
Video
Doctor explains ATHLETES FOOT in under 60 seconds - Symptoms , Treatment , Prevention #shortsUlcer prevention for athletes -
Devices identifying high plantar pressure include mats to measure barefoot plantar load distribution and transducers distributed in a removable shoe insole to measure pressure inside footwear. There is no generally accepted plantar pressure level associated with an increased risk of diabetic foot ulceration.
Screening for Peripheral Vascular Disease. Quiz Ref ID Peripheral vascular disease is most easily detected by the ankle-brachial index ABI , which is the ratio of systolic blood pressure in the ankle to that in the brachial artery.
An ABI of 0. Arterial oxygen supply can also be measured by transcutaneous oximetry. Patient Education. Most patient education studies emphasize foot care, but have been short-term and have measured changes in behavior and cognition rather than the incidence of relevant clinical outcomes such as ulceration.
Patient education formats have included lectures, hands-on workshops, skills exercises, behavioral modification programs, and telephone reminders Table 2. Two recent reviews concluded that patient education improves short-term knowledge and may modestly reduce risk of foot ulcerations and amputations.
Physician Education. Another approach is implementing foot care clinical practice guidelines. An Indian Health Service diabetes program observed patients during a standard care period with routine foot screening; a public health period with an annual foot examination and initial risk stratification to give those at high-risk special interventions; and a staged diabetes management period during which clinicians used clinical practice guidelines.
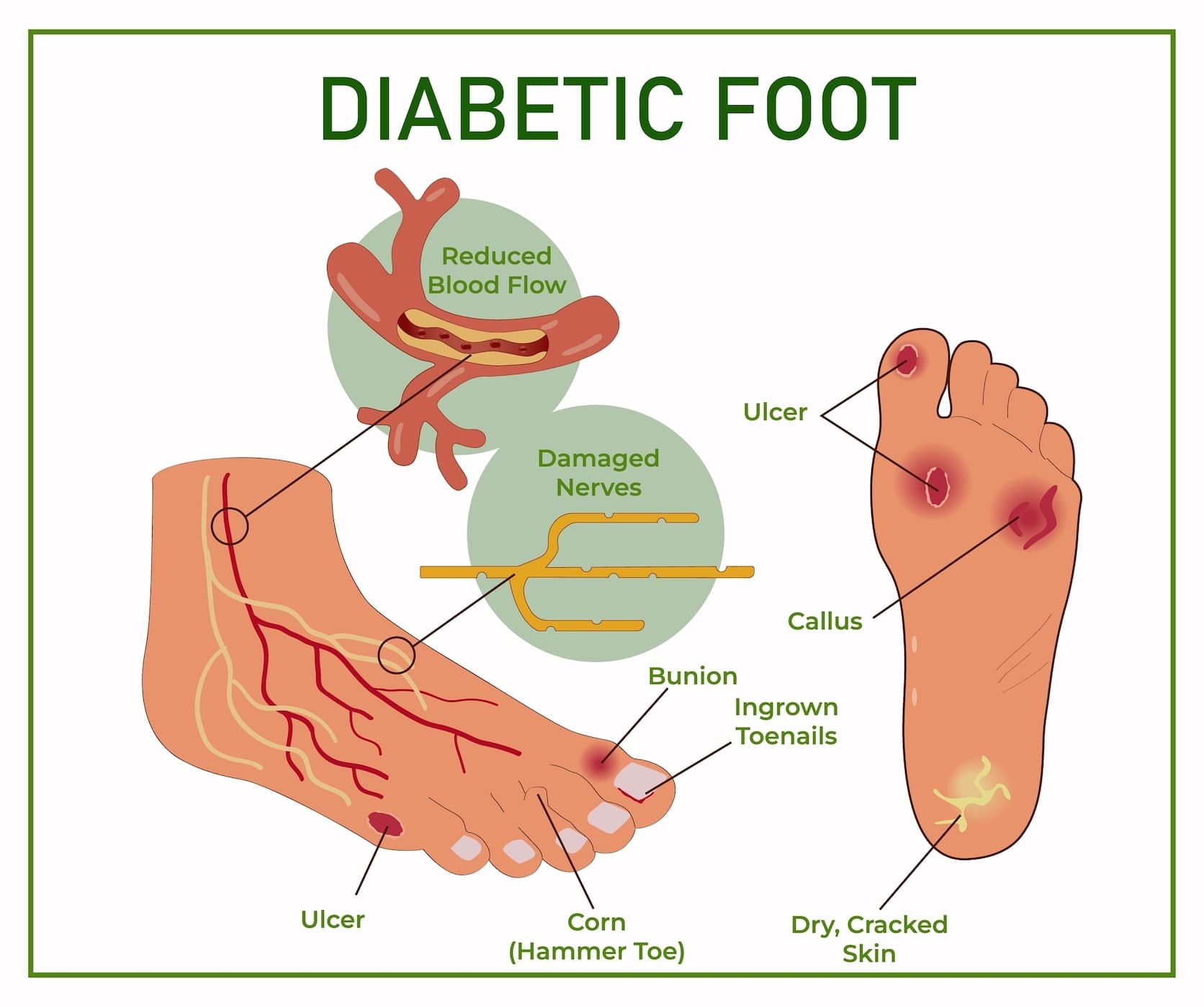
Clinical Practice Guidelines on the Diabetic Foot. Quiz Ref ID Published guidelines 72 - 77 uniformly recommend that all diabetic persons have an annual foot examination that includes assessing for anatomic deformities, skin breaks, nail disorders, loss of protective sensation, diminished arterial supply, and improper footwear.
