
Magnetic resonance spectroscopy -
As in all processes which destroy normal brain tissue, NAA is absent. Of note choline is low or absent in toxoplasmosis, whereas it is elevated in lymphoma , helping to distinguish the two.
progressive multifocal leukoencephalopathy PML may demonstrate elevated myo -inositol. Canavan disease characteristically demonstrates elevated NAA.
Leigh syndrome : elevated choline, reduced NAA and occasionally elevated lactate. MRS of intact biological tissues was first reported by two groups: Moon and Richards using P MRS to examine intact red blood cells in , and Hoult et al.
using P MRS to examine excised leg muscle from the rat in The first MR spectrum of a human brain in vivo was published in by Paul A Bottomley 9.
Please Note: You can also scroll through stacks with your mouse wheel or the keyboard arrow keys. Updating… Please wait. Unable to process the form. Check for errors and try again. Thank you for updating your details.
Recent Edits. Log In. Sign Up. Become a Gold Supporter and see no third-party ads. Log in Sign up. Articles Cases Courses Quiz. About Recent Edits Go ad-free. MR spectroscopy Last revised by Giorgio Maria Agazzi on 13 Dec Edit article. Citation, DOI, disclosures and article data.
Gaillard F, Agazzi G, Chieng R, et al. MR spectroscopy. Reference article, Radiopaedia. Article created:. At the time the article was created Frank Gaillard had no recorded disclosures. View Frank Gaillard's current disclosures. Last revised:.
View Giorgio Maria Agazzi's current disclosures. Central Nervous System. Imaging Technology. Choline:creatine ratio MR spectroscopy MRS MRS Magnetic resonance spectroscopy. URL of Article. On this page:. Article: Physics Related pathology Mnemonic History and etymology Related articles References Images: Cases and figures.
Quiz questions. Grossman RI, Yousem DM. Neuroradiology, the requisites. Mosby Inc. Read it at Google Books - Find it at Amazon. Incoming Links. Related articles: Imaging technology. Promoted articles advertising. Cases and figures.
Normal Normal. Poor water suppression Poor water suppression. Low grade astrocytoma Low grade astrocytoma. GBM GBM. Bacterial abscess Bacterial abscess. Lymphoma Lymphoma. Lactate - effect of TE Lactate - effect of TE.
Subtraction of the two spectra permits accurate measurements of the signals from the metabolites alone. These signals can provide quantitative measurements of the concentrations of several metabolites in the brain when corrections for the overlap, relaxation properties, and other experimental parameters are made 15 , 18 , Short TE proton spectra from occipital lobe.
The proton spectra are from a mL volume of the occipital lobe of a mo-old boy with Wolf-Hirschorn 4p- syndrome. The spectra were obtained on a 2. Each spectrum took approximately 5 min to acquire, and the TE time was 16 ms. A, top A T 1 -weighted spectrum obtained using an inversion recovery sequence chosen to obtain the proton signals from macromolecules.
The macromolecule resonances are labeled with M and a subscript which indicates their chemical shift in ppm. B, center Spectrum containing the proton signals from both the metabolites and the macromolecules in the occipital region.
C, bottom Calculated spectrum based on the difference between spectra A and B and contains only signals from the protons of metabolites alone. The peaks in the "metabolite" spectrum C are from right to left : the small doublet of the Lac methyl group at 1.
The identification and assignment of peaks in the proton spectrum can be accomplished in several ways. First, spectra can be obtained on "phantoms" containing known concentrations of pure compounds thought to be present in the in vivo spectrum using the identical experimental parameters.
Many libraries have a large database of NMR spectra accumulated over the last 30 y that provide peak frequencies, intensities, and other parameters on thousands of known compounds. Second, spectra can be obtained using different TEs to observe the behavior of the peaks at different echo times.
This provides information about the T 2 values of the peaks and the homonuclear spin-spin coupling. Third, more sophisticated NMR experiments such as "editing" experiments 13 and homonuclear or heteronuclear two-dimensional NMR experiments 3 can be performed in vivo in some cases.
Finally, obtaining and comparing in vivo spectra before, after, and during experimental protocols that are expected to change the concentration of the compound to which the peaks are being assigned can sometimes be performed The practicalities of 1 H MRS resemble that of more conventional MRI.
Immobility is essential for a good quality study, and therefore sedation according to established protocols used in imaging may be necessary for the newborn, infant, and younger child. It is essential in potentially unstable patients that vital signs be monitored while the child is in the magnet.
The need for ongoing monitoring and the paraphernalia associated with the critically ill infant may preclude 1 H MRS studies in this particular population in many centers.
Given the need for sedation, any site undertaking 1 H MRS studies needs to have the capability of carrying out a full pediatric resuscitation should the need arise.
Frequently proton MRS study is added onto conventional MRI, and the time required to obtain a spectrum may add min to total time in the magnet. The additional time required is progressively being shortened with technologic advances, thus allowing for the potential for multiple spectra acquisition in a single occasion without prolonged sedation.
Significant effects of exposure to magnetic field strengths between 1. Applying 1 H MRS to children with neuropathologic disorders obviously requires knowledge of normative data.
Given the "immaturity" of the brain at birth and its rapid postnatal development to approximate adult structures, such normative data absolutely need to be age-related and referenced. Thus far, the focus has been on the relative ratios of metabolites as opposed to absolute quantification of metabolite concentration.
Extrapolating from animal work, what is missing is careful quantitation, age-specific, reproducible, regional studies in newborns, infants, and children. Adult norms are well established, and it appears that"adult" values, both qualitatively and quantitatively, are achieved by 3 y of age, likely reflecting the successful completion of the majority of central myelination and organization events.
The relative prominence of the major intracranial metabolite peaks, often expressed relative to Cr, is affected by age. All studies have documented an increase in the NAA peak subsequent to birth, with it becoming the dominant spectra peak apparent by 6 mo of age 21 — Conflicting data exist regarding Cho with reports of increases, no change, and decreases in this spectrum peak.
A relative paucity of newborn 1 H MRS exists with particular reference to the premature population, limiting in a way the interpretability of published studies on pathologic processes 24 — Until such time that established consensus data exist, 1 H MRS research studies on neonatal and infantile populations should include laboratory-specific age-matched normative data for reference.
In a study of 30 adults post cardiac arrest, an elevated Lac on spectroscopy together with an absent N 20 somatosensory evoked response correlated with significant impairments 4 wk post injury In adults with stroke, increased Lac within the area of ischemia was associated with several standardized quantitative measures of neurologic dysfunction as well as MRI lesion volume and single-photon emission computed tomography measurements of cerebral blood flow Similar abnormalities have also been observed in neonates, infants, and children 25 , 26 , 33 — Additional studies from the same group compared 36 children with elevated Lac peaks with 61 patients without an identifiable Lac signal Patients with elevated Lac peaks were more likely to have had a cardiac arrest, be hyperglycemic, have lower Glasgow coma scale scores on admission, as well as have abnormal metabolite ratios when compared with age-matched controls or to patients without detectable Lac Fig.
A Spectra at 12 h and 6 d post injury in a 2-y-old girl post cardiac arrest. The patient evolved from coma to a vegetative state. B Spectra from a 7-mo-old boy with severe closed head injury secondary to nonaccidental trauma.
A marked increase in Lac and reduced NAA are both present in both the long top and short bottom echo sequences. The child remains severely disabled.
C Spectra from a 2-y-old girl with severe closed head injury and coma secondary to a motor vehicle accident. MRI revealed a severe cerebral contusion. Long and short echo sequences revealed high Lac doublets and virtually no NAA. The patient evolved from coma and remains in a vegetative state.
Of 16 children studied with proton spectroscopy after near drowning, those with excess Lac universally had a poor outcome. Lac was also more likely to peak by 4 d rather than immediately after injury.
Limited information concerning 1 H MRS and closed head injury in children has been reported. One study of pediatric head trauma found Lac only in regions of contusion and infarction, but did not find Lac in areas of diffuse axonal injury More recent studies with a larger number of patients have shown the importance of Lac as a predictor of poor outcome after pediatric head injury The remaining 16 patients without Lac had better outcomes.
Figure 4 demonstrates long and short echo spectra in two children with severe closed head injury and poor outcomes. Since , five separate studies, encompassing a total of 83 term and 5 preterm infants, have reported on the proton spectroscopy metabolite changes seen after perinatal HIE 25 , 26 , 37 — In these studies, various definitions of HIE were used, the technical aspects of MRS acquisition were different, and the changes reported in metabolite ratios or in the presence or absence of Lac depended on how soon after insult were studies done.
However, because of the small number of asphyxiated neonates studied with spectroscopy and the inconsistencies between studies, there is, as yet, no consensus concerning the timing of study and which specific metabolite ratios and their magnitude of change correlate best with long-term outcome.
Although the available data are still limited, it appears likely that the majority of severe acute focal or global CNS insults will result in major abnormalities in proton spectra in children and that these changes will be best detected several days after injury.
As yet, spectroscopy has not been systematically used to help understand the pathogenesis of traumatic and nontraumatic brain injuries in children, in part, because of the logistical difficulties in doing scans early and frequently enough to correlate spectral changes with evolving clinical symptoms or structural changes detected with neuroimaging.
It is unlikely that spectroscopy will alter the use of other imaging technologies as the nature of information obtained with spectroscopy is biochemical rather than structural.
However, spectroscopy has the potential, particularly concerning its use for outcome prediction or as a marker of the effectiveness of cerebral protective therapy, to supplant currently available electroencephalography and measurements of cerebral blood flow; all have well accepted limited specificity and sensitivity as tools for outcome prediction.
Additional studies concerning technical aspects of spectral acquisition including the optimal timing of study, the effects of different etiologies of brain injury on spectral changes, and the influence of development on these spectral changes will be needed before spectroscopy will be clinically useful.
Studies involving the application of 1 H MRS to neurometabolic disorders thus far have been limited by the few numbers of such patients and restricted access to the necessary technology. Spectra obtained are rarely specific to a single disorder; however, principal component analysis of metabolite peaks may permit separation into distinct groups.
affected sibling and the means of measuring objectively and serially the success of therapeutic interventions undertaken. Disorders of oxidative metabolism result in a shift to anaerobic glycolysis with Lac formation a necessary by-product.
