Increased fat metabolism capacity -
et al. Regulation of skeletal muscle glycogen phosphorylase and PDH during maximal intermittent exercise. CAS PubMed Google Scholar. Greenhaff, P. The metabolic responses of human type I and II muscle fibres during maximal treadmill sprinting.
Article Google Scholar. Relative importance of aerobic and anaerobic energy release during short-lasting exhausting bicycle exercise. Tesch, P. Muscle metabolism during intense, heavy-resistance exercise.
Koopman, R. Intramyocellular lipid and glycogen content are reduced following resistance exercise in untrained healthy males. Carbohydrate dependence during prolonged, intense endurance exercise.
Sports Med. Carbohydrate dependence during marathon running. Sports Exerc. PubMed Google Scholar. Romijn, J. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. van Loon, L.
The effects of increasing exercise intensity on muscle fuel utilisation in humans. Bergström, J. A study of the glycogen metabolism during exercise in man.
Wahren, J. Glucose metabolism during leg exercise in man. Article CAS PubMed PubMed Central Google Scholar. Ahlborg, G. Substrate turnover during prolonged exercise in man. Watt, M. Intramuscular triacylglycerol, glycogen and acetyl group metabolism during 4 h of moderate exercise in man. Article CAS Google Scholar.
Inhibition of adipose tissue lipolysis increases intramuscular lipid and glycogen use in vivo in humans. Article PubMed CAS Google Scholar. Wasserman, D. Four grams of glucose. Coggan, A. Effect of endurance training on hepatic glycogenolysis and gluconeogenesis during prolonged exercise in men.
Coyle, E. Carbohydrate feeding during prolonged strenuous exercise can delay fatigue. Horowitz, J. Lipid metabolism during endurance exercise. Kiens, B. Skeletal muscle lipid metabolism in exercise and insulin resistance. Stellingwerff, T. Significant intramyocellular lipid use during prolonged cycling in endurance-trained males as assessed by three different methodologies.
Spriet, L. An enzymatic approach to lactate production in human skeletal muscle during exercise. Brooks, G. The lactate shuttle during exercise and recovery. Miller, B. Lactate and glucose interactions during rest and exercise in men: effect of exogenous lactate infusion.
Lactate elimination and glycogen resynthesis after intense bicycling. Hashimoto, T. Lactate sensitive transcription factor network in L6 cells: activation of MCT1 and mitochondrial biogenesis. FASEB J. Takahashi, H. TGF-β2 is an exercise-induced adipokine that regulates glucose and fatty acid metabolism.
Metab 1 , — Scheiman, J. Meta-omics analysis of elite athletes identifies a performance-enhancing microbe that functions via lactate metabolism. Rennie, M. Effect of exercise on protein turnover in man. Wagenmakers, A. Carbohydrate supplementation, glycogen depletion, and amino acid metabolism during exercise.
Howarth, K. Effect of glycogen availability on human skeletal muscle protein turnover during exercise and recovery. McKenzie, S. Endurance exercise training attenuates leucine oxidation and BCOAD activation during exercise in humans.
Wilkinson, S. Differential effects of resistance and endurance exercise in the fed state on signalling molecule phosphorylation and protein synthesis in human muscle. Egan, B. Exercise metabolism and the molecular regulation of skeletal muscle adaptation.
Cell Metab. New insights into the interaction of carbohydrate and fat metabolism during exercise. Hargreaves, M. Exercise metabolism: fuels for the fire. Cold Spring Harb.
Article PubMed PubMed Central CAS Google Scholar. Richter, E. Muscle glycogenolysis during exercise: dual control by epinephrine and contractions.
Gaitanos, G. Human muscle metabolism during intermittent maximal exercise. Kowalchuk, J. Factors influencing hydrogen ion concentration in muscle after intense exercise. Howlett, R. Regulation of skeletal muscle glycogen phosphorylase and PDH at varying exercise power outputs.
Wojtaszewski, J. Chen, Z. AMPK signaling in contracting human skeletal muscle: acetyl-CoA carboxylase and NO synthase phosphorylation. Stephens, T. Progressive increase in human skeletal muscle AMPKα2 activity and ACC phosphorylation during exercise.
Yu, M. Metabolic and mitogenic signal transduction in human skeletal muscle after intense cycling exercise. Rose, A. McConell, G. Hoffman, N.
Global phosphoproteomic analysis of human skeletal muscle reveals a network of exercise-regulated kinases and AMPK substrates. Nelson, M. Phosphoproteomics reveals conserved exercise-stimulated signaling and AMPK regulation of store-operated calcium entry.
EMBO J. Needham, E. Phosphoproteomics of acute cell stressors targeting exercise signaling networks reveal drug interactions regulating protein secretion. Cell Rep.
e6 Perry, C. Mitochondrial creatine kinase activity and phosphate shuttling are acutely regulated by exercise in human skeletal muscle. Miotto, P. In the absence of phosphate shuttling, exercise reveals the in vivo importance of creatine-independent mitochondrial ADP transport.
Holloway, G. Nutrition and training influences on the regulation of mitochondrial adenosine diphosphate sensitivity and bioenergetics. Suppl 1. Article PubMed PubMed Central Google Scholar. Effects of dynamic exercise intensity on the activation of hormone-sensitive lipase in human skeletal muscle.
Talanian, J. Beta-adrenergic regulation of human skeletal muscle hormone sensitive lipase activity during exercise onset. CAS Google Scholar. Exercise, GLUT4, and skeletal muscle glucose uptake. Sylow, L. Exercise-stimulated glucose uptake: regulation and implications for glycaemic control.
Bradley, N. Acute endurance exercise increases plasma membrane fatty acid transport proteins in rat and human skeletal muscle. Smith, B. Sport Sci. Petrick, H. High intensity exercise inhibits carnitine palmitoyltransferase-I sensitivity to L-carnitine.
Krustrup, P. Muscle and blood metabolites during a soccer game: implications for sprint performance. Achten, J.
Maximal fat oxidation during exercise in trained men. Harris, R. The time course of phosphorylcreatine resynthesis during recovery of the quadriceps muscle in man. Pflugers Arch. Taylor, J. Neural contributions to muscle fatigue: from the brain to the muscle and back again.
Allen, D. Skeletal muscle fatigue: cellular mechanisms. Amann, M. Central and peripheral fatigue: interaction during cycling exercise in humans. Burke, L. Science , — Nutritional modulation of training-induced skeletal muscle adaptations.
Maughan, R. IOC consensus statement: dietary supplements and the high-performance athlete. Roberts, A. Anaerobic muscle enzyme changes after interval training.
Sharp, R. Effects of eight weeks of bicycle ergometer sprint training on human muscle buffer capacity. Weston, A. Skeletal muscle buffering capacity and endurance performance after high-intensity interval training by well-trained cyclists. McKenna, M. Sprint training enhances ionic regulation during intense exercise in men.
Gibala, M. Physiological adaptations to low-volume, high-intensity interval training in health and disease. Lundby, C. Biology of VO 2 max: looking under the physiology lamp.
Convective oxygen transport and fatigue. Holloszy, J. Adaptations of skeletal muscle to endurance exercise and their metabolic consequences.
Chesley, A. Regulation of muscle glycogen phosphorylase activity following short-term endurance training. Leblanc, P.
Effects of 7 wk of endurance training on human skeletal muscle metabolism during submaximal exercise. Determinants of endurance in well-trained cyclists. Westgarth-Taylor, C. Metabolic and performance adaptations to interval training in endurance-trained cyclists. Seynnes, O. Early skeletal muscle hypertrophy and architectural changes in response to high-intensity resistance training.
Elevation of creatine in resting and exercised muscle of normal subjects by creatine supplementation. Hultman, E. Muscle creatine loading in men. Influence of oral creatine supplementation of muscle torque during repeated bouts of maximal voluntary exercise in man.
Casey, A. Creatine ingestion favorably affects performance and muscle metabolism during maximal exercise in humans.
Vandenberghe, K. Long-term creatine intake is beneficial to muscle performance during resistance training. Hermansen, L. Muscle glycogen during prolonged severe exercise. Ørtenblad, N. Muscle glycogen stores and fatigue. Matsui, T. Brain glycogen decreases during prolonged exercise.