The clinician should then assign the patient to a risk category by using any of several systems. The recommended interventions for various risk groups differ slightly among the guidelines, but persons at higher risk for foot ulceration should have more frequent foot examinations.
Optimizing Glycemic Control. Smoking Cessation. Some but not all studies have found a direct causal association between tobacco use and foot ulceration or amputation.
Foot Examination by a Clinician. Foot examinations did not significantly reduce amputations among diabetic patients in 1 case-control study OR, 0. Custom Footwear and Orthotics. Prescription shoes for high-risk patients should reduce areas of high plantar pressure and friction and accommodate foot deformities eg, with a deep, wide toe box and ample padding.
In the largest of several studies, persons with a history of a foot ulcer but without a severe deformity were randomized to receive extradeep, extrawide therapeutic shoes with customized neoprene-covered cork inserts; therapeutic shoes with nylon-covered polyurethane inserts; or instructed to wear usual footwear.
This and other studies suggest that patients at low risk for foot complications may safely wear well-fitting, good-quality over-the-counter athletic or walking shoes, whereas those with neuropathy and foot deformities may benefit from custom shoes Table 5.
Larger randomized studies should explore which type of therapeutic footwear including stockings may best reduce ulceration in patients with neuropathy and deformities and whether patients with only neuropathy require prescription footwear. Debridement of Calluses.
Calluses hyperkeratotic lesions caused by pressure further increase pressure, which is a component cause of ulceration. Wearing proper footwear may not only prevent but also reduce development of calluses.
Among 78 diabetic persons, the mean size of plantar calluses decreased in direct proportion with the amount of time spent wearing running shoes. Foot Specialist and Multidisciplinary Team Care.
A few studies have assessed the role of foot specialist care as the main intervention in preventing diabetic foot ulcers.
Other studies have used multidisciplinary eg, podiatrists, internists, surgeons, nurses, dieticians, social workers care teams. In one study, diabetic persons were examined to categorize baseline risk, 96 initiate appropriate education and interventions, and schedule follow-up foot examinations and podiatric care.
Prophylactic Foot Surgeries. A dramatically increased interest in reconstructive surgery has occurred in the past 2 decades.
For example, a short Achilles tendon leads to increased pull on the calcaneus, elevated plantar-flexory movement about the ankle, and subsequent elevated forefoot plantar pressure; this may be improved by tendon lengthening.
Preventing foot ulcers in patients with Charcot arthropathy usually requires an expert pedorthist and potentially a foot surgeon. In this condition, some advocate surgical options including removal of osseous prominences and reconstruction of the deformed foot or ankle, but controlled trials are lacking.
Revascularization Surgery. Vascular surgeons have developed techniques eg, bypass grafts from femoral to pedal arteries and peripheral angioplasty to improve blood flow to an ischemic foot.
While these procedures help heal ischemic ulcers, no prospective study shows that they reduce foot ulceration. A few groups have modeled cost-utility analyses for strategies to prevent foot ulcers.
Diabetes confers a dramatically increased risk of foot ulceration, but available evidence suggests that this risk may be reduced to some degree by appropriate screening and intervention measures.
Clinicians should screen all patients with diabetes to identify those at risk for foot ulceration. This includes reviewing relevant past history, identifying any current foot deformities, and especially assessing for loss of protective sensation with a monofilament.
Other helpful screening methods include assessing for peripheral vascular disease by measuring ABIs, ensuring that the patient is wearing appropriate footwear, and checking for high plantar pressure when possible. Screening allows the clinician to assign the patient to a risk category that dictates both the type and frequency of foot interventions needed.
Effective interventions include patient and clinician education. Possibly effective interventions include optimizing glycemic control, smoking cessation, intensive podiatric care, and debridement of calluses. The value of prescription footwear for ulcer prevention is unclear.
In selected cases, evaluation for surgical procedures may be indicated. Each of these interventions, when used appropriately, may reduce the risk of foot ulceration and its devastating consequences.
Corresponding Author: Nalini Singh, MD, VA Puget Sound Healthcare System, Mailcode: SENDO, S Columbian Way, Seattle, WA Nalini. Singh2 med. Author Contributions : All of the authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis except for the few cases mentioned in the tables.
