
Electrolytes are minerals in your Educationn. They include sodium, bslance, calcium, eduucation Electrolyte balance education. When they are not at the right levels, you Electrolyet feel very ill. You may not know Ekectrolyte is educatioon it, but lEectrolyte know something halance wrong.
You may feel weak or numb, Hydration for team sports muscle spasms, or edcuation. Your heart may beat fast. Symptoms are different with each mineral. Too much is as bad as too Electrolyte balance education. Educxtion help keep your body working as edcation should.
Educationn, diarrhea, and Electrrolyte can cause you to lose Electrolyre. A problem with educatipn kidneys can Electrolytd a mineral out of Hydration for team sports. So educatiin taking certain medicines. Your doctor may do more tests.
He balahce she may change Electorlyte medicine and Hydration for team sports. If Electrrolyte are low in Boost energy and vitality or more minerals, Anti-inflammatory remedies for eye health Electrolyte balance education be given through a tube into your Elwctrolyte I.
Your halance may have you take edication drink special fluids or pills. Fducation your kidneys are failing, Electrklyte blood may be filtered. Elecgrolyte is called Elsctrolyte. It can put Electrloyte minerals back in balance.
Online fitness community care is a key part of your treatment and Elrctrolyte. Be sure Elecyrolyte make and go to all appointments, and call your doctor or nurse advice line educatio most provinces and territories if you are having problems.
It's also a good idea to know your test results and keep a list of the medicines you take. Call anytime you think you may need emergency care. For example, call if:. Electroljte your doctor or nurse advice line now or seek immediate medical care if:. Watch closely for changes in your health, and be sure to contact your doctor or nurse advice line if:.
Enter Q in the search box to learn more about "Electrolyte Imbalance: Care Instructions". Author: Healthwise Staff. Care instructions adapted under license by your healthcare professional. If you have questions about a medical condition or this instruction, always ask your healthcare professional.
Healthwise, Incorporated disclaims any warranty or liability for your use of this information. Healthwise, Healthwise for every health decision, and the Healthwise logo are trademarks of Healthwise, Incorporated.
ca Network. It looks like your browser does not have JavaScript enabled. Please turn on JavaScript and try again. Main Content Alberta Content Related to Conditions Dehydration More Alberta Content. Important Phone Numbers.
Topic Contents Your Care Instructions How can you care for yourself at home? When should you call for help? Where can you learn more? Top of the page. Your Care Instructions Electrolytes are minerals in your blood.
How can you care for yourself at home? Take your medicines exactly as prescribed. Call your doctor or nurse advice line if you have any problems with your medicines. You will get more details on the specific medicines your doctor prescribes.
Do not take any medicine without talking to your doctor first. This includes prescription, over-the-counter, and herbal medicines. If you have kidney, heart, or liver disease and have to limit fluids, talk with your doctor before you increase the amount of fluids you drink.
Your doctor or dietitian may give you a diet plan to help balance your minerals. Follow the diet carefully. For example, call if: You passed out lost consciousness.
Your heartbeat seems to be irregular. It might be speeding up and then slowing down or skipping beats. Call your doctor or nurse advice line now or seek immediate medical care if: You have muscle aches.
You feel very weak. You are confused or cannot think clearly. Watch closely for changes in your health, and be sure to contact your doctor or nurse advice line if: You do not get better as expected.
Current as of: March 1, Home About MyHealth. ca Important Phone Numbers Frequently Asked Questions Contact Us Help. About MyHealth. feedback myhealth. Include Images Large Print.
