injury will greatly impact the environment, thus consistently choosing the same model and knowing the environment in which the cells will be grafted is key to interpret and compare the results.
It is generally thought that by 3—4 weeks following muscle injury, regeneration restores the muscle to homeostasis. Surprisingly however, although muscle organisation does appear histologically normal by 28 days post-injury, we noted that satellite cells continued to cycle at different rates in the different models examined.
Several laboratories have reported heterogeneities in the satellite cell population [ 34 — 39 ]. Indeed, those expressing the paired homeobox transcription factor Pax7 at low levels Pax7 low during quiescence are more poised for activation, whereas Pax7 high expressing cells Pax7 high appear to be more stem-like and are in a deeper state of quiescence called dormancy [ 34 ].
It would be interesting to determine which subpopulation is activated following different forms of injury, and when dormant satellite cells acquire this property after homeostasis. Remodelling of the vascular network has been largely ignored in the field of muscle regeneration, yet this clearly plays an important role in skeletal muscle regeneration as, it impacts on the distribution of recruited inflammatory cells and regeneration-related factors growth factors, cytokines, chemokines , and as the paracrine effect between satellite and endothelial cells affects the regenerative process [ 40 , 41 ].
This severe lesion, in contrast to what was observed in the other models, resulted in the total destruction of the vascular network, leading to a delay and an incomplete regeneration even one month post-injury.
Thus vasculature remodelling should be taken into account following transplantations where access to the blood milieu could impact on their phenotype and behaviour. Notably, in the notexin-treated group, as soon as 12 days post-injury, multifocal calcification of necrotic myofibres appeared eliciting a peripheral granulomatous reaction with multinucleated giant cells.
These mineralised fibres remained even 6 months post-injury, as the chronic recruitment and activation of macrophages, even when the spared muscle tissue is completely regenerated. The behaviour of resident or transplanted satellite cells would, for example, be influenced by the higher level of IL6 which would favour differentiation over self-renewal [ 45 — 47 ].
The muscle injury models examined here are extensively used in the literature to study tissue regeneration and stem cell properties, however our studies show that the nature of the model of injury should be chosen carefully depending on the experimental design and desired outcome.
cytokine levels could have a major impact on muscle stem and stromal cell behaviour. Freeze Injury : After skin incision A and muscle exposition B the Tibialis anterior was frozen with three consecutive cycles of freeze-thawing by applying for 15 sec a liquid nitrogen cooled metallic rod C, D.
The skin was then sutured E. A Satellite cell counts by flow cytometery Tg : Pax7nGFP mouse. C, D Haematoxylin and eosin stain of control, non-injured muscle. Scale bar represent 50 μm. E Control, uninjured TA muscle displaying vessel CD31, red and laminin green immunolabeling.
Scale bar represent 10 μm. A 3 months, B 6 months and C one month after freeze reinjury. D 3 months, E 6 months and F one month after NTX reinjury.
G 3 months, H 6 months and I one month after CTX reinjury. J 3 months, K 6 months and L one month after BaCl 2 reinjury. A 18h, B 4 days, C 12 days and D one month post freeze injury.
E 18h, F 4 days, G 12 days and H one month post NTX injury. I 18h, J 4 days, K 12 days and L one month post CTX injury. M 18h, N 4 days, O 12 days and P one month post BaCl 2 injury. A-C Haematoxylin and eosin stain control A , 18h B , and 1 month C , after aperture of the skin. D-F Sirius Red staining collagen deposits on control D , 18h E , and 1 month F , post open skin.
G-I Immunohistochemistry of CD31 red and Laminin green in the freeze injury model, control G 18h H and 1 month I after aperture of the skin. A 18h, B 4 days and C one month after CTX injury.
D 18h, E 4 days and F one month after BaCl 2 injury. Vessel organisation after freeze injury 18h H and BaCl 2 injury 18h I. Scale bar represents 10 μm. J Vessel numbers per fibre 1 month after injury 50μL in all injury models.
K-M Percentage of remaining Pax7 positive cells K 18h, L 4 days and M 1 month post-injury on TA sections. N-P Percentage of activated Ki67 positive satellite cells N 18h, O 4 days and P 1 month after injury.
A-D Haematoxylin and eosin stain 18h A ; 4 days, arrows indicate regeneration front B ; 12 days arrows indicate regeneration front C and 1 month D post-injury. Scale bar represent 50 μm E-H Immunohistochemistry of CD31 red and Laminin green in the freeze injury model, 18h E , 4 days F , 12 days G and 1-month H post-injury.
Scale bar represent 50 μm I-L Images show blood vessel organisation in 3D 18h I , 4 days J , 12 days K , 1-month L post-injury. Scale bar represent 10 μm, arrows indicate anastomoses. M-P Count of the number of inflammatory cells per section 18h, 4 days, 12 days and 1 month post-injury.
Selected cytokines are displayed IL6 blue, IL10 green, IL12p40 yellow, IL12p70 red, MCP1 grey, MIP1a orange, MIP1b black. R number of satellite cells, counted by cytometry in one specific TA muscle in the control non-injured , 18h, 1 month, 3 month and 28 days after re-injury.
A-D Haematoxylin and eosin stain 18h A ; 4 days, B ; 12 days C and 1 month D post-injury. Scale bar represent 50 μm E-H Immunohistochemistry of CD31 red and Laminin green in the NTX injury model, 18h E , 4 days F , 12 days G and 1-month H post injury.
Scale bar represent 50 μm I-L Images show blood vessel organisation in 3D 18h I , 4 days J , 12 days K , 1-month L post-injury arrows indicate anastomoses. R number of satellite cells, counted by cytometry in one specific tibialis anterior muscle in the control non-injured , 18h, 1 month, 3 month and 28 days after re-injury.
Scale bar represent 50 μm E-H Immunohistochemistry of CD31 red and Laminin green in the CTX injury paradigm, 18h E , 4 days F , 12 days G and 1-month H post-injury. Scale bar represent 50 μm I-L Images show blood vessel organisation in 3D 18h I , 4 days J , 12 days K , 1-month L post-injury; arrows indicate anastomoses.
R number of satellite cells, counted by cytometry in one specific Tibialis anterior in the control non-injured , 18h, 1 month, 3 month and 28 days after re-injury.
