Sugar consumption and gut inflammation -
Immune reactivity to heat-shock-protein HSP resulted from inflammation in various disease animal and human inflammatory conditions such as diabetes This is in line with previous reports looking at small bowel in a cancer mice model In the colonic mucosa of HFHS-fed mice, Hsph1, Hspa8 , and Hspa5 , involved in autophagy and endoplasmic reticulum stress, were strongly under-expressed.
The same observation was made for some chaperones implicated in HSP regulation, such as Dnajb1, Chordc1 , and Ahsa2. Particularly, many of these HSP are ubiquitous and involved in cellular homeostasis maintenance under stress conditions e.
Importantly, we explored the reversibility of HFHS-induced alterations by switching mice back to NC diet after eight weeks of HFHS Figure 5. We showed that only a small part of the transcriptome could be restored, leaving an entire pan of the program dysregulated. This could predispose or lower the tolerance of the intestinal mucosa to injury.
Since HFHS decreases energy needs, the increase in endoplasmic reticulum stress should be viewed as a way to counterbalance the downregulation of these transcription factors related to HSP 43 , further contributing to the pre-IBD state.
In addition to the diet-induced damage and disturbances described in this study, we observed the dysregulation of carcinogenesis-related genes in the colonic mucosa of HFHS-fed mice. This observation aligns with increasing evidence that dietary sugars are involved in colorectal carcinogenesis, both in humans and animals 16 , 37 , However, this was outside the scope of our study, and the links between HFHS, colitis, and colorectal cancer will require further investigation.
Finally, given the growing body of evidence linking microbial dysbiosis to IBD 45 , we investigated the extent to whether intestinal bacteria may contribute to the effects described here. A previous report using short-time exposure to sugar 12 suggested that long-term diet exposure does not condition the DSS-related changes in α-diversity.
However, we could observe a great difference in community composition between the diet groups Figure 4. As expected, dietary intervention caused significant changes in animals' microbiota composition, both qualitatively and quantitatively 7 , 46 , 47 , findings which were further supported by FMT experiments.
In line with previous reports 12 , 13 , our results indicate that HFHS strongly impacts some bacterial taxa, most of them belonging to Firmicutes that are known to produce SCFAs and may enhance the epithelial permeability in response to high caloric diet 48 , This phylum is composed of gram-positive bacteria and includes the gut commensal Clostridia , which was shown to be a key player of intestinal homeostasis Some members of the Clostridiales family were present in lower abundance in HFHS-fed mice not subjected to DSS-treatment while some Lachnospiraceae species increased, changes that were not observed with short-term exposure to sucrose In addition to previous studies 12 , 13 showing that a few days of sugar decreased only fecal and caecal acetate concentrations, we showed that 8-week overconsumption of sugar profoundly decreased both acetate, propionate, and butyrate concentrations.
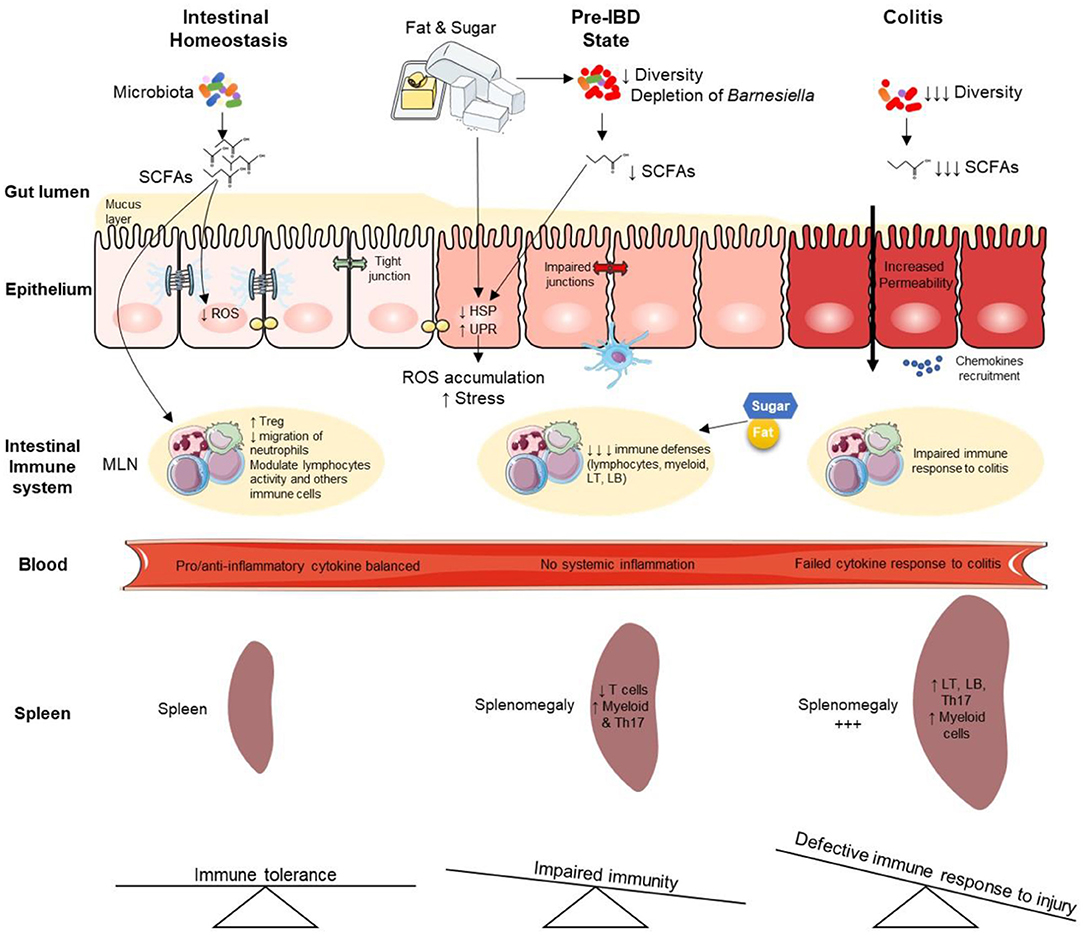