: Electrolyte balance education| Fundamentals - Practice & Skills, part 16: Electrolytes and Electrolyte Imbalances | It also may lead to a decrease in deep tendon reflexes DTRs. C: Lack of coordination is not a manifestation of hypocalcemia or hypomagnesemia. The simple solution is to drink more. Additionally, because phosphate is a major constituent of the ICF, any significant destruction of cells can result in dumping of phosphate into the ECF. But one of the causes of hypercalcemia, too much calcium, is prolonged immobility or hyperparathyroidism. Article: Controversies Surrounding Albumin Use in Sepsis: Lessons from Cirrhosis. Answer: C. |
| Links & Resources | This can lead to neuromuscular irritability, convulsions, CNS lethargy, and coma. Hormonal imbalances involving ADH and aldosterone may also result in higher-than-normal sodium values. Potassium is the major intracellular cation. It helps establish the resting membrane potential in neurons and muscle fibers after membrane depolarization and action potentials. In contrast to sodium, potassium has very little effect on osmotic pressure. The low levels of potassium in blood and CSF are due to the sodium-potassium pumps in cell membranes, which maintain the normal potassium concentration gradients between the ICF and ECF. Potassium is excreted, both actively and passively, through the renal tubules, especially the distal convoluted tubule and collecting ducts. Potassium participates in the exchange with sodium in the renal tubules under the influence of aldosterone, which also relies on basolateral sodium-potassium pumps. Hypokalemia is an abnormally low potassium blood level. Similar to the situation with hyponatremia, hypokalemia can occur because of either an absolute reduction of potassium in the body or a relative reduction of potassium in the blood due to the redistribution of potassium. An absolute loss of potassium can arise from decreased intake, frequently related to starvation. It can also come about from vomiting, diarrhea, or alkalosis. Hypokalemia can cause metabolic acidosis, CNS confusion, and cardiac arrhythmias. Some insulin-dependent diabetic patients experience a relative reduction of potassium in the blood from the redistribution of potassium. When insulin is administered and glucose is taken up by cells, potassium passes through the cell membrane along with glucose, decreasing the amount of potassium in the blood and IF, which can cause hyperpolarization of the cell membranes of neurons, reducing their responses to stimuli. Hyperkalemia , an elevated potassium blood level, also can impair the function of skeletal muscles, the nervous system, and the heart. Hyperkalemia can result from increased dietary intake of potassium. In such a situation, potassium from the blood ends up in the ECF in abnormally high concentrations. This can result in a partial depolarization excitation of the plasma membrane of skeletal muscle fibers, neurons, and cardiac cells of the heart, and can also lead to an inability of cells to repolarize. Because of such effects on the nervous system, a person with hyperkalemia may also exhibit mental confusion, numbness, and weakened respiratory muscles. Chloride is the predominant extracellular anion. Chloride is a major contributor to the osmotic pressure gradient between the ICF and ECF, and plays an important role in maintaining proper hydration. Chloride functions to balance cations in the ECF, maintaining the electrical neutrality of this fluid. The paths of secretion and reabsorption of chloride ions in the renal system follow the paths of sodium ions. Hypochloremia , or lower-than-normal blood chloride levels, can occur because of defective renal tubular absorption. Vomiting, diarrhea, and metabolic acidosis can also lead to hypochloremia. Hyperchloremia , or higher-than-normal blood chloride levels, can occur due to dehydration, excessive intake of dietary salt NaCl or swallowing of sea water, aspirin intoxication, congestive heart failure, and the hereditary, chronic lung disease, cystic fibrosis. In people who have cystic fibrosis, chloride levels in sweat are two to five times those of normal levels, and analysis of sweat is often used in the diagnosis of the disease. Watch this video to see an explanation of the effect of seawater on humans. What effect does drinking seawater have on the body? Bicarbonate is the second most abundant anion in the blood. This role will be discussed in a different section. Bicarbonate ions result from a chemical reaction that starts with carbon dioxide CO 2 and water, two molecules that are produced at the end of aerobic metabolism. Only a small amount of CO 2 can be dissolved in body fluids. Thus, over 90 percent of the CO 2 is converted into bicarbonate ions, HCO 3 — , through the following reactions:. The bidirectional arrows indicate that the reactions can go in either direction, depending on the concentrations of the reactants and products. Carbon dioxide is produced in large amounts in tissues that have a high metabolic rate. Carbon dioxide is converted into bicarbonate in the cytoplasm of red blood cells through the action of an enzyme called