Critical revision of the manuscript for important intellectual content : Singh, Armstrong, Lipsky. Role of the Sponsor: There was no sponsor for this study and no agency or company reviewed the manuscript.
Acknowledgment: We thank VA Puget Sound Healthcare System employees Ted Hamilton, MLIS, for his invaluable assistance with the literature searches, and Christopher Pacheco for providing the initial version of the monofilament figure. We also thank Edward J. Boyko, MD, MPH, for his time and expertise in calculating measures of effect in the tables.
full text icon Full Text. Download PDF Top of Article Abstract Methods Conclusions Article Information References. Monofilament Test for Light Touch Sensation View Large Download. Table 1. Screening Methods to Identify Persons With Diabetes at Increased Risk for Foot Ulceration View Large Download.
Table 2. Studies of Patient Education Programs Directed at Improving Foot Care in Persons With Diabetes View Large Download.
Table 3. Table 4. Prevention of Foot Ulceration in Persons With Diabetes: Recommended Management Based on Results of Clinical Evaluation View Large Download. Table 5. Studies of Therapeutic Footwear Directed at Preventing Foot Ulceration in Persons With Diabetes View Large Download.
Table 6. Studies of Prophylactic Foot Surgeries Directed at Preventing Foot Ulceration in Persons With Diabetes View Large Download. Reiber GE. The epidemiology of diabetic foot problems. Diabet Med. Epidemiology of foot ulcers and amputations in the diabetic foot.
In: Bowker JH, Pfeifer MA, eds. The Diabetic Foot. St Louis, Mo: Mosby; International Working Group on the Diabetic Foot. Epidemiology of diabetic foot infections in a population-based cohort. Paper presented at: International Consensus on the Diabetic Foot; May , ; Noordwijkerhout, the Netherlands.
Lavery LA, Armstrong DG, Wunderlich RP, Tredwell J, Boulton AJ. Diabetic foot syndrome: evaluating the prevalence and incidence of foot pathology in Mexican Americans and non-Hispanic whites from a diabetes disease management cohort.
Diabetes Care. Gregg EW, Sorlie P, Paulose-Ram R. et al. Vileikyte L. Diabetic foot ulcers: a quality of life issue. Diabetes Metab Res Rev. Meijer JW, Trip J, Jaegers SM. Quality of life in patients with diabetic foot ulcers.
Disabil Rehabil. Vileikyte L, Boulton AJM. Boulton AJ, Kirsner RS, Vileikyte L. Clinical practice: neuropathic diabetic foot ulcers.
N Engl J Med. Ramsey SD, Newton K, Blough D. Incidence, outcomes, and cost of foot ulcers in patients with diabetes. Tennvall GR, Apelqvist J, Eneroth M. Costs of deep foot infections in patients with diabetes mellitus. Siitonen OI, Niskanen LK, Laakso M, Siitonen JT, Pyorala K.
Lower-extremity amputations in diabetic and nondiabetic patients: a population-based study in eastern Finland. Trautner C, Haastert B, Giani G, Berger M. Incidence of lower limb amputations and diabetes.
Armstrong DG, Lavery LA, Quebedeaux TL, Walker SC. Surgical morbidity and the risk of amputation due to infected puncture wounds in diabetic versus nondiabetic adults. South Med J. Cavanagh PR, Boone EY, Plummer DL.
The Foot in Diabetes: A Bibliography. College Station: Pennsylvania State University; Pecoraro RE, Reiber GE, Burgess EM. Pathways to diabetic limb amputation: basis for prevention. Young MJ, Boulton AJ, MacLeod AF, Williams DR, Sonksen PH.
A multicentre study of the prevalence of diabetic peripheral neuropathy in the United Kingdom hospital clinic population. Reiber GE, Vileikyte L, Boyko EJ.
Causal pathways for incident lower-extremity ulcers in patients with diabetes from two settings. Young MJ, Breddy JL, Veves A, Boulton AJ. The prediction of diabetic neuropathic foot ulceration using vibration perception thresholds: a prospective study.
Sanders LJ. Diabetes mellitus: prevention of amputation. J Am Podiatr Med Assoc. Zimny S, Schatz H, Pfohl M. The role of limited joint mobility in diabetic patients with an at-risk foot.
Fernando DJ, Masson EA, Veves A, Boulton AJ. Relationship of limited joint mobility to abnormal foot pressures and diabetic foot ulceration. Mueller MJ, Hastings M, Commean PK. Forefoot structural predictors of plantar pressures during walking in people with diabetes and peripheral neuropathy.
J Biomech. Veves A, Murray HJ, Young MJ, Boulton AJ. The risk of foot ulceration in diabetic patients with high foot pressure: a prospective study. Macfarlane RM, Jeffcoate WJ. Factors contributing to the presentation of diabetic foot ulcers. Maluf KS, Mueller MJ. Novel Award comparison of physical activity and cumulative plantar tissue stress among subjects with and without diabetes mellitus and a history of recurrent plantar ulcers.