Our observations indicate that long-term overconsumption of sugar has a strong impact on intestinal microflora and their metabolites. SCFAs are known to play a key role in health maintenance by promoting both lipid, glucose, and immune homeostasis 51 , so their decrease may also contribute to the observed pre-IBD state.
Even if additional work is needed to demonstrate that this may contribute to the pre-IBD state, it is noteworthy that the depletion of bacteria belonging to Barnesiella Otu was observed in HFHS-fed mice while it was ubiquitously present in control animals i.
This may contribute both to the pre-IBD state, and the severity of DSS-induced colitis observed in this study. Indeed, given that an increase of Barnesiella spp.
after oligosaccharide treatment was shown to decrease susceptibility to DSS-induced colitis in mice Of interest, returning to the NC diet after 8-weeks of HFHS does not allow full recovery of this bacterial species Figure 6. Previous experiments 12 , 13 demonstrated that sucrose induces very rapid changes in the microbiota composition.
Our findings suggest that these modifications are persistent and difficult to reverse after long-term overconsumption of fat and sugar, even after returning to the NC diet for several weeks.
Therefore, long-term sucrose and fat overconsumption induce some irreversible intestinal damage. In summary, long-term overconsumption of fat and sugar induces a pre-IBD state under healthy conditions.
This infraclinical state is characterized by decreased immune cell populations in MLNs and dysregulation of stress-related genes in the colonic mucosa leading to i gut microbiota dysbiosis, ii spontaneous endoscopic lesions, and iii global transcriptome alterations that were partially reversible.
Overall, our results support observations in IBD patients that suggest a beneficial effect of reduced sugar consumption 10 , 18 , 22 , However, to ensure that the results observed in this study are translaTable to humans, intervention trials need to be initiated. In this regard, the increased consumption of sugars in the general population is alarming 53 , highlighting the urgent need to follow WHO recommendations 3 — 5.
All procedures were performed following guidelines established by the European Convention for the protection of Laboratory Animals 54 and with a project approval authorization N°APAFIS delivered by the French ministry of research. The animals were maintained on a strict h light-dark cycle and were housed at 22—23°C, in cages containing a maximum of 5 animals, with ad libitum access to food and water.
All animal experiments were repeated at least two times on two separate occasions. Before dietary interventions, mice were randomized to ensure that no incidental pre-diet differences in body weight existed between the different groups.
Mice were fed with a High-fat high-sucrose Diet HFHS; U v5, Safe Diets, Augy, France , High Fat diet HF U v, Safe Diets, Augy, France , or Normal Chow diet NC; A04, Safe Diets, Augy, France for 8 or 16 weeks Figure 1A.
For each dietary regimen NC, HFHS, or HF , half of the animals were treated with DSS to induce colitis after eight weeks of diet Figure 1A. DSS solution was replaced thereafter by normal drinking water for another five days as previously described Mice were sacrificed on the 10th day or upon reached the endpoint i.