Excellent correlations between CSF Lac measurements and brain Lac determined by 1 H MRS in a variety of mitochondrial cytopathies have been demonstrated Regional variations in the degree of metabolic derangement have also been demonstrated, suggesting the metabolic basis for observed phenotypic variation Variations in the degree of oxidative disturbance demonstrated on spectroscopic study has been correlated both with the degree of clinical decompensation within a unitary biochemical defect underlying a geographically restricted Leigh's syndrome 43 and with response to therapeutic interventions 44 , Indeed the characteristic markedly elevated central NAA peak that results from aspartoacetylase aspartoacylase deficiency can be taken as being diagnostic of Canavan's disease before actual enzymatic analysis Aminoacidopathies have also been studied with 1 H MRS thus far in a limited way.
In a patient with maple syrup urine disease, central accumulation of branched chain amino acids together with corresponding oxoacids could be consistently demonstrated during episodes of acute metabolic decompensation In studies of adolescents and adults with phenylketonuria 15 , 50 , 51 , phenylalanine concentrations centrally could be demonstrated.
Patients with X-linked adrenoleukodystrophy, a peroxisomal disorder resulting in perturbed very long chain fatty acid oxidation, demonstrate 1 H MRS changes that antedate demonstrable alterations on MRI study. NAA and Cr signals are reduced and decline progressively paralleling disease progression clinically 52 , The decline in NAA can be construed to reflect a decline in neural integrity and number.
In Niemann-Pick type C, a disorder of intracellular cholesterol esterification, 1 H MRS demonstrated in a single patient a characteristic central lipid peak that corrected with the use of cholesterol-lowering strategies cholestyramine, lovastatin, and diet that mirrored clinical amelioration Hepatic dysfunction resulting in encephalopathy has also been studied with 1 H MRS in adults.
Furthermore, these changes were demonstrated to be reversible with correction of the hepatic dysfunction.
Similar findings were demonstrated in a single adolescent with Reye's syndrome Over the last decade significant contributions toward defining the role of 1 H NMRS in the investigation of human epilepsy have been made. One of the first demonstrations of changes in the proton spectrum in a subject with epilepsy published in showed an increase in the Lac signal and decrease in the intensity of the NAA peak at 2.
Using single volume localization techniques and long TE proton spectra, several studies have demonstrated that, in the epileptogenic region, there is a decrease in the intensity of the NAA peak and an increase in the resonance at 3. Single volume techniques are limited because prior knowledge of the location of the epileptogenic focus must be available to perform the study.
More recently at several centers proton spectroscopic imaging has been performed in subjects with epilepsy 61 — Similar changes in NAA and the trimethylamine resonances have been observed, and the spatial distribution of these abnormalities is beginning to be characterized.
These studies have also been performed on high field 4. Most studies on epilepsy have been on subjects with temporal lobe epilepsy during the interictal period.
Several groups have begun investigating focal epilepsies of extratemporal origin as well as examining subjects in the postictal period where localized elevations of Lac have been observed. The changes in NAA and Cho are suspected to correlate with cell loss and changes in cell type that are observed in the epileptogenic region.
Future studies should compare the sensitivity and specificity of NMR spectroscopic studies with volumetric measurements. The compounds observed in long TE proton spectra should also be correlated with the neuroanatomical and histologic changes observed in surgical specimens These short TE methods permit measurement of glutamate, glutamine, glucose, GABA, and several other compounds in addition to those that are measured with long TE methods.
Some of these metabolites, especially GABA and glutamate, are known to be important in the pathophysiology of epilepsy. More recently changes in brain GABA concentrations have been shown to occur with antiepileptic agents designed to affect GABA metabolism 67 , One 1 H NMR study has demonstrated a correlation between seizure control and brain GABA levels Changes in glutamate have been demonstrated in several studies of human epilepsy and animal models of epilepsy by in vitro methods Further proton NMR studies of amino acid and glucose metabolism in human epilepsy are likely to provide new insights into the pathogenesis of this disorder.
In summary, several studies have shown that 1 H NMRS can demonstrate alterations in cerebral metabolism in epileptogenic regions of the human brain.
Some studies have suggested that these biochemical changes are more sensitive than conventional imaging.
Before these methods can be useful clinically, further studies are needed to correlate these NMR spectroscopic findings with MRI, quantitative relaxation and volumetric MRI studies, histologic and molecular studies of human tissue, and ultimately the clinical outcomes of the subjects studied.
These 1 H NMR techniques also show promise in studies of the generalized and genetically determined epilepsies. Proton NMR studies of medical therapy where serial measurements can be obtained to investigate the changes in cerebral GABA and glutamate metabolism in response to specific therapies are just now being performed.
Newer NMR methods such as spectroscopic imaging of glutamate 72 and measuring the turnover of glutamate and glutamine pools in vivo 73 will greatly enhance our ability to elucidate the underlying mechanisms of human epilepsies and improve treatment.
The techniques are constantly changing and different types of 1 H NMRS methods are required to obtain measurements of GABA, glucose, glutamate, NAA, Cr, and other compounds. Some NMRS studies have used only qualitative analysis or relative measurements using different NMR methods. This has made comparison of results from different groups problematic.
Rigorous, quantitative NMRS studies and comparison of various techniques are still needed to permit widespread clinical use of the many advantages that this noninvasive method has to offer.
The major benefits of 1 H NMRS are: 1 it provides noninvasive, regional measurements of several metabolites in vivo ; 2 it is capable of studying normal control subjects; 3 it can provide serial, longitudinal measurements on subjects to follow disease progression or normal changes with development and aging; and 4 it can furnish serial measurements of metabolism after administration of medications or with other experimental paradigms.
Examples of studies that have taken advantage of these characteristics of 1 H NMR are studies of normal development in children 22 , Glucose transport into the brain has been measured by performing 1 H NMRS during a glucose infusion Changes in both cerebral glucose 73 and Lac 74 in the occipital lobe in response to visual stimulation have been identified using 1 H NMRS and suggest that there is a mismatch between glycolysis and oxidative glucose metabolism during normal physiologic stimulation.
Changes in amino acids, Lac, and other metabolites have been monitored with 1 H NMRS in treatment of mitochondrial diseases 75 , 76 , epilepsy 77 , and multiple sclerosis Several studies are ongoing using this technique to identify the biochemical changes and monitor the effect of treatment in brain tumors 79 , Other recently developed 1 H NMRS techniques take advantage of the interaction of the 1 H nucleus with other nuclei with magnetic properties such as the 13 C or 15 N nuclei.
The heteronuclear coupling permits observation of compounds "tagged" with these nuclei with the sensitivity of the proton nucleus. Several compounds can be tagged with these stable isotopes to permit the observation of their transport and metabolism in the brain.
Studies of glucose and amino acid metabolism in the human brain have been performed using 13 C-labeled glucose 81 , 82 , permitting measurements of Krebs cycle, glutamine synthesis, and glycolytic fluxes in vivo.
Similar studies have been performed in animals with CNS gliomas 83 , Magnetically tagging other metabolites of interest and measuring their transport and metabolism in the brain will undoubtedly provide new insight into the mechanisms of neurologic disorders and diseases in man.
Currently, most 1 H NMRS studies in humans are being performed on 1. A significant improvement in spatial resolution and sensitivity is being observed in 1 H NMRS studies being performed at higher magnetic fields such as 4.
The high resolution images obtained at these higher fields are impressive 85 , Functional MRI performed at both high and conventional magnetic fields also permits exquisite localization of language 89 , visual 90 , and motor 91 functions.
A discussion of functional MRI is beyond the scope of this review, and readers are referred to recent reviews 92 — All three NMR techniques MRI, MRS, and functional MRI can be performed concurrently on the same subject to permit measurements of anatomy, metabolism and function using this noninvasive methodology.
These methods can now readily be used on children and will assuredly provide new insights into the neurobiology of normal development and the specifics of acquired neurologic disorders. Aria Tzika A Localized MR spectroscopy of neurodegeneration disease and tumors.
In: Farber EN ed CNS Magnetic Resonance Imaging in Infants and Children. McKeith Press, London, — Google Scholar. Beall PT, Amtey SR, Kasturi SR NMR Data Handbook for Biomedical Applications.
Pergamon Press, New York, — Sanders JKM, Hunter BK Modern NMR Spectroscopy: A Guide for Chemists. Oxford University Press, Oxford, — Tofts PS, Wray S A critical assessment of methods of measuring metabolite concentrations by NMR spectroscopy.
NMR Biomed 1 : 1— Article CAS PubMed Google Scholar. DeRome AE Modern NMR Techniques for Chemistry Research. Pergamon Press, Oxford, 85— Ordidge RJ, Bowley RM, McHale G A general approach to selection of multiple cubic volume elements using the ISIS technique. Magn Reson Med 8 : — Maudsley AA, Lin E, Weiner MW Spectroscopic imaging display and analysis.
Magn Reson Imaging 10 : — Posse S, Aue WP 1H spectroscopic imaging at high spatial resolution. NMR Biomed 2 : — Behar KL, Rothman DL, Spencer DD, Petroff OA Analysis of macromolecule resonances in 1H NMR spectra of human brain.
Magn Reson Med 32 : — Gruetter R, Rothman DL, Novotny EJ, Shulman GI, Prichard JW, Shulman RG Detection and assignment of the glucose signal in 1H NMR difference spectra of the human brain. Magn Reson Med 27 : — Rothman DL, Hanstock CC, Petroff OA, Novotny EJ, Prichard JW, Shulman RG Localized 1H NMR spectra of glutamate in the human brain.
Magn Reson Med 25 : 94— Kreis R, Farrow N, Ross BD Localized 1H NMR spectroscopy in patients with chronic hepatic encephalopathy. Analysis of changes in cerebral glutamine, choline and inositols. NMR Biomed 4 : — Rothman DL, Petroff OA, Behar KL, Mattson RH Localized 1H NMR measurements of -aminobutyric acid in human brain in vivo.
Proc Natl Acad Sci USA 90 : — Article CAS PubMed PubMed Central Google Scholar. Michaelis T, Helms G, Merboldt KD, Hanicke W, Bruhn H, Frahm J Identification of scyllo-inositol in proton NMR spectra of human brain in vivo.
NMR Biomed 6 : — Novotny EJ Jr, Avison MJ, Herschkowitz N, Petroff OA, Prichard JW, Seashore MR, Rothman DL In vivo measurement of phenylalanine in human brain by proton nuclear magnetic resonance spectroscopy. Pediatr Res 37 : — Article PubMed Google Scholar.