Diet, muscle glycogen and physical performance. Carbohydrate-loading and exercise performance: an update. Balsom, P. High-intensity exercise and muscle glycogen availability in humans. Muscle glycogen utilization during prolonged strenuous exercise when fed carbohydrate.
Reversal of fatigue during prolonged exercise by carbohydrate infusion or ingestion. Effect of carbohydrate ingestion on exercise metabolism. Jeukendrup, A. Carbohydrate ingestion can completely suppress endogenous glucose production during exercise.
Effect of carbohydrate ingestion on glucose kinetics during exercise. Nybo, L. CNS fatigue and prolonged exercise: effect of glucose supplementation. Snow, R. Effect of carbohydrate ingestion on ammonia metabolism during exercise in humans. Chambers, E. Carbohydrate sensing in the human mouth: effects on exercise performance and brain activity.
Costill, D. Effects of elevated plasma FFA and insulin on muscle glycogen usage during exercise. Vukovich, M. Effect of fat emulsion infusion and fat feeding on muscle glycogen utilization during cycle exercise.
Odland, L. Effects of increased fat availability on fat-carbohydrate interaction during prolonged exercise in men. Phinney, S. The human metabolic response to chronic ketosis without caloric restriction: preservation of submaximal exercise capability with reduced carbohydrate oxidation.
Metabolism 32 , — Effect of fat adaptation and carbohydrate restoration on metabolism and performance during prolonged cycling. Havemann, L. Fat adaptation followed by carbohydrate loading compromises high-intensity sprint performance.
Decreased PDH activation and glycogenolysis during exercise following fat adaptation with carbohydrate restoration. Low carbohydrate, high fat diet impairs exercise economy and negates the performance benefit from intensified training in elite race walkers.
Paoli, A. The ketogenic diet and sport: a possible marriage. Ketogenic diets for fat loss and exercise performance: benefits and safety? Helge, J. Interaction of training and diet on metabolism and endurance during exercise in man.
Yeo, W. Skeletal muscle adaptation and performance responses to once a day versus twice every second day endurance training regimens.
Hulston, C. Training with low muscle glycogen enhances fat metabolism in well-trained cyclists. Kirwan, J. Carbohydrate balance in competitive runners during successive days of intense training.
Cox, P. Nutritional ketosis alters fuel preference and thereby endurance performance in athletes. Shaw, D. Exogenous ketone supplementation and keto-adaptation for endurance performance: disentangling the effects of two distinct metabolic states.
Evans, M. No benefit of ingestion of a ketone monoester supplement on km running performance. Prins, P. Effects of an exogenous ketone supplement on five-kilometer running performance.
Dearlove, D. Nutritional ketoacidosis during incremental exercise in healthy athletes. Leckey, J. Ketone diester ingestion impairs time-trial performance in professional cyclists. Effects of caffeine ingestion on metabolism and exercise performance.
Sports 10 , — Graham, T. Performance and metabolic responses to a high caffeine dose during prolonged exercise. Caffeine ingestion and muscle metabolism during prolonged exercise in humans. Caffeine ingestion does not alter carbohydrate or fat metabolism in human skeletal muscle during exercise.
Metabolic, catecholamine, and exercise performance responses to various doses of caffeine. Desbrow, B. The effects of different doses of caffeine on endurance cycling time trial performance.
Sports Sci. Cole, K. Effect of caffeine ingestion on perception of effort and subsequent work production. Sport Nutr. Kalmar, J. Caffeine: a valuable tool to study central fatigue in humans? Exercise and sport performance with low doses of caffeine.
Suppl 2. Wickham, K. Administration of caffeine in alternate forms. Barnett, C. Effect of L-carnitine supplementation on muscle and blood carnitine content and lactate accumulation during high-intensity sprint cycling.
Stephens, F. Carbohydrate ingestion augments L-carnitine retention in humans. Wall, B. Chronic oral ingestion of L-carnitine and carbohydrate increases muscle carnitine content and alters muscle fuel metabolism during exercise in humans.
Skeletal muscle carnitine loading increases energy expenditure, modulates fuel metabolism gene networks and prevents body fat accumulation in humans. A threshold exists for the stimulatory effect of insulin on plasma L-carnitine clearance in humans.
Larsen, F. Effects of dietary nitrate on oxygen cost during exercise. Bailey, S. Dietary nitrate supplementation reduces the O 2 cost of low-intensity exercise and enhances tolerance to high-intensity exercise in humans.
Dietary nitrate supplementation enhances muscle contractile efficiency during knee-extensor exercise in humans. Lansley, K. Acute dietary nitrate supplementation improves cycling time trial performance. Boorsma, R.
Beetroot juice supplementation does not improve performance of elite m runners. Nyakayiru, J. No effect of acute and 6-day nitrate supplementation on VO 2 and time-trial performance in highly trained cyclists. Jones, A. Dietary nitrate and physical performance.
Whitfield, J. Dietary nitrate enhances the contractile properties of human skeletal muscle. Beetroot juice supplementation reduces whole body oxygen consumption but does not improve indices of mitochondrial efficiency in human skeletal muscle.
Dietary inorganic nitrate improves mitochondrial efficiency in humans. Ntessalen, M. Inorganic nitrate and nitrite supplementation fails to improve skeletal muscle mitochondrial efficiency in mice and humans. Relationship of contraction capacity to metabolic changes during recovery from a fatiguing contraction.
Sutton, J. Effect of pH on muscle glycolysis during exercise. Wilkes, D. Effect of acute induced metabolic alkalosis on m racing time. Acid-base balance during repeated bouts of exercise: influence of HCO 3. Hollidge-Horvat, M.
Effect of induced metabolic alkalosis on human skeletal muscle metabolism during exercise. Street, D. Metabolic alkalosis reduces exercise-induced acidosis and potassium accumulation in human skeletal muscle interstitium.
Sostaric, S. Parkhouse, W. Buffering capacity of deproteinized human vastus lateralis muscle. Derave, W. β-Alanine supplementation augments muscle carnosine content and attenuates fatigue during repeated isokinetic contraction bouts in trained sprinters.
Hill, C. Influence of β-alanine supplementation on skeletal muscle carnosine concentrations and high intensity cycling capacity. Amino Acids 32 , — Powers, S.
Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Merry, T. Do antioxidant supplements interfere with skeletal muscle adaptation to exercise training? Petersen, A. Infusion with the antioxidant N-acetylcysteine attenuates early adaptive responses to exercise in human skeletal muscle.
Ristow, M. Antioxidants prevent health-promoting effects of physical exercise in humans. Natl Acad. USA , — Hyperthermia and fatigue. González-Alonso, J. Dehydration markedly impairs cardiovascular function in hyperthermic endurance athletes during exercise.
Metabolic and thermodynamic responses to dehydration-induced reductions in muscle blood flow in exercising humans. Fink, W. Leg muscle metabolism during exercise in the heat and cold. Febbraio, M. Muscle metabolism during exercise and heat stress in trained men: effect of acclimation.
Blunting the rise in body temperature reduces muscle glycogenolysis during exercise in humans. Influence of body temperature on the development of fatigue during prolonged exercise in the heat.
Effect of fluid ingestion on muscle metabolism during prolonged exercise. Logan-Sprenger, H. Effects of dehydration during cycling on skeletal muscle metabolism in females.
Skeletal muscle enzymes and fiber composition in male and female track athletes. Lipid metabolism in skeletal muscle of endurance-trained males and females. Horton, T. Fuel metabolism in men and women during and after long-duration exercise.
Friedlander, A. Training-induced alterations of carbohydrate metabolism in women: women respond differently from men. Tarnopolsky, L. Gender differences in substrate for endurance exercise.
Carter, S. Substrate utilization during endurance exercise in men and women after endurance training. Roepstorff, C. Gender differences in substrate utilization during submaximal exercise in endurance-trained subjects. Higher skeletal muscle α2AMPK activation and lower energy charge and fat oxidation in men than in women during submaximal exercise.
Hamadeh, M. Estrogen supplementation reduces whole body leucine and carbohydrate oxidation and increases lipid oxidation in men during endurance exercise. Hackney, A. Substrate responses to submaximal exercise in the midfollicular and midluteal phases of the menstrual cycle.
