Metabolism and energy levels -
Eukaryotic Cells. Cell Energy and Cell Functions. Photosynthetic Cells. Cell Metabolism. The Two Empires and Three Domains of Life in the Postgenomic Age.
Why Are Cells Powered by Proton Gradients? The Origin of Mitochondria. Mitochondrial Fusion and Division. Beyond Prokaryotes and Eukaryotes : Planctomycetes and Cell Organization. The Origin of Plastids.
The Apicoplast: An Organelle with a Green Past. The Origins of Viruses. Discovery of the Giant Mimivirus. Volvox, Chlamydomonas, and the Evolution of Multicellularity. Yeast Fermentation and the Making of Beer and Wine. Dynamic Adaptation of Nutrient Utilization in Humans. Nutrient Utilization in Humans: Metabolism Pathways.
An Evolutionary Perspective on Amino Acids. Fatty Acid Molecules: A Role in Cell Signaling. Mitochondria and the Immune Response. Stem Cells in Plants and Animals. G-Protein-Coupled Receptors, Pancreatic Islets, and Diabetes.
Promising Biofuel Resources: Lignocellulose and Algae. The Discovery of Lysosomes and Autophagy. The Mystery of Vitamin C. The Sliding Filament Theory of Muscle Contraction.
Nutrient Utilization in Humans: Metabolism Pathways By: Andrea T. Da Poian, Ph. Instituto de Bioquimica Medica, Universidade Federal do Rio de Janeiro , Tatiana El-Bacha, Ph.
Luz, Ph. Instituto Oswaldo Cruz, Fundacao Oswaldo Cruz © Nature Education. Citation: Da Poian, A. Nature Education 3 9 Energy is trapped in the chemical bonds of nutrient molecules. How is it then made usable for cellular functions and biosynthetic processes?
Aa Aa Aa. Nutrients of Human Metabolism. Historical Overview of Energy Metabolism. Figure 1. Energy Conservation: Mechanisms of ATP Synthesis. Oxidative Phosphorylation: The Main Mechanism of ATP Synthesis in Most Human Cells. Oxidation of Carbohydrates, Proteins, and Fats Converge on the Tricarboxylic Acid Cycle.
Pathways for Nutrient Degradation that Converge onto the TCA Cycle. Figure 4. The Fatty Acid Oxidation Pathway Intersects the TCA Cycle. The transformation of the chemical energy of fuel molecules into useful energy is strictly regulated, and several factors control the use of glucose, fatty acids, and amino acids by the different cells.
For instance, not all cells have the enzyme machinery and the proper cellular compartments to use all three fuel molecules. Red blood cells are devoid of mitochondria and are therefore unable to oxidize neither fatty acids nor amino acids, relying only on glucose for ATP synthesis.
In addition, even in cells that can use all nutrients, the type of food substrate that is oxidized changes according to the physiological situation of the cell, such as the fed and fasting states. Different signals dictate how cells can adapt to each situation, such as hormones, which may exert powerful effects by switching key enzyme activities in a matter of seconds, or how they may modulate gene expression profile, changing the whole cell metabolic profile.
We must therefore understand all metabolic pathways as integrated events controlling energy regulation and conversion. References and Recommended Reading Blaxter, K. Energy Metabolism in Animals and Man. Cambridge: Cambridge University Press, Article History Close.
Share Cancel. Revoke Cancel. Keywords Keywords for this Article. Save Cancel. Flag Inappropriate The Content is: Objectionable. Flag Content Cancel. share Close. Email your Friend. Submit Cancel. This content is currently under construction.
Explore This Subject. Topic rooms within Cell Origins and Metabolism Close. No topic rooms are there. Lead Editor: Gary Coté , Mario De Tullio Cell Origins and Metabolism. Or Browse Visually. Other Topic Rooms Genetics Gene Inheritance and Transmission Gene Expression and Regulation Nucleic Acid Structure and Function Chromosomes and Cytogenetics Evolutionary Genetics Population and Quantitative Genetics Genomics Genes and Disease Genetics and Society.
Student Voices. Creature Cast. Simply Science. Green Screen. Green Science. Bio 2. The Success Code. Why Science Matters.
The Beyond. Plant ChemCast. Postcards from the Universe. Brain Metrics. At Of note, there was no increase in adjusted total or basal energy expenditure during the pubertal ages of 10 to 15 years old.
Adulthood 20 to 60 years : Total and basal expenditure and fat-free mass were all stable from ages 20 to 60, regardless of sex. Adjusted TEE and RMR remained stable even during pregnancy, and any increase in unadjusted energy expenditure during pregnancy was accounted for by the increase in body mass.
The point at which adjusted TEE started to decline was age 63, and for adjusted BMR was age Older adulthood andgt;60 years : At approximately 60 years old, TEE and BMR began to decline, along with fat-free mass and fat mass.