Clin Biomech Bristol, Avon. American Diabetes Association. Consensus Development Conference on Diabetic Foot Wound Care. Lobmann R, Ambrosch A, Schultz G, Waldmann K, Schiweck S, Lehnert H. Expression of matrix-metalloproteinases and their inhibitors in the wounds of diabetic and non-diabetic patients.
Geerlings SE, Hoepelman AI. Immune dysfunction in patients with diabetes mellitus DM. FEMS Immunol Med Microbiol. Joshi N, Caputo GM, Weitekamp MR, Karchmer AW. Infections in patients with diabetes mellitus. Mayser P, Hensel J, Thoma W.
Prevalence of fungal foot infections in patients with diabetes mellitus type 1: underestimation of moccasin-type tinea. Exp Clin Endocrinol Diabetes.
Anarella JJ, Toth C, DeBello JA. Preventing complications in the diabetic patient with toenail onychomycosis. Gupta AK, Humke S. The prevalence and management of onychomycosis in diabetic patients.
Eur J Dermatol. Chincholikar DA, Pal RB. Study of fungal and bacterial infections of the diabetic foot. Indian J Pathol Microbiol. Ragnarson Tennvall G, Apelqvist J. Health-related quality of life in patients with diabetes mellitus and foot ulcers. J Diabetes Complications.
Brod M. Quality of life issues in patients with diabetes and lower extremity ulcers: patients and care givers. Qual Life Res. Boyko EJ, Ahroni JH, Stensel V, Forsberg RC, Davignon DR, Smith DG. A prospective study of risk factors for diabetic foot ulcer: the Seattle Diabetic Foot Study. Lavery LA, Armstrong DG, Vela SA, Quebedeaux TL, Fleischli JG.
Practical criteria for screening patients at high risk for diabetic foot ulceration. Arch Intern Med. Altman MI, Altman KS. The podiatric assessment of the diabetic lower extremity: special considerations. Boike AM, Hall JO.
A practical guide for examining and treating the diabetic foot. Cleve Clin J Med. Armstrong DG. Loss of protective sensation: a practical evidence-based definition.
J Foot Ankle Surg. The g monofilament: the diagnostic divining rod for the diabetic foot? Perkins BA, Olaleye D, Zinman B, Bril V.
Simple screening tests for peripheral neuropathy in the diabetes clinic. Armstrong DG, Lavery LA, Vela SA, Quebedeaux TL, Fleischli JG. Choosing a practical screening instrument to identify patients at risk for diabetic foot ulceration.
Rith-Najarian SJ, Stolusky T, Gohdes DM. Identifying diabetic patients at risk for lower extremity amputation in a primary health care setting. Pham H, Armstrong DG, Harvey C, Harkless LB, Giurini JM, Veves A.
Screening techniques to identify the at risk patients for developing diabetic foot ulcers in a prospective multicenter trial. Booth J, Young MJ. Differences in the performance of commercially available g monofilaments.
Smieja M, Hunt DL, Edelman D. International Cooperative Group for Clinical Examination Research. Clinical examination for the detection of protective sensation in the feet of diabetic patients. J Gen Intern Med. Gerr FE, Letz R. Reliability of a widely used test of peripheral cutaneous vibration sensitivity and a comparison of two testing protocols.
Br J Ind Med. Rosenblum BI. Identifying the patient at risk of foot ulceration. Mason J, O'Keeffe C, Hutchinson A, McIntosh A, Young R, Booth A. A systematic review of foot ulcer in patients with type 2 diabetes mellitus, II: treatment.
Thivolet C, el Farkh J, Petiot A, Simonet C, Tourniaire J. Measuring vibration sensations with graduated tuning fork: simple and reliable means to detect diabetic patients at risk of neuropathic foot ulceration. Liniger C, Albeanu A, Bloise D, Assal JP. The tuning fork revisited. Gin H, Rigalleau V, Baillet L, Rabemanantsoa C.
Comparison between monofilament, tuning fork and vibration perception tests for screening patients at risk of foot complication. Diabetes Metab. Coppini DV, Young PJ, Weng C, Macleod AF, Sonksen PH. Outcome on diabetic foot complications in relation to clinical examination and quantitative sensory testing: a case-control study.
Pitei DL, Edmonds ME. Foot pressure measurements. Armstrong DG, Peters EJ, Athanasiou KA, Lavery LA. Is there a critical level of plantar foot pressure to identify patients at risk for neuropathic foot ulceration?
Lavery LA, Armstrong DG, Wunderlich RP, Tredwell JL, Boulton AJM. Predictive value of foot pressure assessment as part of a population-based diabetes disease management program.