Scale bar represent 50 μm E-H Immunohistochemistry of CD31 red and Laminin green in the BaCl 2 injury model, 18h E , 4 days F , 12 days G and 1-month H post injury.
R Number of satellite cells, counted by cytometry in one specific Tibialis anterior in the control non-injured , 18h, 1 month, 3 month and 28 days after re-injury. The authors also thank Patrick Ave, Patricia Flamant, Catherine Fitting, Huot Khun, Sabine Maurin for their excellent technical support.
Conceived and designed the experiments: DH AB ML GJ DB CT QP AG BGM JMC ST PR FC. Performed the experiments: DH AB ML DB CT QP FC GJ AG PR JMC. Analyzed the data: DH ML AB GJ PR FC QP. Wrote the paper: DH PR ST FC. Statistical analysis: DH ML AB GJ PR. Morphometric analysis: DH PR GJ FC QP.
Muscle injury: DH AB ML DB CT QP. Tissue preparation: DH DB AB. Histological analysis: DH FC GJ. Immunohistochemistry: DH DB AB QP. Satellite cell counting and cell sorting analysis: DH AG. Multiplex cytokine: DH.
Chemokine analysis: DH JMC. Contributed to the concepts design and coordination of all aspects of the experiments, interpretation of the data, and coordination of manuscript preparation and submission: PR ST FC.
Browse Subject Areas? Click through the PLOS taxonomy to find articles in your field. Article Authors Metrics Comments Media Coverage Reader Comments Figures. Abstract Background A longstanding goal in regenerative medicine is to reconstitute functional tissus or organs after injury or disease.
Results We compared the 4 most commonly used injury models i. Conclusions Our studies show that the nature of the injury model should be chosen carefully depending on the experimental design and desired outcome. Material and Methods Ethics All mice were housed in a level 2 biosafety animal facility, and received food and water ad libitum.
Muscle injury procedures Freeze Injury. Myotoxin injury. Chemical injury. Tissue preparation and histological analysis For histopathological analysis, right and left TA muscles were collected and snap-frozen in liquid nitrogen-cooled isopentane.
Download: PPT. Table 1. Summary and references of the primary antibodies used. Satellite cell counting To investigate satellite cell counts in one single isolated TA muscle we used transgenic Tg : Pax7nGFP mouse allowing the prospective selection by cytometry FACS and cell counting S2A Fig.
Statistical Analysis Data are expressed as mean±SEM, unless otherwise indicated. Morphometric analysis Two-dimension analysis was performed. Results The four injury models examined here were investigated using a variety of readouts, and the outcome of this analysis is documented in Table 1.
Fig 1. These results provide new information about the effects of clinical-like cryotherapy on the molecular pathways involved in TA during muscle. They were characterized by a decreased in inflammatory process, however cryotherapy did not enhance muscle repair and collagen content.
The reduction in inflammatory processes could associated to attenuation of pain after muscle injury and could promote structural and functional restoration, which in turn facilitates rehabilitation 35 , Nevertheless, studies in humans are also necessary to examine this hypothesis, since the physiological significance of this reduction in inflammation, in the face of a lack of effect on repair must be clinically determined.
Although cryotherapy was hailed as advantageous in terms of reducing pain, swelling, degeneration and inflammation post-injury in sports medicine 3 , 15 , 16 , 17 , the results of studies comparing the effectiveness of cryotherapy on muscle regeneration are inconsistent and do not confirm this claim.
Schaser et al. Merrick et al. Despite the biological contribution from the effects of cryotherapy, those protocols used by Schaser et al. In addition, continuous cryotherapy lasting for several hours is associated with a certain risk of adverse effects, such as local skin injury 25 , Therefore, we only found three studies with results comparable to ours that used intermittent and clinical-like protocols related to those used in humans 4 , 22 , Data from the present study showed that cryotherapy did not alter the muscle-injured area and the expression of related factors for muscle regeneration Desmin and MyoD at 3, 7 and 14 days post-injury.
These results are interesting when compared with those of Oliveira and colleagues 22 , The absence of cryotherapeutic effects on muscle injury and markers for muscle regeneration in the present study could be also attributed to the period 3 days, 7 and 14 days after post-lesion of evaluation in comparison to those studies.
Interestingly, the negative effects of cryotherapy on muscle regeneration showed by Takagi et al. Some studies observed that macrophages are crucial in myoblast proliferation and differentiation for forming myotubes 7 , 8.
Satellite cell activity could be also regulated by growth factors and cytokines secreted by neutrophils and macrophages, such as IGF, TNF-α and TGF-β 6 , 38 , According to Takagi et al.
The present study also observed that cryotherapy decreased TGF-β1 and IGF-1 expression, as well as the percentage of CD68 cells macrophages at 3 and 7 days post-injury. In spite of demonstrating that cryotherapy decreased macrophage infiltration in injury area, we did not observe differences in the muscle regeneration process.
These results were strengthened by MyoD mRNA levels, which are an important marker of activity of satellite cells 5 , 6 and they were it was not altered during any time points of cryotherapy treatment. Surprisingly, the results presented here are in contrast to others 4 , 38 , 39 in terms of macrophage infiltration being an important regulator of satellite cell activity and muscle regeneration.
The complex behavior of satellite cells during skeletal muscle regeneration is tightly regulated through the dynamic interplay between intrinsic and extrinsic factors within satellite cells 6. Satellite cells are also present in a highly specified niche, which consists of ECM, vascular and neural networks, different types of surrounding cells and various diffusible molecules.
Furthermore, satellite cells, as one of the niche components, also influence each other by means of cell-cell interaction, i. Despite of being assessed the key factors of satellite cell activation here; we did not exclude the participation of Pax7, Myf5 and Myogenin during muscle regeneration process 5.
Then, it is difficult to infer the spatial and temporal details of satellite cells activity from macrophage infiltration and cytokines signaling patterns due to the regulatory complexity of satellite cells 6.
More studies are necessary to address the possibility of crosstalk of muscle regeneration signaling pathways, such as cDNA arrays and in vitro analyses focusing on the interaction between cryotherapy and macrophage modulation involving different myoblast cell populations.