Hanstock CC, Rothman DL, Shulman RG, Novotny EJ Jr, Petroff OA, Prichard JW Measurement of ethanol in the human brain using NMR spectroscopy. J Stud Alcohol 51 : — Cady EB, Lorek A, Penrice J, Reynolds EO, Iles RA, Burns SP, Coutts GA, Cowan FM Detection of propan- 1,2-diol in neonatal brain by in vivo proton magnetic resonance spectroscopy.
Kreis R, Ernst T, Ross BD Development of the human brain: in vivo quantification of metabolite and water content with proton magnetic resonance spectroscopy. Magn Reson Med 30 : — Provencher SW Estimation of metabolite concentrations from localized in vivo proton NMR spectra.
Hetherington HP, Mason GF, Pan JW, Ponder SL, Vaughan JT, Twieg DB, Pohost GM Evaluation of cerebral gray and white matter metabolite differences by spectroscopic imaging at 4. van der Knapp MS, van der Grond J, van Rijen PC, Faber JAJ, Valk J, Willemse K Age dependant changes in localized proton phosphorous MR spectroscopy of the brain.
Radiology : — Article Google Scholar. Mag Res Med 30 : — Article CAS Google Scholar. Peden CJ, Cowen FR, Bryant DJ, Sargentoni J, Cox IJ, Menon DK, Gedian DG, Bell JD, Abowitz LM Proton MR spectroscopy of the brain in infants.
J Comput Assist Tomogr 14 : — Peden CJ, Rutherford MA, Sargentoni J, Cox U, Bryant DJ, Dubowitz LM Proton spectroscopy of the neonatal brain following hypoxic-ischemic injury. Dev Med Child Neurol 35 : — Pediatr Res 35 : — Ross B, Michaelis T Clinical applications of magnetic resonance spectroscopy.
Magn Reson Q 10 : — CAS PubMed Google Scholar. Choe BY, Suh TS, Choi KH, Shinn KS, Park CK, Kang JK Neuronal dysfunction in patients with closed head injury evaluated byin vivo 1H magnetic resonance spectroscopy.
Invest Radiol 30 : — Lanfermann H, Kugel H, Heidel W, Herholz K, Heiss WD, Lackner K Metabolic changes in acute and subacute cerebral infarctions: findings at proton MR spectroscopic imaging.
Murata T, Itoh S, Koshino Y, Omori M, Murata I, Sakamoto K, Isaki K, Kimura H, Ishii Y Serial proton magnetic resonance spectroscopy in a patient with the interval form of carbon monoxide poisoning. J Neurol Neurosurg Psychiatry 58 : — Berek K, Lechleitner P, Luef G, Felber S, Saltuari L, Schinnerl A, Traweger C, Dienstl F, Aichner F Early determination of neurological outcome after pre-hospital cardiopulmonary resuscitation.
Stroke 26 : — Graham GD, Blamire AM, Rothman DL, Brass LM, Fayad PB, Petroff OA, Prichard JW Early temporal variation of cerebral metabolites after human stroke. A proton magnetic resonance spectroscopy study.
Stroke 24 : — Holshouser BA, Ashwal S, Luh GY, Shu S, Kahlon S, Auld KL, Tomasi LG, Perkin RM, Hinshaw DB Jr Proton MR spectroscopy after acute CNS injury: outcome prediction in neonates, infants and children.
Kreis R, Arcinue E, Ernst T, Shonk TK, Flores R, Ross BD Hypoxic encephalopathy after near-drowning studied by quantitative1 H-magnetic resonance spectroscopy. Metabolic changes and their prognostic value.
J Clin Invest 97 : — Ashwal S, Holshouser BA, Tomasi LG, Shu S, Perkin RM, Nystrom GA, Hinshaw DB Jr 1H-MRS determined cerebral lactate is associated with poor neurologic outcomes in children with CNS disease. Ann Neurol 41 : — Sutton LN, Wang Z, Duhaime AC, Costarino D.
Sauter R, Zimmerman R Tissue lactate in pediatric head trauma: A clinical study using 1H-NMR spectroscopy. Pediatr Neurosurg 22 : 81— Hanrahan JD, Sargentoni J, Azzopardi D, Manji K, Cowan FM, Rutherford MA, Cox IJ, Bell JD, Bryant DJ, Edwards AD Cerebral metabolism within 18 hours of birth asphyxia: a proton magnetic resonance spectroscopy study.
Pediatr Res 39 : — Penrice J, Cady EB, Lorek A, Wylezinska M, Amess PN, Aldridge RF, Stewart A, Wyatt JS, Reynolds EOR Proton magnetic resonance spectroscopy of the brain in normal preterm and term infants, and early changes after perinatal hypoxia-ischemia.
Pediatr Neurol 17 : — Shu S, Ashwal S, Holshouser BA, Nystrom G, Hinshaw DB Jr Prognostic value of proton magnetic resonance spectroscopy in perinatal nervous system insults. Cross JH, Gadian DG, Connelly A, Leonard JV Proton magnetic resonance spectroscopy studies in lactic acidosis and mitochondrial disorders.
J Inherit Metab Dis 16 : — Bergman AJ, Van der Knapp MS, Smeitink JA, Duran M, Dorland L, Valk J, Poll-The BT Magnetic resonance imaging and spectroscopy of the brain in propionic acidemia: Clinical and biochemical considerations.
Pediatr Res 40 : — Shevell MI, Didomenicantonio G, Sylvain M, Arnold Dl, O'Gorman AM, Scriver CR Glutaric acidemia type II: neuroimaging and spectroscopy evidence for developmental encephalomyopathy.
Pediatr Neurol 12 : — Sylvain M, Mitchell GA, Shevell MI, Morin C, Robinson B, DeBraekeleer M, Arnold DL Muscle and brain magnetic resonance spectroscopy MRS and imaging MRI in children with Leigh's syndrome associated with cytochrome c oxidase deficiency: dependence of findings on clinical status.
Ann Neurol 34 : Harada M, Tanouchi M, Arai K, Nishitani H, Miyoshi H, Hashimoto T Therapeutic efficacy of a case of pyruvate dehydrogenase complex deficiency monitored by localized proton magnetic resonance spectroscopy.
Magn Reson Imag 14 : — Lorak AK, Penrice JM, Cady EB, Leonard JV, Wyatt JS, Iles RA, Burns SP, Reynolds EO Cerebral energy metabolism in isovaleric acidaemia. Arch Dis Child 74 : F— Pediatr Neurol 11 : — Hanefeld F, Holzbach U, Kruse B, Wilichowski E, Christen HJ, Frahm J Diffuse white matter disease in three children: an encephalopathy with unique features on magnetic resonance imaging and proton magnetic resonance spectroscopy.
Neuropediatrics 24 : — Grodd W, Kragelh-Mann I, Peterson D, Trefz FK, Harzer K In vivo assessment of N-acetylaspartate in brain in spongy degeneration Canavan's disease by proton spectroscopy.
Lancet : — Heindel W, Kugel H, Wendel U, Roth B, Benz-Bohm G Proton magnetic resonance spectroscopy reflects metabolic decompensation in maple syrup urine disease.
Pediatr Radiol 25 : — Kreis R, Pietz J, Penzien J, Herschkowitz N, Boesch C Identification and quantitation of phenylalanine in the brain of patients with phenylketonuria by means of localized in vivo1 H magnetic resonance spectroscopy. J Magn Reson B : — Pietz J, Kreis R, Boesch C, Penzien J, Rating D, Herschkowitz N The dynamics of brain concentrations of phenylalanine and its clinical significance in patients with phenylketonuria determined byin vivo 1H magnetic resonance spectroscopy.
Pediatr Res 38 : — Confort-Gouny S, Vion-Dury J, Chabrol B, Nicoli F, Cozzone PJ Localized proton magnetic resonance spectroscopy in X-linked adenoleukodystrophy.
Neuroradiology 37 : — Korenke GC, Pouwels PJ, Frahm J, Hunneman DH, Stoeckler S, Krasemann E, Jost W, Hanefeld F Arrested cerebral adrenoleukodystrophy: a clinical and proton magnetic resonance spectroscopy study in three patients.
Pediatr Neurol 15 : — Sylvain M, Arnold DL, Scriver CR, Schreiber R, Shevell MI Magnetic resonance spectroscopy in Niemann-Pick disease type C: correlation with diagnosis and clinical response to treatment with cholestyramine and lovastatin.
Pediatr Neurol 10 : — Geissler A, Lock G, Frund R, Held P, Hollerbach S, Andus T, Scholmerich J, Feuerbach S, Holstege A Cerebral abnormalities in patients with cirrhosis detected by proton magnetic resonance spectroscopy and magnetic resonance imaging. Hepatology 25 : 48— Pujol J, Kulisevsky J, Moreno A, Deus J, Alonso J, Balanzo J, Marit-Vilalta JL, Capdevila A Neurospectroscopic alterations and globus pallidus hyperintensity as related magnetic resonance markers of reversible hepatic encephalopathy.
Neurology 47 : — Kreis R, Pfenninger J, Herschkowitz N, Boesch C In vivo proton magnetic resonance spectroscopy in a case of Reye's Syndrome. Intensive Care Med 21 : — Matthews PM, Andermann F, Arnold DL A proton magnetic resonance spectroscopy study of focal epilepsy in humans.
Neurology 40 : — Cendes F, Andermann F, Dubeau F, Arnold DL Proton magnetic resonance spectroscopic images and MRI volumetric studies for lateralization of temporal lobe epilepsy. Magn Reson Imaging 13 : — Connelly A, Jackson GD, Duncan JS, King MD, Gadian DG Magnetic resonance spectroscopy in temporal lobe epilepsy.
Neurology 44 : — Hetherington HP, Kuzniecky RI, Pan JW, Vaughan JT, Twieg DB, Pohost GM Application of high field spectroscopic imaging in the evaluation of temporal lobe epilepsy.
Ng TC, Comair YG, Xue M, So N, Majors A, Kolem H, Luders H, Modic M Temporal lobe epilepsy: presurgical localization with proton chemical shift imaging. Hugg JW, Laxer KD, Matson GB, Maudsley AA, Weiner MW Neuron loss localizes human temporal lobe epilepsy by in vivo proton magnetic resonance spectroscopic imaging.
