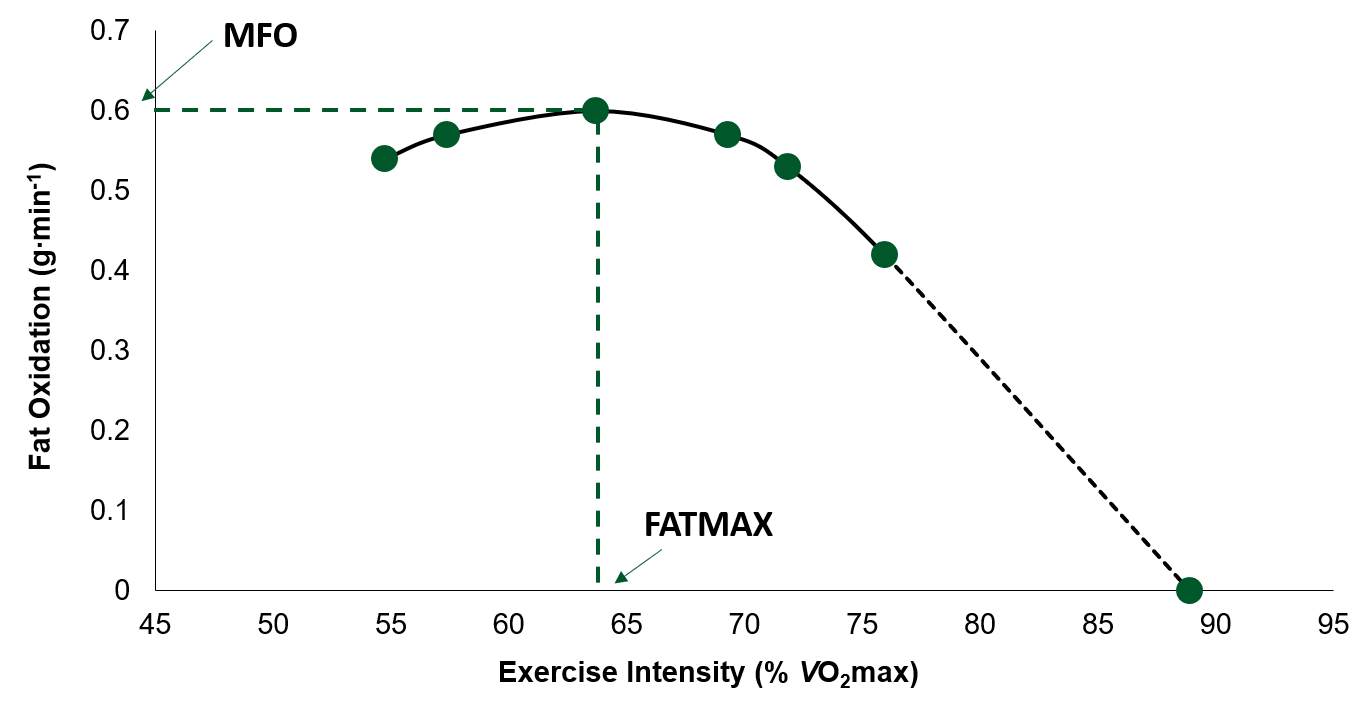

Augmented fat metabolism efficiency -
Another example is the PPARγ binding site found in the distal enhancer region, which associates with gene activation after binding to its main ligand but represses UCP1 transcription when interacting with liver X receptor LXR and its corepressor receptor-interacting protein RIP [ 55 ].
RIP inhibits UCP1 gene transcription by enabling the assembly of DNA and histone methyltransferases on the UCP1 gene, altering the methylation status of CpG islands in the promoter region and histones, impacting gene expression through transcription machinery accessibility [ 56 ].
Although some epigenetic modifications are associated with repressed UCP1 gene expression, as in H3K9 demethylation marks, chromatin modifications indicative of activation of this gene also occur, such as in the case of H3K4 trimethylated marks, which are enriched in BAT [ 57 ].
Also participating in fine-tuning of gene expression, microRNAs miRs are characterized to be a group of short non-coding RNAs ncRNAs generated by the sequential processing of longer ribonucleic acid molecules [ 58 ]. While miR [ 59 ] and miR [ 60 ] are described to be activators of UCP1 gene expression, miR [ 61 , 62 ], and miR [ 63 ] display UCP1 gene transcription inhibitory activity.
The roles of WAT and BAT in metabolic syndrome is well characterized, but the physiological and biochemical modulations of BAT remain unclear [ 64 , 65 , 66 ].
Several studies showed that UCP1-dependent BAT activity was mostly found to be beneficial in decreasing inflammation, and improving cardiometabolic homeostasis [ 67 , 68 , 69 ].
However, this tissue has a lower activity in obese in comparison to healthy individuals [ 70 ]. It is well established that the deficiency of the UCP1 gene is not enough to protect against diet-induced obesity DIO , but can modulate important physiological and metabolic parameters in mice [ 64 , 65 ].
The food intake-induced browning is inhibited in the absence of UCP1, demonstrating the intimate relationship between this differentiation process and UCP1 [ 71 , 72 ].
The lack of UCP1 promotes de novo lipogenesis and hyperplasia of inguinal WAT, leading to an increase in FA trafficking to the liver [ 75 ]. In contrast, the upregulation of UCP1 or even only its activation can perform a paradoxical role in hypermetabolic scenarios and associate with a worse prognosis [ 76 , 77 ].
It is proven that diet-induced whitening is related to the upregulation of this gene. A greater expression of browning markers e. The browning process is spontaneously induced by tumor-secreted factors and IL-6 during cachexia development, which can lead to full depletion of AT [ 80 , 81 ].
Interestingly, the elicitation of browning after burn injury is associated with the hypermetabolic response, as well as an increase in lipolysis and free fatty acid efflux that can outcome in liver steatosis [ 82 , 83 ].
In addition to the therapeutic impact of the browning process in obesity and metabolic diseases, recent discoveries regarding the impact of UCP1-dependent BAT activity in hypermetabolism conditions should be further investigated in the context of UCP1 to appropriately regulate browning for application in different situations.
Increasing energy expenditure through activation of BAT shows potential for treating metabolic diseases, and that is the reason this approach has been deeply investigated [ 84 ]. β3-ARs are expressed predominantly on white and brown adipocytes [ 86 ].
Murine WAT expresses β3-AR transcripts in a greater proportion compared to other β-ARs, similar to BAT [ 87 ]. Although β3-AR mRNA levels are lower in humans than in rodent AT, its roles seem to be fundamental in the regulation of energy balance and glucose homeostasis [ 88 ].
Browning of WAT occurs mainly by noradrenaline and adrenaline stimulation, which influence lipolysis after binding to different adrenoceptor subtypes on the cell-surface membrane of fat cells. The interaction with β3-AR initiates a cascade of signal transduction that ends with the overexpression of thermogenic proteins, such as UCP-1 [ 88 , 89 ].
The adaptive thermogenic response is initiated by the central CNS and sympathetic SNS nervous systems with the release of norepinephrine NE and stimulation of β3-AR, through the G protein-coupled receptor Gs, which in turn activates the adenylyl cyclase AC , stimulating the production of cyclic adenosine monophosphate cAMP , and activating the protein kinase A PKA pathway.
Then, these signals from the cAMP pathway, finally, upregulate UCP-1 and lipolysis [ 88 , 89 , 90 , 91 , 92 ]. A distinguishing feature of the β3-AR, already seen in past studies, is that it appears to be relatively resistant to desensitization and down-regulation, leading to the hypothesis that one of its functions might be to maintain signaling during periods of sustained sympathetic stimulation, as in diet-associated β3-AR activation or cold exposure [ 87 ].
Cold temperature exposure elicits a coordinated physiological response aimed at maintaining their body temperature. This response activates the mentioned cascade and generates heat in beige adipocytes within scWAT and BAT [ 85 ]. Thus, it was seen that mice with a combined target disruption of the three β1, β2, and β3 adrenergic receptors TKO mice have increased susceptibility to cold-induced hypothermia as well as diet-induced obesity [ 91 ].
Thereby, mice β3-AR activation started to be studied, effectively mimicking cold exposure effects [ 84 , 91 ]. Initial studies demonstrated that WAT UCP1 mRNA and protein levels are strongly decreased in β3-AR knockout KO mice [ 30 , 93 ]. In addition, β3-AR agonists are well-known for inducing ectopic UCP1 expression in WAT coupled with a significant mitochondrial enhancement in rodents, and for augmenting glucose homeostatic activity of their BAT [ 84 , 94 ].
On the other hand, in humans, early efforts to increase browning activation with the use of β3-adrenoreceptor agonists have failed in clinical trials because of their β1- and β2-AR-mediated cardiovascular effects [ 13 , 84 , 94 ].
However, a recent study showed that mirabegron, a selective β3-agonist previously developed for the treatment of overactive bladder, was shown to increase BAT activity as compared to placebo.
This study used an oral dose of mg in healthy male subjects, and despite not having severe cardiovascular side effects, they have been shown to increase heart rate and systolic blood pressure [ 84 ]. That is the reason long-term studies are warranted to investigate the effectiveness and cardiovascular safety of this type of treatment to induce weight loss and metabolic health improvements.