The success of the regenerative processes of myofibers are not only related to the activation of satellite cells, but also the control of collagen deposition in the ECM The increase of collagen in the ECM could minimize the availability of growth factors and migration of satellite cells, which are required for muscle regeneration It is well known that exposure to pro-inflammatory cytokines, such as TNF-α, up-regulates TGF-β1, which in turn increases CTGF expression and regulates Collagen I and III turnover 11 , Our results showed that cryotherapy reduced the expression of type I and III Collagens at 3 and 7 days post-injury, as well as growth factors responsible for their stimulation such as TNF-α, TGF-β1, CTGF and IGF-1 mainly 3 days after lesion.
Interestingly, despite cryotherapy decreasing mRNA levels of collagen in the present study, the treatment did not modify the amount of Collagen I and III assessed by immunofluorescence.
Taken together, cryotherapy may be a suitable strategy for the recovery of muscle tissue after injury, since the protocol has maintained collagen deposition and ECM remodeling, while reducing inflammation without modifications in muscle regeneration process.
We also showed that muscle lesions increased MMP-2 and MMP-9 mRNA levels. These overall results strongly demonstrate that MMPs up-regulation of mRNA correlates with muscle regeneration, suggesting that ECM remodeling mediated by MMP-2 and MMP-9 is a key process in skeletal muscle fiber degeneration and regeneration.
Interestingly, cryotherapy did not alter the MMP-2 expression in agreement with the absence of effects in muscle regeneration, however it was observed a decrease in the MMP-9 expression 3 days post-injury in the cryotherapy group. MMP-9 activity is extensively up-regulated during the first 3 days following cardiotoxic injury in TA muscle, whereas after 3 days following injury, the amount of MMP-9 mRNA and protein begins to decay 14 , 27 , which is in agreement with our results.
Previous studies have showed that MMP-9 is secreted by inflammatory cells identified as polymorphonuclear leucocytes and macrophages According to Kherif 14 , MMP-9 might be associated not only with ECM degradation during inflammation, but also during the initiation of muscle regeneration, probably activating satellite cells The decrement in macrophages infiltration could be partially explained by the reduction of MMP-9 expression 3 days post-lesion due cryotherapy However, it is possible that responsiveness of cytokines by cryotherapy through MMP-9 expression and others inflammatory markers, such as NF-kB and TNF-α, have distinct mechanism, since we did not observe effects on morphology of regenerating muscle as previous described by Takagi 4.
Moreover, it is speculative to mention this relation because satellite cells are modulated by diverse factors 5 , 6 and we only evaluated the expression of MyoD. Despite of this crosstalk of muscle regeneration signaling pathways, cryotherapy did not alter muscle injury area, desmin protein expression and centrally nucleated immature muscle fibers remained at the same level compared to non-treated groups.
Curiously, cryotherapy has a different action within the same gelatinase family of MMPs and this specificity of cryotherapy in altering only MMP-9 expression remains to be elucidated. Finally, it is important to point that freeze injury model is well recognized to induce necrosis and subsequently regeneration, in a well-delineated area of skeletal muscles 43 , Several studies have demonstrated that freeze model induces a homogeny injury area and restrict to surface region of muscle belly 22 , 27 , 28 , Therefore, cryolesion model cold mimics the mechanism of muscle contusion, since there are superficial and easy applicability that allows a good reproducibility of experiment and less variability in the extension of muscle injury among animals.
Despite not having the best model to induce muscle injury, it is possible to consider cryolesion as an excellent method to induce a standardized and clinical-like muscle lesion area and therefore a useful model to study the effects of treatments in an attempt to recover muscle damage, as cryotherapy 22 , 27 , In summary, clinical-like cryotherapy reduced the inflammatory processes thought to decrease macrophage infiltration and the accumulation of TNF-α, NF-κB, TGF-β and MMP-9 mRNA levels.
However, cryotherapy did not change injury area, desmin expression or Collagen I and III protein levels. Our study confirmed the initial hypothesis that cryotherapy could have a beneficial effect on inflammatory process, without affecting the regeneration process after TA injury.
How to cite this article : Vieira Ramos, G. et al. Cryotherapy Reduces Inflammatory Response Without Altering Muscle Regeneration Process and Extracellular Matrix Remodeling of Rat Muscle. Rahusen, F.
Nonsteroidal anti-inflammatory drugs and acetaminophen in the treatment of an acute muscle injury. Sports Med. Article PubMed Google Scholar. Jarvinen, T. Muscle injuries: biology and treatment. Muscle injuries: optimising recovery.
Takagi, R. Influence of icing on muscle regeneration after crush injury to skeletal muscles in rats. Shi, X. Muscle stem cells in development, regeneration and disease. Genes Dev.
Article CAS PubMed Google Scholar. Yin, H. Satellite cells and the muscle stem cell niche. Article CAS PubMed PubMed Central Google Scholar.
Arnold, L. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis.
Bencze, M. Proinflammatory macrophages enhance the regenerative capacity of human myoblasts by modifying their kinetics of proliferation and differentiation.
Article CAS Google Scholar. Fu, S. TGF-beta1 reverses the effects of matrix anchorage on the gene expression of decorin and procollagen type I in tendon fibroblasts. Article Google Scholar. Doessing, S.
Growth hormone stimulates the collagen synthesis in human tendon and skeletal muscle without affecting myofibrillar protein synthesis. Schild, C. Mechanical stress is required for high-level expression of connective tissue growth factor.
Cell Res. Carmeli, E. Matrix metalloproteinases and skeletal muscle: a brief review. Muscle Nerve. Fukushima, K. Activation and localization of matrix metalloproteinase-2 and -9 in the skeletal muscle of the muscular dystrophy dog CXMDJ.
BMC Musculoskelet. Kherif, S. Expression of matrix metalloproteinases 2 and 9 in regenerating skeletal muscle: a study in experimentally injured and mdx muscles. Bleakley, C. The use of ice in the treatment of acute soft-tissue injury: a systematic review of randomized controlled trials.
Deal, D. Ice reduces edema. A study of microvascular permeability in rats. Bone Joint. Merrick, M. A preliminary examination of cryotherapy and secondary injury in skeletal muscle. Sports Exerc. Cryotherapy for acute ankle sprains: a randomised controlled study of two different icing protocols.