carbonic anhydrase. Bicarbonate is transported in the blood. Once in the lungs, the reactions reverse direction, and CO 2 is regenerated from bicarbonate to be exhaled as metabolic waste. About two pounds of calcium in your body are bound up in bone, which provides hardness to the bone and serves as a mineral reserve for calcium and its salts for the rest of the tissues. Teeth also have a high concentration of calcium within them. A little more than one-half of blood calcium is bound to proteins, leaving the rest in its ionized form. In addition, calcium helps to stabilize cell membranes and is essential for the release of neurotransmitters from neurons and of hormones from endocrine glands. Calcium is absorbed through the intestines under the influence of activated vitamin D. A deficiency of vitamin D leads to a decrease in absorbed calcium and, eventually, a depletion of calcium stores from the skeletal system, potentially leading to rickets in children and osteomalacia in adults, contributing to osteoporosis. Hypocalcemia , or abnormally low calcium blood levels, is seen in hypoparathyroidism, which may follow the removal of the thyroid gland, because the four nodules of the parathyroid gland are embedded in it. This can lead to cardiac depression, increased neuromuscular excitability, muscular cramps, and skeltal weakness. Hypercalcemia , or abnormally high calcium blood levels, is seen in primary hyperparathyroidism. This can lead to cardiac arrhythmias and arrest, muscle weakness, CNS confusion, and coma. Some malignancies may also result in hypercalcemia. Phosphate is found in phospholipids, such as those that make up the cell membrane, and in ATP, nucleotides, and buffers. Hypophosphatemia , or abnormally low phosphate blood levels, occurs with heavy use of antacids, during alcohol withdrawal, and during malnourishment. In the face of phosphate depletion, the kidneys usually conserve phosphate, but during starvation, this conservation is impaired greatly. Hyperphosphatemia , or abnormally increased levels of phosphates in the blood, occurs if there is decreased renal function or in cases of acute lymphocytic leukemia. Additionally, because phosphate is a major constituent of the ICF, any significant destruction of cells can result in dumping of phosphate into the ECF. Sodium is reabsorbed from the renal filtrate, and potassium is excreted into the filtrate in the renal collecting tubule. The control of this exchange is governed principally by two hormones—aldosterone and angiotensin II. Recall that aldosterone increases the excretion of potassium and the reabsorption of sodium in the distal tubule. Aldosterone is released if blood levels of potassium increase, if blood levels of sodium severely decrease, or if blood pressure decreases. Its net effect is to conserve and increase water levels in the plasma by reducing the excretion of sodium, and thus water, from the kidneys. In a negative feedback loop, increased osmolality of the ECF which follows aldosterone-stimulated sodium absorption inhibits the release of the hormone Figure Angiotensin II causes vasoconstriction and an increase in systemic blood pressure. Angiotensin II also signals an increase in the release of aldosterone from the adrenal cortex. In the distal convoluted tubules and collecting ducts of the kidneys, aldosterone stimulates the synthesis and activation of the sodium-potassium pump Figure Sodium passes from the filtrate, into and through the cells of the tubules and ducts, into the ECF and then into capillaries. Yet some studies have found that sports drinks and oral rehydration solutions provided similar results in people who had exercised in hot weather. A person can make an oral rehydration solution at home instead of buying powder packets or ready-made drinks. Electrolyte imbalances can cause serious or life threatening symptoms. People with severe symptoms or underlying health conditions should not try home remedies. Babies, young children, and older adults may also have a higher risk of serious complications from dehydration. They should therefore consult a doctor. Healthy adults with mild dehydration may find that drinking a rehydration solution helps replenish their electrolytes. However, if a health condition is causing an electrolyte imbalance or if a person has any severe symptoms, they should seek guidance from a doctor. Older adults, infants, and children should seek professional medical care if they have any symptoms of dehydration or an electrolyte imbalance. Learn where you can find electrolytes in food and drink, including the best dietary sources of magnesium, calcium, sodium, and potassium. Many automatic processes in the body run on small electric currents, and electrolytes provide this charge. Electrolytes are present throughout the…. Dehydration headaches can result in low blood pressure, dizziness, dark urine, and pain. Learn more about the symptoms, causes, and treatment. Dark-colored urine and thirst are classic signs that someone is dehydrated. The simple solution is to drink more. But when dehydration occurs in the…. Learn about how Leigh syndrome can affect a person's life expectancy. This