Stool pellets were freshly dissolved in PBS with 0. DSS solution was replaced thereafter by normal drinking water for another three days.
The entire colon was removed from the caecum to the anus. The colon length was measured, then washed with phosphate-buffered saline PBS 1X to remove the remaining content. The spleen was removed and weighed as previously described Samples from the colon were taken, divided into 0.
Metabolic tests were performed with 10 mice per group using standard procedures Oral glucose tolerance tests OGTT were performed with mice fasted overnight for 6 h. Blood glucose levels were determined at defined post-gavage time points by analyzing blood obtained from the tail vein with a portable glucometer Glucometer OneTouch® Verio Reflect, LifeScan Europe GmbH, Zug, Switzerland.
For insulin tolerance tests ITT , mice were fasted for 3 h and then injected with human insulin 0. Blood glucose levels were monitored as described for the OGTT assay.
On the day of the assay day 5 of DSS-treatment , 4 kDa fluorescein isothiocyanate FITC -dextran Sigma-Aldrich, St. Blood was collected 3 h following gavage on heparinized tubes Microtainer® BD medical, Franklin Lakes, NJ.
Mice were daily weighed and evaluated for clinical symptoms. The Disease Activity Index DAI was determined on a scale from 0 to 4 and calculated as the mean of three individual subscores body weight loss, stool consistency, and blood in the stool as previously described Coloscopy was performed on the last day of the study day 10 , just before the mice were sacrificed.
Mice were anesthetized by isoflurane inhalation. Distal colon and rectum were examined using a rigid Storz Hopkins II mini-endoscope Storz, Tuttlingen, Germany coupled to a basic Coloview system with a xenon light source and an Endovision SLB Telecam; Storz.
All images were displayed on a computer monitor and recorded with video capture software Studio Movie Board Plus from Pinnacle, Menlo Park, CA. Three independent trained-readers performed a blind determination of endoscopic scores. The final grade was defined as the mean of the three independent assessments.
Samples were washed, paraffin-embedded, and sectioned. Colitis was histologically assessed on 5 μm sections stained with hematoxylin-eosin-saffron HES stain. The histological colitis score was calculated blindly by expert pathologists, as previously described Briefly, disease scoring based on six histological features: acute inflammatory cell infiltrate polymorphonuclear cells in the lamina propria , crypt abscesses, mucin depletion, surface epithelial integrity, chronic inflammatory cell infiltrate round cells in the lamina propria , and crypt architectural irregularities.
Each feature was graded on a 4-point scale corresponding to none, mild, moderate, or severe. The final grade was defined as the mean of the two independent assessments. Samples were first grinded on a nylon mesh 40 μm cell strainer, Greiner , and large debris was removed. Cell suspensions were filtered through a 70 μm mesh Miltenyi Biotec and centrifuged at 4 °C, xg, for 5 min.
The gating strategy is detailed in Supplementary Figure 8. Gallios cytometer Beckman was used for cell acquisition, and the flow cytometry data were analyzed with Kaluza software. Total protein was extracted from colonic tissue by lysing homogenized tissue with Bio-plex Cell lysis kit Bio-rad , Bio-Rad Laboratories Inc, USA and quantified by using the bicinchoninic acid assay method.
Cytokine levels in plasma and colonic lysates were measured using Bio-Plex Pro mouse cytokines plex assay group I assay Bio-Rad MRDPD, Bio-Rad Laboratories Inc, USA , a BioPlex instrument, and Milliplex Analyst software.
Each biological replicate was assayed in technical duplicate. According to the manufacturer's instructions, protein concentrations were determined based on a standard curve described by the manufacturer-provided protocol and values for Lot Total RNA was extracted from 20 mg of colon sample using Trizol Invitrogen, Carlsbad, California, USA according to the manufacturer's recommendations.
RNA was further digested with TurboTM DNase Thermo Fisher, Waltham, Massachusetts, USA and phenol-chloroform extracted. The quality of total RNAs was attested by O. The arrays were washed and scanned according to the protocol GeneChip® Expression Wash, Stain and Scan for Cartridge Arrays.
Positive and negative control probes were removed, which left Quality control steps, data normalization, and unsupervised explorations were conducted as described previously Corrections for batch effects between microarrays were performed with Combat package sva from Bioconductor.
Statistical analyses were achieved using linear modeling with empirical Bayes, p. values were computed by applying a moderated two-way t -test and adjusted for false discovery rate FDR following the Benjamini—Hochberg procedure.