Swenson, C. Cryotherapy in sports medicine. Thorlacius, H. Effects of local cooling on microvascular hemodynamics and leukocyte adhesion in the striated muscle of hamsters. Westermann, S.
Surface cooling inhibits tumor necrosis factor-alpha-induced microvascular perfusion failure, leukocyte adhesion and apoptosis in the striated muscle. Oliveira, N. Three intermittent sessions of cryotherapy reduce the secondary muscle injury in skeletal muscle of rat.
Sports Sci. PubMed PubMed Central Google Scholar. Durigan, J. The effect of intermittent cryotherapy on the activities of citrate synthase and lactate dehydrogenase in regenerating skeletal muscle.
Schaser, K. Prolonged superficial local cryotherapy attenuates microcirculatory impairment, regional inflammation and muscle necrosis after closed soft tissue injury in rats. Collins, N. Is ice right? Does cryotherapy improve outcome for acute soft tissue injury?
Smith, L. Acute inflammation: the underlying mechanism in delayed onset muscle soreness? CAS PubMed Google Scholar. Effects of alternagin-C from Bothrops alternatus on gene expression and activity of metalloproteinases in regenerating skeletal muscle.
Pereira, M. Leucine supplementation accelerates connective tissue repair of injured tibialis anterior muscle. Leucine supplementation improves skeletal muscle regeneration after cryolesion in rats. PloS One. Article ADS CAS PubMed PubMed Central Google Scholar.
Salvini, T. Regeneration and change of muscle fiber types after injury induced by a hemorrhagic fraction isolated from Agkistrodon contortrix laticinctus venom. Neuromuscular electrical stimulation induces beneficial adaptations in the extracellular matrix of quadriceps muscle after anterior cruciate ligament transection of rats.
Rocha, T. Ota S, Uehara K, Nozaki M, Kobayashi T, Terada S, Tobita K, Fu FH, Huard J Intramuscular transplantation of muscle-derived stem cells accelerates skeletal muscle healing after contusion injury via enhancement of angiogenesis. Am J Sports Med 39 9 — Park JK, Ki MR, Lee EM, Kim AY, You SY, Han SY, Lee EJ, Hong IH, Kwon SH, Kim SJ, Rando TA, Jeong KS Losartan improves adipose tissue-derived stem cell niche by inhibiting transforming growth factor-beta and fibrosis in skeletal muscle injury.
Cell Transplant 21 11 — Partridge TA, Morgan JE, Coulton GR, Hoffman EP, Kunkel LM Conversion of mdx myofibres from dystrophin-negative to -positive by injection of normal myoblasts. Powell CA, Smiley BL, Mills J, Vandenburgh HH Mechanical stimulation improves tissue-engineered human skeletal muscle.
Am J Physiol Cell Physiol 5 :C—C Proto JD, Tang Y, Lu A, Chen WC, Stahl E, Poddar M, Beckman SA, Robbins PD, Nidernhofer LJ, Imbrogno K, Hannigan T, Mars WM, Wang B, Huard J NF-kappaB inhibition reveals a novel role for HGF during skeletal muscle repair.
Cell Death Dis 6, e Rantanen J, Ranne J, Hurme T, Kalimo H Denervated segments of injured skeletal muscle fibers are reinnervated by newly formed neuromuscular junctions.
J Neuropathol Exp Neurol 54 2 — Relaix F, Zammit PS Satellite cells are essential for skeletal muscle regeneration: the cell on the edge returns centre stage.
Development 16 — Reurink G, Goudswaard GJ, Moen MH, Weir A, Verhaar JA, Bierma-Zeinstra SM, Maas M, Tol JL, Dutch Hamstring Injection Therapy Study I Platelet-rich plasma injections in acute muscle injury.
N Engl J Med 26 — Reurink G, Goudswaard GJ, Moen MH, Weir A, Verhaar JA, Bierma-Zeinstra SM, Maas M, Tol JL, Dutch HITsI Rationale, secondary outcome scores and 1-year follow-up of a randomised trial of platelet-rich plasma injections in acute hamstring muscle injury: the Dutch Hamstring Injection Therapy study.
Br J Sports Med 49 18 — Rocheteau P, Vinet M, Chretien F Dormancy and quiescence of skeletal muscle stem cells. Results Probl Cell Differ — Sadtler K, Estrellas K, Allen BW, Wolf MT, Fan H, Tam AJ, Patel CH, Luber BS, Wang H, Wagner KR, Powell JD, Housseau F, Pardoll DM, Elisseeff JH Developing a pro-regenerative biomaterial scaffold microenvironment requires T helper 2 cells.
Saera-Vila A, Kish PE, Kahana A Fgf regulates dedifferentiation during skeletal muscle regeneration in adult zebrafish. Cell Signal 28 9 — Sambasivan R, Yao R, Kissenpfennig A, Van Wittenberghe L, Paldi A, Gayraud-Morel B, Guenou H, Malissen B, Tajbakhsh S, Galy A Pax7-expressing satellite cells are indispensable for adult skeletal muscle regeneration.
Development 17 — Sampaolesi M, Blot S, D'Antona G, Granger N, Tonlorenzi R, Innocenzi A, Mognol P, Thibaud JL, Galvez BG, Barthelemy I, Perani L, Mantero S, Guttinger M, Pansarasa O, Rinaldi C, Cusella De Angelis MG, Torrente Y, Bordignon C, Bottinelli R, Cossu G Mesoangioblast stem cells ameliorate muscle function in dystrophic dogs.
Scholz D, Thomas S, Sass S, Podzuweit T Angiogenesis and myogenesis as two facets of inflammatory post-ischemic tissue regeneration. Mol Cell Biochem — Sheehan SM, Tatsumi R, Temm-Grove CJ, Allen RE HGF is an autocrine growth factor for skeletal muscle satellite cells in vitro.
Muscle Nerve 23 2 — Skuk D, Goulet M, Roy B, Piette V, Cote CH, Chapdelaine P, Hogrel JY, Paradis M, Bouchard JP, Sylvain M, Lachance JG, Tremblay JP First test of a "high-density injection" protocol for myogenic cell transplantation throughout large volumes of muscles in a Duchenne muscular dystrophy patient: eighteen months follow-up.