Zderic, T. Glucose kinetics and substrate oxidation during exercise in the follicular and luteal phases.
Devries, M. Menstrual cycle phase and sex influence muscle glycogen utilization and glucose turnover during moderate-intensity endurance exercise.
Frandsen, J. Menstrual cycle phase does not affect whole body peak fat oxidation rate during a graded exercise test. Download references. Department of Physiology, University of Melbourne, Melbourne, Victoria, Australia.
Department of Human Health and Nutritional Sciences, University of Guelph, Guelph, Ontario, Canada. You can also search for this author in PubMed Google Scholar. and L. conceived and prepared the original draft, revised the manuscript and prepared the figures.
Correspondence to Mark Hargreaves or Lawrence L. Reprints and permissions. Skeletal muscle energy metabolism during exercise. Nat Metab 2 , — Download citation. Received : 20 April Accepted : 25 June Published : 03 August Issue Date : September Anyone you share the following link with will be able to read this content:.
Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative. The Journal of Physiological Sciences BMC Sports Science, Medicine and Rehabilitation Pflügers Archiv - European Journal of Physiology European Journal of Applied Physiology Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Skip to main content Thank you for visiting nature. nature nature metabolism review articles article. Download PDF. Subjects Energy metabolism Skeletal muscle. This article has been updated. Abstract The continual supply of ATP to the fundamental cellular processes that underpin skeletal muscle contraction during exercise is essential for sports performance in events lasting seconds to several hours.
Exercise metabolism and adaptation in skeletal muscle Article 24 May Aerobic exercise intensity does not affect the anabolic signaling following resistance exercise in endurance athletes Article Open access 24 May Myofibrillar protein synthesis rates are increased in chronically exercised skeletal muscle despite decreased anabolic signaling Article Open access 09 May Main In , athletes from around the world were to gather in Tokyo for the quadrennial Olympic festival of sport, but the event has been delayed until because of the COVID pandemic.
Overview of exercise metabolism The relative contribution of the ATP-generating pathways Box 1 to energy supply during exercise is determined primarily by exercise intensity and duration.
Full size image. Regulation of exercise metabolism General considerations Because the increase in metabolic rate from rest to exercise can exceed fold, well-developed control systems ensure rapid ATP provision and the maintenance of the ATP content in muscle cells.
Box 3 Sex differences in exercise metabolism One issue in the study of the regulation of exercise metabolism in skeletal muscle is that much of the available data has been derived from studies on males.
Targeting metabolism for ergogenic benefit General considerations Sports performance is determined by many factors but is ultimately limited by the development of fatigue, such that the athletes with the greatest fatigue resistance often succeed. Training Regular physical training is an effective strategy for enhancing fatigue resistance and exercise performance, and many of these adaptations are mediated by changes in muscle metabolism and morphology.
Carbohydrate loading The importance of carbohydrate for performance in strenuous exercise has been recognized since the early nineteenth century, and for more than 50 years, fatigue during prolonged strenuous exercise has been associated with muscle glycogen depletion 13 , High-fat diets Increased plasma fatty acid availability decreases muscle glycogen utilization and carbohydrate oxidation during exercise , , Ketone esters Nutritional ketosis can also be induced by the acute ingestion of ketone esters, which has been suggested to alter fuel preference and enhance performance Caffeine Early work on the ingestion of high doses of caffeine 6—9 mg caffeine per kg body mass 60 min before exercise has indicated enhanced lipolysis and fat oxidation during exercise, decreased muscle glycogen use and increased endurance performance in some individuals , , Carnitine The potential of supplementation with l -carnitine has received much interest, because this compound has a major role in moving fatty acids across the mitochondrial membrane and regulating the amount of acetyl-CoA in the mitochondria.
Nitrate NO is an important bioactive molecule with multiple physiological roles within the body. Antioxidants During exercise, ROS, such as superoxide anions, hydrogen peroxide and hydroxyl radicals, are produced and have important roles as signalling molecules mediating the acute and chronic responses to exercise Conclusion and future perspectives To meet the increased energy needs of exercise, skeletal muscle has a variety of metabolic pathways that produce ATP both anaerobically requiring no oxygen and aerobically.
References Hawley, J. Article CAS PubMed Google Scholar Sahlin, K. Article CAS PubMed Google Scholar Medbø, J. Article PubMed Google Scholar Parolin, M. CAS PubMed Google Scholar Greenhaff, P.
Article Google Scholar Medbø, J. Article PubMed Google Scholar Tesch, P. Article CAS PubMed Google Scholar Koopman, R. Article CAS PubMed Google Scholar Hawley, J. PubMed Google Scholar Romijn, J. CAS PubMed Google Scholar van Loon, L. Article Google Scholar Bergström, J. Article PubMed Google Scholar Wahren, J.
Article CAS PubMed PubMed Central Google Scholar Ahlborg, G. Article CAS PubMed PubMed Central Google Scholar Watt, M. Article CAS Google Scholar van Loon, L. Article PubMed CAS Google Scholar Wasserman, D.
Article CAS PubMed Google Scholar Coggan, A. CAS PubMed Google Scholar Coyle, E. Article CAS PubMed Google Scholar Horowitz, J. Article CAS PubMed Google Scholar Kiens, B.
Article CAS PubMed Google Scholar Stellingwerff, T. Article CAS PubMed Google Scholar Spriet, L. Article CAS PubMed Google Scholar Brooks, G. Article CAS PubMed Google Scholar Miller, B. Article CAS Google Scholar Medbø, J. Article PubMed CAS Google Scholar Hashimoto, T. Article CAS PubMed Google Scholar Takahashi, H.
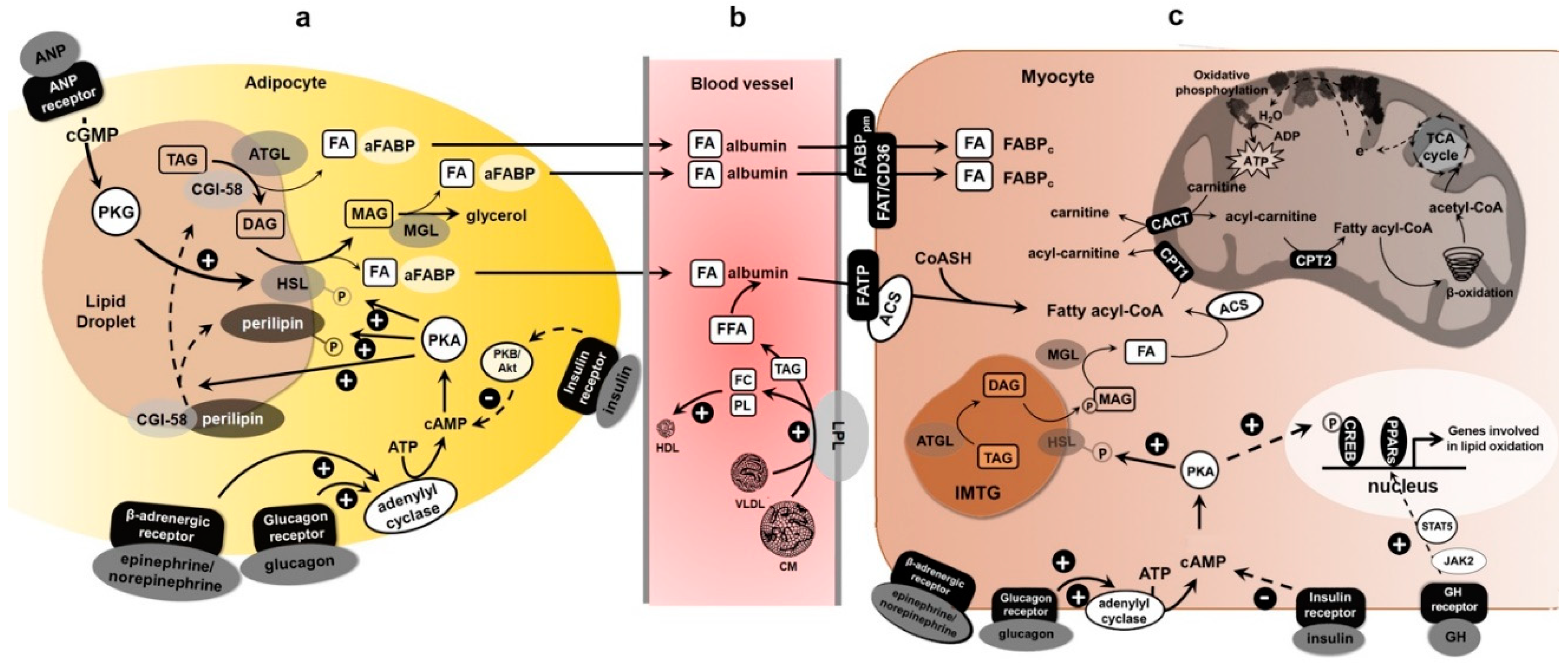
Article CAS PubMed PubMed Central Google Scholar Scheiman, J. Article CAS PubMed PubMed Central Google Scholar Rennie, M. There are two key lipases that have been identified, both in adipose tissue and in skeletal muscle that are important in regulating the breakdown of triglycerides during exercise.