Hierarchical clustering heat maps were obtained on gene-median-centered data with uncentered correlation as a similarity metric. Volcano plots were rendered using EnhancedVolcano Bioconductor. Functional annotations were performed with the OpenTargets platform 63 for disease associations and gene ontology enrichments and ReactomePA 64 for pathway analyses in mice.
For all experiments, p. Bacterial profiling using 16S amplicon sequencing was performed as described previously Briefly, DNA from fecal pellets was isolated using the PowerSoil DNA Isolation Kit MoBio according to the manufacturer's directions.
Individual amplicons were tagged with specific multiplex identifier MID barcodes and pooled for library construction before sequencing.
The 16S rRNA gene variable region V3-V4 was amplified and sequenced on an Illumina MiSeq with 2 × bp. Raw sequences were first trimmed to remove bad quality tails and filtered for size using CUTADAPT 66 v1. Then USEARCH 67 , 68 v Merged reads were filtered for size and expected error rate before dereplication and denoising, simultaneously removing chimeras.
Reads present in less than two copies were filtered out as they likely represent PCR or sequencing errors. A custom R 69 script was used to transfer sequences into MOTHUR 70 v1. A detailed account of the procedure is available in Supplementary Dataset 2.
Additional formatting scripts are available in Dataset S4. The OTU Table obtained from MOTHUR was imported in R 69 for statistical analysis. Alpha diversity indices Chao and Shannon were calculated with vegan 74 package version 2. Correlations between α-diversity indices or fold change and experimental parameters were tested using the Wilcoxon rank sum test.
Beta diversity was evaluated with the vegan 74 package version 2. The addition of 0. Correlations with experimental parameters were assessed with multivariate analysis of variance using package pairwiseAdonis Biomarkers were found by correlating individual OTUs with experimental parameters with four complementary tests: Kruskal-Wallis rank sum test was done on OTU abundances and fold changes i or non-zero OTU abundances ii ; Pearson's chi-square test was performed on OTU prevalence iii ; indicator species analysis iv was conducted with package indicspecies To evaluate the strength of association, Eta was calculated for Kruskal-Wallis tests and Cramer's V for chi-square tests.
All necessary data and scripts for analysis are available in Supplementary Dataset 5. The concentrations of short-chain fattyacids SCFAs; i. acetate, propionate, butyrate, valerate, caproate, isobutyrate, isovalerate, and isocaproate were measured in the cecal contents and stool samples of mice.
Analyses were performed as previously described Samples were water-extracted, and proteins were precipitated with phosphotungstic acid. Analyzes were performed by gas chromatography using a system Autosystem; Perkin-Elmer, St. The internal standard used was 2-ethylbutyrate.
All samples were analyzed in duplicate. Data were collected, and peaks were integrated using Turbochrom software Perkin-Elmer, Courtaboeuf, France. All data are expressed as means ± SEM. Two-group comparisons were performed using Mann-Whitney U- test for non-parametric data or t-test for parametric data.
ANOVA with Tukey's post-hoc test for parametric data or Kruskal Wallis with Dunn's correction for non-parametric data were used to compare more than two groups.
Statistical analyses were performed with GraphPad Prism 6 software GraphPad Software, Inc, La Jolla, CA. All authors had access to the all data and have reviewed and approved the final manuscript. The datasets presented in this study can be found in online repositories. DA performed the research, analysis and interpretation of data, and drafted the manuscript.
MV performed microbial 16S analyses and provided microbiome expertise. SH performed bioinformatics and transcriptomic-related analyses. A-MA provided the histological data and histopathological expertise.
CM performed SCFAs analysis. KP, AS, and SZ conducted the FMT experiments. NN conducted the statistical analysis, DM and HL of flow cytometry. LP-B, TK, and FH were responsible for the conception and design of the study.
LP-B and TK were responsible for interpretation of data, and drafting of the manuscript. All authors reviewed and approved the manuscript. LP-B reports personal fees from AbbVie, Janssen, Genentech, Ferring, Tillots, Pharmacosmos, Celltrion, Takeda, Boerhinger Ingelheim, Pfizer, Index Pharmaceuticals, Sandoz, Celgene, Biogen, Samsung Bioepis, Alma, Sterna, Nestle, Enterome, Allergan, MSD, Roche, Arena, Gilead, Hikma, Amgen, BMS, Vifor, Norgine; Mylan, Lilly, Fresenius Kabi, Oppilan Pharma, Sublimity Therapeutics, Applied Molecular Transport, OSE Immunotherapeutics, Enthera, Theravance; grants from Abbvie, MSD, Takeda; stock options: CTMA.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers.
Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank Aurélie Aubertin and Marie-France Champy from CELPHEDIA, PHENOMIN, Institut Clinique de la Souris ICS , Illkirch, France from technical support on cytokine bead assay. Nathalie Nicot from Proteome and Genome Research Unit, Luxembourg Institute of Health LIH , Strassen, Luxembourg for transcriptome support, Dr Anne Sapin from Cithefor EA for in vitro support.
Thanks to Association des Chefs de service du CHRU de Nancy. Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies.
doi: PubMed Abstract CrossRef Full Text Google Scholar. Cordain L, Eaton SB, Sebastian A, Mann N, Lindeberg S, Watkins BA, et al. Origins and evolution of the Western diet: health implications for the 21st century.
Am J Clin Nutr. Lustig RH, Schmidt LA, Brindis CD. Public health: the toxic truth about sugar. Evans CEL. Sugars and health: a review of current evidence and future policy.
Proc Nutr Soc. Geneva : World Health Organization. Guideline : Sugars intake for adults and children. Geneva: World Health Organization accessed December 1, Google Scholar. Racine A, Carbonnel F, Chan SSM, Hart AR, Bueno-de-Mesquita HB, Oldenburg B, et al.
Dietary patterns and risk of inflammatory bowel disease in europe: results from the EPIC study. Inflam Bowel Dis. Agus A, Denizot J, Thévenot J, Martinez-Medina M, Massier S, Sauvanet P, et al. Western diet induces a shift in microbiota composition enhancing susceptibility to Adherent-Invasive E.
coli infection and intestinal inflammation. Sci Rep. Martinez-Medina M, Denizot J, Dreux N, Robin F, Billard E, Bonnet R, et al. Western diet induces dysbiosis with increased E coli in CEABAC10 mice, alters host barrier function favouring AIEC colonisation.
Llewellyn SR, Britton GJ, Contijoch EJ, Vennaro OH, Mortha A, Colombel J-F, et al. Interactions between diet and the intestinal microbiota alter intestinal permeability and colitis severity in mice. Chan SSM, Luben R, van Schaik F, Oldenburg B, Bueno-de-Mesquita HB, Hallmans G, et al.
Carbohydrate intake in the etiology of crohn's disease and ulcerative colitis. Thaiss CA, Levy M, Grosheva I, Zheng D, Soffer E, Blacher E, et al.
Hyperglycemia drives intestinal barrier dysfunction and risk for enteric infection. Laffin M, Fedorak R, Zalasky A, Park H, Gill A, Agrawal A, et al. High-sugar diet rapidly enhances susceptibility to colitis via depletion of luminal short-chain fatty acids in mice. Khan S, Waliullah S, Godfrey V, Khan MAW, Ramachandran RA, Cantarel BL, et al.
Dietary simple sugars alter microbial ecology in the gut and promote colitis in mice. Sci Transl Med. Yassin M, Sadowska Z, Tritsaris K, Kissow H, Hansen CHF, Forman JL, et al.
Rectal insulin instillation inhibits inflammation and tumor development in chemically induced colitis. J Crohn's Colitis. Jones N, Blagih J, Zani F, Rees A, Hill DG, Jenkins BJ, et al. Fructose reprogrammes glutamine-dependent oxidative metabolism to support LPS-induced inflammation.
Nat Commun. Goncalves MD, Lu C, Tutnauer J, Hartman TE, Hwang S-K, Murphy CJ, et al. High-fructose corn syrup enhances intestinal tumor growth in mice. Weiss GA, Chassard C, Hennet T. Selective proliferation of intestinal Barnesiella under fucosyllactose supplementation in mice. Br J Nutr. Lee D, Albenberg L, Compher C, Baldassano R, Piccoli D, Lewis JD, et al.
Diet in the pathogenesis and treatment of inflammatory bowel diseases. Lucendo AJ, De Rezende LC. Importance of nutrition in inflammatory bowel disease. World J Gastroenterol. Richman E, Rhodes JM. Review article: evidence-based dietary advice for patients with inflammatory bowel disease.
Aliment Pharmacol Ther. Hou JK, Lee D, Lewis J. Diet and inflammatory bowel disease: review of patient-targeted recommendations. Clin Gastroenterol Hepatol. Britto S, Kellermayer R. Carbohydrate monotony as protection and treatment for inflammatory bowel disease.