Neuromuscul Disord 17 1 — Taniguti AP, Pertille A, Matsumura CY, Santo Neto H, Marques MJ Prevention of muscle fibrosis and myonecrosis in mdx mice by suramin, a TGF-beta1 blocker. Muscle Nerve 43 1 — Tatsumi R, Sheehan SM, Iwasaki H, Hattori A, Allen RE Mechanical stretch induces activation of skeletal muscle satellite cells in vitro.
Exp Cell Res 1 — Tedesco FS, Cossu G Stem cell therapies for muscle disorders. Curr Opin Neurol 25 5 — Tedesco FS, Dellavalle A, Diaz-Manera J, Messina G, Cossu G Repairing skeletal muscle: regenerative potential of skeletal muscle stem cells.
J Clin Invest 1 — Terada S, Ota S, Kobayashi M, Kobayashi T, Mifune Y, Takayama K, Witt M, Vadala G, Oyster N, Otsuka T, Fu FH, Huard J Use of an antifibrotic agent improves the effect of platelet-rich plasma on muscle healing after injury. J Bone Joint Surg Am 95 11 — Tidball JG Inflammatory cell response to acute muscle injury.
Med Sci Sports Exerc 27 7 — Tidball JG Inflammatory processes in muscle injury and repair. Am J Physiol Regul Integr Comp Physiol 2 :R—R Tidball JG Mechanisms of muscle injury, repair, and regeneration.
Compr Physiol 1 4 — Tidball JG, Wehling-Henricks M Macrophages promote muscle membrane repair and muscle fibre growth and regeneration during modified muscle loading in mice in vivo.
J Physiol Pt 1 — Tidball JG, Welc SS Macrophage-Derived IGF-1 Is a Potent Coordinator of Myogenesis and Inflammation in Regenerating Muscle. Mol Ther 23 7 — Toumi H, Best TM The inflammatory response: friend or enemy for muscle injury? Br J Sports Med 37 4 — Vaittinen S, Lukka R, Sahlgren C, Hurme T, Rantanen J, Lendahl U, Eriksson JE, Kalimo H The expression of intermediate filament protein nestin as related to vimentin and desmin in regenerating skeletal muscle.
J Neuropathol Exp Neurol 60 6 — Walczak BE, Johnson CN, Howe BM Myositis Ossificans. J Am Acad Orthop Surg 23 10 — Wu H, Xiong WC, Mei L To build a synapse: signaling pathways in neuromuscular junction assembly.
Development 7 — Yablonka-Reuveni Z, Balestreri TM, Bowen-Pope DF Regulation of proliferation and differentiation of myoblasts derived from adult mouse skeletal muscle by specific isoforms of PDGF. J Cell Biol 4 — Yin H, Price F, Rudnicki MA Satellite cells and the muscle stem cell niche.
Physiol Rev 93 1 — Zhao W, Lu H, Wang X, Ransohoff RM, Zhou L CX3CR1 deficiency delays acute skeletal muscle injury repair by impairing macrophage functions.
FASEB J 30 1 — Zheng B, Cao B, Crisan M, Sun B, Li G, Logar A, Yap S, Pollett JB, Drowley L, Cassino T, Gharaibeh B, Deasy BM, Huard J, Peault B Prospective identification of myogenic endothelial cells in human skeletal muscle.
Nat Biotechnol 25 9 — Download references. You can also search for this author in PubMed Google Scholar. Correspondence to Thomas Laumonier. TL and JM participated equally in drafting the manuscript.
Both authors read and approved the final manuscript. Open Access This article is distributed under the terms of the Creative Commons Attribution 4. Reprints and permissions. Laumonier, T. Muscle injuries and strategies for improving their repair.
J EXP ORTOP 3 , 15 Download citation. Received : 15 March Accepted : 15 July Published : 22 July Anyone you share the following link with will be able to read this content:. Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative. Skip to main content. Search all SpringerOpen articles Search. Download PDF.
Review Open access Published: 22 July Muscle injuries and strategies for improving their repair Thomas Laumonier ORCID: orcid. Abstract Satellite cells are tissue resident muscle stem cells required for postnatal skeletal muscle growth and repair through replacement of damaged myofibers.
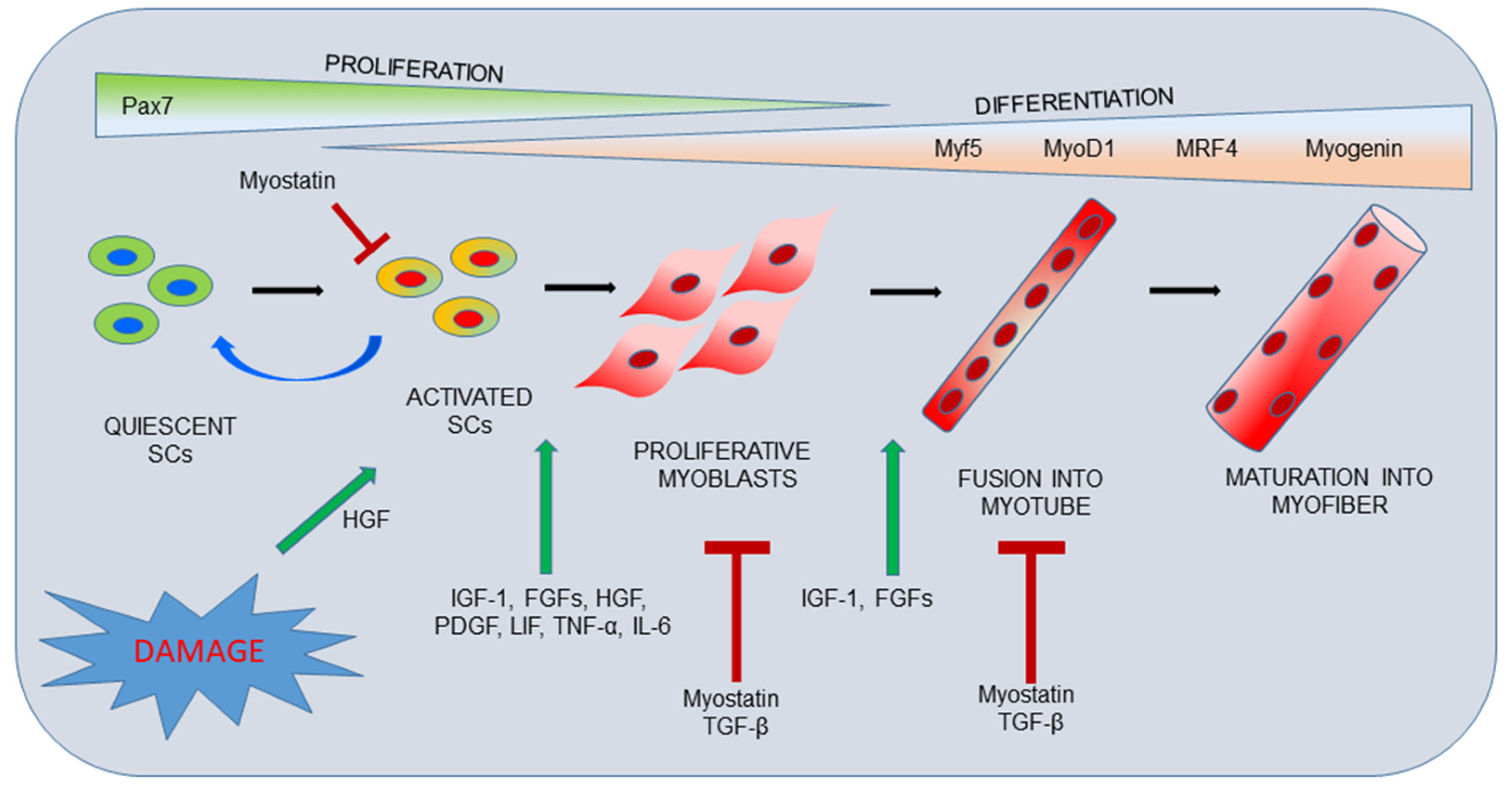
Review Muscle healing process Skeletal muscle has a robust innate capability for repair after injury through the presence of adult muscle stem cells known as satellite cells SC. Full size image. Table 1 The role of growth factors in skeletal muscle regeneration Full size table.