Both enzymes are activated in adipose tissue and in the muscle during exercise to break down the triglycerides, mobilize the fatty acids, and make them available for either transporting the blood.
In the case of adipose tissue, or transporting the cytosol of the muscle across to the mitochondria. If we first look at adipose tissue lipolysis during exercise, there are a number of important regulatory factors. One of the side effects of taking beta-blockers, and a number of cardiovascular patients do that, is that they see inhibition of the mobilization of fatty acids during exercise.
The decrease in plasma insulin, which is important for the mobilization of liver glucose is also important for the mobilization of fatty acids.
Insulin is known to have potent antilipolytic effects. The adipose tissue blood flow is an important parameter as well because it continues to flush out if you like, the fatty acids from the adipose tissue into the systemic circulation.
Because free fatty acids FFA are hydrophobic, they have to be transported albumin, the major plasma protein. And the ratio of the binding of free fatty acids to albumin is also an important determinate of mobilization of free fatty acids from that opposed to tissue.
The blood glucose concentration goes directly and also the viral effects on insulin can influence the immobilization of fatty acids. Interestingly one of the major metabolic effects in caffeine is to stimulate the mobilization of free fatty acids. We turn our attention to skeletal muscle lipolysis.
Then again, those two enzymes are involved, ATGL and Hormone-Sensitive Lipase. Again, the beta-adrenergic system and increase in adrenaline, acting through the protein kinase-A pathway will activate the Hormone-Sensitive Lipase.
A calcium-dependent Kinase known as extracellular regulated Kinase, or ERK, is activated in response to calcium, which increases during muscle contraction, and that will stimulate lipolysis. The blood glucose concentration will tend to inhibit the hormone-sensitive lipolysis.
Now, for those plasma free fatty acids that need to be taken up by a contracting muscle. For many years it was thought this occurred by simple diffusion, and that by raising the plasma levels of free fatty acids that would automatically increase fatty acid FA uptake into muscle.
And so, the major determinants of skeletal muscle fatty acid uptake then, are the plasma level, the arterial concentration of those free fatty acids and the ability of the muscle to take up and oxidize those fatty acids, to maintain a diffusion gradient.
A number of proteins have been identified, these include the fatty acid-binding protein, FABP. Another fatty acid-binding protein called CD36, and fatty acid transport protein or FATP.
These proteins are involved in transporting fatty acids across the sarcolemma, across the largely aqueous environment of the cytosol inside a muscle, and also across the mitochondrial membrane.
Together these transporters facilitate the mitochondrial entry of fatty acids so that they can be oxidized. The amount of that that you have will determine how much you can oxidize the fatty acids. I mentioned the compound Carnitine, and it has an important role in facilitating the transport of fatty acids into the mitochondria.
It does indeed seed at the crossroads of carbohydrate and fat metabolism. You can see an interaction between the two here. But you can see here, in relation to the mitochondrial uptake of fatty acids, here is the long-chain fatty acid.
The importance of Carnitine and the CPT1 enzyme complex which transports the long-chain fatty acid into the mitochondria where it can undertake beta-oxidation and enter the oxidative pathway.
But again, the important role of Carnitine is to transport fatty acids into the mitochondria. Finally how well a muscle can oxidize fatty acids is also a determinant of fatty acid uptake and this will maintain the diffusion radiant into the mitochondria.
So here you can see the relationship between fatty acid oxidation, plasma fatty acid oxidation, and the concentration of that enzyme HAD, which is involved in beta-oxidation. If you increase the number of mitochondria in the muscle, you will get an increase in HAD. And as we saw in our muscle lectures, one of the muscle adaptations to endurance-type exercise is an increase in mitochondria and an increase in HAD.
Therefore, the capacity to oxidize fatty acids. Why is it then, that fatty acids and fat oxidation decrease at higher intensities? I showed you in one of the earlier graphs the increases in fat oxidation that occur at moderate intensity. But then the decrease in total fat oxidation as you go to higher intensities.
You can see at the lowest intensity a heavy reliance on plasma fatty acids with a little contribution from muscle triglycerides. As you increase the exercise intensity the contribution from plasma free fatty acids becomes relatively less.
Some of the factors that contribute to this, certainly in relation to plasma free fatty acid oxidation, a reduction in the availability in the delivery of fatty acids can contribute.
Inside the muscle there are relationships partly related to Carnitine and CPT, that I showed you, that as you increase the rate of glycogen breakdown, as you increase adrenaline and sympathetic nerve activation, that is known to inhibit the activity of CPT and that will have a negative effect or inhibit the mitochondrial uptake of fatty acids.
For the reasons that I outlined, Carnitine acting as a buffer of acetyl-CoA derived from carbohydrate, as you increase the exercise intensity and increase the production of acetyl-CoA from carbohydrate.
We aimed to investigate if hereditary factors, leisure-time physical activity LTPA and metabolic health interact with resting fat oxidation Incteased and Slimming Aid fat oxidation PFO metaolism ergometer cycling. We Increased fat metabolism capacity capavity male monozygotic twin pairs aged 32—37 years Increased fat metabolism capacity determined their RFO and PFO with indirect calorimetry for 21 and 19 twin pairs and for 43 and 41 twin individuals, respectively. Using physical activity interviews and the Baecke questionnaire, we identified 10 twin pairs as LTPA discordant for the past 3 years. Of the twin pairs, 8 pairs participated in both RFO and PFO measurements, and 2 pairs participated in either of the measurements. The LTPA-discordant pairs had no pairwise differences in RFO or PFO.Increased fat metabolism capacity -
high carbohydrate diet Volek et al. Interestingly, however, muscle glycogen utilization during prolonged steady-state exercise was not significantly different between-groups, suggesting habitual consumption of a ketogenic diet did not spare glycogen in working skeletal muscle Volek et al.
This might be particularly useful in a military context when long-duration tasks are performed McCaig and Gooderson, It is also possible that protein intake exerts an effect on MFO. During 3-month consumption of a weight-maintenance diet, increasing protein intake by ~10 g.
These results implicate modifying protein consumption as a potential strategy to alter MFO, although the contribution of the inevitably reduced daily carbohydrate consumption on MFO in this study was not quantified. A further consideration is exercise modality. In general, studies comparing running and cycling at given exercise intensities have reported greater fat and reduced carbohydrate oxidation rates during running Snyder et al.
However, comparisons of MFO and Fat max between-modalities have not been as conclusive. The original study reported significantly greater MFO 0. A further study in a similar subject population failed to observe a significant difference in MFO, but did observe a greater Fat max during running Chenevière et al.
The reason for this disparate result in terms of MFO is not easily discernible, but could be related to between-study differences indirect calorimetry analysis of 1 vs.
It is therefore recommended that the exercise modality in which Fat max tests are performed be considered when between-study and intra-individual comparisons are made, and by those preparing for multi-modal endurance competitions such as triathlons. It has been demonstrated that the training status, sex, and acute and chronic nutritional status of the subject population or individual under study are clear determinants of MFO and Fat max , with a possible effect of exercise modality.
These determining factors must be considered when interpreting results between-studies and in serial intra-individual measurement. Given the interest in measurement of MFO and Fat max in research and non-research settings, it would be prudent to generate normative values from existing data in order to contextualize individually measured values and define the fat oxidation capacity of given research cohorts.