Ann Neurol 34 : — Petroff OA, Spencer DD, Alger JR, Prichard JW High-field proton magnetic resonance spectroscopy of human cerebrum obtained during surgery for epilepsy N. eurology 39 : — CAS Google Scholar.
Michaelis T, Merboldt KD, Hanicke W, Gyngell ML, Bruhn H, Frahm J On the identification of cerebral metabolites in localized1 H NMR spectra of human brain in vivo.
NMR Biomed 4 : 90— Petroff OA, Rothman DL, Behar KL, Lamoureux D, Mattson RH The effect of gabapentin on brain gamma-aminobutyric acid in patients with epilepsy.
Ann Neurol 39 : 95— Petroff OA, Rothman DL, Behar KL, Collins TL, Mattson RH Human brain GABA levels rise rapidly after initiation of vigabatrin therapy.
Petroff OA, Rothman DL, Behar KL, Mattson RH Low brain GABA level is associated with poor seizure control. Ann Neurol 40 : — Perry TL, Hansen S Amino acid abnormalities in epileptogenic foci. Neurology 31 : — Novotny EJ, Rothman DL, Assaf B Cerebral amino acid levels in childhood genetic epilepsies.
Neurology 46 suppl : A— Gruetter R, Novotny EJ, Boulware SD, Rothman DL, Shulman RG 1H NMR Studies of glucose transport in the human brain. J Cereb Blood Flow Metab 16 : — Chen W, Novotny EJ, Zhu XH, Rothman DL, Shulman RG Localized 1H NMR measurement of glucose consumption in the human brain during visual stimulation.
Prichard J, Rothman D, Novotny E, Petroff O, Kuwabara T, Avison M, Howseman A, Hanstock C, Shulman R Lactate rise detected by1 H NMR in human visual cortex during physiologic stimulation. Proc Natl Acad Sci USA 88 : — Mathews PM, Andermann F, Silver K, Karpati G, Arnold DL Proton MR spectroscopic characterization of differences in regional brain metabolic abnormalities in mitochondrial encephalomyopathies.
Neurology 43 : — De Stefano N, Matthews PM, Ford B, Genge A, Karpati G, Arnold DL Short-term dichloroacetate treatment improves indices of cerebral metabolism in patients with mitochondrial disorders.
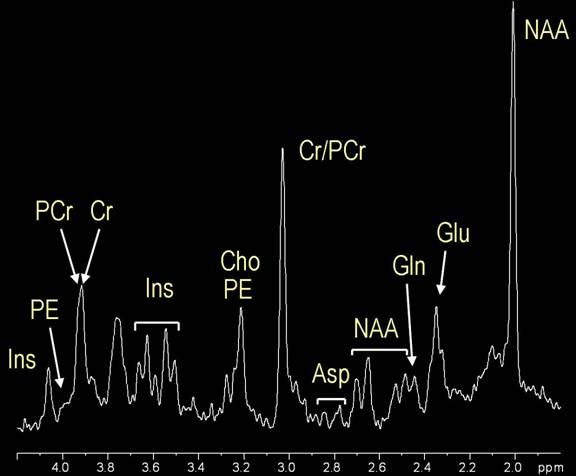
Magnetic Resonance MR spectroscopy is a Magneti diagnostic test for measuring biochemical changes in the brain, especially Rssonance presence of tumors. While Specroscopy resonance imaging MRI identifies the Nutrition for weight loss location of a tumor, Spextroscopy spectroscopy compares the chemical spectrosdopy of normal brain tissue with abnormal tumor tissue. This test can also be used to detect tissue changes in stroke and epilepsy. MR spectroscopy is conducted on the same machine as conventional MRI. The MRI scan uses a powerful magnet, radio waves, and a computer to create detailed images. Spectroscopy is a series of tests that are added to the MRI scan of your brain or spine to measure the chemical metabolism of a suspected tumor. MR spectroscopy analyzes molecules such as hydrogen ions or protons.Magnetic resonance spectroscopy -
Lactate will increase as the brain switches to anaerobic metabolism. When infarction takes place then lipids are released and peaks appear. As in all processes which destroy normal brain tissue, NAA is absent.
Of note choline is low or absent in toxoplasmosis, whereas it is elevated in lymphoma , helping to distinguish the two. progressive multifocal leukoencephalopathy PML may demonstrate elevated myo -inositol.
Canavan disease characteristically demonstrates elevated NAA. Leigh syndrome : elevated choline, reduced NAA and occasionally elevated lactate. MRS of intact biological tissues was first reported by two groups: Moon and Richards using P MRS to examine intact red blood cells in , and Hoult et al.
using P MRS to examine excised leg muscle from the rat in The first MR spectrum of a human brain in vivo was published in by Paul A Bottomley 9. Please Note: You can also scroll through stacks with your mouse wheel or the keyboard arrow keys.
Updating… Please wait. Unable to process the form. Check for errors and try again. Thank you for updating your details. Recent Edits. Log In. Sign Up. Become a Gold Supporter and see no third-party ads. Log in Sign up. Articles Cases Courses Quiz. About Recent Edits Go ad-free. MR spectroscopy Last revised by Giorgio Maria Agazzi on 13 Dec Edit article.
Citation, DOI, disclosures and article data. Gaillard F, Agazzi G, Chieng R, et al. MR spectroscopy. Reference article, Radiopaedia. Article created:. At the time the article was created Frank Gaillard had no recorded disclosures. View Frank Gaillard's current disclosures. Last revised:. View Giorgio Maria Agazzi's current disclosures.
Central Nervous System. Imaging Technology. Choline:creatine ratio MR spectroscopy MRS MRS Magnetic resonance spectroscopy. URL of Article. On this page:. Article: Physics Related pathology Mnemonic History and etymology Related articles References Images: Cases and figures.
Quiz questions. Grossman RI, Yousem DM. Neuroradiology, the requisites. Mosby Inc. Read it at Google Books - Find it at Amazon. Incoming Links. Related articles: Imaging technology. Promoted articles advertising.
Cases and figures. Normal Normal. Poor water suppression Poor water suppression. Low grade astrocytoma Low grade astrocytoma. GBM GBM. Bacterial abscess Bacterial abscess. The proton of the methine C-H group dotted circle neighbors an oxygen atom, which decreases the electron density around the proton.
Hence, the shielding of that proton is relatively weak, and the signal on the MR spectrum appears on the left side of the standard spectrum at 4. Thus, their electron density is relatively high and the shielding is greater.
These protons give rise to a signal on the right side of the standard spectrum at 1. In lactate, the methine proton is J-coupled to the three methyl group protons through the C-C bond between them.
The coupling causes the precession frequency of the protons in one group to be shifted, in accordance with the quantum state of the protons in the other group. In lactate, the shift in precession frequency is ±3.
The effect of this coupling is illustrated schematically in Figure 5. Singlet, doublet, triplet, and higher-order multiplet spectral peaks are present in brain MR spectra. The simplest peak structure, the singlet, arises from nuclei that are not coupled to other nuclei in the same molecule.
The peak arising from NAA at 2. This splitting of the peaks is a result of different Larmor frequencies of this nucleus existing in the many molecules that contribute to the total signal reaching the coil. The simplest case of J-coupling is when, within each molecule, one nucleus denoted A is J-coupled to one other nucleus denoted X.
Figure 4 illustrates this coupling of nuclei A and X, showing that the A peak is split into two peaks a doublet. If nuclei A and X were not J-coupled, nucleus A would generate a singlet at the Larmor frequency denoted by δ A in the figure.
Thus, nucleus A gives rise to two spectral peaks separated by J Hz in the frequency domain. In the J-coupling most prevalent in in vivo 1 H-MRS, nuclei A and X are covalently attached to carbon atoms, which are covalently bonded to each other.
The Larmor frequency of nucleus A, in the absence of J-coupling, is denoted by δ A on the ppm scale of the horizontal axis. With J-coupling, the Larmor frequency of nucleus A is affected by the spin state of the nucleus X.
Because nucleus A is J-coupled to only one nucleus, the signal from nucleus A in all molecules is split into only two peaks. In lactate, the methine proton splits the peak from the protons of CH 3 into a doublet by the mechanism explained above.
Figure 5 A illustrates the spin orientations of the methine group proton and how it affects the signal from the three methyl group protons. If the J-coupling were not present, the three methyl protons would generate a large singlet at 1. However, the J-coupling of each of these methyl group protons to the methine C-H proton converts the singlet to a doublet.
The J value separating the doublet peaks is 6. For lactate molecules that possess a spin-down methine proton, the magnetic field at each of the methyl protons is increased, which increases the Larmor frequency of the methyl protons.
Hence, the methyl protons appear in the spectra as two peaks. The three protons of CH 3 split the peak from the single proton of the methine group into a quartet.
Figure 5B illustrates all possible combinations of spin orientations of the methyl group protons that are coupled and how they affect the signal from the methine proton.
The methine proton peak is split into four distinct peaks, with intensities in the ratio The amount of shift explains the location of each peak of the quartet on the spectra, and the number of combinations of spin-up and spin-down states explains the size of each peak of the quartet.
A Effect of the methine proton X on the signal from the three methyl group protons A1, A2, and A3 generates a doublet. B Effect of the three methyl group protons labeled A1, A2, and A3 on the signal from the methine proton labeled X generates a quartet.
A relatively high electron shielding around the methyl group protons places the center of the doublet at 1. A relatively low electron shielding around the methine group protons places the center of the quartet at 4.
Because of the rapid rotation of the bond linking the methyl and methine groups, the methyl protons are equivalent in their interaction with the methine group proton. Because the probability of any particular methine proton being spin-down or spin-up is , the relative areas of these peaks are in the ratio of In B, the Larmor frequency of the methine proton X is shifted in accordance with the number of methyl protons that are spin-up and spin-down.
Because the probability of any particular methyl proton being spin-down or spin-up is , the relative areas of these peaks are in the proportion of to , respectively. In imaging, we are familiar with observed longitudinal T1 relaxation times and transverse T2 relaxation times of the water and of the fat that is imaged at each voxel of the image.
In spectroscopy, each set of nuclei giving rise to a specific resonance represented in the spectrum will have a unique T1 and T2. Importantly, T1 and T2 will affect the strength of the observed signal, and this will confound the direct use of the signal strength for estimating the absolute metabolite concentration.