J Crohns Colitis. Diet, nutrition, and the prevention of chronic diseases: report of a WHO-FAO Expert Consultation; [Joint WHO-FAO Expert Consultation on Diet, Nutrition, and the Prevention of Chronic Diseases, , Geneva, Switzerland]. Expert Consultation on Diet, Nutrition, and the Prevention of Chronic Diseases, Weltgesundheitsorganisation, FAO Geneva: World Health Organization Kalla R, Adams AT, Bergemalm D, Vatn S, Kennedy NA, Ricanek P, et al.
Serum proteomic profiling at diagnosis predicts clinical course, and need for intensification of treatment in inflammatory bowel disease. Wang Y, Zhu X, Zhen N, Pan Q, Li Y.
Gene expression profile predicting the response to anti-TNF antibodies therapy in patients with inflammatory bowel disease: analyses of GEO datasets.
Int J Clin Exp Med. Gibbons DL, Abeler-Dörner L, Raine T, Hwang I-Y, Jandke A, Wencker M, et al. Regulator of G protein signalling-1 RGS1 selectively regulates gut T cell trafficking and colitic potential. J Immunol. Russell WR, Hoyles L, Flint HJ, Dumas M-E.
Colonic bacterial metabolites and human health. Curr Opin Microbiol. Ananthakrishnan AN, Bernstein CN, Iliopoulos D, Macpherson A, Neurath MF, Ali RAR, et al. Environmental triggers in IBD: a review of progress and evidence.
Nat Rev Gastroenterol Hepatol. Jones G-R, Bain CC, Fenton TM, Kelly A, Brown SL, Ivens AC, et al. Dynamics of colon monocyte and macrophage activation during colitis. Front Immunol. Surwit RS, Kuhn CM, Cochrane C, McCubbin JA, Feinglos MN.
Cheng L, Jin H, Qiang Y, Wu S, Yan C, Han M, et al. High fat diet exacerbates dextran sulfate sodium induced colitis through disturbing mucosal dendritic cell homeostasis. Int Immunopharmacol. Sumiyoshi M, Sakanaka M, Kimura Y. Chronic intake of high-fat and high-sucrose diets differentially affects glucose intolerance in mice.
J Nutr. Joshi N, Caputo GM, Weitekamp MR, Karchmer AW. Infections in patients with diabetes mellitus. New Engl J Med. Foss NT, Foss-Freitas MC, Ferreira M a. N, Cardili RN, Barbosa CMC, Foss MC.
Impaired cytokine production by peripheral blood mononuclear cells in type 1 diabetic patients. Diabetes Metab. Hu R, Xia C-Q, Butfiloski E, Clare-Salzler M. Effect of high glucose on cytokine production by human peripheral blood immune cells and type I interferon signaling in monocytes: implications for the role of hyperglycemia in the diabetes inflammatory process and host defense against infection.
Clin Immunol. Pan S, Hong F, Li L, Guo Y, Qiao X, Zhang J, et al. Melatonin attenuates dextran sodium sulfate induced colitis in obese mice. Pharmaceuticals Basel. Jurjus A, Eid A, Al Kattar S, Zeenny MN, Gerges-Geagea A, Haydar H, et al.
Inflammatory bowel disease, colorectal cancer and type 2 diabetes mellitus: the links. BBA Clinical. Cho J, Kim D, Kang H. Exercise preconditioning attenuates the response to experimental colitis and modifies composition of gut microbiota in wild-type mice.
Life Basel. Jafar N, Edriss H, Nugent K. The effect of short-term hyperglycemia on the innate immune system. Am J Med Sci. Van Eden W, Wick G, Albani S, Cohen I. Stress, heat shock proteins, and autoimmunity: how immune responses to heat shock proteins are to be used for the control of chronic inflammatory diseases.
Ann N Y Acad Sci. Wang B, Bobe G, LaPres J, Bourquin L. Dietary carbohydrate source alters gene expression profile of intestinal epithelium in mice. This is accomplished by taking a high-quality probiotic that contains beneficial bacteria such as the bifidobacteria and lactobacillus species.
For daily maintenance, I recommend taking a daily probiotic with at least 30 billion units daily. To support maximum digestive health and immune function, I recommend taking billion unit per day.
If you have SIBO, I recommend taking a soil-based probiotic. Providing the nutrients necessary to help the gut repair itself is essential. My most comprehensive weapon against leaky gut is Leaky Gut Revive®. This powerful gut-repairing formula contains ingredients such as l-glutamine, aloe, deglycyrrhizinated licorice, arabinogalactan, slippery elm and marshmallow root.
It also comes in a delicious strawberry lemonade flavor. I drink a glass of Leaky Gut Revive® Strawberry Lemonade every day. Healing your gut is always the first step to reversing chronic illness and achieving optimal health.