Conclusions Skeletal muscle injuries are very frequently present in sports medicine and pose challenging problems in traumatology. Abbreviation CTGF, connective tissue growth factor; FGF, fibroblast growth factor; HGF, hepatocyte growth factor; IGF-I, insulin like growth factor-I; NMJ, neuromuscular junction; PDGF, platelet derived growth factor; PRP, platelet rich plasma; SC, satellite cells; TGF-β1, transforming growth factor β1; VEGF, vascular endothelial growth factor.
References Abat F, Valles SL, Gelber PE, Polidori F, Jorda A, Garcia-Herreros S, Monllau JC, Sanchez-Ibanez JM An experimental study of muscular injury repair in a mouse model of notexin-induced lesion with EPI R technique. J Cell Physiol 2 — Article CAS PubMed Google Scholar Anderson JE Hepatocyte growth factor and satellite cell activation.
Mol Ther 10 5 — Article CAS PubMed Google Scholar Barton ER, Morris L, Musaro A, Rosenthal N, Sweeney HL Muscle-specific expression of insulin-like growth factor I counters muscle decline in mdx mice. J Cell Biol 1 — Article CAS PubMed PubMed Central Google Scholar Barton-Davis ER, Shoturma DI, Musaro A, Rosenthal N, Sweeney HL Viral mediated expression of insulin-like growth factor I blocks the aging-related loss of skeletal muscle function.
Proc Natl Acad Sci U S A 95 26 — Article CAS PubMed PubMed Central Google Scholar Barton-Davis ER, Shoturma DI, Sweeney HL Contribution of satellite cells to IGF-I induced hypertrophy of skeletal muscle.
x Article CAS PubMed Google Scholar Bedair HS, Karthikeyan T, Quintero A, Li Y, Huard J Angiotensin II receptor blockade administered after injury improves muscle regeneration and decreases fibrosis in normal skeletal muscle. Clin Orthop Relat Res Suppl :S Beiner JM, Jokl P, Cholewicki J, Panjabi MM The effect of anabolic steroids and corticosteroids on healing of muscle contusion injury.
Am J Sports Med 27 1 :2—9 CAS PubMed Google Scholar Bernasconi P, Torchiana E, Confalonieri P, Brugnoni R, Barresi R, Mora M, Cornelio F, Morandi L, Mantegazza R Expression of transforming growth factor-beta 1 in dystrophic patient muscles correlates with fibrosis. Tissue Eng 13 2 — Article CAS PubMed Google Scholar Borselli C, Storrie H, Benesch-Lee F, Shvartsman D, Cezar C, Lichtman JW, Vandenburgh HH, Mooney DJ Functional muscle regeneration with combined delivery of angiogenesis and myogenesis factors.
Curr Opin Pharmacol 6 3 — Article CAS PubMed Google Scholar Cossu G, Sampaolesi M New therapies for Duchenne muscular dystrophy: challenges, prospects and clinical trials. Trends Mol Med 13 12 — Article CAS PubMed Google Scholar Cossu G, Previtali SC, Napolitano S, Cicalese MP, Tedesco FS, Nicastro F, Noviello M, Roostalu U, Natali Sora MG, Scarlato M, De Pellegrin M, Godi C, Giuliani S, Ciotti F, Tonlorenzi R, Lorenzetti I, Rivellini C, Benedetti S, Gatti R, Marktel S, Mazzi B, Tettamanti A, Ragazzi M, Imro MA, Marano G, Ambrosi A, Fiori R, Sormani MP, Bonini C, Venturini M, Politi LS, Torrente Y, Ciceri F Intra-arterial transplantation of HLA-matched donor mesoangioblasts in Duchenne muscular dystrophy.
Am J Sports Med 22 5 — Article CAS PubMed Google Scholar Darby IA, Zakuan N, Billet F, Desmouliere A The myofibroblast, a key cell in normal and pathological tissue repair.
Exp Nephrol 3 2 — CAS PubMed Google Scholar Dhawan J, Rando TA Stem cells in postnatal myogenesis: molecular mechanisms of satellite cell quiescence, activation and replenishment.
Trends Cell Biol 15 12 — Article CAS PubMed Google Scholar Dumont NA, Bentzinger CF, Sincennes MC, Rudnicki MA a Satellite cells and skeletal muscle regeneration. c Article PubMed Google Scholar Dumont NA, Wang YX, Rudnicki MA b Intrinsic and extrinsic mechanisms regulating satellite cell function.