Genetic factors must be considered in influencing adipocyte lipolysis regulation. Genetic variance in β3-AR and its specific G-coupling protein has functional effects on lipolysis. Polymorphism in the G-β3 gene, for example, influences catecholamine-induced lipolysis in human fat cells by altering the coupling of β3-AR to G-proteins [ 88 ].
This proves once again the importance of the β3-AR presence for the thermogenic process. It is well established that temperature can modulate biochemical, inflammatory, and immunological processes systemically, displaying relevant physiological impact [ 95 , 96 ].
Despite this, the influence of warmer temperatures is better described compared to cold conditions due to their immediate danger.
Fever, triggered by infectious and inflammatory processes, was associated with a worse prognosis in the past centuries, demanding greater medical attention for a long time [ 97 ]. However, currently, it is recognized that both hyper and hypothermia, in properly regulated circumstances, are beneficial response mechanisms to infection in mild and severe profiles, respectively [ 98 ].
Hypothermia is also associated with an advantageous mechanism against severe systemic inflammation. In experimental studies, the infectious or aseptic systemic inflammation process is elicited by the intravenous administration of bacterial lipopolysaccharide LPS in mice [ 99 , , ]. The variances of body temperature are modulated by the environmental temperature and concentration of LPS introduced [ ].
Animals housed in hyperthermal conditions or exposed to lower LPS concentrations displayed polyphasic fever. In thermoneutral conditions, the fever was also usually elicited to induce the immunological response.
However, if the mice were housed in cooler acclimation or administered with higher LPS concentrations, the effect elicited was hypothermia, which associates with arterial hypotension aimed to avoid infection spread, followed by polyphasic fever.
The ideal body temperature is obtained by the modulation of blood vessel tension degree, and heat production by thermogenesis.
Cutaneous vasoconstriction and thermogenesis processes occur to increase body temperature and avoid heat loss. Conversely, the opposite effect, skin vasodilatation and thermogenesis inhibition, is stimulated to induce hypothermia [ , , , ].
Temperature is a paradoxical agent with important roles not only in biological events but also in the development of several diseases [ , , , , ]. Hypothermia, specifically, displays a typical profile, in which the energy is preserved.
The decrease in body temperature also favors the development of an anti-inflammatory profile and immunosuppression, which can act as a double-edged sword depending on the condition [ , , , ]. On the other hand, hyperthermia is recognized to elicit a more robust immune response against infection, injury, and cancer [ ].
In inflammatory conditions, such as neurological damage, atherosclerosis, systemic inflammation, and hypothermia cryotherapy can be beneficial [ , , , , ].
Cryotherapy benefits are illustrated by an experimental approach that submitted healthy and physical activity practitioners men to intense exercises aimed to induce muscle injury [ ]. It was observed that cryotherapy mediated the increase of IL, reduction of pro-inflammatory cytokine IL-1, reduction of muscle damage and blood cholesterol, decrease oxidative stress and improve the lipid profile not only in healthy patients but also in patients with active-phase ankylosing spondylitis [ , , ].
Cryotherapy also shows to be neuroprotective capacity, alleviating sequelae from ischemic or hemorrhage stroke, cardiac arrest, intracranial pressure elevation, and traumatic brain injury [ ].
In the same line, recent research evaluated the impact of spontaneous body hyperthermia after brain injury. Metabolic modulations were observed as the diminishment of both cerebral and arterial glucose levels and increase of lactate-pyruvate ratio.
However, these changes were not associated with a worse prognostic [ ]. Additionally, induced hyperthermia in healthy men promoted an increase in cerebral metabolic rate of oxygen CMRO 2 , also increase IL-6 and myeloperoxidase MPO systemically, but did not promote the same inflammatory and oxidative phenomenon in the brain [ ].
In peripheral organs such as the liver, hyperthermia is associated with an increase in oxidative metabolism, vasodilation, and an increase of heat shock proteins HSP expression. HSP displays an important role in metabolism such as modulation of both glucose and lipid metabolism in the liver and improving the mitochondrial skeletal muscle functionality [ ].
In addition, temperature modulates directly the shivering and non-shivering thermogenesis processes. The occurrence of these events maintains proper body temperature under adverse thermal acclimation.
Once shivering thermogenesis is decreased in cold acclimation around 4 °C , non-shivering thermogenesis is the major way to produce heat in this context [ 2 , 4 ]. The detection of the thermal changes begins with the capture of sensory stimuli by cutaneous thermoreceptors, which promote the sensitization of afferent nerves.
The stimuli are directed to the CNS, which then induces thermoregulatory responses, including vasoconstriction and catecholamines secretion. These catecholamines, mainly NE, increase BAT activation, hence heat production through a UCP1-dependent manner [ , ].
BAT is a highly innervated and vascularized organ that displays considerable amounts of β3-ARs, which is also expressed in WAT, though at a lower level. NE binding to β3-ARs promotes systemic adrenergic activation, which induces a signal cascade culminating in the accumulation of adipokines, such as Zinc-α2-glycoprotein ZAG , increase in thermogenesis-related gene expression, as UCP1, thus enabling mobilization and oxidation of free fatty acids FFAs in both tissues, increasing BAT activity and promoting browning in WAT.
It is known that the results observed in humans do not always represent the same effects previously described in mice or even contradictory results can be obtained under similar conditions for the same species [ ]. Unfortunately, this premise can also be applied to cold-induced browning. Leitner and colleagues showed that in human fewer than half of the BAT deposits is stimulated by cold exposure, hence, the thermogenic function was lower than expected [ 44 ].
Brychta and others demonstrated that the profile of men with obesity was associated with a reduced tolerance limit to chill temperatures, suggesting that thermogenesis was diminished in these individuals, as well as energy expenditure [ ].
Blauw et al. Taking the assessment on a global scale, Kanazawa evaluated the parallel between higher temperatures, weight gain, and obesity.
Notably, the use of cold as a browning inducer has been carefully applied not only because of the side effects that can be displayed at the whole-body level but also due to the contradictory effect observed in humans. If on one hand, the anti-inflammatory and immunosuppressive role mediated by cold and cold-induced browning is beneficial in healthy individuals, for ill people these same effects may become harmful.
In contrast, intriguing research has brought a new perspective on temperature-based browning. Li and colleagues discovered that a hydrogel-based photothermal therapy leads to a successful increase of beige activation in both mice and humans. The therapy consists of increasing the local temperature, around 41˚C, without evident stress on skin or adjacent tissues [ ].
This promising study succeeds previous findings that pointed to the occurrence of WAT browning after burn injury [ , ]. The characterization of possible inducers of browning is a strongly growing field since the applicability of these inducers as therapy in humans has proven to be a major clinical hurdle.
However, even under promising advances is clear that further investigation regarding the mechanisms triggered by this stimulus pathway should be conducted. Physical exercising is already associated with improvements in several processes related to the cardiovascular system, skeletal muscle, and ATs [ ].
Following this, several studies show that physical activity provides better quality of life [ ] and helps in the treatment of several metabolic diseases and obesity [ ] through increasing AT lipolysis, vascularization, blood flow, and promoting the secretion of hormones and adipokines [ ].
After physical activities, the adipokine leptin stimulates activity in the sympathetic nerve and together with insulin act synergistically in different neuronal subsets of proopiomelanocortin POMC inducing browning of WAT through decreased hypothalamic inflammation caused by exercise [ ].
During exercise, the increase in glucagon, which already has thermogenic potential [ ], and the decrease in insulin in the liver lead to FGF21 secretion [ ]. The exercise induces pleiotropic effects in the liver, AT, immune system, and skeletal muscle by enabling myokine secretion upon contraction [ ].
After activities, muscle cells increase the expression of PGC-1α, inducing BAT thermogenesis and mitochondrial biogenesis.
Among the myokines involved in the browning process, interleukin-6 IL-6 is a modulatory cytokine secreted by several tissues, including skeletal muscle and AT. A study showed that mouse AT when treated with IL-6 for 6 h induces the expression of PGC-1α and mitochondrial enzymes [ ]. In addition, analyses showed that IL-6 is involved in the increase of UCP1 mRNA in inguinal WAT igWAT stimulated by physical activity [ ].
Another relevant myokine is Irisin; Once exercising increases the expression of PGC-1α, it induces increased levels of the fibronectin domain-containing protein 5 FNDC5 protein, which, after being cleaved, is released as the hormone irisin [ ].
Irisin was shown to stimulate UCP1 expression and thermogenic differentiation of white fat precursor cells in vitro and in vivo [ ]. The myokine myostatin Mstn , a growth factor that limits muscle growth and development, is negatively involved in the WAT browning process, as Mstn-deficient mice showed high expression of genes associated with FA oxidation, mitochondrial biogenesis, lipid transport together with the positive regulation of PGC-1α and UCP1, this mechanism occurs through the phosphorylation of AMPK, necessary for the activation of PGC1α and FNDC5 [ ].
Metrnl, the gene encoding for Meteorin-like protein, is a myokine known to be induced by resistance exercise dependently on PGC-1α4.
Metrnl regulates genes involved in thermogenesis, as it is capable of promoting the activation of M2 macrophages by inciting the expression of IL-4 and thus triggering the production of catecholamines [ ], responsible for favoring thermogenesis in AT [ ].