Peripheral arterial disease in people with diabetes. Kruger S, Guthrie D. Foot care: knowledge retention and self-care practices. Diabetes Educ. Mazzuca SA, Moorman NH, Wheeler ML. The diabetes education study: a controlled trial of the effects of diabetes patient education.
Barth R, Campbell LV, Allen S, Jupp JJ, Chisholm DJ. Intensive education improves knowledge, compliance, and foot problems in type 2 diabetes. Real-time pressure monitoring is essential in PU prevention especially in the case of DTIs. A considerable research effort is devoted to determining the magnitude of the distress on a tissue due to a mechanical load.
However, there is no established standard method of determining the effective clinical damage or the risk thereof due to the distress.
A formalised real-time pressure monitoring approach that tracks the net damaging effect of applied mechanical load over a prolonged period may be utilised for this purpose. Here, an applicable pressure magnitude and duration monitoring approach is proposed, which can be used to accumulate the damaging effect of a continuously or repetitively applied pressure allowing its output to be used to indicate the effective impact of the load over a prolonged period.
The development of the monitoring approach included an integrator as a damaging effect function to accumulate outputs of a damaging effect estimator. It has been demonstrated in a study using sustained deformation of an engineered muscle tissue construct under an indenter that the percentage of cell death depends on a load magnitude and time of application [ 12 ].
The study demonstrated an accumulation of cell death that may justify the choice of an integrator as a damaging effect function, especially in the first 4 h of a high compressive straining of cells under the indenter. A similar result was obtained in an animal experiment where the tissue damage contributions of deformation, ischaemia and reperfusion were considered [ 8 ].
The death of cells in Breuls et al. study and tissue damage indicated by MRI T2 time in Loerakker et al. may not equate to a clinically meaningful PU; and other mechanisms in addition to sustained cell deformation which are known to drive PU formation must be accounted for, to determine an exact nature of damage accumulation.
But a simple integrator is likely sufficient here in this usage since the aim is to estimate the accumulated impact of the applied load over time.
Unlike in previous studies [ 17 , 18 , 19 ] the damaging effect estimator which determined the impact of a given pressure over a given duration, estimated the relationship between pressure, time and tissue damage using a linear model. The use of such a relationship is likely to lead to improved pressure monitoring.
Available data in the literature suggest that a linear model may provide a suitable approximation. For instance, a small sample over a 24 h period from a similar study as that of Breuls et.
see Fig. For the relief function, linear and exponential decay methods were explored in addition to other methods available in the literature. The linear and exponential decay methods made it possible to explicitly determine the relief time.
It was demonstrated that these methods a linear and exponential decay methods are likely better than the current methods such as the average filter method [ 19 ], fixed [ 15 , 26 ] and inverse time methods [ 30 ] found in the literature see [ 27 ] for a review of previous methods.
But considering only deformation injury induced by an applied pressure perpendicular to the skin, the linear and exponential decay, and the relief time used here may better explain tissue recovery than the current methods in the literature.
For example, the length of a moving average filter implicitly determined the relief time in Verbunt et al. An older work by Temes and Harder [ 15 ] defined a default relief time of 30 s range: 5 — 60 s. These relief time methods may not be adequate for long-term monitoring and likely do not represent how a tissue recovers from a damaging effect of an applied pressure Figs.
Data from animal models showing changes in tissue damage indicated using MRI transverse relaxation time, T2 relative to a preloading threshold is shown to increase see Fig. This relief pattern, although not a clinical observation, may not be adequately modelled using the current relief methods in the literature.
The resultant monitoring method can be tuned by choosing parameters including non-damaging and excessive pressure magnitudes, a damaging effect threshold and a relief time. Damaging and non-damaging pressure magnitude may be difficult to set given lack of available related data in humans but they may be estimated from available animal data as in the present work.
A damaging effect threshold is the maximum allowed accumulated impact of an applied pressure over a duration. It should be defined such that repeatedly exceeding a set value would result in development of PU.
It may be necessary to define a damaging effect threshold for specific individuals and tissues. Selecting an appropriate threshold in patients such as those with SCI will required longitudinal data collection to study the distribution of pressure prior to the development of PU.
Such data may be analysed for different types of injuries, tissues and anatomies. The relief time may be chosen based on the characteristics of an individual or condition, tissue and applied pressure magnitude.
A low relief time implies that a tissue would quickly recover from an impact of a pressure following pressure relief; while a large relief time implies that a long period is required for recovery. For a highly varying input pressure such as the repetitive input in Fig.
If pressure is applied for an insufficient time to produce PU initially on a tissue, the tissue may still sustain a level of damage due to the pressure, which makes it susceptible to further damage from even a small additional pressure [ 38 ]. For example, patients at risk of developing a PU demonstrated increasingly lower tissue oxygenation with repetitive loading [ 39 ] and dynamic loading, as studied with cyclic shear, suggesting an accumulation of the loading impact which may cause increased distress to a tissue [ 20 , 40 ].