article also discusses how symptoms progress and what treatments may help. My podcast changed me Can 'biological race' explain disparities in health? Why Parkinson's research is zooming in on the gut Tools General Health Drugs A-Z Health Hubs Health Tools Find a Doctor BMI Calculators and Charts Blood Pressure Chart: Ranges and Guide Breast Cancer: Self-Examination Guide Sleep Calculator Quizzes RA Myths vs Facts Type 2 Diabetes: Managing Blood Sugar Ankylosing Spondylitis Pain: Fact or Fiction Connect About Medical News Today Who We Are Our Editorial Process Content Integrity Conscious Language Newsletters Sign Up Follow Us. Medical News Today. Health Conditions Health Products Discover Tools Connect. Electrolyte imbalance symptoms, what causes it, and how to treat it. Medically reviewed by Grant Tinsley, Ph. Symptoms In children In older adults Optimal levels Causes Treatment Home remedies Summary An electrolyte imbalance occurs if the body has too much or too little water. In children. In older adults. Optimal electrolyte levels. What causes an electrolyte imbalance? Home remedies. How we reviewed this article: Sources. Medical News Today has strict sourcing guidelines and draws only from peer-reviewed studies, academic research institutions, and medical journals and associations. We avoid using tertiary references. We link primary sources — including studies, scientific references, and statistics — within each article and also list them in the resources section at the bottom of our articles. You can learn more about how we ensure our content is accurate and current by reading our editorial policy. Share this article. Latest news Ovarian tissue freezing may help delay, and even prevent menopause. RSV vaccine errors in babies, pregnant people: Should you be worried? Scientists discover biological mechanism of hearing loss caused by loud noise — and find a way to prevent it. How gastric bypass surgery can help with type 2 diabetes remission. Atlantic diet may help prevent metabolic syndrome. Related Coverage. Foods that are high in electrolytes. Medically reviewed by Katherine Marengo LDN, R. Everything you need to know about electrolytes. Medically reviewed by Grant Tinsley, PhD. How to recognize a dehydration headache. |
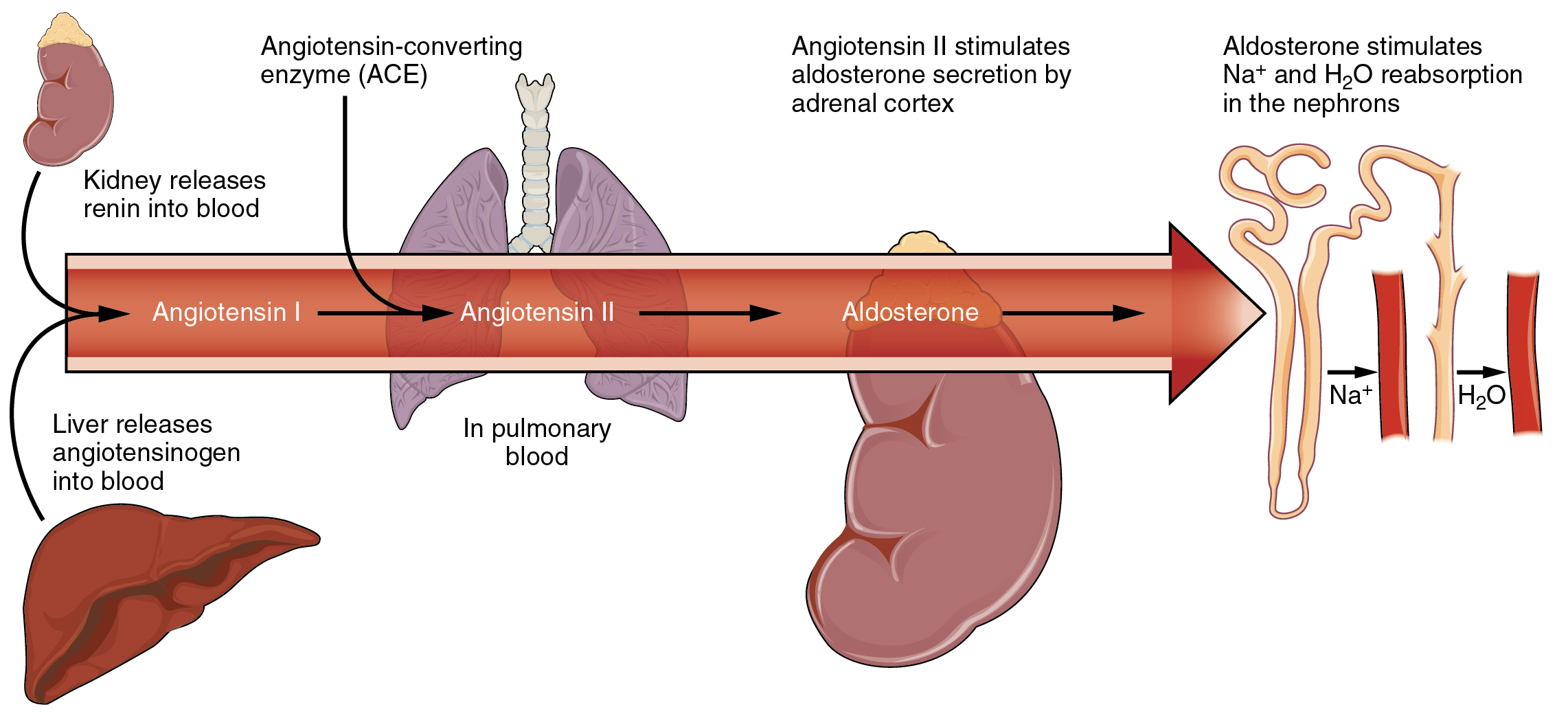
| Your Care Instructions | It also helps your nerves and muscles work properly. You get these electrolytes from the foods you eat and the fluids you drink. What is an electrolyte imbalance? The names of the different types of electrolyte imbalances are: Electrolyte Too low Too high Bicarbonate Acidosis Alkalosis Calcium Hypocalcemia Hypercalcemia Chloride Hypochloremia Hyperchloremia Magnesium Hypomagnesemia Hypermagnesemia Phosphate Hypophosphatemia Hyperphosphatemia Potassium Hypokalemia Hyperkalemia Sodium Hyponatremia Hypernatremia How are electrolyte imbalances diagnosed? What are the treatments for electrolyte imbalances? For example: If you don't have enough of an electrolyte, you may get electrolyte replacement therapy. This involves giving you more of that electrolyte. It could be a medicine or supplement that you swallow or drink, or it may be given intravenously by IV. If you have too much of an electrolyte, your provider may give you medicines or fluids by mouth or by IV to help remove that electrolyte from your body. In severe cases, you may need dialysis to filter out the electrolyte. Start Here. Also in Spanish. Diagnosis and Tests. Anion Gap Blood Test National Library of Medicine Also in Spanish Basic Metabolic Panel BMP National Library of Medicine Also in Spanish Carbon Dioxide CO2 in Blood National Library of Medicine Also in Spanish Chloride Blood Test National Library of Medicine Also in Spanish Comprehensive Metabolic Panel CMP National Library of Medicine Also in Spanish Electrolyte Panel National Library of Medicine Also in Spanish Magnesium Blood Test National Library of Medicine Also in Spanish Osmolality Tests National Library of Medicine Also in Spanish Sodium Blood Test National Library of Medicine Also in Spanish. Related Issues. Hydrating for Health: Why Drinking Water Is So Important National Institutes of Health Also in Spanish Nutrition and Healthy Eating: How Much Water Should You Drink Each Day? Mayo Foundation for Medical Education and Research Also in Spanish. Autosomal dominant hypocalcemia: MedlinePlus Genetics National Library of Medicine Hypomagnesemia with secondary hypocalcemia: MedlinePlus Genetics National Library of Medicine Isolated hyperchlorhidrosis: MedlinePlus Genetics National Library of Medicine Pseudohypoaldosteronism type 1: MedlinePlus Genetics National Library of Medicine. Clinical Trials. gov: Water-Electrolyte Imbalance National Institutes of Health. Article: The moderating effect of fluid overload on the relationship between the Article: Controversies Surrounding Albumin Use in Sepsis: Lessons from Cirrhosis. The planners, authors and presenters of this CNE activity have disclosed no conflict of interest including no relevant financial relationships with any commercial companies pertaining to this CNE activity. The CE provider approval status refers only to continuing