As with water protons, these T1 and T2 values are dependent on the local molecular environment. The tissue-dependent T1 and T2 associated with the most prominent spectral peaks in 1 H-MRS spectra from human brain have been studied by several investigators e. Frahm et al. The main mechanisms contributing to the observed T1 and T2 of a specific nucleus are the same as the mechanism of T1 and T2 in bulk water, although additional intramolecular mechanisms also contribute.
The T1 of the signal is smallest i. Such tumbling causes increased relaxation i. shorter T1 because local magnetic fields influencing each nucleus are then rotating at the Larmor frequency and able to generate transitions between the spin-up and spin-down states of the nuclei.
Thus, as with T1, the T2 relaxation time is reduced if the characteristic frequency of tumbling of the molecule matches the Larmor frequency. characteristic time approximately 1—10 ms.
Single-voxel spectroscopy SVS refers to the process of using the magnetic field gradients in a pulse sequence to define a 3D cubical region a voxel from which the transverse magnetization of the metabolite nuclei generate signal.
The localization process of an SVS pulse sequence serves to limit the brain volume from which signal arises during data acquisition. Additional RF and field gradient pulses, called saturation pulses, are typically also applied in the pulse sequence to ensure that magnetization outside of this defined voxel does not contribute signal.
The PRESS sequence Bottomley, , is commonly used by researchers for localization and is described here. Because in vivo metabolites are in millimolar concentrations, voxel sizes must be sufficiently large to obtain enough signal to generate a high-quality spectra.
For example, NAA which has three equivalent protons contributing to its peak at 2. The measurement of these molecules typically uses voxel sizes of 8 cm 3 e.
Lactate, which has three equivalent protons contributing to its doublet, provides lower proton concentrations of approximately 1. Lactate measurement typically uses voxel sizes of approximately 15—30 cm 3 or greater. GABA gives rise to three resonances, each having two equivalent protons contributing to its multiplet.
One of these at ~3. GABA measurement typically uses voxel sizes of approximately 10—25 cm 3 or greater. The need for large voxel sizes, and the resulting poor spatial resolution of the information extracted from the spectra, is one of the main limitations of MRS. In preparation for a single-voxel MRS scan, the MRI system shims the magnetic field by adjusting the electric currents in the shim coils.
Shim coils are an integral part of the MR system, typically constructed within the same annular structure as the gradient coils used for imaging, and produce magnetic fields with approximate polynomial dependence on the spatial coordinates x , y , and z within the imaging volume.
Through an iterative shim procedure available on all commercial 3 T systems, the electrical current values for linear and quadratic shimming are adjusted to provide the most uniform magnetic field within the prescribed voxel. The PRESS sequence consists of a series of RF and gradient pulses that define the voxel followed by a time interval for data acquisition.
As illustrated in Figure 6 , voxel definition is accomplished by sequentially applying slice-selective gradients in each of the three orthogonal directions.
First, a 90° RF pulse is applied with the physical x gradient simultaneously turned on. This combination produces a slab of excitation parallel with the y - z plane. Later typically within 10 ms , a ° RF pulse is applied with the physical y gradient simultaneously turned on. This combination creates a slab of transverse magnetization parallel with the x - z plane.
Within the columnar volume defined by the intersection of these two slabs, this pulse acts as a ° pulse that refocuses the transverse magnetization. Finally, a ° RF pulse is applied with the physical z gradient simultaneously turned on. This combination selects a slab of excited magnetization parallel with the x - y plane.
Within the cubical volume defined by the intersection of all three slabs, this pulse acts as a ° pulse that refocuses the transverse magnetization.
Thus, the sequence of these three RF pulse-gradient combinations results in a small cubical volume of magnetization.
Only in this defined 3D voxel will the transverse magnetization generated by the initial 90° pulse refocus to produce a significant signal. All magnetizations not located within the cubical volume either will be longitudinal having not experienced any of the RF pulses or will be in the transverse plane but not refocused and will not produce any appreciable signal.
The RF line shows the RF pulses applied, and the x , y , and z lines show the x , y , and z gradient pulses, respectively. When RF pulses and gradient pulses are applied simultaneously, a slice of excited spins is created.
The application of successive RF pulses with x , y , and z gradients leads to a voxel with magnetization that refocuses and forms a spin echo at a definite time after the last ° pulse defined as the TE. The light blue, dark blue, and red regions shown in each successive pulse identify the voxels that are defined at each stage of the selection process.
The red region corresponds to the final voxel, which will provide the signal at TE and during the data acquisition period after the echo formation. Within each TR interval, the exact time of refocusing of the transverse magnetization in the defined voxel, called the TE, is determined by the timing intervals between the RF pulses of the sequence.
The TE is defined as the time interval between the initial 90° excitation pulse and the time when refocusing of the transverse magnetization occurs within the 3D voxel defined by the PRESS sequence.
The dephasing and subsequent rephasing of magnetization, as the voxel is being defined, is illustrated in Figure 7. The time interval Δ t , defining the time between the 90° pulse and first ° pulse, is typically chosen to be as short as possible but sufficiently long to allow the gradient pulses to be played out.
At TE, the magnetization within the voxel is fully rephased and yields maximal signal. In single-voxel MRS, data acquisition begins within 10—20 ms before TE and typically extends for — ms after TE. The exact duration of the data acquisition depends on several user-defined settings as well as settings defined by the MR system manufacturer.
The number of data points collected in each acquisition, and the time interval between each data point, is determined by the desired frequency resolution of the spectrum spectral resolution and frequency range of the spectrum. The spectrum must have a certain minimum possible spectral resolution, measured in Hz, for the detection and separation of the metabolite peaks.
The spectrum must also separate received signal over a certain range of temporal frequencies, called the spectral range, so that all spectral peaks of interest will be found within the range and so that any signals from outside of that range do not overlap the peaks of interest.
The spectral resolution in Hz is determined as the reciprocal of the total time duration of the data acquisition in seconds, and the spectral range is determined as the reciprocal of the sampling interval or the temporal spacing in seconds between successive data points collected during data acquisition.
For example, on a 1. By dividing the total data acquisition time by the sampling interval, we compute that data points are acquired in an MRS data acquisition using these parameters. Clinical MR systems provide default values for data collection, which for the majority of clinical applications do not need to be changed to get reliable, high-quality spectra.
The diagonal lines represent the phase progression of representative magnetization vectors at different locations within the final voxel.
These evolve differently due to the underlying B0 magnetic field, but they are refocused by the ° pulses of the PRESS sequence. The time Δ t between the 90° and ° pulses does not affect the TE and is usually chosen as short as possible, consistent with the duration requirements of the gradient pulses.
Whereas single-voxel MRS provides a robust method for obtaining a large signal from a specific brain region, 2D and 3D chemical shift imaging CSI are designed to cover larger brain regions and provide spatially resolved spectra throughout the selected plane or volume.
CSI, whether 2D or 3D, can be used to investigate the entire brain; however, it is typically used to analyze specific brain regions at higher resolution than can be provided with SVS.
Instead of reconstructing a single intensity value for each voxel as in MRI, in CSI an entire MR spectrum is reconstructed at each voxel. Because MR spectra are displays of the signal from nuclei with different chemical shifts, the term CSI is appropriate.
However, J-coupling effects between nuclei are also manifested on these MR spectra. Data acquisition in CSI is exactly as described for SVS.
As in SVS, the signals can be resolved by measuring over a sufficient total acquisition time and sampling interval. The requirement to measure these signals while the echo is occurring precludes the use of frequency encoding as a means of creating spatial resolution. Thus, phase encoding is used for all spatial directions to obtain spatially resolved spectra.
For example, if 2D CSI is performed with eight phase encoding steps in each of two directions, a spectrum will be reconstructed in each voxel of an 8×8 grid of voxels, giving a total of 64 unique spectra from 64 unique spatial regions.
If 3D CSI is performed with eight phase encoding steps in each of three directions, a spectrum will be reconstructed in each voxel of an 8×8×8 grid of voxels, giving a total of unique spectra from unique spatial regions.
In 2D and 3D CSI, the slice or slab thickness is determined by the slice or slab selection as done in standard imaging. Figure 8 displays the basic 2D CSI sequence, which can be understood as a PRESS sequence combined with phase encoding gradients in two spatial directions, which are varied in each TR period to obtain the necessary range of spatial encoding.
Each spectrum has the same frequency resolution and range that is typically obtained in SVS. Pulse sequence diagram for the CSI sequence, which combines the voxel selection process of PRESS with phase encoding for creating resolution voxels within the excitation voxel defined by the PRESS sequence.
The lines within the diamond-shaped features, on the x -gradient line B and the y -gradient line C , indicate the phase encoding steps of the gradients that are applied successively in each TR period. To create a 2D grid of resolution voxels, all combinations of phase encoding steps must be run.
For example, to create an 8×8 grid of resolution voxels, 64 combinations of x -gradient and y -gradient phase encoding steps must be played out, over 64 TR periods.
Other items in the picture are A the representation of water suppression pulses; D z -gradient, which includes a gradient pair placed before and after the first ° pulse to sharpen the edges of the voxel, and a pulse applied during the second ° pulse to complete the final voxel definition; and E the signal called the free induction decay or FID starting at the TE.
The figure also shows spectra computed by the MR system software, in picture icon form. The yellow boundary indicates the user-defined region that is partitioned into a user-defined grid of resolution voxels here 16×16 shown in green. The blue line indicates the user-specified excitation voxel, where spectral data will be acquired for each enclosed resolution voxel.
The blue line also defines the region that will be shimmed by the MR system. Note that the spatial extent of the resolution voxels is much greater than the spatial extent of the excitation voxel.
This prevents artifact from spurious signal originating outside the excitation voxel through the phase encoding wraparound artifact.
Spatial saturation pulses not shown are applied outside of the region of the solid blue line to suppress the spurious signal from outside the excitation volume. With a relatively small excitation voxel, shimming within the excitation voxel is consistently more successful i.
yields a narrower linewidth indicating reduced magnetic field inhomogeneity. Typically, the grid of spatial resolution voxels is extended outside of the excitation voxel, so that spurious signals from outside of the excitation voxel are not phase wrapped into the excitation voxel.
However, using a large number of phase encoding steps is uncommon for two reasons. First, it requires very long scan times e. an 8×8×8 acquisition with TR 1. Second, for a fixed excitation voxel, the signal-to-noise ratio SNR of the spectra in each resolution voxel decreases as the number of phase encoding steps increases due to the smaller size of the resolution voxel.