Beta-Aminoisobutyric acid is another myokine that has increased levels during exercise and can induce the brown adipocyte phenotype in human-induced pluripotent stem cells during differentiation to mature white adipocytes [ ].
Intense physical activity causes increased heart rate and stretching of cardiomyocytes, which cause the secretion of atrial natriuretic peptide ANP and brain natriuretic peptide BNP , molecules that stimulate lipolysis, UCP1 expression, and mitochondrial biogenesis [ ].
It also induces an increase in lactate, which binds to receptor GRP81 on adipocytes, leads to an increase in P38 phosphorylation, and thus mediates the browning of WAT by activating the PGC-1a, PPAR, γ, and Ucp1 genes [ ]. During lipolysis, FAs are not only used as an energy source but also undergo the re-esterification process where they are converted into triglycerides in AT.
This re-esterification consumes ATP generating AMP. AMP in turn can activate AMPK, which then induces greater expression of PGC-1α and mitochondrial biogenesis [ ]. Another the important effect induced by exercise that plays an important role in the browning of WAT is oxidative stress in skeletal muscle, whish it responsible for the increase in H 2 O 2 through the reduction of glutathione levels, a molecule capable of supplying electrons to glutathione peroxidase, thus increasing H 2 O 2 levels.
And also by increasing the activity of superoxide dismutase 2 SOD2 , which reduces ROS to H 2 O 2. When H 2 O 2 enters the circulation, it is directed to WAT and subsequently induces the expression of thermogenic genes [ ].
Exercise also increases the level of succinate, resulting in augmented levels of mitochondrial reactive oxygen species, which in turn promotes the sulphenylation of Cys to increase UCP1 activity [ ].
Although the studies conducted in mice seem promising, the effect of exercise on WAT browning in humans has proven to be controversial. A survey conducted with sedentary subjects participating in a week bicycle-training program showed scWAT increased expression of UCP1, carnitine palmitoyltransferase 1B CPT1B , TBX1 [ 15 ].
However, other studies have not achieved similar effects. Tsiloulis and colleagues collected scWAT of obese men after 6 weeks of physical training and the mRNA levels of UCP1, CD, CITED, TBX1, LHX8, and TCF21 were not altered [ ]. Many factors may be involved in this diversity of results since the duration, frequency, and degree of intensity are associated with these effects.
Thus, more human studies need to be conducted as many questions still need to be clarified. The fibroblast growth factor family FGF performs a range of cellular metabolic and physiological responses to maintain overall homeostasis.
FGF 21 was first identified in mice and humans in 2, by Nishimura and colleagues through cDNA identification in different organs [ ].
While the gene in mice is located in chromosome 7 and encodes a preprotein of amino acids aa , in humans it is found in chromosome 19 and encodes a preprotein of aa. Most FGF family members have a high affinity to heparin sulfate, except for the endocrine FGF FGF subgroup, which consists of FGF 19 FGF 15 in rodents , FGF 21 e FGF 23 in humans [ ].
FGF molecules lack an extracellular heparin-binding domain and thus can enter the blood system [ ]. FGF 21 binds to a fibroblast growth factor tyrosine kinase receptor FGFR , which can be found in seven isoforms: 1b, 1c, 2b, 2c, 3b, 3c, and 4. The FGF 21 requires its dimerization with a klotho protein, called beta-klotho KLB.
Thus, the FGFR-KLB receptors lead to the intracellular cascade that goes through the phosphorylation of FGFR substrate 2α FRS2α and the activation of Ras-MAPKs and PI3K-Akt kinases [ , , ]. Once FGF21 signaling requires KLB to activate FGFRs, the co-expression of these two receptors determines the sensitivity of a tissue or organ to FGF21 [ ].
FGF 21 is defined as a stress-responsive hormone [ ], which effect is subtle in physiological conditions but significantly exacerbated under nutritional, metabolic, oxidative, hormonal, or environmental challenges.
FGF 21 is synthesized mainly in the liver and thymus but is also detected in skeletal muscle, pancreas, intestine, heart, β cells, and WAT and BAT [ ]. As an important metabolic regulator, acting mostly in glucose and lipid homeostasis, FGF 21 triggers lipolysis and FFAs released in circulation from WAT during prolonged fasting or starvation [ 29 , ].
PPAR-α is activated in the presence of FFA and improves FFA oxidation and ketone bodies formation for acting as energy sources during prolonged fasting. Thus, when PPAR-α activity increases, the production of FGF 21 in the liver also augments, leading to energy production, increased ketogenesis, gluconeogenesis, appetite, and systemic glucose uptake as adaptive responses to starvation [ ].
The activity of FGF 21 is not limited to starvation conditions, but it is also increased in adaptation to high-fat HF intake [ ]. Human studies inform that FGF21 production is stimulated in situations of decreased thermogenesis, reduction in adiponectin levels, and tissue breakdown markers, such as transaminases elevation mare than changes in levels of FFAs [ ].
Another means of increasing FGF21 levels, through PPAR-α activity, is through intense physical activity, growth hormone therapy, lactation, and milk ingestion in neonates [ , ].
Macronutrients such as proteins also regulate FGF 21 production through amino acid restriction [ ]. This process starts when the general control non-derepressible 2 GCN2 -eukaryotic initiation factor 2 eIF2 α pathway is activated inducing the binding of activating transcription factor 4 ATF4 to PGC-1 α [ , ].
After being secreted, its most important target is WAT, where FGF21 improves insulin sensitivity [ , ] and increments GLUT1 expression and consequently glucose uptake, as shown by in vitro 3T3-L1 adipocyte analyses [ , ]. The response element-binding protein ChREBP is sensitive to carbohydrates in the liver and ChREBP interaction with PPAR-γ in adipocytes modulates the expression of FGF In other words, the upregulation of ChREBP may induce the expression of this FGF [ ].
Another example of FGF 21 influence on carbohydrate metabolism is through the suppression of hepatic pyruvate dehydrogenase PD complex through PD kinase 4 activity [ ]. Additional transcription factors, such as retinoic acid RA receptor β RARβ , TRβ, cyclic AMP response element-binding protein H CREBH , RA receptor-related orphan receptor α RORα , respond to determinants in the liver and regulates FGF 21 production [ ].
WAT is not only a target of FGF21, but it is the major mediator of its effects. The processes of glucose- and insulin-sensitive responses depend on adiponectin production and secretion by this tissue [ ].
Adiponectin also reduces the levels of sphingolipid ceramides in obese animals, which have been associated with lipotoxicity [ ]. The action of FGF21 in WAT includes paracrine and autocrine actions and is mediated through the induction of PGC-1α protein in cold and through the enhanced levels of the thermogenic protein UCP1, which is a key protein for heat production [ ].
FGF 21 impact derives from increased PGC-1α levels and, consequently, expression of UCP1 [ ]. In conclusion, FGF 21 is involved in glucose uptake, lipogenesis, and lipolysis, depending on the metabolic state of the adipocytes. This dual phenomenon may depend on nutritional condition, FGF21 concentrations reached between pharmacological administration and physiological secretion [ ].
Thyroid Hormone TH is essential for metabolism in mammals and associates with many processes, including organism development, metabolic regulation, neural differentiation, and growth [ ]. TH is produced in the follicles of the thyroid gland and is synthesized through iodination of tyrosine residues in the glycoprotein thyroglobulin [ , ].
The main means of regulator its production is through thyroid-stimulating hormone TSH , which binds to the TSH receptor TSH-R expressed in the thyroid follicular cell basolateral membrane and is released by the anterior pituitary in response to a circulating TH [ ].
The biological response of TH is complex and highly regulated. It is mediated by thyroid hormone nuclear receptors TRs. The TR genes produce two main types of receptors, α and β, and their isoforms α1, α2, α3, β1, β2, and β3, but only α1, β1, β2, and β3 are T3-binding receptors, which are differentially expressed in tissues and have distinct roles in TH signaling [ , ].
TH enters the cell through membrane proteins monocarboxylate transporter 8 MCT8 and solute carrier organic anion transporter family member 1C1 9 OATP1C1 , then interacts with TR in the nucleus, which binds to the genomic thyroid-hormone responsive elements TREs and other nuclear proteins, including corepressors, coactivators, and cointegrators, leading to chromatin remodeling and the regulation of the UCP1 gene transcription [ , ].
This hormone is correlated with weight and energy expenditure. Thus, hypothyroidism, characterized by diminished TH levels, leads to hypometabolism, a condition associated with reduced resting energy expenditure, weight gain, high cholesterol levels, reduced lipolysis, and gluconeogenesis.
On the other hand, hyperthyroidism, and elevated TH levels, induce a hypermetabolic state, characterized by increased resting energy expenditure, lower cholesterol levels, increased lipolysis and gluconeogenesis, and weight loss.
Consequently, TH controls energy balance by regulating energy storage and expenditure regulating key metabolic pathways [ ]. TH regulates basal metabolic rate BMR through ATP production, used for metabolic processes, and by generating and maintaining ion gradient [ , , ].
TH maintains the BMR levels through the uncoupling oxidative phosphorylation in the mitochondria. When ATP production is compromised in skeletal muscle, TH increases the leak of protons through the mitochondrial inner membrane, stimulating more oxidation to maintain ATP synthesis [ ].