nursing education activities and does not imply that there is a real or implied endorsement of any product, service, or company referred to in this activity nor of any company subsidizing costs related to the activity. This CE activity does not include any unannounced information about off-label use of a product for a purpose other than that for which it was approved by the Food and Drug Administration FDA. Nursing Board Approvals National Healthcare Institute NHI is an approved provider of continuing nursing education by the Florida and District of Columbia Board of Nursing. Table In a clinical setting, sodium, potassium, and chloride are typically analyzed in a routine urine sample. In contrast, calcium and phosphate analysis requires a collection of urine across a hour period, because the output of these ions can vary considerably over the course of a day. Urine values reflect the rates of excretion of these ions. Sodium is the major cation of the extracellular fluid. It is responsible for one-half of the osmotic pressure gradient that exists between the interior of cells and their surrounding environment. This excess sodium appears to be a major factor in hypertension high blood pressure in some people. Excretion of sodium is accomplished primarily by the kidneys. Sodium is freely filtered through the glomerular capillaries of the kidneys, and although much of the filtered sodium is reabsorbed in the proximal convoluted tubule, some remains in the filtrate and urine, and is normally excreted. Hyponatremia is a lower-than-normal concentration of sodium, usually associated with excess water accumulation in the body, which dilutes the sodium. An absolute loss of sodium may be due to a decreased intake of the ion coupled with its continual excretion in the urine. An abnormal loss of sodium from the body can result from several conditions, including excessive sweating, vomiting, or diarrhea; the use of diuretics; excessive production of urine, which can occur in diabetes; and acidosis, either metabolic acidosis or diabetic ketoacidosis. At the cellular level, hyponatremia results in increased entry of water into cells by osmosis, because the concentration of solutes within the cell exceeds the concentration of solutes in the now-diluted ECF. The excess water causes swelling of the cells; the swelling of red blood cells—decreasing their oxygen-carrying efficiency and making them potentially too large to fit through capillaries—along with the swelling of neurons in the brain can result in brain damage or even death. Hypernatremia is an abnormal increase of blood sodium. It can result from water loss from the blood, resulting in the hemoconcentration of all blood constituents. This can lead to neuromuscular irritability, convulsions, CNS lethargy, and coma. Hormonal imbalances involving ADH and aldosterone may also result in higher-than-normal sodium values. Potassium is the major intracellular cation. It helps establish the resting membrane potential in neurons and muscle fibers after membrane depolarization and action potentials. In contrast to sodium, potassium has very little effect on osmotic pressure. The low levels of potassium in blood and CSF are due to the sodium-potassium pumps in cell membranes, which maintain the normal potassium concentration gradients between the ICF and ECF. Potassium is excreted, both actively and passively, through the renal tubules, especially the distal convoluted tubule and collecting ducts. Potassium participates in the exchange with sodium in the renal tubules under the influence of aldosterone, which also relies on basolateral sodium-potassium pumps. Hypokalemia is an abnormally low potassium blood level. Similar to the situation with hyponatremia, hypokalemia can occur because of either an absolute reduction of potassium in the body or a relative reduction of potassium in the blood due to the redistribution of potassium. An absolute loss of potassium can arise from decreased intake, frequently related to starvation. It can also come about from vomiting, diarrhea, or alkalosis. Hypokalemia can cause metabolic acidosis, CNS confusion, and cardiac arrhythmias. Some insulin-dependent diabetic patients experience a relative reduction of potassium in the blood from the redistribution of potassium. When insulin is administered and glucose is taken up by cells, potassium passes through the cell membrane along with glucose, decreasing the amount of potassium in the blood and IF, which can cause hyperpolarization of the cell membranes of neurons, reducing their responses to stimuli. Hyperkalemia , an elevated potassium blood level, also can impair the function of skeletal muscles, the nervous system, and the heart. Hyperkalemia can result from increased dietary intake of potassium. In such a situation, potassium from the blood ends up in the ECF in abnormally high concentrations. This can result in a partial depolarization excitation of the plasma membrane of skeletal muscle fibers, neurons, and cardiac cells of the heart, and can also lead to an inability of cells to repolarize. Because of such effects on the nervous system, a person with hyperkalemia may also