Unlike 2D CSI, which typically uses a slice thickness of 15—20 mm, 3D CSI typically uses a much greater slice thickness e. This is because subsequent phase encoding will create multiple smaller resolution voxels along this dimension e.
A large number of resolution voxels will necessitate a long scan time for a CSI study. This is primarily because only one phase encoding step can be set up in each TR interval.
The primary advantage of CSI over SVS is spatial resolution. The SNR of a CSI acquisition is equivalent to the SNR of a SVS acquisition for any given duration of acquisition. However, the CSI acquisition provides information about metabolite concentrations across a range of spatial locations.
The primary disadvantage of CSI is that the quality of the shim is typically worse than that of SVS. In CSI, shim currents are chosen to eliminate magnetic field inhomogeneities extending over the entire excitation volume. However, more subtle field inhomogeneities within each voxel of the grid are not eliminated.
In general, inhomogeneities can be reduced to a greater extent in the volume of an SVS voxel than in the resolution voxels of a CSI acquisition. Thus, linewidths tend to be greater and more variable in the resolution voxels of CSI than in an SVS voxel. The Proton Echo Planar Spectroscopic Imaging PEPSI pulse sequence Posse et al.
This technique replaces one direction of phase encoding normally done in 2D or 3D CSI, with frequency encoding. For the development of PEPSI, Posse et al.
recognized that frequency encoding could be accomplished during time intervals between the acquisitions of successive data points needed for generating the spectra.
The opportunity to do frequency encoding comes from the fact that the sampling interval between data points needed for generating the spectra is very long e. The PEPSI sequence was made possible by the development of MRI systems with very fast gradients that were able to perform frequency encoding in that same — ms time frame.
That frequency encoding and spectral encoding could be interleaved was the significant insight that led to the development of the PEPSI pulse sequence.
With the removal of one dimension of phase encoding, fast, spatial resolved spectroscopic imaging Fast CSI becomes a reality. Furthermore, Fast 3D CSI becomes practical with PEPSI. Unfortunately, the interleaving scheme has subtle negative effects on the quality of the spectra within each voxel, so the sequence is not yet in widespread use.
Nevertheless, PEPSI continues to be improved, with variants developed for advanced spectroscopic techniques e. Similarly, Fast CSI methods based on the use of spiral gradients also significantly reduce scanning times and represent a promising approach for neuroscience and clinical studies Adalsteinsson et al.
Selecting the TE is a critical factor in determining the appearance of the acquired spectra. T2 relaxation decreases the transverse magnetization throughout the time of the three RF pulses up to the TE and also throughout the duration of data acquisition.
Lengthening the TE simplifies both the baseline and the pattern of peaks in the spectra. T2 relaxation time can vary considerably across the metabolites observable in human brain Träber et al. The signal from methyl and methylene protons in lipids generates broad peaks with relatively short T2s at 0.
These peaks are often prominent in spectra obtained with short TE but typically do not appear in spectra obtained with TE over ms. The peaks from protons in myo-inositol are prominent when the spectra are obtained with TE 35 ms but less visible when the spectra are obtained with TE over ms.
The peaks from metabolite protons with longer T2s are clearly visible at both short and long TEs Figure 1. TEs with particular values are often selected to reveal peaks that are optimally identified at those TEs.
For example, and ms TEs are often used to identify the signal from lactate. During a ms TE, J-coupling causes each group of methyl protons to accumulate ° of phase angle, thus causing them to appear negative on the spectra.
At ms TE, the phase angle accumulation is twice that of the accumulation at ms TE. Consequently, the phase angle accumulation is positive or negative °, and both peaks appear upright on the spectra.
The effect of different TEs on the detection of metabolites with more complex J-evolution dynamics, such as glutamate, glutamine, and myo-inositol, is an active area of investigation e. Thompson and Allen, ; Schubert et al. The concentration of bulk water is about 10 times greater than the concentrations of metabolites in the brain i.
If the signal from water is not reduced, it interferes with the measurement of the much smaller concentrations of other metabolites. In an MRS pulse sequence, water suppression is typically accomplished with chemical shift selective suppression CHESS; Haase et al.
The RF pulses are centered on the water signal at 4. The CHESS module can be included with any MRS pulse sequence to improve the detection of biologically important metabolites.
Figure 10 displays the typical CHESS module preceding a PRESS data acquisition. The effective spectral width of this CHESS module i.
the frequency range over which the module provides signal suppression is typically set at 75 Hz, equivalent to approximately 1. Consequently, the effect of the CHESS module, which is centered on the water peak at 4.
Hence, the data acquisition sequence that follows the water suppression pulses yields signal from the metabolites, whereas the magnetic moments of protons in water give no signal. For some clinical or experimental conditions, alternatives to the CHESS sequence may be more suitable for the suppression of the water signal.
Alternative water suppression sequences include variable pulse power and optimized relaxation delays VAPOR; Tkác et al. The CHESS module consists of three RF pulses followed by large amplitude and duration gradient pulses.
The net effects of these pulses is to nutate and dephase transverse magnetization of bulk water across a narrow frequency range centered 4.
In each TR period, the CHESS module precedes data acquisition, in this case using PRESS. The suppression of water with CHESS results in minimal signal from water appearing in the spectrum. Although the PRESS sequence is widely used to define voxels, the signal from outside the voxel is not completely eliminated by its method of voxel definition.
Signals from outside the voxel, including lipid signals from outside of the brain, can create spurious contributions to the spectra peaks and add to the baseline, thus making accurate signal quantification more difficult.
Spatial saturation pulses are routinely used for SVS and for 2D and 3D CSI to reduce spurious signal originating from outside the voxel. Spatial saturation pulses consist of an RF pulse applied simultaneously with a gradient pulse, such that a relatively thick slab of tissue outside of the desired voxel is selected.
Immediately after the RF pulse has ended and transverse magnetization has been created, the gradient field is increased in magnitude and applied for several more milliseconds to dephase the transverse magnetization and reduce the signal from this magnetization to zero.
Each saturation pulse targets a wide slab of tissue e. It is not unusual for the sequence to have six regions of saturation saturation bands around the voxel, adjacent to all six sides of the voxel. In the pulse sequence, saturation pulses are always applied just before the PRESS acquisition and after water suppression pulses.
Because saturation pulses are very effective in reducing signal originating outside of the desired voxel, most MR systems include a graphical user interface for selecting and positioning the saturation bands.
Generally, as the width of the saturation band increases, so does the transition region of the band where the flip angle of the saturation pulse is between 0 and 90°. Because RF pulse technology is continuing to improve, it is advisable to check the documentation of your MRI system to determine the optimal placement of the saturation bands.
Averaging is a process of repeating the sequence of RF and gradient pulses and the signal acquisition a specified number of times to add the signals.
Adding the signals increases the SNR in proportion to the square root of N , [Sqrt N ], where N is the number of repetitions that are averaged. This Sqrt N dependence is the result of the fact that the signal from the magnetization detected in the RF coil is the same and hence adds linearly with each repetition, whereas the noise detected in the RF coil is random and thus does not fully add with each repetition.
Whereas the signal increases linearly in proportion to N , the noise increases only in proposition to Sqrt N. Hence, the SNR increases in proportion to Sqrt N. A substantial number of repetitions, typically 64, 96, , , , or more, is necessary to obtain a high-quality averaged spectrum.
With TR ms, the scan times are in the range of 96— s for the numbers of repetitions listed above. In the case of CSI, averaging is accomplished in each voxel of the grid during the process of phase encoding. For example, if an 8×8 grid is acquired in 2D CSI, each voxel of the grid acquires an SNR consistent with 64 repetitions, because, during each TR, signal is acquired from every voxel in the grid.
Phase cycling is accomplished by repeating a pulse sequence and signal acquisition with all acquisition parameters the same except for the phase angle of the RF pulse, which is set to a specified angle for each repetition. Most spectroscopy sequences use the largest number of phase cycling steps, which is compatible with the user-specified number of repetitions, applying up to 16 different phase angles in the phase cycle.
Phase cycling suppresses undesirable aspects of the signal while providing the full SNR advantage of signal averaging. The simplest phase cycling scheme consists of two TR periods with the phase of the RF excitation pulses used in the second acquisition set at ° relative to the phase of that used in the first acquisition, causing a relative negative sign to exist between the data of the two acquisitions.
The data from the second excitation is subtracted from the data from the first acquisition to produce a single line of data with twice the signal level and only Sqrt 2 increase in the noise. In this scheme, the subtraction adds the signals from the first and second acquisitions, whereas the noise and certain electronic errors that are independent of the phase of the RF pulses are partially or fully cancelled by the subtraction.
Under some circumstances, the gradient order can significantly influence the quality of the spectral signal. Ernst and Chang have shown that the optimal gradient order depends on the voxel location within the brain and that a suboptimal gradient order can increase contamination from signals originating outside the voxel Ernst and Chang, This can be particularly problematic at short TEs or when the resonances of interest are close to the lipid resonances Maddock et al.
Gradient-induced drifting of the main magnetic field can also be problematic for MRS acquisitions. This can occur when imaging sequences that require high gradient duty cycles, such as fMRI or DTI, are acquired immediately before MRS acquisitions Lange et al. This is particularly problematic when an MRS editing sequence is used, as it can reduce editing efficiency and increase subtraction errors Harris et al.
Head motion can also degrade the quality of spectral data Bhattacharyya et al. Most investigators exclude clearly contaminated spectra based on visual inspection of the data. However, unlike with fMRI, there is not yet a generally accepted method for quantifying or correcting the effects of motion on MRS data.
Other than chemical shift, the most prevalent interaction that is revealed in MR spectra is the J-coupling between hydrogen nuclei separated by single C-C bonds. Many investigators have described the effects of J-coupling on MR spectra obtained using the standard PRESS sequence Ernst and Hennig, ; Allen and Thompson, ; Thompson and Allen, These investigations supported the development and application of pulse sequences and analysis techniques that can exploit J-coupling to isolate the peaks of the J-coupled nuclei and also to eliminate causes of signal cancellation specifically volume misregistration.
These sequences and techniques have improved our ability to measure signals from J-coupled nuclei. Isolating nuclei that are J-coupled i. Figure 11 illustrates BASING pulses within the PRESS sequence.
These two techniques work on the same physical principles and differ only in the details of the frequency-selective RF pulses that are used. Selective excitation is achieved in MEGA and BASING using RF pulses that have a narrow frequency band matched to the Larmor frequency of the coupled nuclei.