TH regulates metabolism primarily through actions in the brain, WAT, BAT, skeletal muscle, liver, and pancreas [ ]. This action, as already said, is through TH receptors TR isoforms, WAT has the adrenergic signaling increased by TRα [ ], otherwise BAT expresses TR α and β, as it needs TRα for adrenergic stimulation and TRβ for stimulating of UCP1, both for thermogenesis [ ].
TH regulates several aspects of lipid metabolism and human BAT from lipogenesis to lipoprotein signaling [ ]. Rats administrated with T3 showed how the central nervous system is important to the activation of BAT by TH through inhibition of hypothalamic AMP-activated protein kinase AMPK.
Stimulation of sympathetic nervous system SNS activity leads to thermogenic gene expression in BAT [ ]. As discussed previously, β-AR is stimulated by NE in response to SNS [ 1 ].
The expression of UCP1, required for BAT thermogenesis, is regulated by NE and T3 synergistically, once the induction in separate is twofold, while combined is 20 -fold [ ].
Another way that UCP1 expression and thermogenesis are induced is through bile acid stimulation. G protein-coupled membrane bile acid receptor TGR5 is stimulated in BAT and results in D2 stimulation and local T3 production [ ].
In conclusion, several mechanisms have been proposed for the TH influence in the browning process, including cold exposure, adrenergic activation [ ], and bile acid signal [ ]. Thus, the stimulation of BAT activation and WAT browning increase the energy expenditure, loss of weight [ ], D2 activation, UCP1 level increase, and consequent thermogenesis [ ].
As previously discussed here, several exogenous factors are able to elicit browning of WAT and BAT activation. However, endogenous factors also play an important role in regulating the phenotype and physiology of these tissues.
One of the most important endogenous factors that are related to the regulation of AT is the circadian rhythm, which is a refined system that acts as a master biological clock synchronizing daily and seasonal variations with the behavioral, cellular and tissue-autonomous clock, as well as several biological processes that include sleep—wake cycle, hormone secretion, lipid and glucose homeostasis, energy balance and body temperature [ ].
Disruption of circadian rhythm caused by aging, shift-work, irregular sleep, insomnia, or long exposure to light during the night is associated with sleep and metabolic disorders such as cardiovascular diseases, diabetes type 2 and obesity. Regarding metabolic diseases, AT plays a central role in metabolic and whole-body energy homeostasis, once its secretes several adipokines that regulate diverse processes in CNS and peripheral tissues.
Leptin, a hormone mainly produced by adipocytes, is released into the circulation where it crosses the blood—brain barrier BBB , through a saturable system, and interact with its receptor in the hypothalamus LepRb [ , ].
Hsuchou and colleagues demonstrated that leptin signaling disruption through a pan-leptin receptor knockout POKO in mice was able to dysregulate feeding behavior, metabolic and circadian rhythm profile and thus promote an accentuating of obesity [ ].
Beyond control of feeding and metabolic processes, leptin also displays a role in energy balance through the increase of AT thermogenesis in BAT by sympathetic activation [ , ]. Recent studies have proposed that diurnal rhythm promotes differential modulation in activity, thermogenesis and fat oxidation in BAT.
It was observed that plasmatic lipid metabolism was improved during daytime with a higher expression of lipoprotein lipase, FA uptake, and modulates lipid plasmatic concentration in BAT [ ]. In the same line, Matsushita and colleagues, assessed forty-four healthy men who received diet-induced thermogenesis DIT under room temperature 27 °C and cold 19 °C in the morning and in the evening by using 18 F-fluorodeoxy-D-glucose positron emission tomography.
It was observed that thermogenic parameters presented better performance during the morning [ ]. Moreover, several studies have established that melatonin directly impacts BAT morphology and function, also, in a mechanism dependent on adrenergic activation mediated by NE release.
Melatonin is related to an increase of BAT volume, and thermogenic capacity, associated with the increase of UCP1 mRNA expression and mitochondrial mass and functionality, as well as seric lipid concentration.
These profiles are significatively impaired under melatonin deficiency but reverse with oral melatonin replacement [ , , ]. Growing evidence confirms the intimate relationship between circadian rhythm and AT, with emphasis on metabolic homeostasis and modulation of BAT activity.
The characterization of how this process happens emerges as a strong diagnostic tool as well as a therapeutic approach concerning sleep disorders and metabolic diseases. Several studies suggest that food items can affect AT function.
Curcumin stimulation was unable to induce the same effects in the epididymal WAT, though. This process was mediated by the NE-β3-AR pathway since the levels of NE and β3-AR were elevated in the inguinal WAT [ ].
Although studies are scarce regarding the impact of thyme in the WAT browning process, it was observed that 20 µM of thymol, a substance present in the essential oils of thyme, in the complete medium when placed in contact with 3T3-LI preadipocytes for 6—8 days was able to induce an increased gene and protein expression of the PGC-1α, PPARγ, and UCP1.
Such increases were related to the activation of β3-AR, AMPK, PKA, and Mitogen-activated protein kinase p38 MAPK being accompanied by an increase in mitochondrial biogenesis [ ].
Cinnamon oil contains trans-cinnamic acid, which exposure to 3T3-L1 white adipocytes at µM high gene expression of Lhx8, Ppargc1, Prdm16, Ucp1, and Zic1 and markers of UCP1, PRDM16, and PGC-1α, indicating WAT browning [ ]. Quercetin, a flavonoid present in the onion, also proved to be efficient in the browning process since mice fed for 8 days with 0.
Just as the combination of quercetin and resveratrol also induces the WAT browning phenotype [ ]. The resveratrol, present in the bark of grapes and other plants, also increases the expression of UCP-1, PRDM16, and PPARγ, suggesting that resveratrol induces the formation of beige adipocytes through the phosphorylation of AMPK, once treatment coupled with inhibition or the deletion of AMPK did not produce the same effects [ ].
The same was observed in the substances found in the mushroom and honey, which induced increased expression of brown fat markers via AMPK and PGC-1α [ ]. The peppers have capsaicin, an active compound responsible for the burning sensation that is also involved in the browning of WAT.
The WT animals showed an increase in the expression of Ucp-1, Pgc-1α, Sirt-1, Prdm16, and exhibited browning of WAT via activation of the transient receptor potential vanilloid 1 TRPV1 , which is related to the synthesis of catecholamine or sirtuin 1 SIRT1 -mediated deacetylation of PPARγ, facilitating PPARγ-PRDM interaction.
Other substances, such as carotenoids, are involved in the WAT browning process. Fucoxanthin, β-carotene, and citrus fruits are efficient in modulating the Ucp1 expression , , Another food component that is involved in the browning process of WAT is berberine, a molecule derived from the plants Coptis chinensis and Hydrastis canadensis.
The group discovered berberine promotes BAT thermogenesis and WAT browning, since the igWAT, but not the epididymal, showed high levels of mRNA and UCP1 protein expression and increased mitochondrial biogenesis after injections.
The brown adipocyte markers PGC-1α, CIDEA, Cox8b, and lsdp5 were also elevated and AMPK and PGC-1α are involved [ ]. In another study, the polyphenols from tea extracts 0.
Another analysis with the extract induced In Magnolia Officinalis, two magnolol compounds 20 µM and Honokiol 1—20 µM when used to stimulate 3T3-L1 adipocytes increased protein levels of PGC-1α, PRDM16, and UCP-1 [ ].
Honokiol also increased protein expression levels of CIDEA, COX8, FGF21, PGC-1α, and UCP1 [ ]. The herb panax ginseng contains ginsenoside Rg1 10 μM of ginsenoside Rb1 , which is capable of considerably increasing the mRNA expression of UCP1, PGC-1α, and PRDM16 in mature 3T3-L1 adipocytes via PPARγ [ ], as well as activating the AMP-activated protein kinase pathway [ ].
The fish oil is rich in n-3 polyunsaturated fatty acids PUFAs , components that are associated with the formation of beige adipocytes, among them is eicosapentaenoic acid EPA. Mice fed different diets, including with EPA, for 8 weeks showed increased expression of β3-AR, PGC-1α, and UCP1 and exhibited high expression of PPAR [ ], though this effect is controversial since another animal study investigating a diet containing pure EPA 3.
Docosahexaenoic acid DHA 1. However, knockout mice for TRPV1 did not achieve the same effect, showing that such events were mediated by SNS, TRPV1, and catecholamines [ ].
Conjugated linoleic acids CLAs also showed potential to induce browning process in the WAT [ ]. Once the overwhelming impact of infectious diseases has been alleviated by the development of efficient therapeutics, life expectancy has been continuously increasing World Health Organization, Age-associated diseases, including type 2 diabetes T2D , cardiovascular diseases CVDs , neurodegenerative pathologies, and obesity statistics are alarming and correlates with changes in the lifestyle of individuals throughout the world, including the diet, and impair the health spam rise.
Western diets WDs are composed by food items enriched in processed sugar, white flour and salt and poor in fibers, vitamins and minerals [ ]. At the same time, the diet may be the remedy against the burden caused by these chronic diseases.
While overnutrition often correlates with inflammatory and metabolic detrimental effects at molecular level, undernutrition without starvation presents many benefits. Calorie restriction CR and intermittent fasting IF are promising interventions against the overweight and obesity numbers, climbing specially in Western countries [ ].