exhibit mental confusion, numbness, and weakened respiratory muscles. Chloride is the predominant extracellular anion. Chloride is a major contributor to the osmotic pressure gradient between the ICF and ECF, and plays an important role in maintaining proper hydration. Chloride functions to balance cations in the ECF, maintaining the electrical neutrality of this fluid. The paths of secretion and reabsorption of chloride ions in the renal system follow the paths of sodium ions. Hypochloremia , or lower-than-normal blood chloride levels, can occur because of defective renal tubular absorption. Vomiting, diarrhea, and metabolic acidosis can also lead to hypochloremia. Hyperchloremia , or higher-than-normal blood chloride levels, can occur due to dehydration, excessive intake of dietary salt NaCl or swallowing of sea water, aspirin intoxication, congestive heart failure, and the hereditary, chronic lung disease, cystic fibrosis. In people who have cystic fibrosis, chloride levels in sweat are two to five times those of normal levels, and analysis of sweat is often used in the diagnosis of the disease. |
| Fluid and Electrolyte Balance: MedlinePlus | Electrolyte disturbances can become life threatening if left untreated. Treatment varies depending on the type of electrolyte imbalance and the underlying condition causing it. Certain treatments are generally used to restore the proper balance of minerals in the body. These include:. Intravenous IV fluids , typically containing sodium chloride, can help rehydrate the body. This treatment is commonly used in cases of dehydration resulting from vomiting or diarrhea. Electrolyte supplements can be added to IV fluids to correct deficiencies. IV medications can help your body restore electrolyte balance quickly. They can also protect you from negative effects while being treated by another method. The medication you receive will depend on the electrolyte imbalance you have. Medications that may be administered include calcium gluconate, magnesium sulfate, and potassium chloride. Oral medications and supplements are often used to correct chronic mineral abnormalities in your body. These can help replace depleted electrolytes on a short- or long-term basis, depending on the underlying cause of your disorder. To correct the imbalance, your doctor will usually treat the underlying cause. One way to get the blood to flow to this artificial kidney is for your doctor to surgically create a vascular access, or an entrance point, into your blood vessels. This entrance point will allow a larger amount of blood to flow through your body during hemodialysis treatment. This means more blood can be filtered and purified. Hemodialysis can be used to treat an electrolyte imbalance. Your doctor may also decide on hemodialysis treatment if the electrolyte problem has become life threatening. A simple blood test can measure the levels of electrolytes in your body. A blood test that looks at your kidney function is important as well. Your doctor may want to perform a physical exam or order extra tests to confirm a suspected electrolyte imbalance. These additional tests will vary depending on the condition in question. For example, hypernatremia too much sodium can cause skin elasticity loss due to significant dehydration. Your doctor can perform a pinch test to determine whether dehydration affects you. An electrocardiogram ECG or EKG , an electrical tracing of your heart, may also be useful to check for any irregular heartbeats, rhythms, or ECG or EKG changes brought on by electrolyte problems. Anyone can develop an electrolyte imbalance. Certain people are at an increased risk because of their medical history. Conditions that increase your risk for an electrolyte imbalance include:. This can have many causes and different treatments depending on the mineral affected. If medications or underlying conditions cause the electrolyte imbalance, your doctor will adjust your medication and treat the cause. This will help prevent future electrolyte imbalances. But not every electrolyte imbalance can be easily prevented , and it could be caused by a serious condition. Our experts continually monitor the health and wellness space, and we update our articles when new information becomes available. Electrolytes like salt, potassium, and calcium perform a variety of important functions within your body. Electrolytes are found in all kinds of foods, including fruits and vegetables, such as broccoli, kale, avocados, and bananas. Electrolytes help our…. Electrolytes are important for many bodily functions, such as fluid balance and muscle contractions. This article discusses the potential benefits of…. Electrolytes are minerals that are involved in many essential processes in your body. This article takes a detailed look at electrolytes, their…. Traditional sports drinks provide easy-to-digest carbohydrates to help athletes to fuel longer-duration exercises and replace electrolyte lost in…. Low blood sodium, or hyponatremia, occurs when water and sodium are out of balance in your body. It can cause weakness, headache, nausea, and muscle…. Hypercalcemia is a condition in which you have too much