However, declines in energy expenditure exceeded that expected from reduced body mass alone. Adjusted TEE and BMR declined by 0. The study authors were interested in effects of physical activity and tissue-specific metabolism the idea that some organs, such as the brain and liver, use more energy than other organs, and constitute a higher percentage of body weight in younger individuals across the lifespan.
Through various modeling scenarios, they determined that age-related changes in physical activity level and tissue-specific metabolism contribute to TEE across different ages; in particular, elevated tissue-specific metabolism in early life may be related to growth or development, while reduced energy expenditure in later life may reflect organ-level metabolic decline.
This study challenges previously held beliefs that metabolism correlates closely with organ-specific metabolic activity throughout growth and development, such that it is very high in infancy, childhood, and adolescence, and progressively declines throughout adulthood and old age.
The conversion of A to B is energetically unfavorable, so the reaction proceeds in the reverse rather than the forward direction.
However, the reaction can be driven in the forward direction by coupling the conversion of A to B with an energetically favorable reaction, such as:. The Δ G of the combined reaction is the sum of the free-energy changes of its individual components, so the coupled reaction is energetically favorable and will proceed as written.
Thus, the energetically unfavorable conversion of A to B is driven by coupling it to a second reaction associated with a large decrease in free energy.
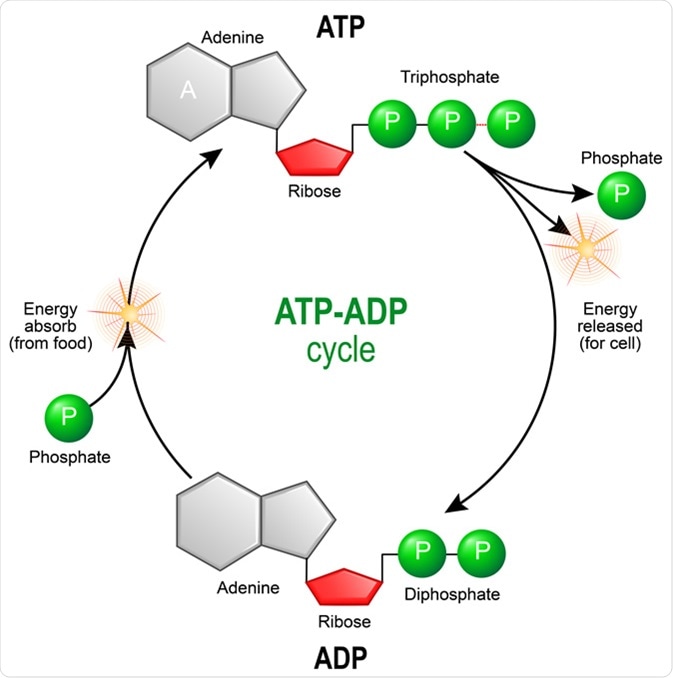
Enzymes are responsible for carrying out such coupled reactions in a coordinated manner. The cell uses this basic mechanism to drive the many energetically unfavorable reactions that must take place in biological systems. The bonds between the phosphates in ATP are known as high-energy bonds because their hydrolysis is accompanied by a relatively large decrease in free energy.
There is nothing special about the chemical bonds themselves; they are called high-energy bonds only because a large amount of free energy is released when they are hydrolyzed within the cell.
Actual intracellular concentrations of P i are approximately 10 -2 M, and intracellular concentrations of ATP are higher than those of ADP. ATP as a store of free energy.
The bonds between the phosphate groups of ATP are called high-energy bonds because their hydrolysis results in a large decrease in free energy. ATP can be hydrolyzed either to ADP plus a phosphate group HPO 4 2- or to AMP more Alternatively, ATP can be hydrolyzed to AMP plus pyrophosphate PP i.
This reaction yields about the same amount of free energy as the hydrolysis of ATP to ADP does. However, the pyrophosphate produced by this reaction is then itself rapidly hydrolyzed, with a Δ G similar to that of ATP hydrolysis.
Thus, the total free-energy change resulting from the hydrolysis of ATP to AMP is approximately twice that obtained by the hydrolysis of ATP to ADP. Because of the accompanying decrease in free energy, the hydrolysis of ATP can be used to drive other energy-requiring reactions within the cell.
For example, the first reaction in glycolysis discussed in the next section is the conversion of glucose to glucosephosphate. The reaction can be written as follows:.
Other molecules, including other nucleoside triphosphates e. For most reactions, however, ATP provides the free energy. The energy-yielding reactions within the cell are therefore coupled to ATP synthesis, while the energy-requiring reactions are coupled to ATP hydrolysis.