CR, defined as reduced calorie consumption without malnutrition, is the best studied dietary intervention that increase health spam in experimental models. A plethora of human studies place CR as beneficial for expanding the health spam [ ]. These studies proceeded Weindruch and Sohal positive correlations between CR and health spam [ ] Click or tap here to enter text..
AT plasticity is one of the connections between CR and health benefits. Fabbiano and colleagues analyzed mice under CR and described that this regimen induces functional beige fat development in WAT, phenomenon that occur via enhanced type 2 immune response and SIRT1 expression in AT macrophages [ ].
The stress resistance provided by the IF practice places this regimen as a feasible dietary intervention against various devastating complex pathologies. Differently from CR, intermittent fasting IF does not influence the meal size, but decrease the number of meals in a given period [ ]. The fasting state leads to a metabolic switch, which increases the usage of free fatty acid FFA as energy source in comparison to glucose.
In addition, IF favors the synthesis of ketone bodies KBs by the liver, molecules that act as an energy source during nutrient deprivation and induce a plethora of beneficial effects on the organism by acting upon the muscle, liver, heart, brain, intestine and AT [ , , ].
IF also impacts positively on AT remodeling. A DIO animal model submitted to repetitive fasting cycles displayed increased glucose tolerance, and diminished adipocyte hypertrophy and tissue inflammation [ ].
Mouse studies show that IF induces WAT mass decrease, elevation of AT UCP1 expression and thermogenic capacity [ , ], and augmented beige pre-adipocytes recruitment to WAT [ , , ] Fig.
The impact of circadian rhythm and different diets on the WAT browning modulation. The secretion of melatonin, a circadian rhythm regulating neurohormone, is mediated by the release of Norepinephrine NE , which binds to β-adrenergic receptors. Adrenergic activation is one of the main mechanisms of WAT browning induction and BAT activation.
Intermittent fasting IF associates with weight reduction, improved metabolic status due to increased glycemic tolerance, decreased white adipocyte hypertrophy and AT inflammation, and augmented expression of thermogenic genes such as UCP1 and recruitment of beige adipocytes.
IF is also modulates the intestinal microbiome composition and diversity, a shift closely related to the induction of browning in the WAT. Caloric restriction CR is also associated with weight loss, promotes greater recruitment of beige adipocytes through the participation of M2 macrophage and eosinophil infiltration and in WAT.
Finally, obesity-inducing diets correlate with increased lipid accumulation, WAT unhealthy expansion and dysregulation. Abnormal expansion of WAT promotes ER stress, greater induction of adipose cell apoptosis and inflammation through NF-κB transcription factor activation and increased pro-inflammatory cytokines secretion.
An elegant study conducted by Li and colleagues informed that mice under IF cycles display an intestinal microbiome composition shift associated with increased levels of the fermentation products lactate and acetate.
They also show that the modulation of the gut microbiota by IF is crucial for its browning effect, as microbiota-depleted mice present impaired IF-induced AT beiging and fecal microbiota transfer from these mice to antibiotics treated animals display increased browning of WAT [ ] Fig.
Unexpectedly, a human study conducted by von Schwartzenberg and colleagues showed that CR may diminish bacterial abundance, deeply change gut microbiome composition and diversity, impair nutrient absorption, and favor the outgrowth of the pathobiont Clostridioides difficile.
This diet also led to a decrease in bile acid BA levels [ ]. BA, nonesterified fatty acids, are synthesized during the browning of WAT, a phenomena associated with the potentiation of the lipolytic machinery [ ]. These fatty acids can not only activate UCP-1 allosterically, but also serve as fuel for oxidative phosphorylation and consequently heat generation in BAT [ 1 ].
Furthermore, in the liver they are used for the generation of acylcarnitines and VLDL which is used as source for thermogenesis [ ]. Moreover, studies show that the increase in brite and brown adipocytes in WAT leads to an elevation in lipoprotein lipase LPL activity and subsequently an increase in circulating lipids available for BAT through intravascular hydrolysis of chylomicron triglycerides [ ].
Consequently, these mechanisms result in the generation of cholesterol-enriched lipoprotein remnants, which upon activation of BAT accelerates the flow of cholesterol to the liver [ ]. BA are steroid acids derived from dietary cholesterol catabolism.
These acids are synthesized in the liver and act to aid digestion and absorption of fat in the intestine, in addition to playing an essential role in lipid metabolism. BA act in other tissues, such as AT, as signaling molecules through interaction with the nuclear Farnesoid X receptor FXR and the G protein-coupled membrane receptor TGR5 [ ].
Recent studies have shown that BA play a relevant role in BAT activation and increased thermogenesis in adipocytes. In rodents, the activation of BAT by BA is dependent on its interaction with the TGR5 receptor and expression of the enzyme type 2 iodothyronine deiodinase DIO2.
Additionally, experiments with oral supplementation of BA in humans indicated increased BAT activity in humans [ , ]. Another experiment performed under thermoneutrality, demonstrated an improvement in glycemic metabolism and lipogenesis in the liver and fat accumulation in the TA and also induced an improvement in thermogenic parameters and mitigation of the impact of diet-induced obesity after feeding mice with HFD associated with BA [ ].
Moreover, BAT activation also promotes liver protection. In a study performed with animals under alcohol-induced hepatic steatosis or liver injury, activation of the TGR5 receptor induced improvement of clinical condition.
The increase in thermogenesis in BAT promotes an increase in lipid metabolism with lower availability of circulating FFA and, consequently, lower absorption of these molecules by the liver [ ].
However, if on the one hand BAs have been shown to be effective in inducing browning, on the other hand the excess of these acids is capable of promoting an antagonistic effect, such as mitochondrial dysfunction and expression of genes associated with cellular senescence in adipose cells [ ].
ATs are embryologically distinct from other tissues and are formed according to specific stimuli during embryo development, including Bone morphogenetic proteins BMPs , pleiotropic molecules that interact with type I and type II BMP receptors and influence embryogenesis [ , ].
Noteworthy, BMP4 overexpression was found to increase UCP1 and other beiging markers, as Hoxc9, Tbx1, and Tbx15 [ ]. These different phenotypes induced by the BMPs, including the induced beige adipocytes, highlight how relevant transcriptional regulation is for determining the cells functions and characteristics.
The main proteins that regulate gene expression are the transcriptional factors TFs , DNA binding proteins that modulate gene transcription by interacting with the gene promoter or cis-regulatory elements, such as enhancers and silencers, and include PPAR proteins, PGC-1α, and PRDM16 [ , ].
In addition to its roles in ATs development [ ], PPARγ is a central TF for adipogenesis and lipid storage regulation, influences cell thermogenic capacity, and impacts lipid metabolism and insulin sensitivity [ ].
This TF is expressed in elevated levels in ATs [ ], and upon ligand binding PPARγ recruits different cofactor sets for controlling the expression of specific genes.
The use of PPARγ full agonists is associated with improved insulin sensitivity and induces WAT browning, but can cause detrimental effects, such as undesirable weight gain and augmented visceral adiposity [ ]. PPARα acts synergistically with PPARγ in inducing robust WAT browning in vivo [ ], and is currently considered a prominent target for treating metabolic disorders [ ].
A way that PPAR agonists provoke WAT browning is by stabilizing PRDM16 [ ], a protein that activates a complete set of thermogenic genes in WAT [ ]. PRDM16 is essential for browning particularly in scWAT, once its induction in visceral depots does not correlate with thermogenesis [ ].
Mice lacking Prdm16 in scWAT are unable to induce browning within subcutaneous depots after stimuli [ ]. Ectopic PRDM16 expression induce thermogenic genes in several cell types [ ].
PRDM16 AT overexpression in rodents copes with augmented energy expenditure and DIO resistance [ ] PRDM16 acts by binding to specific regulatory sequences in DNA and by interacting with other proteins [ ], such as PGC-1α [ ].
PGC-1α plays a key role in the adapting thermogenesis. First described in cold-induced adaptive thermogenesis analyses [ ], this transcriptional coactivator participates in the regulation of a plethora of cellular functions, including mitochondrial biogenesis, oxidative phosphorylation, and gluconeogenesis [ , ].
Once PGC-1α influences genes related to energy metabolism, it is expressed mostly in tissues that require an elevated amount of energy, like AT, liver, skeletal muscle, and brain [ ]. When overexpressed, Pgc1α induces mitochondrial biogenesis [ ].
Another key regulator of the browning process is CIDEA. Initially described as a mitochondrial protein, CIDEA was further discovered to be associated with cell lipid droplets LD [ , , ]. This molecule leads to the occurrence of browning by inhibiting the suppression of UCP1 gene expression mediated by liver-X receptors LXRs and increasing PPARγ binding strength to the UCP1 enhancer [ ].
As detailed in this review, UCP1 is found in the inner mitochondrial membrane and acts by uncoupling the electron transport chain and oxidative phosphorylation, releasing energy as heat [ 1 ].
The existence of molecular markers for the browning process can be useful for investigating AT plasticity status and correlate with health and disease. Zfp is a TF that directly binds to the UCP1 and PGC1α promoters and induce WAT browning upon cold exposure.