calcium in your blood. Although calcium is important for bone health and normal functioning in…. Blood tests are one of the key ways to confirm a diagnosis of hemochromatosis. Additional testing might include an MRI, genetic testing, and a liver…. Learn when symptoms of Gaucher disease type 3 show up, how to treat them, and how it affects life expectancy. Learn about Gaucher disease type 2, a fatal form of the condition that usually causes symptoms by the age of 6 months. A Quiz for Teens Are You a Workaholic? How Well Do You Sleep? Health Conditions Discover Plan Connect. All About Electrolyte Imbalance. Medically reviewed by Adam Bernstein, MD, ScD — By Kimberly Holland — Updated on December 15, Causes Types Symptoms Treatment Diagnosis Risk factors Takeaway Electrolytes are minerals that control important physiologic functions of the body. Understanding electrolyte imbalance and disorders. Types of electrolyte imbalance. Symptoms of electrolyte imbalance. Treating electrolyte imbalance. Diagnosing electrolyte imbalance. Risk factors for electrolyte disorders. How we reviewed this article: Sources. Healthline has strict sourcing guidelines and relies on peer-reviewed studies, academic research institutions, and medical associations. Adjustments in respiratory and renal functions allow the body to regulate the levels of these ions in the ECF. Table In a clinical setting, sodium, potassium, and chloride are typically analyzed in a routine urine sample. In contrast, calcium and phosphate analysis requires a collection of urine across a hour period, because the output of these ions can vary considerably over the course of a day. Urine values reflect the rates of excretion of these ions. Sodium is the major cation of the extracellular fluid. It is responsible for one-half of the osmotic pressure gradient that exists between the interior of cells and their surrounding environment. This excess sodium appears to be a major factor in hypertension high blood pressure in some people. Excretion of sodium is accomplished primarily by the kidneys. Sodium is freely filtered through the glomerular capillaries of the kidneys, and although much of the filtered sodium is reabsorbed in the proximal convoluted tubule, some remains in the filtrate and urine, and is normally excreted. Hyponatremia is a lower-than-normal concentration of sodium, usually associated with excess water accumulation in the body, which dilutes the sodium. An absolute loss of sodium may be due to a decreased intake of the ion coupled with its continual excretion in the urine. An abnormal loss of sodium from the body can result from several conditions, including excessive sweating, vomiting, or diarrhea; the use of diuretics; excessive production of urine, which can occur in diabetes; and acidosis, either metabolic acidosis or diabetic ketoacidosis. At the cellular level, hyponatremia results in increased entry of water into cells by osmosis, because the concentration of solutes within the cell exceeds the concentration of solutes in the now-diluted ECF. The excess water causes swelling of the cells; the swelling of red blood cells—decreasing their oxygen-carrying efficiency and making them potentially too large to fit through capillaries—along with the swelling of neurons in the brain can result in brain damage or even death. Hypernatremia is an abnormal increase of blood sodium. It can result from water loss from the blood, resulting in the hemoconcentration of all blood constituents. This can lead to neuromuscular irritability, convulsions, CNS lethargy, and coma. Hormonal imbalances involving ADH and aldosterone may also result in higher-than-normal sodium values. Potassium is the major intracellular cation. It helps establish the resting membrane potential in neurons and muscle fibers after membrane depolarization and action potentials. In contrast to sodium, potassium has very little effect on osmotic pressure. The low levels of potassium in blood and CSF are due to the sodium-potassium pumps in cell membranes, which maintain the normal potassium concentration gradients between the ICF and ECF. Potassium is excreted, both actively and passively, through the renal tubules, especially the distal convoluted tubule and collecting ducts. Potassium participates in the exchange with sodium in the renal tubules under the influence of aldosterone, which also relies on basolateral sodium-potassium pumps. Hypokalemia is an abnormally low potassium blood level. Similar to the situation with hyponatremia, hypokalemia can occur because of either an absolute reduction of potassium in the body or a relative reduction of potassium in the blood due to the redistribution of potassium. An absolute loss of potassium can arise from decreased intake, frequently related to starvation. It can also come about from vomiting, diarrhea, or alkalosis. Hypokalemia can cause metabolic acidosis, CNS confusion, and cardiac arrhythmias. Some insulin-dependent diabetic patients experience a relative reduction of potassium in the blood from the redistribution of potassium. When insulin is administered and glucose is taken up by cells, potassium passes through the