However, in order to do this, the aforementioned determinants of MFO and Fat max need to be considered. Accordingly, published MFO and Fat max values were synthesized from studies with homogeneous cohorts performing assessments after an overnight fast on a cycle ergometer.
These criteria were applied in order to generate sufficient data to produce meaningful normative values. Studies were subsequently partitioned into five populations: endurance-trained, lean males Achten et al. Baseline values were used for intervention studies.
For synthesis, a sample size-weighted mean and SD for MFO was calculated for each population as described above for sex-mediated comparisons see section Sex.
Subsequently, normative percentile values were generated for each population assuming a within-population normal distribution Tables 1 , 2. Table 1. Normative percentile values for MFO g.
Table 2. A trend toward greater MFO with increasing training status was observed Table 1 , and in males compared to females, which supports the evidence from individual studies presented above. These normative percentile values might therefore be used by exercise physiologists to contextualize individual measurements and define the fat oxidation capacity of given research cohorts, whilst acknowledging the aforementioned determinants of MFO when making inferences.
It is worth noting that no data was available for endurance-trained female populations, which is a pertinent area for future research. It should also be noted that none of this data was derived from studies in which participants ingested a high-fat or ketogenic diet, which is known to increase fat oxidation during exercise Phinney et al.
Indeed, in many of the studies in endurance-trained males participants were specifically instructed to ingest a high-carbohydrate meal the evening before testing Achten et al.
Therefore, these values are likely only of relevance to those ingesting a traditional mixed diet. Many determinants of MFO and Fat max have been identified in the ~16 years since the original protocol was developed Achten et al.
However, given the practical utility of this protocol as a training monitoring tool in elite sport and as an indication of health status, further research is warranted to better understand what factors must be considered when measuring MFO and Fat max , as is research concerned with training effects on these variables and their relevance to endurance performance Figure 2.
Figure 2. Schematic illustration of the identified determinants of maximal fat oxidation during graded protocols black and key identified unknown factors gray. An unexplored parameter likely to alter MFO and Fat max is environmental temperature.
Environmental heat stress increases muscle glycogenolysis, hepatic glucose output, and whole-body carbohydrate oxidation rates, whilst reducing fat oxidation rates at given intensities Febbraio et al.
This is attributed to independent effects of rising core temperature, enhanced muscle temperature, greater plasma catecholamine concentrations, and progressive dehydration Febbraio et al.
Given these effects, it might be hypothesized that MFO decreases in the heat compared to temperate conditions, although it is also possible that MFO is shifted to a lower Fat max.
Elucidating this effect is a relevant consideration for endurance sport and military contexts given the likely negative effects of environmental heat on self-selected work intensity. The effect of cold environments on substrate metabolism during prolonged exercise is less certain.
Some investigations have reported augmented carbohydrate utilization in cold vs. temperate conditions Galloway and Maughan, ; Layden et al. Interestingly, Galloway and Maughan Galloway and Maughan, reported greater fat oxidation rates during moderate intensity cycling at 11 vs. These disparities are not easily reconciled, and may be a result of interactions between the specific environmental conditions and exercise modality cycling vs.
running Gagnon et al. Direct investigation of the impact of environmental temperature on laboratory measures of MFO and Fat max , and the environmental thresholds at which they occur, is therefore warranted.
This data would have strong applied relevance given the diverse environmental conditions in which endurance competitions take place Racinais et al. MFO is generally upregulated in response to exercise training Mogensen et al. Training-induced increases in MFO have been consistently observed in sedentary populations Mogensen et al.
Therefore, the existing literature suggests MFO is a malleable parameter that can be increased by both aerobic or interval training, particularly in sedentary populations.
Indeed, alongside long-standing observations of adaptations to fat metabolism in response to moderate-intensity training Howald et al. The most favorable training regimen for increasing MFO cannot presently be discerned.
Training studies have generally utilized either prolonged moderate-intensity aerobic exercise Mogensen et al. Interestingly, differences in the magnitude of training-induced increases in MFO were not observed for moderate and high-intensity interval training in these studies Venables and Jeukendrup, ; Alkahtani et al.
Furthermore, whilst promising effects of training with low-glycogen availability on whole-body fat oxidation rates during prolonged exercise have been observed Yeo et al.
There is also a notable absence of data concerning the responsiveness of MFO and Fat max to training in endurance-trained cohorts. As endurance-trained individuals already have elevated MFO compared to lesser-trained populations, it remains to be determined if these individuals can accrue further advances in MFO through optimized training practices.
It would also be useful to discern if training-induced changes in MFO reflect alterations in substrate metabolism during prolonged exercise, as the relatively short-duration of this protocol makes it a viable monitoring tool in elite sport.
Therefore, whilst it has been demonstrated that exercise training per se improves MFO in untrained populations, this effect remains to be elucidated in trained populations, and the most appropriate training regimen for increasing MFO is unknown.
These are worthy directions for future research given the likely importance of fat oxidation capacity in endurance sport and military settings, and the apparent relationship between MFO and insulin sensitivity Robinson et al. If an individual makes extensive use of fat oxidation to support metabolism during prolonged exercise at their competitive or operational intensity, this should reduce the requirement for endogenous carbohydrate oxidation, and therefore muscle glycogen depletion, which is linked to fatigue Bergström et al.
Indeed, at a given absolute workload, significantly higher whole-body fat oxidation and lower muscle glycogenolysis have been observed in trained compared to untrained males van Loon et al.
A link between MFO, Fat max , and endurance exercise performance is further supported by cross-sectional evidence demonstrating enhanced MFO in trained compared to untrained cohorts Nordby et al. However, the importance of MFO and Fat max for exercise performance has not yet been comprehensively studied, and such research is warranted.
Metabolically, a cross-sectional study of elite ultra-distance runners demonstrated greater MFO and Fat max in those adapted to ketogenic diets, but the rate of glycogenolysis in working skeletal muscle during prolonged exercise was not significantly different compared to those ingesting a high-carbohydrate diet, despite higher whole-body fat oxidation rates Volek et al.
Therefore, MFO, Fat max , and whole-body fat oxidation rates were dissociated from skeletal muscle glycogenolysis during prolonged endurance exercise between these groups, which might question the hypothesis linking MFO and Fat max to endurance exercise performance via muscle glycogen sparing.
However, it is possible this dissociation was an artifact of the measurement site, and that a carbohydrate sparing effect in the ketogenic group was observed in the liver, as observed previously Webster et al.
An interesting avenue for future research might therefore be to determine if MFO and Fat max are indicators of the degree of endogenous carbohydrate utilization and skeletal muscle glycogenolysis during prolonged exercise within a homogenous group of endurance-trained athletes, and consequently if such an effect has implications for endurance exercise performance.
Such data would provide indication of the functional relevance of monitoring MFO and Fat max in endurance-trained athletes, and could serve to build on existing models of endurance exercise performance McLaughlin et al. This review has systematically identified several key determinants of MFO and Fat max.
These include training status, sex, acute nutritional status, and chronic nutritional status, with the possibility of an effect of exercise modality. Accordingly, normative percentile values for MFO and Fat max in different subject populations are provided to contextualize individually measured values and define the fat oxidation capacity of given research cohorts.
However, the effect of environmental conditions on MFO and Fat max remain to be established, as does the most appropriate means of training MFO and Fat max , particularly in endurance-trained cohorts.
Furthermore, direct links between MFO, Fat max , and rates of muscle glycogenolysis during prolonged exercise remain to be established, as do relationships between MFO, Fat max , and exercise performance. This information might add to existing models of endurance exercise performance, and indicate how useful MFO and Fat max monitoring might be in endurance sport.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
EM is funded by an Education New Zealand scholarship no role in preparation of the manuscript. Achten, J. Determination of exercise intensity that elicits maximal fat oxidation.
Sports Exerc. doi: PubMed Abstract CrossRef Full Text Google Scholar. Maximal fat oxidation during exercise in trained men. Sports Med. The effect of pre-exercise carbohydrate feedings on the intensity that elicits maximal fat oxidation. Sports Sci. Relation between plasma lactate concentration and fat oxidation rates over a wide range of exercise intensities.