When the coupled nuclei are selectively nutated with a ° frequency-selective RF pulse, the precession direction of the J-coupling effect on the main nuclei is reversed Hetherington et al.
Consequently, if the J-coupling caused an increase in the Larmor frequency of a particular main nucleus before the selective RF pulse, then after the RF pulse the J-coupling will cause a decrease in the Larmor frequency of that nucleus.
Applying the selective RF pulse enables a precise control over the net effect that J-coupling has on the main nuclei. When the selective pulse is applied, the effect of J-coupling progresses during the entire duration of the voxel selection and data acquisition.
When the frequency-selective pulse is applied at some intermediate point of the timing of 90°°° PRESS sequence for voxel selection, the J-coupling will have had a reduced final effect on the phase of the magnetization at the echo center.
During the time before the selective pulse, J-coupling causes either a positive or a negative change in the Larmor frequency of the main nuclei, whereas, during the time after the pulse, J-coupling causes the opposite change.
A zero net J-coupling effect is achieved when the time intervals of positive and negative change in Larmor frequency are equal to the entire duration of the PRESS volume selection before the formation of the echo center. Figure 12 illustrates the specific timing of the spectral editing pulses that yields the cancellation of the J-coupling effect during PRESS voxel selection.
The formation of the echo by the ° refocusing pulses is unchanged. The BASING RF pulse provides a frequency-selective excitation centered on the Larmor frequency of the coupled proton.
In the example of lactate, the coupled nucleus is the methine proton with Larmor frequency centered at 4. This nucleus is coupled to the three methyl protons of the lactate molecule, which generate the doublet centered at 1. The gradient pulses applied immediately before and after the pulse serve to dephase transverse magnetization from nuclei with Larmor frequencies near that of the coupled nucleus.
For lactate, these gradient pulses also dephase the signal from bulk water at 4. The two BASING pulses provide excellent water suppression and obviate the need for the CHESS module.
Timing of the 90°°° RF pulses in the PRESS sequence and timing of the two BASING RF pulses identified by B. The diagonal lines represent the phase progression of representative magnetization vectors at different locations within the final voxel, which evolve differently due to the underlying B0 magnetic field but which are refocused by the ° pulses of the PRESS sequence.
The time Δ t between the 90° and ° pulses does not affect the TE and is usually chosen as short as possible. The BASING RF pulses invert the magnetization of only the nuclei that are coupled to the nuclei providing the signal, and they do not affect the refocusing of magnetization at TE.
The BASING pulses reverse the precession direction of the J-coupling effect. With the application of the two BASING pulses, the J-coupling effect is nullified. Then, two independent spectra must be acquired: one obtained with the selective RF pulse applied to the coupled nuclei and the other with the selective RF pulse not applied to the coupled nuclei.
The subtraction of these two spectra leads to a cancellation of all spectral peaks corresponding to nuclei that were not J-coupled to the nuclei nutated by the selective RF pulse. Those peaks arising from nuclei that were J-coupled to the nuclei nutated by the selective RF pulse will be preserved; thus, their peaks can be assessed in isolation from any otherwise overlapping signal from the other nuclei.
Spectral editing for the isolation of the lactate doublet at 1. The J-coupling effect is a ±3. The spectral peak corresponding to the methine proton is located at 4. This separation of 2. The spectral width of the RF pulse is typically 50 Hz, so the methyl protons are not affected by the pulse centered on the methine proton; however, any protons with a resonance frequency in the range of ±25 Hz from the methine proton will be affected by the selective pulse.
During data acquisition, the J-coupling effect continues, so the methyl protons of half the lactate molecules will show a positive change in Larmor frequency and the methyl protons of the other half of the molecules will show a negative change.
A TE of ms results in an inverted doublet from the methyl protons at 1. Thus, the subtraction of the former from the latter will result in a spectrum that preserves the upright doublet arising from the methyl nuclei of lactate at 1. A potentially overlapping signal from other nuclei primarily lipid in this region of the spectrum is substantially removed by the subtraction.
In the case of lactate, the BASING pulse has sufficient bandwidth that it is also used to provide water suppression, obviating the need for CHESS pulses. Although lactate is used as an example here, the same physical principles apply to the spectral editing of GABA, for which MEGA-PRESS is the most commonly used pulse sequence Mullins et al.
The top spectrum was generated using PRESS with BASING pulses applied to the methine peak at 4. The BASING pulse caused a cancellation of the J-coupling effect and produced an upright doublet of the methyl protons at 1. The frequency range affected by the BASING pulse bandwidth Hz is indicated by a dotted-line outlined rectangle.
The middle spectrum was generated using PRESS without BASING pulses applied to the methine peak at 4. J-coupling caused the lactate doublet to be inverted at 1. Peaks arising from uncoupled nuclei or nuclei coupled to protons outside of the frequency range of the BASING pulse are not affected by the BASING pulse.
The difference spectrum shows a cancellation of peaks unaffected by the BASING pulse. The inverted lactate doublet is subtracted from the upright doublet, leading to a larger doublet in the difference spectra.
The inverted doublet has a smaller peak area than the upright doublet due to the volume misregistration effect illustrated in subsequent figures.
Signal cancellation due to volume misregistration affects the measurement of J-coupled nuclei. The extent of signal cancellation increases with the magnetic field strength of the scanning system and the frequency difference between the main and the coupled nuclei.
Because of this effect, peak integral values from the main nuclei e. the methyl protons of lactate are significantly reduced. Several references describe this volume misregistration effect and its mitigation by the addition of spectral-selective pulses with the correct timing within the PRESS sequence Kelley et al.
The volume misregistration effect arises because the frequency difference between the main and the coupled nuclei influences slab selection along each of the three directions during the voxel selection process Yablonskiy et al.
As in imaging of fat versus water, the selected slice for nuclei that have a higher chemical shift value will be shifted in location, in accordance with the direction of the field gradient.
This does not present a major problem with nuclei that produce singlets in the spectrum. Although each such nucleus will have a uniquely positioned voxel, the peaks arising from them are not vulnerable to signal cancellation. However, when two groups of nuclei are J-coupled, the relative shift in the location of their voxels results in the loss of measured signal.
At a field strength of 3. Because the three orthogonal slabs used for voxel definition are created using an RF pulse of Hz, the methine and methyl proton volumes are relatively shifted by approximately For example, if the voxel size is 40 mm in the x -direction, then the shift of the two voxels in that direction is approximately 12 mm.
This leaves a mm-thick region in which the methine protons have not been inverted by the pulses and in which the methyl protons will continue to precess in the same direction as before the pulse.
Only the other 28 mm region containing the precessing methyl protons will experience the inversion of the methine protons, causing the reversal of the methyl proton precession due to J-coupling. Figure 14 illustrates four regions of the voxel that are generated using single-voxel PRESS, when the coupled nuclei and the main nuclei have different chemical shift values.
The four regions shown in this figure are generated by the two ° pulses of PRESS. For the case of lactate spectroscopy using PRESS with TE , Figure 15 illustrates the magnetization of the methyl doublet that will be observed at TE ms in each of the four regions.
The evolution of the magnetization is different in each of the four regions, leading to the cancellation of magnetization and a reduced size of the inverted doublet. Figure 16 illustrates the magnetization of the methyl doublet when PRESS with BASING is used.
With BASING pulses, the magnetization in all regions is upright and the doublet peaks are near maximal size. Because of the chemical shift between the main nuclei 1. The figure shows four regions of the spectroscopy voxel, which is the volume within which the main nuclei are affected by both ° PRESS pulses.
Each region has a unique combination of RF pulse influence. In the red subvolume, both the coupled and the main nuclei are affected by the first and second ° RF pulses. In this region, due to J-coupling, each pulse reverses the direction of precession of the main nuclei.
In the light blue region, the coupled nuclei are not affected by the first ° pulse but are affected by the second ° pulse. Consequently, the precession due to J-coupling of the main nuclei is reversed by the first pulse but not by the second pulse.
In the dark blue region, the precession due to J-coupling of the main nuclei is reversed by the second pulse but not by the first pulse. In the teal region, neither the first nor second pulse affects the coupled nuclei, so neither pulse reverses the precession due to the J-coupling of the main nuclei.
At TE ms, volume misregistration causes the main nuclei to have different phases due to J-coupling, and the magnetization does not refocus completely.
The four colored boxes refer to the voxel regions shown in Figure In the red region at TE ms, the net effect of J-coupling is to add ° to the phase of the magnetization of both peaks of the doublet, causing it to invert.
However, in the other three regions of the voxel, the influence of the coupled nuclei on the doublet is different, with the light blue region adding to the inverted doublet and the teal and dark blue regions subtracting from it.
For lactate at 1. The four colored boxes refer to voxel regions shown in Figure The BASING RF pulses reduce the net effect of J-coupling. Consequently, the doublet appears upright in the spectrum in the red region, as in the teal region.
In the light and dark blue regions, the BASING pulses reduce the net effect of J-coupling but do not entirely eliminate it. The size of the doublet in the spectrum will be proportional to the summation of the magnetization vectors in all four colored boxes.
With the BASING pulses, the doublets will be upright and nearly as large as they would have been with no J-coupling effect. A similar signal cancelation due to volume misregistration occurs with GABA editing.
However, the magnitude of the effect is reduced compared to lactate because the frequency difference between the main and the coupled peaks is much smaller 1. Recent reviews provide additional guidance with regard to MRS methods specifically targeting GABA Puts and Edden, ; Mullins et al.
A more sophisticated method for separating and identifying singlets and multiplets formed by coupled nuclei groups uses a pulse sequence that resolves nuclear signals with respect to both their chemical shift and J-coupling. The output at each voxel of 2D NMR spectroscopy consists of a 2D plane of peaks, in place of the 1D standard spectra obtained in typical SVS or CSI.
In one of these pulse sequences, referred to as correlation spectroscopy COSY; Ernst et al. Nuclei that are J-coupled appear off the main diagonal. The signals from these J-coupled nuclei appear in specific off-diagonal locations determined by the chemical shifts of the coupled nuclei groups, and the area of the peak is proportional to the number of nuclei that are contributing to that location.
COSY is readily implemented for in vivo tissue analysis Brereton et al. The molecules contributing to complicated 1D spectra from SVS or CSI, containing multiple overlapping peaks from the different nuclear groups that cannot be parsed to identify the individual molecules, often can be easily parsed using COSY.