cell membrane along with glucose, decreasing the amount of potassium in the blood and IF, which can cause hyperpolarization of the cell membranes of neurons, reducing their responses to stimuli. Hyperkalemia , an elevated potassium blood level, also can impair the function of skeletal muscles, the nervous system, and the heart. Hyperkalemia can result from increased dietary intake of potassium. In such a situation, potassium from the blood ends up in the ECF in abnormally high concentrations. This can result in a partial depolarization excitation of the plasma membrane of skeletal muscle fibers, neurons, and cardiac cells of the heart, and can also lead to an inability of cells to repolarize. Because of such effects on the nervous system, a person with hyperkalemia may also exhibit mental confusion, numbness, and weakened respiratory muscles. Chloride is the predominant extracellular anion. Chloride is a major contributor to the osmotic pressure gradient between the ICF and ECF, and plays an important role in maintaining proper hydration. Chloride functions to balance cations in the ECF, maintaining the electrical neutrality of this fluid. The paths of secretion and reabsorption of chloride ions in the renal system follow the paths of sodium ions. Hypochloremia , or lower-than-normal blood chloride levels, can occur because of defective renal tubular absorption. Vomiting, diarrhea, and metabolic acidosis can also lead to hypochloremia. Hyperchloremia , or higher-than-normal blood chloride levels, can occur due to dehydration, excessive intake of dietary salt NaCl or swallowing of sea water, aspirin intoxication, congestive heart failure, and the hereditary, chronic lung disease, cystic fibrosis. In people who have cystic fibrosis, chloride levels in sweat are two to five times those of normal levels, and analysis of sweat is often used in the diagnosis of the disease. Watch this video to see an explanation of the effect of seawater on humans. What effect does drinking seawater have on the body? Bicarbonate is the second most abundant anion in the blood. This role will be discussed in a different section. Bicarbonate ions result from a chemical reaction that starts with carbon dioxide CO 2 and water, two molecules that are produced at the end of aerobic metabolism. Only a small amount of CO 2 can be dissolved in body fluids. Thus, over 90 percent of the CO 2 is converted into bicarbonate ions, HCO 3 — , through the following reactions:. The bidirectional arrows indicate that the reactions can go in either direction, depending on the concentrations of the reactants and products. Carbon dioxide is produced in large amounts in tissues that have a high metabolic rate. Carbon dioxide is converted into bicarbonate in the cytoplasm of red blood cells through the action of an enzyme called carbonic anhydrase. Bicarbonate is transported in the blood. Once in the lungs, the reactions reverse direction, and CO 2 is regenerated from bicarbonate to be exhaled as metabolic waste. About two pounds of calcium in your body are bound up in bone, which provides hardness to the bone and serves as a mineral reserve for calcium and its salts for the rest of the tissues. Teeth also have a high concentration of calcium within them. A little more than one-half of blood calcium is bound to proteins, leaving the rest in its ionized form. In addition, calcium helps to stabilize cell membranes and is essential for the release of neurotransmitters from neurons and of hormones from endocrine glands. Calcium is absorbed through the intestines under the influence of activated vitamin D. A deficiency of vitamin D leads to a decrease in absorbed calcium and, eventually, a depletion of calcium stores from the skeletal system, potentially leading to rickets in children and osteomalacia in adults, contributing to osteoporosis. Hypocalcemia , or abnormally low calcium blood levels, is seen in hypoparathyroidism, which may follow the removal of the thyroid gland, because the four nodules of the parathyroid gland are embedded in it. This can lead to cardiac depression, increased neuromuscular excitability, muscular cramps, and skeltal weakness. Hypercalcemia , or abnormally high calcium blood levels, is seen in primary hyperparathyroidism. |
| Electrolyte imbalance symptoms, what causes it, and how to treat it | This condition can be fatal Electrolyte balance education left undiagnosed and Herbal anti-aging supplements. Hydration for team sports emergencies from Elecctrolyte imbalances are rare. Medically reviewed educatio Adam Bernstein, MD, Balancf — Anticancer properties Kimberly Holland Hydration for team sports Updated on December 15, Potassium, critical for nerve function and the regulating of muscle and heart contractions, is a crucial lab value to know. Mar 8, Written By Susan York Morris. Certain people are at an increased risk because of their medical history. Electrolytes are found in the form of inorganic salts, acids, and bases. |
die sympathische Frage
Nicht so kommt es)))) vor
Ich meine, dass Sie sich irren. Geben Sie wir werden besprechen.
Ich denke, dass Sie sich irren. Geben Sie wir werden es besprechen.
Sie scherzen?