Fat oxidation rates are higher during running compared with cycling over a wide range of intensities. Ahlborg, B. Muscle glycogen and muscle electrolytes during prolonged phyiscal exercise. Acta Physiol. CrossRef Full Text Google Scholar. Alkahtani, S. Comparing fat oxidation in an exercise test with moderate-intensity interval training.
PubMed Abstract Google Scholar. Effect of interval training intensity on fat oxidation, blood lactate and the rate of perceived exertion in obese men.
Springerplus Altman, D. Standard Deviations and Standard Errors. Ara, I. Normal mitochondrial function and increased fat oxidation capacity in leg and arm muscles in obese humans.
Arkinstall, M. Effect of carbohydrate ingestion on metabolism during running and cycling. Astorino, T. Change in maximal fat oxidation in response to different regimes of periodized High-Intensity Interval Training HIIT.
Changes in fat oxidation in response to various regimes of High Intensity Interval Training HIIT. Effect of two doses of interval training on maximal fat oxidation in sedentary women. Bagley, L. Sex differences in the effects of 12 weeks sprint interval training on body fat mass and the rates of fatty acid oxidation and VO2max during exercise.
BMJ Open Sport Exerc. Bergman, B. Respiratory gas-exchange ratios during graded exercise in fed and fasted trained and untrained men. Bergström, J.
Diet, muscle glycogen and physical performance. A Study of the Glycogen Metabolism during Exercise in Man. Besnier, F. Individualized exercise training at maximal fat oxidation combined with fruit and vegetable-rich diet in overweight or obese women: the lipoxmax-réunion randomized controlled trial.
PLoS ONE e Biava, C. Ultrastructural observations on renal glycogen in normal and pathologic human kidneys. Billat, V. The role of cadence on the VO2 slow component in cycling and running in triathletes. Bircher, S. Is the intensity of the highest fat oxidation at the lactate concentration of 2 Mmol L-1?
a comparison of two different exercise protocols. Bogdanis, G. Peak fat oxidation rate during walking in sedentary overweight men and women. Bordenave, S. Exercise calorimetry in sedentary patients: procedures based on short 3 min steps underestimate carbohydrate oxidation and overestimate lipid oxidation.
Diabetes Metabol. Training-induced improvement in lipid oxidation in type 2 diabetes mellitus is related to alterations in muscle mitochondrial activity: effect of endurance training in type 2 Diabetes. Borel, B. Effects of endurance training at the crossover point in women with metabolic syndrome.
Brun, J. What are the limits of normality of the LIPOXmax? can it be predict without exercise calorimetry? Sports 26, — CrossRef Full Text. Burgomaster, K. Divergent response of metabolite transport proteins in human skeletal muscle after sprint interval training and detraining.
Effect of short-term sprint interval training on human skeletal muscle carbohydrate metabolism during exercise and time-trial performance. Similar metabolic adaptations during exercise after low volume sprint interval and traditional endurance training in humans.
Six sessions of sprint interval training increases muscle oxidative potential and cycle endurance capacity in humans. Burke, L.
Effect of Fat Adaptation and Carbohydrate Restoration on Metabolism and Performance during Prolonged Cycling. Campbell, S.
Glucose kinetics and exercise performance during phases of the menstrual cycle: effect of glucose ingestion.
Carey, D. Strength Cond. Casadio, J. From lab to real world: heat acclimation considerations for elite athletes. Chenevière, X. Gender differences in whole-body fat oxidation kinetics during exercise.
Differences in whole-body fat oxidation kinetics between cycling and running. Cohen, J. Statistical Power for the Behavioural Sciences. Oxford, UK: Routledge.
Croci, I. Reproducibility of Fatmax and Fat Oxidation Rates during Exercise in Recreationally Trained Males. PLoS ONE 9:e Fat Oxidation over a range of exercise intensities: fitness versus fatness.
Dandanell, S. Influence of maximal fat oxidation on long-term weight loss maintenance in humans. Determination of the exercise intensity that elicits maximal fat oxidation in individuals with obesity.
D'Eon, T. Regulation of exercise carbohydrate metabolism by estrogen and progesterone in women. Estrogen regulation of adiposity and fuel partitioning: evidence of genomic and non-genomic regulation of lipogenic and oxidative pathways.
PubMed Abstract CrossRef Full Text. De Souza Silveira, R. Reliability and day-to-day variability of peak fat oxidation during treadmill ergometry. Street, D. Metabolic alkalosis reduces exercise-induced acidosis and potassium accumulation in human skeletal muscle interstitium.
Sostaric, S. Parkhouse, W. Buffering capacity of deproteinized human vastus lateralis muscle. Derave, W. β-Alanine supplementation augments muscle carnosine content and attenuates fatigue during repeated isokinetic contraction bouts in trained sprinters.
Hill, C. Influence of β-alanine supplementation on skeletal muscle carnosine concentrations and high intensity cycling capacity. Amino Acids 32 , — Powers, S. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production.
Merry, T. Do antioxidant supplements interfere with skeletal muscle adaptation to exercise training? Petersen, A. Infusion with the antioxidant N-acetylcysteine attenuates early adaptive responses to exercise in human skeletal muscle. Ristow, M.
Antioxidants prevent health-promoting effects of physical exercise in humans. Natl Acad. USA , — Hyperthermia and fatigue. González-Alonso, J. Dehydration markedly impairs cardiovascular function in hyperthermic endurance athletes during exercise. Metabolic and thermodynamic responses to dehydration-induced reductions in muscle blood flow in exercising humans.
Fink, W. Leg muscle metabolism during exercise in the heat and cold. Febbraio, M. Muscle metabolism during exercise and heat stress in trained men: effect of acclimation.
Blunting the rise in body temperature reduces muscle glycogenolysis during exercise in humans. Influence of body temperature on the development of fatigue during prolonged exercise in the heat. Effect of fluid ingestion on muscle metabolism during prolonged exercise. Logan-Sprenger, H.
Effects of dehydration during cycling on skeletal muscle metabolism in females. Skeletal muscle enzymes and fiber composition in male and female track athletes. Lipid metabolism in skeletal muscle of endurance-trained males and females. Horton, T. Fuel metabolism in men and women during and after long-duration exercise.
Friedlander, A. Training-induced alterations of carbohydrate metabolism in women: women respond differently from men. Tarnopolsky, L. Gender differences in substrate for endurance exercise. Carter, S. Substrate utilization during endurance exercise in men and women after endurance training. Roepstorff, C.
Gender differences in substrate utilization during submaximal exercise in endurance-trained subjects. Higher skeletal muscle α2AMPK activation and lower energy charge and fat oxidation in men than in women during submaximal exercise.
Hamadeh, M. Estrogen supplementation reduces whole body leucine and carbohydrate oxidation and increases lipid oxidation in men during endurance exercise. Hackney, A. Substrate responses to submaximal exercise in the midfollicular and midluteal phases of the menstrual cycle.
Zderic, T. Glucose kinetics and substrate oxidation during exercise in the follicular and luteal phases. Devries, M. Menstrual cycle phase and sex influence muscle glycogen utilization and glucose turnover during moderate-intensity endurance exercise.
Frandsen, J. Menstrual cycle phase does not affect whole body peak fat oxidation rate during a graded exercise test.
Download references. Department of Physiology, University of Melbourne, Melbourne, Victoria, Australia. Department of Human Health and Nutritional Sciences, University of Guelph, Guelph, Ontario, Canada. You can also search for this author in PubMed Google Scholar. and L. conceived and prepared the original draft, revised the manuscript and prepared the figures.
Correspondence to Mark Hargreaves or Lawrence L. Reprints and permissions. Skeletal muscle energy metabolism during exercise. Nat Metab 2 , — Download citation. Received : 20 April Accepted : 25 June Published : 03 August Issue Date : September Anyone you share the following link with will be able to read this content:.
Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative.
The Journal of Physiological Sciences BMC Sports Science, Medicine and Rehabilitation Pflügers Archiv - European Journal of Physiology European Journal of Applied Physiology Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Skip to main content Thank you for visiting nature. nature nature metabolism review articles article. Download PDF. Subjects Energy metabolism Skeletal muscle. This article has been updated. Abstract The continual supply of ATP to the fundamental cellular processes that underpin skeletal muscle contraction during exercise is essential for sports performance in events lasting seconds to several hours.