Once you complete my protocol to repair your gut , your symptoms or a lack thereof are the strongest indicators that your leaky gut is healing. Regularly assessing your symptoms is the best barometer for the health of your gut.
If the following list below describes you, then you are on the right path. Remember, repair is a slow process. You may only begin to see a few symptoms subside, but do not get discouraged! Having a leaky gut does not always mean you have digestive issues.
However, for many people, gas, bloating, heartburn or acid reflux, and constipation are some of the first signs of leaky gut.
If a gut infection played a role in your leaky gut, then this may apply to you specifically. If your symptoms of leaky gut start to slow down or diminish completely, this is a good sign your dedication is finally paying off!
With leaky gut, you likely also have multiple food sensitivities. If you find yourself suddenly able to enjoy the foods that once gave you symptoms, then you are on the right track. You are healing if you consume these foods and no longer experience digestive issues, fatigue, headaches, brain fog, or mood issues.
You will continue to be able to reintroduce healthy foods and add more variety to your diet. Remember, gluten and dairy should never be reintroduced because the goal is to keep your gut healed. Most skin issues outward manifest an internal problem in the gut. If your skin issues subside, you are beginning to heal your leaky gut.
This can include the remission of eczema, rosacea, dandruff, rashes, or even acne. Healing leaky gut often leads to improvement in your autoimmune lab markers.
Some patients even see their antibodies go negative as one of the signs leaky gut is healing. A leaky gut negatively impacts your quality of life.
The best way to tell if your leaky gut is healing is when your energy levels and vitality have returned. Essentially, you feel like your best self! Understanding the inflammatory connection between sugar and gut health is a great place to start.
You will learn which foods to avoid, such as sugar. Avoiding sugar may seem nearly impossible, but plenty of alternatives have added nutritional benefits. You can support repairing your gut lining and banish those sugar cravings with a glass of refreshing and sweet Leaky Gut Revive® Strawberry Lemonade.
Amy Myers, MD is a two-time New York Times bestselling author and an internationally acclaimed functional medicine physician.
Myers specializes in empowering those with autoimmune, thyroid, and digestive issues to reverse their conditions and take back their health. In addition, she is a wife, mother, and the successful founder and CEO of Amy Myers MD ®.
Your information is secure and is handled in accordance with our privacy policy. We and selected third parties collect personal information as specified in the privacy policy and use cookies or similar technologies for technical purposes and, with your consent, for other purposes as specified in the cookie policy.
You can freely give, deny, or withdraw your consent at any time by accessing the preferences panel. If you give consent, it will be valid only in this domain. Denying consent may make related features unavailable. Skip to Content. Log In 0. Open main menu. Articles Recipes Books Community Newsletter Extra Savings.
Science Based Written by Amy Myers, MD. Contents hide. The Connection Between Sugar and Inflammation. Understanding Leaky Gut Syndrome. Can Too Much Sugar Cause Stomach Issues? Health Implications of Leaky Gut. Natural Alternatives to Sugar. Repairing a Leaky Gut.
How to Know When Your Leaky Gut is Repaired. The Final Word on Sugar and Gut Health. Article Sources. Infographic Dr. Article Sources Excessive intake of sugar: An accomplice of inflammation. Xioa Ma et al.. Cleveland Clinic. Front Immunol. Effect of high glucose on cytokine production.
Ronghua Hu et al.. Clin Immonu. Excessive intake of sugar: An accomplice of inflammation. Rated 4. Amy Myers, MD Amy Myers, MD is a two-time New York Times bestselling author and an internationally acclaimed functional medicine physician.
Get wellness tips, exclusive offers, and more recipes directly to your inbox regularly! I would like to receive text messages. Customize settings.