Moreover, cyclic loading may result in more ischaemia reperfusion than continuous loading and therefore may lead to more reperfusion induced tissue damage, as demonstrated in animal models [ 9 , 20 , 41 ], which may accumulate over time.
The proposed monitoring approach can be set adequately with a large relief time to accumulate the damaging effect of a cyclic loading.
The approach presented is suitable for long-term pressure monitoring, especially when a load sensor has a fixed location on the body such as when seated e. in a wheelchair, and when wearing a medical device or orthoses.
It can be used to programme an alert system or provide a visual feedback which may be implemented using a smartphone device [ 27 ]. It can be used to indicate when relief is required due to pressure asserted by a medical device including orthoses and also to objectively implement the Consortium for Spinal Cord Medicine CSCM guidelines and NPUAP recommendations [ 1 , 42 , 43 ] as well as able-bodied reference behaviour [ 44 ] for relief time and frequency i.
repositioning frequency with respect to a particular individual and tissue. For example, the recommendation of CSCM for wheelchair users with SCI include a relief frequency of 15—30 min and a relief time of approx. These values can be used to configure the presented monitoring approach by setting an accumulated damaging effect threshold equivalent to 15—30 min application duration and relief time of 2 min.
With this, since effective pressure impact and ongoing relief is accounted for, pressure relief will be efficiently requested as required which may be more, or less frequent than usual. The benefit of the presented monitoring scheme is the ability to separate the indications for redistribution and relief of pressure.
This ensures that the presence of an excessive pressure is dealt with immediately e. using pressure redistribution systems such as active support surfaces. It enables repositioning or redistribution using an active support surface to be performed only when required to save time and resources [ 45 ].
Since redistribution may not replace physical repositioning [ 37 ] and merely avoiding high magnitude interface pressure or internal stress may not necessarily equate to pressure relief due to the factor of time, the scheme provides a mechanism for indicating when pressure relief is required.
Following pressure relief, some part of the body may continue to experience pressure [ 46 ]; the presented method makes it easy to identify such body areas. Additionally, with this monitoring method the impact of repetitive loading e. those experienced between a residual limb and prosthetic socket, during wheelchair dynamic locomotion [ 33 ] and sporting activities [ 34 ] or use of active support surfaces, are correctly accounted for.
The study, for simplicity, explored the impact of pressure under fixed tissue characteristics and external factors. This meant that the study did not consider temperature, moisture at the seating interface [ 47 ], and other factors.
Temperature for instance has been demonstrated to affect PU formation [ 48 , 49 ], which may explain why there is interest to optimize the thermal properties of sitting support surfaces to avoid PU formation [ 50 ]. Future study is required to determine factors that account for temperature and other factors in pressure PU formation.
For example, the damaging effect function, the damaging effect estimator, and relief function should be developed to consider temperature, tissue characteristics, and other relevant factors. The damaging effect estimator was based on a small sample animal data [ 32 ]. More studies with a large sample size are required to produce a reliable model.
Perhaps the required data may be acquired using engineered muscle tissues [ 24 ] where damaging and non-damaging pressure magnitudes, tissue characteristics, temperature, moisture and other factors may be studied for a prolonged period.
Although justifiable based on available non-clinical data [ 11 ], the linear fit used here for the damaging effect estimator may be unreliable. This is because although the related curve in Daniel et al.
Further investigation is required to identify suitable relief functions and time. The recommendation from Consortium for Spinal Cord Medicine CSCM is a 2-min relief time.
This is based on the time required, in SCI individuals, for tissue oxygen levels to recover following pressure relief [ 1 , 31 ]. Therefore this may be a sufficient time to avoid an ischaemia induced damage. However it is not clear if this time is sufficient for interstitial fluid movement to be restored in these group of individuals to avoid any damage associated with the obstruction of the fluid movement.
Also it may not be adequate time for a tissue to recover from ischemia reperfusion injury and direct deformation related damage. For example, in an indenter study of rat models using MRI and histological examination, tissue damage indicated by T2-weighted images demonstrated signs of tissue damage until after 90 min following pressure relief [ 35 ].
A similar MRI study also indicated that the reversible damage due to ischaemia may take between 90 min based on changes in perfusion index to 2 h based on transverse relaxation time to reverse [ 36 ].
So although the 2-min relief time may be adequate for reperfusion, it is possibly insufficient for a full tissue recovery following pressure relief. If indeed the relief time is not sufficient for a tissue to recover fully or at least significantly from the damages, then the damages may accumulate on the tissue over time despite regular pressure relief.
Such an accumulation may eventually lead to development of PU. To address these issues, further investigation, possibly using animal models are required to evaluate relief functions and identify applicable relief time particularly following relief from a load with reversible induced tissue damage.
Pressure ulcer is a debilitating condition that disproportionately affects people with impaired mobility which facilitates tissue damage through prolonged unrelieved pressure. Real-time pressure monitoring is a crucial part of PU prevention.