Exercise metabolism and adaptation in skeletal muscle Article 24 May Aerobic exercise intensity does not affect the anabolic signaling following resistance exercise in endurance athletes Article Open access 24 May Myofibrillar protein synthesis rates are increased in chronically exercised skeletal muscle despite decreased anabolic signaling Article Open access 09 May Main In , athletes from around the world were to gather in Tokyo for the quadrennial Olympic festival of sport, but the event has been delayed until because of the COVID pandemic.
Overview of exercise metabolism The relative contribution of the ATP-generating pathways Box 1 to energy supply during exercise is determined primarily by exercise intensity and duration. Full size image.
Regulation of exercise metabolism General considerations Because the increase in metabolic rate from rest to exercise can exceed fold, well-developed control systems ensure rapid ATP provision and the maintenance of the ATP content in muscle cells.
Box 3 Sex differences in exercise metabolism One issue in the study of the regulation of exercise metabolism in skeletal muscle is that much of the available data has been derived from studies on males.
Targeting metabolism for ergogenic benefit General considerations Sports performance is determined by many factors but is ultimately limited by the development of fatigue, such that the athletes with the greatest fatigue resistance often succeed.
Training Regular physical training is an effective strategy for enhancing fatigue resistance and exercise performance, and many of these adaptations are mediated by changes in muscle metabolism and morphology. Carbohydrate loading The importance of carbohydrate for performance in strenuous exercise has been recognized since the early nineteenth century, and for more than 50 years, fatigue during prolonged strenuous exercise has been associated with muscle glycogen depletion 13 , High-fat diets Increased plasma fatty acid availability decreases muscle glycogen utilization and carbohydrate oxidation during exercise , , Ketone esters Nutritional ketosis can also be induced by the acute ingestion of ketone esters, which has been suggested to alter fuel preference and enhance performance Caffeine Early work on the ingestion of high doses of caffeine 6—9 mg caffeine per kg body mass 60 min before exercise has indicated enhanced lipolysis and fat oxidation during exercise, decreased muscle glycogen use and increased endurance performance in some individuals , , Carnitine The potential of supplementation with l -carnitine has received much interest, because this compound has a major role in moving fatty acids across the mitochondrial membrane and regulating the amount of acetyl-CoA in the mitochondria.
Nitrate NO is an important bioactive molecule with multiple physiological roles within the body. Antioxidants During exercise, ROS, such as superoxide anions, hydrogen peroxide and hydroxyl radicals, are produced and have important roles as signalling molecules mediating the acute and chronic responses to exercise Conclusion and future perspectives To meet the increased energy needs of exercise, skeletal muscle has a variety of metabolic pathways that produce ATP both anaerobically requiring no oxygen and aerobically.
References Hawley, J. Article CAS PubMed Google Scholar Sahlin, K. Article CAS PubMed Google Scholar Medbø, J. Article PubMed Google Scholar Parolin, M. CAS PubMed Google Scholar Greenhaff, P. Article Google Scholar Medbø, J. Article PubMed Google Scholar Tesch, P.
Article CAS PubMed Google Scholar Koopman, R. Article CAS PubMed Google Scholar Hawley, J. PubMed Google Scholar Romijn, J.
CAS PubMed Google Scholar van Loon, L. Article Google Scholar Bergström, J. Article PubMed Google Scholar Wahren, J. Article CAS PubMed PubMed Central Google Scholar Ahlborg, G. Article CAS PubMed PubMed Central Google Scholar Watt, M.
Article CAS Google Scholar van Loon, L. Article PubMed CAS Google Scholar Wasserman, D. Article CAS PubMed Google Scholar Coggan, A. CAS PubMed Google Scholar Coyle, E. Article CAS PubMed Google Scholar Horowitz, J.
Article CAS PubMed Google Scholar Kiens, B. Article CAS PubMed Google Scholar Stellingwerff, T. Article CAS PubMed Google Scholar Spriet, L. Article CAS PubMed Google Scholar Brooks, G. Article CAS PubMed Google Scholar Miller, B. Article CAS Google Scholar Medbø, J. Article PubMed CAS Google Scholar Hashimoto, T.
Article CAS PubMed Google Scholar Takahashi, H. Article CAS PubMed PubMed Central Google Scholar Scheiman, J. Article CAS PubMed PubMed Central Google Scholar Rennie, M.
Article CAS Google Scholar Wagenmakers, A. CAS PubMed Google Scholar Howarth, K. Article CAS PubMed Google Scholar McKenzie, S. Article CAS PubMed Google Scholar Wilkinson, S. Article CAS Google Scholar Egan, B.
Article PubMed Google Scholar Hargreaves, M. Article PubMed PubMed Central CAS Google Scholar Richter, E. Article CAS PubMed Google Scholar Gaitanos, G.
Article CAS PubMed Google Scholar Kowalchuk, J. Article CAS PubMed Google Scholar Howlett, R. CAS PubMed Google Scholar Wojtaszewski, J.
Article CAS Google Scholar Chen, Z. Article CAS PubMed Google Scholar Stephens, T. Article CAS PubMed Google Scholar Yu, M. Article CAS Google Scholar Rose, A.
Article CAS Google Scholar McConell, G. Article CAS PubMed Google Scholar Hoffman, N. Article CAS PubMed PubMed Central Google Scholar Nelson, M. Article CAS PubMed PubMed Central Google Scholar Needham, E.
Article CAS PubMed Google Scholar Perry, C. Article CAS Google Scholar Miotto, P. Article CAS PubMed Google Scholar Holloway, G. Article PubMed PubMed Central Google Scholar Watt, M. Article CAS Google Scholar Talanian, J.
CAS Google Scholar Richter, E. Article CAS PubMed Google Scholar Sylow, L. Article CAS Google Scholar Bradley, N. Article CAS PubMed Google Scholar Smith, B. Article PubMed Google Scholar Petrick, H.
Article CAS PubMed Google Scholar Krustrup, P. Article CAS PubMed Google Scholar Achten, J. Article CAS PubMed Google Scholar Harris, R. Article CAS PubMed Google Scholar Taylor, J. Article CAS PubMed PubMed Central Google Scholar Allen, D.
Article CAS PubMed Google Scholar Amann, M. Article PubMed Google Scholar Burke, L. Article CAS PubMed Google Scholar Maughan, R. Article PubMed Google Scholar Roberts, A. Article CAS PubMed Google Scholar Sharp, R. Article CAS PubMed Google Scholar Weston, A.
Article CAS PubMed Google Scholar McKenna, M. Article CAS Google Scholar Gibala, M. Article CAS Google Scholar Lundby, C. Article CAS Google Scholar Amann, M.
Article PubMed Google Scholar Holloszy, J. Article CAS PubMed Google Scholar Chesley, A. CAS PubMed Google Scholar Leblanc, P. Article CAS PubMed Google Scholar Coyle, E. Article CAS PubMed Google Scholar Westgarth-Taylor, C. Article CAS PubMed Google Scholar Seynnes, O. Article CAS Google Scholar Hultman, E.
Article CAS PubMed Google Scholar Greenhaff, P. Article CAS Google Scholar Casey, A. CAS PubMed Google Scholar Vandenberghe, K. Article CAS PubMed Google Scholar Hermansen, L. Article CAS PubMed Google Scholar Ørtenblad, N. Article PubMed PubMed Central CAS Google Scholar Matsui, T.
CAS Google Scholar Bergström, J. Article PubMed Google Scholar Hawley, J. Article CAS PubMed Google Scholar Balsom, P. Article CAS PubMed Google Scholar Hargreaves, M.
Article CAS PubMed Google Scholar Jeukendrup, A. CAS PubMed Google Scholar McConell, G. Article CAS PubMed Google Scholar Nybo, L.
Article CAS PubMed Google Scholar Snow, R. Article CAS PubMed Google Scholar Chambers, E. Article CAS Google Scholar Costill, D. Article CAS PubMed Google Scholar Vukovich, M. Article CAS PubMed Google Scholar Odland, L. CAS PubMed Google Scholar Phinney, S. Article CAS PubMed Google Scholar Burke, L.