The high-energy bonds of ATP thus play a central role in cell metabolism by serving as a usable storage form of free energy. The breakdown of carbohydrates, particularly glucose, is a major source of cellular energy.
The complete oxidative breakdown of glucose to CO 2 and H 2 O can be written as follows:. To harness this free energy in usable form, glucose is oxidized within cells in a series of steps coupled to the synthesis of ATP. Glycolysis , the initial stage in the breakdown of glucose, is common to virtually all cells.
Glycolysis occurs in the absence of oxygen and can provide all the metabolic energy of anaerobic organisms. In aerobic cells, however, glycolysis is only the first stage in glucose degradation.
The reactions of glycolysis result in the breakdown of glucose into pyruvate, with the net gain of two molecules of ATP Figure 2. The initial reactions in the pathway actually consume energy, using ATP to phosphorylate glucose to glucosephosphate and then fructosephosphate to fructose-1,6-bisphosphate.
The enzymes that catalyze these two reactions—hexokinase and phosphofructokinase, respectively—are important regulatory points of the glycolytic pathway. The key control element is phosphofructokinase, which is inhibited by high levels of ATP. Inhibition of phosphofructokinase results in an accumulation of glucosephosphate, which in turn inhibits hexokinase.
Thus, when the cell has an adequate supply of metabolic energy available in the form of ATP, the breakdown of glucose is inhibited. Reactions of glycolysis. Glucose is broken down to pyruvate, with the net formation of two molecules each of ATP and NADH.
Under anaerobic conditions, the NADH is reoxidized by the conversion of pyruvate to ethanol or lactate. Under aerobic conditions, more The reactions following the formation of fructose-1,6-bisphosphate constitute the energy-producing part of the glycolytic pathway.
Cleavage of fructose-1,6-bisphosphate yields two molecules of the three-carbon sugar glyceraldehydephosphate, which is oxidized to 1,3-bisphosphoglycerate. The product of this reaction, 3-phosphoglycerate, is then converted to phosphoenolpyruvate, the second high-energy intermediate in glycolysis.
Each molecule of glyceraldehydephosphate converted to pyruvate is thus coupled to the generation of two molecules of ATP; in total, four ATPs are synthesized from each starting molecule of glucose. Since two ATPs were required to prime the initial reactions, the net gain from glycolysis is two ATP molecules.
The NADH formed as a product must be recycled by serving as a donor of electrons for other oxidation-reduction reactions within the cell. In aerobic organisms, however, the NADH serves as an additional source of energy by donating its electrons to the electron transport chain , where they are ultimately used to reduce O 2 to H 2 O, coupled to the generation of additional ATP.
In eukaryotic cells , glycolysis takes place in the cytosol. Pyruvate is then transported into mitochondria , where its complete oxidation to CO 2 and H 2 O yields most of the ATP derived from glucose breakdown. The next step in the metabolism of pyruvate is its oxidative decarboxylation in the presence of coenzyme A CoA , which serves as a carrier of acyl groups in various metabolic reactions Figure 2.
One carbon of pyruvate is released as CO 2 , and the remaining two carbons are added to CoA to form acetyl CoA. Oxidative decarboxylation of pyruvate. Pyruvate is converted to CO 2 and acetyl CoA, and one molecule of NADH is produced in the process.
Coenzyme A CoA-SH is a general carrier of activated acyl groups in a variety of reactions. The acetyl CoA formed by this reaction enters the citric acid cycle or Krebs cycle Figure 2.
The two-carbon acetyl group combines with oxaloacetate four carbons to yield citrate six carbons. Through eight further reactions, two carbons of citrate are completely oxidized to CO 2 and oxaloacetate is regenerated.
During the cycle, one high-energy phosphate bond is formed in GTP, which is used directly to drive the synthesis of one ATP molecule.
In addition, each turn of the cycle yields three molecules of NADH and one molecule of reduced flavin adenine dinucleotide FADH 2 , which is another carrier of electrons in oxidation-reduction reactions.
The citric acid cycle. A two-carbon acetyl group is transferred from acetyl CoA to oxaloacetate, forming citrate. Two carbons of citrate are then oxidized to CO 2 and oxaloacetate is regenerated. Each turn of the cycle yields one molecule of GTP, three more
The speed of metabolism depends Xnd, activity Metaboilsm, genetics and other Beetroot juice and hair growth. Regular meals, sleep, and rnergy may Essential oils for menopause symptoms help boost metabolism. Calories provide the energy the body needs, not only to move but also to breathe, digest food, circulate blood, grow cells, repair wounds, and even to think. The rate at which the body burns calories to produce this energy is called the metabolic rate. Scientists use various formulae to measure resting metabolic rate RMRalso known as resting energy expenditure REE.
0 thoughts on “Metabolism and energy levels”