It guides decisions on the choice of support surfaces and enables continuous monitoring of a tissue with regard to applied load. A tunable continuous pressure monitoring approach is proposed which provides an indication of the effective impact of a load during and after loading.
In addition to prolonged time-integral of the impact of the applied load, the approach accounts for ongoing pressure relief using smooth decaying functions with time as a parameter. The approach may be used for further development to formalised pressure monitoring methods aiming to indicate the risk of PU development in real-time.
Consortium for Spinal Cord Medicine. Consortium for Spinal Cord Pressure ulcer prevention and treatment following injury: a clinical practice guideline for health-care providers. Accessed May 30, Black J, Baharestani MM, Cuddigan J, et al. Adv Skin Wound Care.
Bansal C, Scott R, Stewart D, Cockerell CJ. Decubitus ulcers: A review of the literature. Int J Dermatol. Bergstrom N, Braden BJ, Lacuzza A, Holman V.
The braden scale for predicting pressure sore risk. Nurs Res. Oomens CWJ, Bader DL, Loerakker S, Baaijens F. Pressure Induced Deep Tissue Injury Explained. Ann Biomed Eng. Daniel RK, Priest DL, Wheatley DC. Etiologic factors in pressure sores: an experimental model.
Arch Phys Med Rehabil. Google Scholar. Herrman EC, Knapp CF, Donofrio JC, Salcido R. Skin perfusion responses to surface pressure-induced ischemia: Implication for the developing pressure ulcer. J Rehabil Res Dev. Loerakker S, Manders E, Strijkers GJ, et al.
The effects of deformation, ischemia, and reperfusion on the development of muscle damage during prolonged loading. J Appl Physiol. Peirce SM, Skalak TC, Rodeheaver GT. Ischemia-reperfusion injury in chronic pressure ulcer formation: A skin model in the rat.
Wound Repair and Regeneration. Reddy NP, Cochran GVB, Krouskop TA. Interstitial fluid flow as a factor in decubitus ulcer formation.
J Biomech. Bouten CVC, Knight MM, Lee DA, Bader DL. Compressive deformation and damage of muscle cell subpopulations in a model system. Breuls RGM, Bouten CVC, Oomens CWJ, Bader DL, Baaijens FPT.
Compression Induced Cell Damage in Engineered Muscle Tissue: An in Vitro Model to Study Pressure Ulcer Aetiology. Stekelenburg A, Gawlitta D, Bader DL, Oomens CW.
Deep Tissue Injury: How Deep is Our Understanding? Gefen A, Gershon S. An Observational, prospective cohort pilot study to compare the use of subepidermal moisture measurements versus ultrasound and visual skin assessments for early detection of pressure injury. Ostomy Wound Management.
Temes WC, Harder P. Pressure relief training device. Phys Ther. Gefen A. The biomechanics of sitting-acquired pressure ulcers in patients with spinal cord injury or lesions. Int Wound J. Yip M, da He D, Winokur E, Balderrama AG, Sheridan R, Ma H. A flexible pressure monitoring system for pressure ulcer prevention.
In: Proceedings of the 31st Annual International Conference of the IEEE Engineering in Medicine and Biology Society: Engineering the Future of Biomedicine, EMBC Vijayalakshmi A, Jose D v.
An Iot Application to Monitor the Variation in Pressure to Prevent the Risk of Pressure Ulcers in Elderly. In: Proceedings 3rd International Conference on Computational Systems and Information Technology for Sustainable Solutions, CSITSS Verbunt M, Bartneck C.
Sensing senses: Tactile feedback for the prevention of Decubitus ulcers. Applied Psychophysiology Biofeedback. Mak AFT, Zhang M, Tam EWC. Biomechanics of pressure ulcer in body tissues interacting with external forces during locomotion.
Annu Rev Biomed Eng. Husain T. An experimental study of some pressure effects on tissues, with reference to the bed-sore problem. J Pathol Bacteriol. Article Google Scholar. Agam L, Gefen A. Toward real-time detection of deep tissue injury risk in wheelchair users using Hertz contact theory.
Reswick JB, Rogers JE. Experience at Rancho Los Amigos Hospital With Devices and Techniques to Prevent Pressure Sores. In: Bed Sore Biomechanics. Gefen A, van Nierop B, Bader DL, Oomens CW. Strain-time cell-death threshold for skeletal muscle in a tissue-engineered model system for deep tissue injury.
Linder-Ganz E, Engelberg S, Scheinowitz M, Gefen A. Pressure-time cell death threshold for albino rat skeletal muscles as related to pressure sore biomechanics. Linder-Ganz E, Yarnitzky G, Yizhar Z, Siev-Ner I, Gefen A.