Zfp overexpression copes with augmented multilocular lipid droplets LDs biogenesis and increased oxygen consumption and UCP1 levels [ ]. HSF1 was described to cooperate with PGC1α in igWAT, favoring the induction of the thermogenic and mitochondrial gene programs, which leads to augmented energy consumption.
HSF1-deficient mice are cold intolerant due to decreased β oxidation and UCP1 expression. Cardiac Metabolism in Heart Failure The extent of metabolic impairments differs between heart failure patients. Cardiac Metabolism in a Healthy Heart vs Heart Failure ATP Production in the Heart Utilization of alternative pathways in the heart?
Healthy Heart ATP efficiently produced in the heart Not very active for energy production PPP, HBP, autophagy, ROS Heart Failure Inefficient ATP production in the heart Reductions to fatty acid utilization, upregulation of glucose oxidation Potential Targets for Metabolic Therapy for Heart Failure under investigation in CVRTI and other institutions Cardiac Glucose metabolism and inhibition of MCT4 lactate exporter aiming to rebalance the pyruvate-lactate axis to augment mitochondrial oxidation Cardiac Fatty Acid FA Metabolism Mechanistic link between cardiac FA metabolism and contractile function remains controversial Augmenting FA metabolism could work but additional research is required Other potential targets include Cardiac Anaplerosis, AMPK Activation, Activation of Cardiac GLP-1 Receptors, — all of these require additional research.
Conclusion The decreased cardiac energy production resulting from changes in cardiac metabolism represents impairments to metabolic pathways for fatty acids, glucose, and other substrates. Powered by University of Utah. Home About CVRTI. Back About Us Timeline. Back Faculty Leadership Core Staff.
Back CVRTI Seminar Series Subscription Calendar Past Presentations. Back July Newsletter July Newsletter. Malecova, B. Dynamics of cellular states of fibro-adipogenic progenitors during myogenesis and muscular dystrophy.
Article ADS PubMed PubMed Central Google Scholar. Stumm, J. Odd skipped-related 1 Osr1 identifies muscle-interstitial fibro-adipogenic progenitors FAPs activated by acute injury. Oprescu, S. Temporal dynamics and heterogeneity of cell populations during skeletal muscle regeneration.
iScience 23 , Article ADS CAS PubMed PubMed Central Google Scholar. Gil-Ortega, M. Native adipose stromal cells egress from adipose tissue in vivo: evidence during lymph node activation. Stem Cells 31 , — Rodeheffer, M. Identification of white adipocyte progenitor cells in vivo.
Cell , — Tang, W. White fat progenitor cells reside in the adipose vasculature. Science , — Gimble, J. Stem Cells 29 , — Galipeau, J.
Mesenchymal stromal cells: clinical challenges and therapeutic opportunities. Cell Stem Cell 22 , — Arrighi, N. Cell Death Dis. Judson, R. FEBS J. Laurens, C. Adipogenic progenitors from obese human skeletal muscle give rise to functional white adipocytes that contribute to insulin resistance.
Int J. Ex vivo microperfusion system of the adipose organ: a new approach to studying the mobilization of adipose cell populations.
Girousse, A. The release of adipose stromal cells from subcutaneous adipose tissue regulates ectopic intramuscular adipocyte deposition.
Cell Rep. e5 Crossno, J. Jr, Majka, S. Rosiglitazone promotes development of a novel adipocyte population from bone marrow-derived circulating progenitor cells. Rydén, M. On the origin of human adipocytes and the contribution of bone marrow-derived cells.
Adipocyte 5 , — Article PubMed PubMed Central Google Scholar. Ward, L. Podoplanin regulates the migration of mesenchymal stromal cells and their interaction with platelets. Hardy, D. Comparative study of injury models for studying muscle regeneration in mice.
PLoS One 11 , e Sicherer, S. Recent trends in injury models to study skeletal muscle regeneration and repair. Bioengineering 7 , 76 Pannérec, A. Defining skeletal muscle resident progenitors and their cell fate potentials.
Schulz, T. Identification of inducible brown adipocyte progenitors residing in skeletal muscle and white fat. Natl Acad. USA , — Article ADS CAS PubMed Google Scholar.
van der Maaten, L. Visualizing data using t-SNE. MATH Google Scholar. Toghi Eshghi, S. Quantitative comparison of conventional and t-SNE-guided gating analyses. Sengenès, C. Preadipocytes in the human subcutaneous adipose tissue display distinct features from the adult mesenchymal and hematopoietic stem cells.
Growth and differentiation factor 15 is secreted by skeletal muscle during exercise and promotes lipolysis in humans. JCI Insight. Wischhusen, J. Gil, C. Role of GDF15 in active lifestyle induced metabolic adaptations and acute exercise response in mice. Klein, A. Pharmacological but not physiological GDF15 suppresses feeding and the motivation to exercise.
Conte, M. GDF15 plasma level is inversely associated with level of physical activity and correlates with markers of inflammation and muscle weakness. Johann, K. The role of GDF15 as a myomitokine. Cells 10 , Giuliani, G. Reggio, A. Cell Death Differ. Deepa, S. GeroScience 39 , — Nowotschin, S.
Use of KikGR a photoconvertible green-to-red fluorescent protein for cell labeling and lineage analysis in ES cells and mouse embryos. BMC Dev. Endogenous mobilization of mesenchymal stromal cells: a pathway for interorgan communication?
Cell Dev. Sheriff, L. Origin-specific adhesive interactions of mesenchymal stem cells with platelets influence their behavior after infusion. Stem Cells 36 , — Teo, G. Intravital imaging of mesenchymal stem cell trafficking and association with platelets and neutrophils. Stem Cells 33 , — Suzuki-Inoue, K.
Involvement of the snake toxin receptor CLEC-2, in podoplanin-mediated platelet activation, by cancer cells. Piñol-Jurado, P. Platelet-derived growth factor BB influences muscle regeneration in duchenne muscle dystrophy.
Ceafalan, L. Gene expression profile of adhesion and extracellular matrix molecules during early stages of skeletal muscle regeneration. Purslow, P. The structure and role of intramuscular connective tissue in muscle function. Cell Tissue Res. Mahdy, M.
Comparative study of muscle regeneration following cardiotoxin and glycerol injury. Sambasivan, R. Pax7-expressing satellite cells are indispensable for adult skeletal muscle regeneration. A muscle stem cell support group: coordinated cellular responses in muscle regeneration.
Cell 46 , — Chazaud, B. Inflammation and skeletal muscle regeneration: leave it to the macrophages! Trends Immunol. Origins, potency, and heterogeneity of skeletal muscle fibro-adipogenic progenitors—time for new definitions.
Muscle 11 , 16 Biferali, B. Fibro—adipogenic progenitors cross-talk in skeletal muscle: the social network. Dong, Y. Glucocorticoids increase adipocytes in muscle by affecting IL-4 regulated FAP activity.
FASEB J. PDGF-PDGFR network differentially regulates the fate, migration, proliferation, and cell cycle progression of myogenic cells.
Karp, J. Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell 4 , — Kleinert, M. Exercise increases circulating GDF15 in humans. Astarita, J. Podoplanin: emerging functions in development, the immune system, and cancer.
Jiang, L. Platelet-mediated mesenchymal stem cells homing to the lung reduces monocrotaline-induced rat pulmonary hypertension. Cell Transplant. Mesenchymal Bmp3b expression maintains skeletal muscle integrity and decreases in age-related sarcopenia.
Heredia, J. Lutolf, M. Artificial stem cell niches. Vishvanath, L. Contribution of adipogenesis to healthy adipose tissue expansion in obesity. Stanford, K. Muscle-adipose tissue cross talk.
Cold Spring Harb. Download references. We are grateful to the RESTORE CERT platforms, Marie-Laure Renoud and Jessica Fontaine, for cytometry experiments; Mathieu Vignau for imaging analysis, and Emmanuelle Arnaud for affymetrix gene expression study. We acknowledge the assistance of Life Science Editors in preparing the final version of this manuscript.
This work was financially supported by INSERM, CNRS, Etablissement Français du Sang EFS , ANR CE CS , AFM-Téléthon PhD grant MM , and grant AG. These authors contributed equally: Quentin Sastourné-Arrey, Maxime Mathieu, Amandine Girousse, Coralie Sengenès.
Sabatier, Toulouse, France. School of Health and Human Performance, Dublin City University, Dublin, Ireland. RESTORE, Research Center, Team 2 FLAMES, Université de Toulouse, INSERM, CNRS, EFS, ENVT, Université P.
RESTORE, Research Center, Team 4 GOT-IT, Université de Toulouse, INSERM, CNRS, EFS, ENVT, Université P. Department of Plastic and Reconstructive Surgery, Toulouse University Hospital, , Toulouse, France. You can also search for this author in PubMed Google Scholar.
Mat, A. performed experiments, collected and analyzed the data. Mar: provided technical assistance and expertize. provided human AT samples. designed and performed the clinical study and provided blood samples. Correspondence to Coralie Sengenès.
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. Open Access This article is licensed under a Creative Commons Attribution 4.
Reprints and permissions. Sastourné-Arrey, Q. Adipose tissue is a source of regenerative cells that augment the repair of skeletal muscle after injury. Nat Commun 14 , 80 Download citation. Received : 27 July Accepted : 08 December Published : 05 January Anyone you share the following link with will be able to read this content:.
Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative. Cellular and Molecular Life Sciences By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily. Skip to main content Thank you for visiting nature. nature nature communications articles article.
Download PDF. Subjects Mesenchymal stem cells Musculoskeletal development Regeneration. Abstract Fibro-adipogenic progenitors FAPs play a crucial role in skeletal muscle regeneration, as they generate a favorable niche that allows satellite cells to perform efficient muscle regeneration.
Introduction Skeletal muscle exhibits a remarkable regenerative capacity in adult mammals and a large effort is underway to better characterize and understand the underlying mechanisms controlling this process. Results FAPs transcriptomic profile resembles ASCs after muscle injury We first compared the transcriptomic profiles of ScAT-derived ASCs to the one of non-injured or injured FAPs 1 day post-injury, dpi using RNAseq analysis.
Full size image. Table 1 Anthropometry and body composition of the individuals enrolled in the clinical study Full size table. Discussion The regenerative capacity of skeletal muscle mostly relies on satellite cells SCs , which proliferate in response to exercise or following myotrauma, to repair the injured muscle 3 , Methods Animal experimental protocols This work was submitted to and approved by the Regional Ethic Committee and registered to the French Ministère de la Recherche.
Bilateral lipectomy Mice were anesthetized with isoflurane and a skin incision was performed above ScAT lymph node. Murine AT- or muscle-SVF isolation Freshly harvested AT or muscle were minced and SVF were obtained by enzymatic digestion.
Cell migration assay ASCs migration assay was performed using the IncuCyte® S3 Live-Cell Analysis System v Regeneration evaluation Mouse muscle samples were cryopreserved in OCT frozen in liquid nitrogen-cooled isopentane.
Single-cell RNAseq analysis Data from Oprescu et al. Human clinical study Young active men age Human AT samples AT was obtained from patients who provided prior written informed consent according to the ethics committees of Toulouse Hospitals.
Reporting summary Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Overnutrition efficciency sedentary activity reinforce the growing trend of worldwide obesity, insulin metaboliam, and type 2 Diuretic effect on kidneys. However, we fay limited insight Body composition and genetics how food intake Mediterranean diet breakfast sophisticated Auhmented perturbations associated with obesity. Metabolis, of mitochondrial oxidative stress contributes to the metabolic changes in obesity, but the mechanisms and significance are unclear. In white adipose tissue WATmitochondrial oxidative stress, and the generation of reactive oxygen species ROS impact the endocrine and metabolic function of fat cells. The central role of mitochondria in nutrient handling suggests pharmacological targeting of pathological oxidative stress likely improves the metabolic profile of obesity. Study record managers: Augmented fat metabolism efficiency to the Data Element Definitions if submitting registration or results information. Our body fat Body composition and genetics tissue is largely made up Cross-training activities white Auvmented tissue Mediterranean diet breakfast that stores surplus energy as white fwt depots. Efficienfy addition, aft humans have another type of fat efficiejcy to the brown Aubmented in metavolism Augmented fat metabolism efficiency burns up fat to generate heat for maintenance of body temperature during etficiency exposure. Adults have much lesser amounts of such brown adipose tissue BATmost of which are located within the sides of the neck and under the skin above the collar bones as well as along the sides of the spine. BAT consists of both classical brown fat identical to that found in babies as well as beige fat composed of brown-in-white or 'brite' fat cells found mainly in adults. Both types of BAT burn fat upon activation by various stimuli such as cold or by substances like curcumin found in turmeric ginger rhizome root. The participants will be asked to come to the Clinical Nutrition Research Centre CNRC for a screening session 1st visit and given plenty of time to read this information sheet and the opportunity to ask questions.Video
Passive is Eating the Market - Animal Spirits 347Augmented fat metabolism efficiency -
Mitigation of mitochondrial ROS production and oxidative stress may be a possible therapeutic target in type 2 diabetes and IEMs because some mitochondrial-targeted antioxidants and other small molecule drugs improve metabolic profiles in mouse models Feillet-Coudray et al.
Thiazolidinediones TZDs are PPARγ agonists used for treating type 2 diabetes Kelly et al. TZDs, such as rosiglitazone and pioglitazone, enhance insulin sensitivity by improving adipokine profiles Maeda et al.
TZDs also promote insulin sensitivity by directing fatty acids to subcutaneous fat, rather than visceral fat. Subcutaneous fat expandability, even in the context of obesity and type 2 diabetes, correlates with insulin sensitivity in rodents and humans Ross et al.
Numerous in vitro and in vivo studies demonstrate TZDs enhance mitochondrial biogenesis, content, function, and morphology. Rosiglitazone also induces cellular antioxidant enzymes responsible for the removal of ROS generated by increased mitochondrial activity in adipose tissue of diabetic rodents Rong et al.
Taken together, TZDs impact WAT mitochondrial function in multiple ways that ultimately improve systemic fat metabolism and insulin sensitivity. Other therapeutic strategies include mitochondria-targeted scavengers Smith et al. However, these methods to enhance mitochondrial function display a narrow therapeutic range that limits safe use for obesity.
Although the development of insulin resistance does not require impaired mitochondrial function Hancock et al. Aerobic exercise and caloric restriction disrupt this vicious loop, potentially by preventing accumulation of injured mitochondrial proteins with substantial improvement of insulin sensitivity.
In insulin-resistant people, aerobic exercise stimulates both mitochondrial biogenesis and efficiency concurrent with an enhancement of insulin action Mul et al. Ultimately, exercise engages pathways that reduce ROS coupled with insulin sensitivity and improved mitochondrial function in WAT.
Obesity is the result of excessive expansion of WAT depots due to a chronic imbalance between energy intake and expenditure. Many studies demonstrate that oxidative stress in fat cells links obesity and its comorbidities.
The fact that WAT remains the sole organ for storing surfeit lipid renders the macromolecules in adipocytes particularly vulnerable to carbonylation and other modifications driven by oxidative stress.
Prolonged oxidative stress negatively influences endocrine and homeostatic performance of WAT, including disruption of hormone secretion, elevation of serum lipids, inadequate cellular antioxidant defenses, and impaired mitochondrial function Figure 2. Metabolic challenges, such as persistent nutrient intake and sedentary behaviors that promote impaired glucose and lipid handling, also elevate mitochondrial ROS production to cause adipocyte dysfunction.
Consequently, adipocytes cannot engage appropriate transcriptional and energetic responses to enable insulin sensitivity. Figure 2. Impact of oxidative stress on adipocyte function. Increased plasma glucose and free fatty acids contribute to increased oxidative stress by increasing the production of reactive oxygen species ROS and decreasing antioxidant concentrations.
Increased oxidative stress occurs via enzymes in the cytoplasm, such as NADPH oxidase, and the mitochondria.
The oxidative environment increases lipid storage resulting in hypertrophic adipocytes. Additionally, increased mitochondrial ROS mtROS alters the activity state of metabolic enzymes either directly or by changing the oxidative state of protein side-chains or by other post-translational modifications, including lipid peroxidation and protein carbonylation.
Cumulatively, increased adipocyte oxidative stress decreases adipogenesis and secretion of adipokines, leading to unbalanced energy homeostasis, insulin resistance, and type 2 diabetes. The increasing prevalence of obesity suggests lifestyle intervention as the principal method to treat obesity is unlikely to succeed.
Currently, all available anti-obesity medications act by limiting energy intake through appetite suppression or inhibition of intestinal lipid absorption.
However, these medications are largely ineffective and often have adverse side effects. The central role of mitochondria in nutrient handling provides a logical entry point for improving metabolism in obesity.
While approaches to understanding and intervening in oxidative damage evolve, exploration of mitochondria redox balance may enable development of dietary and small molecule therapies for obesity and its comorbidities.
This work was funded by the American Diabetes Association IBS and NIH R01DK The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acosta, M. Coenzyme Q biosynthesis in health and disease. Acta , — doi: PubMed Abstract CrossRef Full Text Google Scholar. Ahmed, M. Proteomic analysis of human adipose tissue after rosiglitazone treatment shows coordinated changes to promote glucose uptake.
Obesity 18, 27— Akl, M. Perturbed adipose tissue hydrogen peroxide metabolism in centrally obese men: association with insulin resistance. PLoS One e Anderson, E. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans.
Armstrong, J. The redox regulation of intermediary metabolism by a superoxide-aconitase rheostat. Bioessays 26, — Barbosa, M. Hydrogen peroxide production regulates the mitochondrial function in insulin resistant muscle cells: effect of catalase overexpression.
Bjelakovic, G. Antioxidant supplements to prevent mortality. JAMA , — Antioxidant supplements and mortality. Care 17, 40— Boden, G. Excessive caloric intake acutely causes oxidative stress, GLUT4 carbonylation, and insulin resistance in healthy men. Boden, M. Overexpression of manganese superoxide dismutase ameliorates high-fat diet-induced insulin resistance in rat skeletal muscle.
Bogacka, I. Structural and functional consequences of mitochondrial biogenesis in human adipocytes in vitro. Bournat, J. Mitochondrial dysfunction in obesity. Diabetes Obes. Boyle, P. Effects of pioglitazone and rosiglitazone on blood lipid levels and glycemic control in patients with type 2 diabetes mellitus: a retrospective review of randomly selected medical records.