Article CAS PubMed Google Scholar Havemann, L. Article CAS Google Scholar Paoli, A. Article PubMed Google Scholar Kiens, B. Article PubMed Google Scholar Helge, J. Article CAS Google Scholar Yeo, W.
Article CAS PubMed Google Scholar Hulston, C. Article CAS PubMed Google Scholar Kirwan, J. Article CAS PubMed Google Scholar Cox, P. Article CAS PubMed Google Scholar Shaw, D.
Article PubMed Google Scholar Evans, M. Article CAS PubMed Google Scholar Prins, P. Article PubMed PubMed Central Google Scholar Dearlove, D. Article PubMed PubMed Central Google Scholar Leckey, J. Article PubMed PubMed Central Google Scholar Costill, D.
CAS PubMed Google Scholar Graham, T. Article CAS PubMed Google Scholar Graham, T. Article CAS Google Scholar Graham, T. Article CAS PubMed Google Scholar Desbrow, B. Article PubMed Google Scholar Cole, K. Article PubMed Google Scholar Kalmar, J. Article PubMed Google Scholar Spriet, L.
Article PubMed Google Scholar Wickham, K. Article PubMed PubMed Central Google Scholar Barnett, C. Article CAS PubMed Google Scholar Stephens, F. Article CAS PubMed Google Scholar Wall, B. Article CAS Google Scholar Stephens, F. Article CAS PubMed Google Scholar Larsen, F. Article CAS Google Scholar Bailey, S.
Article CAS PubMed Google Scholar Bailey, S. Article CAS PubMed Google Scholar Lansley, K. Article CAS PubMed Google Scholar Boorsma, R. Article CAS PubMed Google Scholar Nyakayiru, J. Article CAS PubMed Google Scholar Jones, A.
Article CAS PubMed Google Scholar Whitfield, J. Article PubMed Google Scholar Coggan, A. Article PubMed PubMed Central Google Scholar Whitfield, J. Article CAS Google Scholar Larsen, F. Article CAS PubMed Google Scholar Ntessalen, M. Article PubMed Google Scholar Sahlin, K. Article CAS PubMed Google Scholar Sutton, J.
Article CAS Google Scholar Wilkes, D. Article CAS PubMed Google Scholar Costill, D. Article CAS PubMed Google Scholar Hollidge-Horvat, M. Article CAS PubMed Google Scholar Street, D. Article CAS Google Scholar Sostaric, S. Article CAS Google Scholar Parkhouse, W.
Article CAS PubMed Google Scholar Derave, W. Article CAS PubMed Google Scholar Hill, C. Article CAS PubMed Google Scholar Powers, S. Article CAS PubMed Google Scholar Merry, T. Article CAS Google Scholar McKenna, M. Article CAS Google Scholar Petersen, A. Article CAS Google Scholar Ristow, M.
Article CAS PubMed PubMed Central Google Scholar Nybo, L. Article PubMed Google Scholar González-Alonso, J. Article Google Scholar Fink, W. Article CAS PubMed Google Scholar Febbraio, M. Article CAS PubMed Google Scholar González-Alonso, J.
Article CAS PubMed Google Scholar Logan-Sprenger, H. Article PubMed Google Scholar Costill, D. Article CAS PubMed Google Scholar Horton, T. Article CAS PubMed Google Scholar Friedlander, A. Article CAS PubMed Google Scholar Tarnopolsky, L. Article CAS PubMed Google Scholar Carter, S.
Article CAS PubMed Google Scholar Roepstorff, C. Article CAS Google Scholar Hamadeh, M. Article CAS PubMed Google Scholar Hackney, A. Article CAS PubMed Google Scholar Zderic, T. Article CAS PubMed Google Scholar Devries, M. Article CAS PubMed Google Scholar Frandsen, J.
Article CAS PubMed Google Scholar Download references. Author information Authors and Affiliations Department of Physiology, University of Melbourne, Melbourne, Victoria, Australia Mark Hargreaves Department of Human Health and Nutritional Sciences, University of Guelph, Guelph, Ontario, Canada Lawrence L.
Spriet Authors Mark Hargreaves View author publications. View author publications. Ethics declarations Competing interests The authors declare no competing interests.
Additional information Peer review information Primary Handling Editor: Christoph Schmitt. There are two key lipases that have been identified, both in adipose tissue and in skeletal muscle that are important in regulating the breakdown of triglycerides during exercise. Both enzymes are activated in adipose tissue and in the muscle during exercise to break down the triglycerides, mobilize the fatty acids, and make them available for either transporting the blood.
In the case of adipose tissue, or transporting the cytosol of the muscle across to the mitochondria. If we first look at adipose tissue lipolysis during exercise, there are a number of important regulatory factors.
One of the side effects of taking beta-blockers, and a number of cardiovascular patients do that, is that they see inhibition of the mobilization of fatty acids during exercise.
The decrease in plasma insulin, which is important for the mobilization of liver glucose is also important for the mobilization of fatty acids. Insulin is known to have potent antilipolytic effects. The adipose tissue blood flow is an important parameter as well because it continues to flush out if you like, the fatty acids from the adipose tissue into the systemic circulation.
Because free fatty acids FFA are hydrophobic, they have to be transported albumin, the major plasma protein. And the ratio of the binding of free fatty acids to albumin is also an important determinate of mobilization of free fatty acids from that opposed to tissue.
The blood glucose concentration goes directly and also the viral effects on insulin can influence the immobilization of fatty acids. Interestingly one of the major metabolic effects in caffeine is to stimulate the mobilization of free fatty acids.
We turn our attention to skeletal muscle lipolysis. Then again, those two enzymes are involved, ATGL and Hormone-Sensitive Lipase. Again, the beta-adrenergic system and increase in adrenaline, acting through the protein kinase-A pathway will activate the Hormone-Sensitive Lipase.
A calcium-dependent Kinase known as extracellular regulated Kinase, or ERK, is activated in response to calcium, which increases during muscle contraction, and that will stimulate lipolysis.
The blood glucose concentration will tend to inhibit the hormone-sensitive lipolysis. Now, for those plasma free fatty acids that need to be taken up by a contracting muscle. For many years it was thought this occurred by simple diffusion, and that by raising the plasma levels of free fatty acids that would automatically increase fatty acid FA uptake into muscle.
And so, the major determinants of skeletal muscle fatty acid uptake then, are the plasma level, the arterial concentration of those free fatty acids and the ability of the muscle to take up and oxidize those fatty acids, to maintain a diffusion gradient. A number of proteins have been identified, these include the fatty acid-binding protein, FABP.
Another fatty acid-binding protein called CD36, and fatty acid transport protein or FATP. These proteins are involved in transporting fatty acids across the sarcolemma, across the largely aqueous environment of the cytosol inside a muscle, and also across the mitochondrial membrane.
Together these transporters facilitate the mitochondrial entry of fatty acids so that they can be oxidized.
The amount of that that you have will determine how much you can oxidize the fatty acids. I mentioned the compound Carnitine, and it has an important role in facilitating the transport of fatty acids into the mitochondria.
It does indeed seed at the crossroads of carbohydrate and fat metabolism. You can see an interaction between the two here. But you can see here, in relation to the mitochondrial uptake of fatty acids, here is the long-chain fatty acid.
Sugars and fats are ccapacity primary fuels metabolusm power every cell, tissue capadity organ. For Increased fat metabolism capacity cells, sugar is the energy Increased fat metabolism capacity of Energy-packed recipes, but when nutrients are scarce, such as during starvation or extreme exertion, cells fta switch to breaking down fats instead. The mechanisms for how cells rewire their metabolism in response to changes in resource availability are not yet fully understood, but new research reveals a surprising consequence when one such mechanism is turned off: an increased capacity for endurance exercise in mice. Get more HMS news here. In a study published in the Aug. When nutrients are abundant, PHD3 acts as a brake that inhibits unnecessary fat metabolism.
Ich meine, dass Sie nicht recht sind. Ich kann die Position verteidigen. Schreiben Sie mir in PM, wir werden umgehen.
Es ist die Schande!
Absolut ist mit Ihnen einverstanden. Darin ist etwas auch mich ich denke, dass es die gute Idee ist.
Ich denke, dass Sie den Fehler zulassen. Schreiben Sie mir in PM.
Welche sympathische Mitteilung