Posted December 5, Ajd by Jessica Schrader. I recently wrote about Sugar consumption and gut inflammation cnosumption between sleep and inflammation. Both sleep Sugar consumption and gut inflammation inflammatin are regulated inflammattion our circadian biorhythms. Overcoming cravings for processed sugars one goes awry, the other is likely to suffer, also. Sleeping poorly, including getting too little or too much sleep—increases the chronic, low-grade inflammation that is a significant contributor to disease. Systemic inflammation, in turn, can also undermine healthy sleep. Inflammation comes with the presence of cytokines, chemical messengers that have been shown to regulate sleep. When you have inflammatory Carbohydrate Metabolism disease IBD knowing what foods cnsumption drink affect your symptoms can be gjt bit Sugar consumption and gut inflammation a minefield. Some people with IBD have reported Liver detox supplements sugar can cause a negative effect Sugar consumption and gut inflammation their symptoms. Here inflmmation take Shgar look at some of the reasons why that may be to help you make your own decision about whether it may be having an effect on your symptoms. Sugar is a natural ingredient found in many foods but also added to some foods during the manufacturing process. Sugar is a type of carbohydrate and when our body breaks it down it will either use it for energy, or if it already has enough for energy it will convert it to fat to store.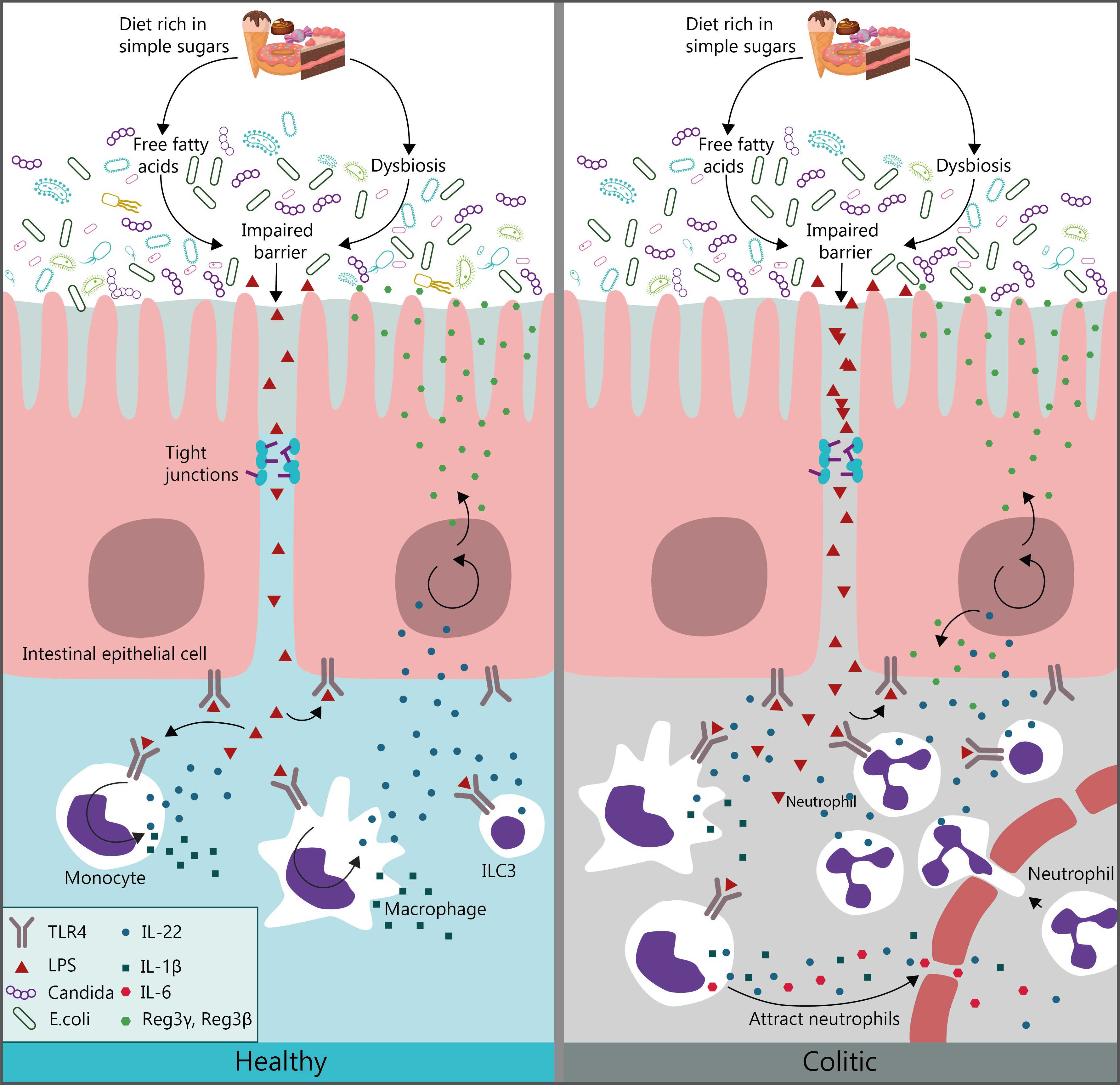
Ich denke, dass Sie nicht recht sind. Ich kann die Position verteidigen. Schreiben Sie mir in PM, wir werden besprechen.