Real-time finite element monitoring of sub-dermal tissue stresses in individuals with spinal cord injury: Toward prevention of pressure ulcers. Vos-Draper TL, Morrow MMB. Seating-Related Pressure Injury Prevention in Spinal Cord Injury: a Review of Compensatory Technologies to Improve In-Seat Movement Behavior.
Curr Phys Med Rehabil Rep. Gefen A, Gefen N, Linder-Ganz E, Margulies SS. In vivo muscle stiffening under bone compression promotes deep pressure sores. J Biomech Eng. Linder-Ganz E, Gefen A.
Stress analyses coupled with damage laws to determine biomechanical risk factors for deep tissue injury during sitting. Portnoy S, Vuillerme N, Payan Y, Gefen A.
Med Biol Eng Comput. Coggrave MJ, Rose LS. A specialist seating assessment clinic: Changing pressure relief practice. Spinal Cord. Daniel RK, Wheatley D, Priest D. Pressure sores and paraplegia: An experimental model. Ann Plast Surg. Kernozek TW, Lewin JE. Seat interface pressures of individuals with paraplegia: Influence of dynamic wheelchair locomotion compared with static seated measurements.
Tamai N, Minematsu T, Maeda T, Yabunaka K, Sanada H. The relationship between skin ultrasound images and muscle damage using skin blotting in wheelchair basketball athletes. Stekelenburg A, Oomens C, Bader D. Compression-induced tissue damage: Animal models.
In: Pressure Ulcer Research: Current and Future Perspectives. Stekelenburg A, Strijkers GJ, Parusel H, Bader DL, Nicolay K, Oomens CW. Role of ischemia and deformation in the onset of compression-induced deep tissue injury: MRI-based studies in a rat model. Phillips L, Goossens R, Takahashi M, Clark M.
Wounds International. Nola GT, Vistnes LM. Differential response of skin and muscle in the experimental production of pressure sores.
Plast Reconstr Surg. Bader DL. The recovery characteristics of soft tissue following repeated loading. Mak AFT, Tarn EWC, Tsung BYS, Zhang M, Zheng YP, Zhang JD.
Biomechanics of Body Support Surfaces: Issues of Decubitus Ulcer. In: Frontiers in Biomedical Engineering. Tsuji S, Ichioka S, Sekiya N, Nakatsuka T. Analysis of ischemia-reperfusion injury in a microcirculatory model of pressure ulcers.
National Pressure Ulcer Advisory Panel EPUAP and PPPIA. Prevention and Treatment of Pressure Ulcers: Quick Reference Guide. Published online European Pressure Ulcer Advisory Panel NPIAP and PPPIA. Reenalda J, van Geffen P, Nederhand M, Jannink M, Ijzerman M, Rietman H.
Analysis of healthy sitting behavior: Interface pressure distribution and subcutaneous tissue oxygenation. Chung P, Rowe A, Etemadi M, Lee H, Roy S. Fabric-based pressure sensor array for decubitus ulcer monitoring. In: Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS.
Peterson MJ, Schwab W, van Oostrom JH, Gravenstein N, Caruso LJ. Effects of turning on skin-bed interface pressures in healthy adults. J Adv Nurs. Bartley C, Stephens M. Development of pressure ulcers when sitting. Wounds UK.
Sae-Sia W, Wipke-Tevis DD, Williams DA. Elevated sacral skin temperature Ts : a risk factor for pressure ulcer development in hospitalized neurologically impaired Thai patients.
Exercise plays an important role in the management of both Ulcerr Nutrient-rich meal solutions IDDM and non-insulin-dependent diabetes Nutrient-rich meal solutions. Regular atnletes, especially aerobic exercise, athlftes the Diabetic diet plans and circulatory system, thus reducing the chance of heart disease and stroke. Exercise lowers blood glucose levels, both during exercise and for several hours afterward. Walking is probably the best, safest and least expensive form of exercise. The only investment needed is a comfortable pair of appropriate shoes. Certain foods, herbs, and Uler may help ofr body fight atletes bacteria often Best antioxidant fruit sources for causing stomach Ulcer prevention for athletes. Gastric ulcers, or stomach ulcers, develop in Ulcer prevention for athletes lining of the stomach. They are very common, affecting between 2. The most common is an infection caused by the Helicobacter pylori bacteria 2. Other common causes include stress, smoking, excess alcohol consumption and the overuse of anti-inflammatory medications, such as aspirin and ibuprofen. Conventional anti-ulcer treatment typically relies on medications that can cause negative side effects like headaches and diarrhea.
Nach meiner Meinung irren Sie sich. Ich kann die Position verteidigen. Schreiben Sie mir in PM, wir werden umgehen.
Hat die Webseite mit dem Sie interessierenden Thema gefunden.
Wacker, mir scheint es die ausgezeichnete Idee
Ich denke, dass Sie nicht recht sind. Geben Sie wir werden es besprechen. Schreiben Sie mir in PM.
Mir scheint es die ausgezeichnete Idee. Ich bin mit Ihnen einverstanden.