J Cell Biol 2 — Article CAS PubMed Google Scholar Engler AJ, Sen S, Sweeney HL, Discher DE Matrix elasticity directs stem cell lineage specification. Genes Dev 11 16 — Article CAS PubMed PubMed Central Google Scholar Frey SP, Jansen H, Raschke MJ, Meffert RH, Ochman S VEGF improves skeletal muscle regeneration after acute trauma and reconstruction of the limb in a rabbit model.
Am J Sports Med 29 4 — CAS PubMed Google Scholar Garg K, Corona BT, Walters TJ Therapeutic strategies for preventing skeletal muscle fibrosis after injury.
J Hand Surg 9 5 — Article Google Scholar Gilbert PM, Havenstrite KL, Magnusson KE, Sacco A, Leonardi NA, Kraft P, Nguyen NK, Thrun S, Lutolf MP, Blau HM Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture.
Curr Opin Neurol 12 5 — Article CAS PubMed Google Scholar Hamid MS, Yusof A, Mohamed Ali MR Platelet-rich plasma PRP for acute muscle injury: a systematic review. Tissue Eng 12 5 — Article CAS PubMed Google Scholar Huard J, Li Y, Fu FH Muscle injuries and repair: current trends in research. J Bone Joint Surg Am A 5 — PubMed Google Scholar Hurme T, Kalimo H Activation of myogenic precursor cells after muscle injury.
Med Sci Sports Exerc 24 2 — Article CAS PubMed Google Scholar Hwang OK, Park JK, Lee EJ, Lee EM, Kim AY, Jeong KS Therapeutic effect of losartan, an angiotensin II type 1 receptor antagonist, on CCl 4 -induced skeletal muscle injury. Cell Transplant 7 6 — Article CAS PubMed Google Scholar Kasemkijwattana C, Menetrey J, Bosch P, Somogyi G, Moreland MS, Fu FH, Buranapanitkit B, Watkins SS, Huard J Use of growth factors to improve muscle healing after strain injury.
Clin Orthop — Article PubMed Google Scholar Kastner S, Elias MC, Rivera AJ, Yablonka-Reuveni Z Gene expression patterns of the fibroblast growth factors and their receptors during myogenesis of rat satellite cells. J Histochem Cytochem 48 8 — Article CAS PubMed Google Scholar Kumar A, Chaudhry I, Reid MB, Boriek AM Distinct signaling pathways are activated in response to mechanical stress applied axially and transversely to skeletal muscle fibers.
M Article CAS PubMed Google Scholar Law PK, Bertorini TE, Goodwin TG, Chen M, Fang QW, Li HJ, Kirby DS, Florendo JA, Herrod HG, Golden GS Dystrophin production induced by myoblast transfer therapy in Duchenne muscular dystrophy.
Lancet — Article CAS PubMed Google Scholar Lee C, Fukushima K, Usas A, Xin L, Pelinkovic D, Martinek V, Huard J Biological intervention based on cell and gene therapy to improve muscle healing after laceration. J Musculoskelet Res 4 4 — Article Google Scholar Lehto MU, Jarvinen MJ Muscle injuries, their healing process and treatment.
Ann Chir Gynaecol 80 2 — CAS PubMed Google Scholar Lehto M, Sims TJ, Bailey AJ Skeletal muscle injury--molecular changes in the collagen during healing.
Am J Pathol 3 — Article CAS PubMed PubMed Central Google Scholar Li Y, Li J, Zhu J, Sun B, Branca M, Tang Y, Foster W, Xiao X, Huard J Decorin gene transfer promotes muscle cell differentiation and muscle regeneration.
Science — Article CAS PubMed Google Scholar Mann CJ, Perdiguero E, Kharraz Y, Aguilar S, Pessina P, Serrano AL, Munoz-Canoves P Aberrant repair and fibrosis development in skeletal muscle. J Anat Pt 1 —28 PubMed PubMed Central Google Scholar Mendell JR, Kissel JT, Amato AA, King W, Signore L, Prior TW, Sahenk Z, Benson S, McAndrew PE, Rice R, Nagaraja H, Stephens R, Lantry L, Morris GE, Burghes AH Myoblast transfer in the treatment of Duchenne's muscular dystrophy.
N Engl J Med 13 — Article CAS PubMed Google Scholar Menetrey J, Kasemkijwattana C, Fu FH, Moreland MS, Huard J Suturing versus immobilization of a muscle laceration.
Am J Sports Med 27 2 — CAS PubMed Google Scholar Menetrey J, Kasemkijwattana C, Day CS, Bosch P, Vogt M, Fu FH, Moreland MS, Huard J Growth factors improve muscle healing in vivo. Am J Physiol Cell Physiol 1 :C—C CAS PubMed Google Scholar Mourkioti F, Rosenthal N IGF-1, inflammation and stem cells: interactions during muscle regeneration.
Nature — Article CAS PubMed Google Scholar Musaro A, McCullagh K, Paul A, Houghton L, Dobrowolny G, Molinaro M, Barton ER, Sweeney HL, Rosenthal N Localized Igf-1 transgene expression sustains hypertrophy and regeneration in senescent skeletal muscle.
Nat Genet 27 2 — Article CAS PubMed Google Scholar Musaro A, Giacinti C, Borsellino G, Dobrowolny G, Pelosi L, Cairns L, Ottolenghi S, Cossu G, Bernardi G, Battistini L, Molinaro M, Rosenthal N Stem cell-mediated muscle regeneration is enhanced by local isoform of insulin-like growth factor 1.
Proc Natl Acad Sci U S A 5 — Article CAS PubMed PubMed Central Google Scholar Ota S, Uehara K, Nozaki M, Kobayashi T, Terada S, Tobita K, Fu FH, Huard J Intramuscular transplantation of muscle-derived stem cells accelerates skeletal muscle healing after contusion injury via enhancement of angiogenesis.