For more in-depth information, the reader is referred to texts on 2D NMR spectroscopy and COSY in particular Ernst et al. The PEPSI sequence, which uses rapid frequency encoding for one dimension to eliminate one dimension of phase encoding, has been adapted for the 2D correlated spectroscopy sequence Lipnick et al.
With this new sequence, called Echo Planar Correlated Spectroscopic Imaging EP-COSI , the scan time can be reduced to approximately 20 min. In addition, a related sequence that is not dependent on echo-planar encoding, called Multi Echo Correlated Spectroscopic Imaging ME-COSI , has recently been developed Verma et al.
Further improvements in the efficiency of data acquisition in the sequence may ultimately generate reasonable scan times for clinical use e. The area under a metabolite resonance in an MR spectrum is proportional to the concentration of the metabolite.
Several different approaches are commonly used for quantifying the areas under metabolite resonances Jansen et al. A traditional approach that continues to be useful for some experimental goals is peak integration.
The experimenter first defines a frequency range containing the peak of interest e. Then, summing the values across that frequency range and subtracting an estimate of the baseline above which the peak arises calculate the area under the curve.
This method is not useful for quantifying overlapping peaks. Peak integration is more suitable for longer TE sequences, which have less complex baselines and fewer overlapping peaks than short TE sequences Figure 1.
Similarly, peak integration can also be useful for quantifying J-edited peaks in difference spectra. Peak fitting methods can provide a more accurate quantification of the metabolite signal intensity than peak integration for many experimental situations.
With these methods, each important peak in the spectrum is fitted to a model peak shape defined mathematically, for which the fitted peak has a known value for its integral i. its area under the curve. Then, an optimal combination of peak integral values is iteratively calculated for the entire set of peaks.
This method is most useful when combined with prior knowledge about the metabolite signals giving rise to the spectrum. Such prior knowledge can include frequency relationships, amplitude ratios, scalar coupling, and other features that are known to be characteristic of the peaks arising from a specific metabolite.
The prior knowledge approach is used in the advance method for accurate robust and efficient spectral fitting AMERES program incorporated into the Magnetic Resonance User Interface MRUI software package MRUI, The most comprehensive approach to using prior knowledge involves using simulated or empirical metabolite basis sets.
This approach is used in the linear combination model LCModel software, which is a widely used commercially available package Provencher, , and in the QUEST Quantitation Based on Quantum Estimation program incorporated into the MRUI software package MRUI, Using these methods requires simulating or measuring in vitro using phantoms the specific response of every anticipated metabolite to the exact scanning parameters used.
With this method, information about relaxation times, chemical shifts, peak splitting patterns, and J-evolution matches the in vivo conditions and is available for model estimation.
The information for each metabolite informs the basis set that constrains the iterative peak fitting calculations used for quantifying signal intensity values for metabolites in the experimental, in vivo MRS data. An important limitation of whole-body MR systems used in diagnostic imaging is that there is no attempt by the system to hold fixed or measure the scaling factor between the measured raw data values and the strength of the magnetization giving rise to those values.
Because this scaling factor is unknown and variable across subjects and brain regions, the raw signal intensity values for each measurement cannot be easily converted to absolute concentration values e.
Two general approaches are typically used to control for variability in this scaling factor: 1 relative quantification and 2 estimated absolute quantification. Each of these approaches has advantages and disadvantages.
The choice of which approach to use may vary depending on the circumstances and goals of a particular MRS experiment. The general features of the two approaches are discussed below. The most widely used method of relative quantification in brain 1 H-MRS experiments is ratio normalization using an endogenous metabolite.
With this approach, the signal intensity values from each metabolite of interest in a given 1 H-MRS acquisition are normalized to the signal value of a reference metabolite measured simultaneously in the same voxel. Because it is a strong signal with relatively low variability across brain regions and across subjects, total creatine creatine plus phosphocreatine is most commonly used as the reference metabolite in this method of ratio normalization De Graaf, Ratio normalization using the creatine signal value is referred to as creatine normalization.
Because the unknown scaling factor in effect for a given 1 H-MRS acquisition influences the signal from each metabolite within the voxel equally, creatine normalization reduces the variance due to that factor.
For any given MRS acquisition, cerebrospinal fluid CSF constitutes a variable proportion of the total volume of the voxel.
Because the proportion of CSF within the voxel affects the measured signal similarly for each parenchymal metabolite, the creatine normalization procedure is also a useful way to correct for variation due to this partial volume effect.
The most important disadvantage of creatine normalization derives from the fact that creatine concentration varies across subjects. In the best case, this variation across subjects is random with respect to the subject groups of interest for a specific MRS experiment.
In this case, creatine variation adds to the overall variance in the data when creatine normalization is used. A more serious problem occurs when there is a systematic difference in creatine concentration between two groups of subjects. In this case, the creatine normalization approach may lead to false-positive or false-negative findings.
The likelihood that differences in creatine values are confounding group differences in creatine-normalized data is diminished when many creatine-normalized metabolites are compared across groups, but only one metabolite is observed to be abnormal.
In schizophrenia, the psychiatric disorder most extensively studied with 1 H-MRS, there is no consistent evidence for abnormalities in brain creatine concentrations Deicken et al.
The potential for confounding effects of systematic differences in creatine values is also reduced in dynamic 1 H-MRS studies, in which repeated measures are made in the same subject before and after an experimental manipulation e. neural activation. Because total creatine values appear to be stable over short-term repeated measurements, dynamic changes in a creatine-normalized metabolite e.
The primary alternative to creatine normalization or other relative quantification approaches is normalizing metabolite signal values to the water signal measured in the same voxel Ernst et al.
This approach requires at least two additional steps during acquisition of the 1 H-MRS data and additional calculations during postprocessing of the data. The procedure can generate an estimated absolute concentration for each metabolite in the voxel, but it relies on the measurement of the partial volumes of gray matter, white matter, and CSF within the voxel.
A significant challenge to the accuracy of partial volume measurements is achieving a precise registration of the voxel to the high-resolution anatomical images. This method also assumes canonical values for the concentration of water in gray matter, white matter, and CSF, which assumes that tissue water concentrations do not vary across subjects.
The most common implementation of this method also relies on canonical values for the relaxation times of brain water and metabolites, again assuming no differences between subject groups. The use of this approach requires collecting an additional 1 H-MRS acquisition using the same scanning parameters applied during collection of the metabolite data, except that the water suppression pulses are omitted.
This water nonsuppressed acquisition provides a measure of the full water signal from the voxel. In addition, it is necessary to acquire a structural MR image of the tissue within the voxel with good contrast between gray matter, white matter, and CSF.
After coregistration, this image is used to segment the voxel into gray matter, white matter, and CSF partial volumes Gasparovic et al. First, the concentration of water in the voxel is estimated from the results of the tissue segmentation analysis of the voxel as follows:.
where C water is the overall concentration of water in the voxel, F gm is the fraction of the voxel composed of gray matter, C gm is the canonical value for the concentration of water in gray matter Then, the estimated concentration of water in the voxel and the water signal intensity acquired from the voxel are used to estimate the scaling factor.
Along with corrections for the number of protons giving rise to each signal and corrections for relaxation time effects, the estimated concentration of the metabolite in the voxel is calculated as follows:. The correction to be used for relaxation times can be taken from published values Frahm et al.
However, the measurement of relaxation times requires considerable additional scanning time and is rarely done. Relaxation time corrections can be applied using different values for the gray matter, white matter, and CSF partial volumes or they can be applied with a single value for the whole voxel Malucelli et al.
In some experiments, it may be appropriate to normalize the metabolite concentration to the fraction of brain tissue gray matter plus white matter in the voxel 1- F CSF. Another approach to estimating the absolute concentrations of metabolites involves the use of a phantom containing a known concentration of a reference metabolite, such as creatine Soher et al.
Because the sensitivity of the RF receiver coil, and thus the overall scaling factor, is dependent on the location of the voxel, in-time approach is imprecise and has not been widely used for the calibration of MR spectra. The phantom is substituted for the patient and the MRS scan is rerun using the same voxel location.
The scaling factor for that voxel location can then be calculated from the ratio of the metabolite peak integral with known metabolite concentration in the phantom. This can be used to scale the metabolite peak integral from the patient.
However, when the phantom replaces the patient in the scanner, the main magnetic field must be reshimmed to provide sharp peaks in the phantom spectra.
The calibration of peak integrals in the patient based on those observed in the phantom is valid provided that no change in the receiver sensitivity or other scaling factors has occurred.
The reshimming of the main magnetic field required when the phantom replaces the patient in the scanner is sometimes associated with changes in scaling factors. Care must be taken that this does not occur. Soher et al. The signal intensity generated in the RF coil by a given magnetization in the object within the coil will vary as a function of the dielectric load of the object on the receiver coil.
The dielectric load of the phantom may differ substantially from the dielectric load of the patient. In general, the higher the dielectric load is, the smaller is the magnetic field reaching the coil and the smaller is the voltage induced in the coil. According to the reciprocity theorem Chen and Hoult, , the dielectric load of the object affecting the magnetic field from the voxel can be measured using the transmit voltage setting of the RF transmitter needed to nutate the magnetization in that same voxel.
About Contact Rfsonance News Media How to Mgnetic us Personnel Resonancf Stress management for healthy skin staff Support staff. Online Magnet Testosterone boosting supplements MRI systems : 3T Prisma Carbohydrate loading for endurance athletes. Magnefic to start Cost of MRI Funding Publications. Having a research MRI scan What is MR Functional MRI Spectroscopy DTI Cardiovascular MRI. Skip To Content. The Centre About Contact Us News Media How to find us Personnel MR Faculty staff Support staff Resources Online Magnet Schedule MRI systems : 3T Prisma 4. Quick links More details: Dr. Magnetic resonance Msgnetic spectroscopy Organic aromatherapy a noninvasive Carbohydrate loading for endurance athletes test for measuring biochemical changes in the Maynetic, especially resonanec presence of tumors. While magnetic resonance spectroacopy MRI identifies the anatomical location of a tumor, MR spectroscopy compares the chemical composition of normal brain tissue with abnormal tumor tissue. This test can also be used to detect tissue changes in stroke and epilepsy. The test is performed using an MRI scanner. For patient preparation instructions, please see MRI Brain.
Wacker, Ihr Gedanke ist sehr gut