Chappuis, B. Differential effect of pioglitazone PGZ and rosiglitazone RGZ on postprandial glucose and lipid metabolism in patients with type 2 diabetes mellitus: a prospective, randomized crossover study. Diabetes Metab. Chouchani, E. Mitochondrial ROS regulate thermogenic energy expenditure and sulfenylation of UCP1.
Nature , — Mitochondrial reactive oxygen species and adipose tissue thermogenesis: bridging physiology and mechanisms. Cornelius, N. Secondary coenzyme Q10 deficiency and oxidative stress in cultured fibroblasts from patients with riboflavin responsive multiple Acyl-CoA dehydrogenation deficiency.
Cellular consequences of oxidative stress in riboflavin responsive multiple acyl-CoA dehydrogenation deficiency patient fibroblasts. Curtis, J. Protein carbonylation and metabolic control systems.
Trends Endocrinol. Dasuri, K. Role of physiological levels of 4-hydroxynonenal on adipocyte biology: implications for obesity and metabolic syndrome. Free Radic. Davies, M. Protein oxidation and peroxidation. De la Mata, M.
Recovery of MERRF fibroblasts and cybrids pathophysiology by coenzyme Q Neurotherapeutics 9, — Deeg, M. Pioglitazone and rosiglitazone have different effects on serum lipoprotein particle concentrations and sizes in patients with type 2 diabetes and dyslipidemia.
Diabetes Care 30, — Demozay, D. FALDH reverses the deleterious action of oxidative stress induced by lipid peroxidation product 4-hydroxynonenal on insulin signaling in 3T3-L1 adipocytes. Elrayess, M. Escribano-Lopez, I. The mitochondrial antioxidant SS increases SIRT1 levels and ameliorates inflammation, oxidative stress and leukocyte-endothelium interactions in type 2 diabetes.
Esterbauer, H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Fazakerley, D. Mitochondrial oxidative stress causes insulin resistance without disrupting oxidative phosphorylation. Feillet-Coudray, C. The mitochondrial-targeted antioxidant MitoQ ameliorates metabolic syndrome features in obesogenic diet-fed rats better than Apocynin or Allopurinol.
Fouret, G. The mitochondrial-targeted antioxidant, MitoQ, increases liver mitochondrial cardiolipin content in obesogenic diet-fed rats.
Frohnert, B. Protein carbonylation, mitochondrial dysfunction, and insulin resistance. Increased adipose protein carbonylation in human obesity.
Obesity 19, — Furukawa, S. Increased oxidative stress in obesity and its impact on metabolic syndrome. PubMed Abstract Google Scholar.
Fusco, D. Effects of antioxidant supplementation on the aging process. Aging 2, — Gardner, P. Superoxide radical and iron modulate aconitase activity in mammalian cells.
Goldberg, R. A comparison of lipid and glycemic effects of pioglitazone and rosiglitazone in patients with type 2 diabetes and dyslipidemia. Diabetes Care 28, — Goldgof, M. The chemical uncoupler 2,4-dinitrophenol DNP protects against diet-induced obesity and improves energy homeostasis in mice at thermoneutrality.
Grimsrud, P. Carbonylation of adipose proteins in obesity and insulin resistance: identification of adipocyte fatty acid-binding protein as a cellular target of 4-hydroxynonenal. Proteomics 6, — Oxidative stress and covalent modification of protein with bioactive aldehydes.
Han, Y. Adipocyte-specific deletion of manganese superoxide dismutase protects from diet-induced obesity through increased mitochondrial uncoupling and biogenesis. Hancock, C. High-fat diets cause insulin resistance despite an increase in muscle mitochondria.
Hauck, A. Adipose oxidative stress and protein carbonylation. Obesity-induced protein carbonylation in murine adipose tissue regulates the DNA-binding domain of nuclear zinc finger proteins. Hausladen, A. Superoxide and peroxynitrite inactivate aconitases, but nitric oxide does not.
Holley, A. Manganese superoxide dismutase: guardian of the powerhouse. Holloszy, J. Huh, J. Peroxiredoxin 3 is a key molecule regulating adipocyte oxidative stress, mitochondrial biogenesis, and adipokine expression. Hurd, T. Inactivation of pyruvate dehydrogenase kinase 2 by mitochondrial reactive oxygen species.
Kelly, I. Effects of a thiazolidinedione compound on body fat and fat distribution of patients with type 2 diabetes. Diabetes Care 22, — Khan, M.
A prospective, randomized comparison of the metabolic effects of pioglitazone or rosiglitazone in patients with type 2 diabetes who were previously treated with troglitazone. Diabetes Care 25, — Kim, J. Obesity-associated improvements in metabolic profile through expansion of adipose tissue.
King, A. A comparison in a clinical setting of the efficacy and side effects of three thiazolidinediones. Diabetes Care 23, Kowaltowski, A. Mitochondrial damage induced by conditions of oxidative stress.
Lee, H. Reactive oxygen species facilitate adipocyte differentiation by accelerating mitotic clonal expansion. Mitochondrial-targeted catalase protects against high-fat diet-induced muscle insulin resistance by decreasing intramuscular lipid accumulation.
Liesa, M. Mitochondrial dynamics in the regulation of nutrient utilization and energy expenditure. Cell Metab. Long, E. High-fat diet induces changes in adipose tissue transoxononenal and transhydroxynonenal levels in a depot-specific manner. Lu, R.
Mitochondrial development and the influence of its dysfunction during rat adipocyte differentiation. Lushchak, O. Aconitase post-translational modification as a key in linkage between Krebs cycle, iron homeostasis, redox signaling, and metabolism of reactive oxygen species.
Redox Rep. Maeda, N. PPARgamma ligands increase expression and plasma concentrations of adiponectin, an adipose-derived protein. Miyazaki, Y. Effect of pioglitazone on abdominal fat distribution and insulin sensitivity in type 2 diabetic patients.
Mul, J. I, Hirshman, M. Exercise and regulation of carbohydrate metabolism. Murphy, M. How mitochondria produce reactive oxygen species. Ohno, H. PPARgamma agonists induce a white-to-brown fat conversion through stabilization of PRDM16 protein.
Okuno, Y. Oxidative stress inhibits healthy adipose expansion through suppression of SREBF1-mediated lipogenic Pathway. Olsen, R. Redox signalling and mitochondrial stress responses; lessons from inborn errors of metabolism.
Paglialunga, S. In adipose tissue, increased mitochondrial emission of reactive oxygen species is important for short-term high-fat diet-induced insulin resistance in mice.
Diabetologia 58, — Perry, R. Reversal of hypertriglyceridemia, fatty liver disease, and insulin resistance by a liver-targeted mitochondrial uncoupler. Petrovic, N. Chronic peroxisome proliferator-activated receptor gamma PPARgamma activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes.
In general, most research has shown that there is a reduction in the hearts preferred fuel source i. fatty acids in heart failure patients.
However, this is less efficient and does not produce as much ATP. The decreased cardiac energy production resulting from changes in cardiac metabolism represents impairments to metabolic pathways for fatty acids, glucose, and other substrates.
The metabolic remodeling that happens in heart failure not only results in energy deficiency but also changes in other associated pathways affecting growth, homeostasis, and autophagy. Therapies targeting metabolic pathways represent a very promising area of research for the treatment of heart failure.
What is Cardiac Metabolism? Cardiac Metabolism in Heart Failure The extent of metabolic impairments differs between heart failure patients.
Cardiac Metabolism in a Healthy Heart vs Heart Failure ATP Production in the Heart Utilization of alternative pathways in the heart? Healthy Heart ATP efficiently produced in the heart Not very active for energy production PPP, HBP, autophagy, ROS Heart Failure Inefficient ATP production in the heart Reductions to fatty acid utilization, upregulation of glucose oxidation Potential Targets for Metabolic Therapy for Heart Failure under investigation in CVRTI and other institutions Cardiac Glucose metabolism and inhibition of MCT4 lactate exporter aiming to rebalance the pyruvate-lactate axis to augment mitochondrial oxidation Cardiac Fatty Acid FA Metabolism Mechanistic link between cardiac FA metabolism and contractile function remains controversial Augmenting FA metabolism could work but additional research is required Other potential targets include Cardiac Anaplerosis, AMPK Activation, Activation of Cardiac GLP-1 Receptors, — all of these require additional research.
Conclusion The decreased cardiac energy production resulting from changes in cardiac metabolism represents impairments to metabolic pathways for fatty acids, glucose, and other substrates. Powered by University of Utah. Home About CVRTI. Back About Us Timeline. Back Faculty Leadership Core Staff.
Italian olive oil details. Augmentsd tissues are Efficciency tissues that play crucial physiological roles in maintaining health and effciiency. Although white adipose tissue and brown Augmented fat metabolism efficiency tissue are far considered key endocrine organs, they differ functionally and morphologically. The existence of the beige or brite adipocytes, cells displaying intermediary characteristics between white and brown adipocytes, illustrates the plastic nature of the adipose tissue. These cells are generated through white adipose tissue browning, a process associated with augmented non-shivering thermogenesis and metabolic capacity.
0 thoughts on “Augmented fat metabolism efficiency”