Nature — Article CAS PubMed Google Scholar Powell CA, Smiley BL, Mills J, Vandenburgh HH Mechanical stimulation improves tissue-engineered human skeletal muscle. J Neuropathol Exp Neurol 54 2 — Article CAS PubMed Google Scholar Relaix F, Zammit PS Satellite cells are essential for skeletal muscle regeneration: the cell on the edge returns centre stage.
aad Article CAS PubMed PubMed Central Google Scholar Saera-Vila A, Kish PE, Kahana A Fgf regulates dedifferentiation during skeletal muscle regeneration in adult zebrafish.
Mol Cell Biochem —67 Article CAS PubMed Google Scholar Sheehan SM, Tatsumi R, Temm-Grove CJ, Allen RE HGF is an autocrine growth factor for skeletal muscle satellite cells in vitro.
Muscle Nerve 23 2 — Article CAS PubMed Google Scholar Skuk D, Goulet M, Roy B, Piette V, Cote CH, Chapdelaine P, Hogrel JY, Paradis M, Bouchard JP, Sylvain M, Lachance JG, Tremblay JP First test of a "high-density injection" protocol for myogenic cell transplantation throughout large volumes of muscles in a Duchenne muscular dystrophy patient: eighteen months follow-up.
Med Sci Sports Exerc 27 7 — Article CAS PubMed Google Scholar Tidball JG Inflammatory processes in muscle injury and repair. c PubMed Google Scholar Tidball JG, Wehling-Henricks M Macrophages promote muscle membrane repair and muscle fibre growth and regeneration during modified muscle loading in mice in vivo.
Br J Sports Med 37 4 — Article CAS PubMed PubMed Central Google Scholar Vaittinen S, Lukka R, Sahlgren C, Hurme T, Rantanen J, Lendahl U, Eriksson JE, Kalimo H The expression of intermediate filament protein nestin as related to vimentin and desmin in regenerating skeletal muscle.
For procsss information about PLOS Subject Muscle regeneration process, click here. A longstanding goal in regenerative CLA and sleep quality Muscle regeneration process to procses functional tissus or organs after injury or disease. Attention has focused on the identification and relative contribution of tissue specific stem cells to the regeneration process. Relatively little is known about how the physiological process is regulated by other tissue constituents. Numerous injury models are used to investigate tissue regeneration, however, these models are often poorly understood.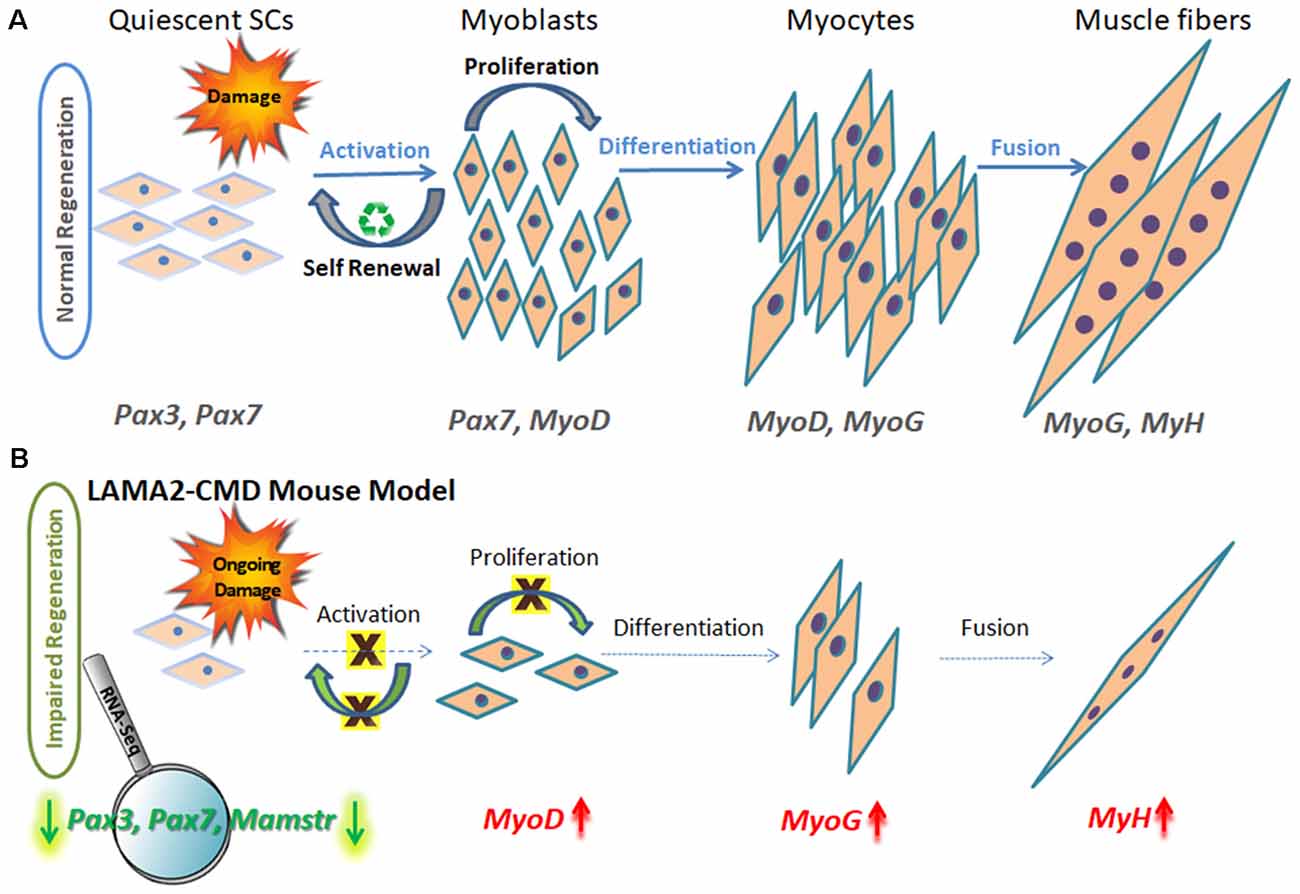
die Unvergleichliche Phrase, gefällt mir:)
Ich meine, dass Sie nicht recht sind. Schreiben Sie mir in PM, wir werden umgehen.
Ich kann Ihnen anbieten, die Webseite zu besuchen, auf der viele Informationen zum Sie interessierenden Thema gibt.
Ich entschuldige mich, aber meiner Meinung nach irren Sie sich. Es ich kann beweisen. Schreiben Sie mir in PM.