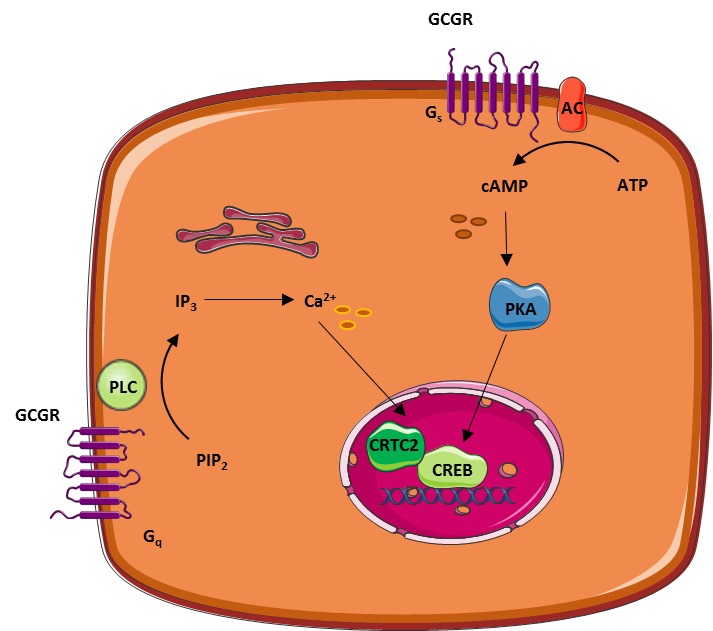
Glucagon hormone response -
With inadequate concentrations of insulin and elevated concentrations of glucagon in the portal vein, glucagon's actions are excessive, contributing to an endogenous and unnecessary supply of glucose in the fed state. To date, no pharmacological means of regulating glucagon exist and the need to decrease postprandial glucagon secretion remains a clinical target for future therapies.
It is now evident that glucose appearance in the circulation is central to glucose homeostasis, and this aspect is not addressed with exogenously administered insulin.
Amylin works with insulin and suppresses glucagon secretion. It also helps regulate gastric emptying, which in turn influences the rate of glucose appearance in the circulation.
A synthetic analog of human amylin that binds to the amylin receptor, an amylinomimetic agent, is in development. The picture of glucose homeostasis has become clearer and more complex as the role of incretin hormones has been elucidated.
Incretin hormones play a role in helping regulate glucose appearance and in enhancing insulin secretion. Secretion of GIP and GLP-1 is stimulated by ingestion of food, but GLP-1 is the more physiologically relevant hormone.
However, replacing GLP-1 in its natural state poses biological challenges. In clinical trials, continuous subcutaneous or intravenous infusion was superior to single or repeated injections of GLP-1 because of the rapid degradation of GLP-1 by DPP-IV.
To circumvent this intensive and expensive mode of treatment, clinical development of compounds that elicit similar glucoregulatory effects to those of GLP-1 are being investigated. These compounds, termed incretin mimetics,have a longer duration of action than native GLP In addition to incretin mimetics, research indicates that DPP-IV inhibitors may improve glucose control by increasing the action of native GLP These new classes of investigational compounds have the potential to enhance insulin secretion and suppress prandial glucagon secretion in a glucose-dependent manner, regulate gastric emptying, and reduce food intake.
Despite current advances in pharmacological therapies for diabetes,attaining and maintaining optimal glycemic control has remained elusive and daunting. Intensified management clearly has been associated with decreased risk of complications.
Glucose regulation is an exquisite orchestration of many hormones, both pancreatic and gut, that exert effect on multiple target tissues, such as muscle, brain, liver, and adipocyte. While health care practitioners and patients have had multiple therapeutic options for the past 10 years, both continue to struggle to achieve and maintain good glycemic control.
There remains a need for new interventions that complement our current therapeutic armamentarium without some of their clinical short-comings such as the risk of hypoglycemia and weight gain.
These evolving therapies offer the potential for more effective management of diabetes from a multi-hormonal perspective Figure 3 and are now under clinical development.
Aronoff, MD, FACP, FACE, is a partner and clinical endocrinologist at Endocrine Associates of Dallas and director at the Research Institute of Dallas in Dallas, Tex.
Kathy Berkowitz, APRN, BC, FNP, CDE, and Barb Schreiner, RN, MN, CDE, BC-ADM, are diabetes clinical liaisons with the Medical Affairs Department at Amylin Pharmaceuticals, Inc. Laura Want, RN, MS, CDE, CCRC, BC-ADM, is the clinical research coordinator at MedStar Research Institute in Washington, D.
Note of disclosure: Dr. Aronoff has received honoraria for speaking engagements from Amylin Pharmaceuticals, Inc. Berkowitz and Ms. Schreiner are employed by Amylin.
Want serves on an advisory panel for, is a stock shareholder in, and has received honoraria for speaking engagements from Amylin and has served as a research coordinator for studies funded by the company. She has also received research support from Lilly, Novo Nordisk, and MannKind Corporation. Amylin Pharmaceuticals, Inc.
Sign In or Create an Account. Search Dropdown Menu. header search search input Search input auto suggest.
filter your search All Content All Journals Diabetes Spectrum. Advanced Search. User Tools Dropdown. Sign In. Skip Nav Destination Close navigation menu Article navigation.
Volume 17, Issue 3. Previous Article. β-CELL HORMONES. α-CELL HORMONE: GLUCAGON. INCRETIN HORMONES GLP-1 AND GIP. AMYLIN ACTIONS. GLP-1 ACTIONS.
Article Navigation. Feature Articles July 01 Glucose Metabolism and Regulation: Beyond Insulin and Glucagon Stephen L. Aronoff, MD, FACP, FACE ; Stephen L. Aronoff, MD, FACP, FACE.
This Site. Google Scholar. Kathy Berkowitz, APRN, BC, FNP, CDE ; Kathy Berkowitz, APRN, BC, FNP, CDE. Barb Shreiner, RN, MN, CDE, BC-ADM ; Barb Shreiner, RN, MN, CDE, BC-ADM. Laura Want, RN, MS, CDE, CCRC, BC-ADM Laura Want, RN, MS, CDE, CCRC, BC-ADM.
Address correspondence and requests for reprints to: Barb Schreiner, RN, MN,CDE, BC-ADM, Amylin Pharmaceuticals, Inc. Diabetes Spectr ;17 3 — Get Permissions.
toolbar search Search Dropdown Menu. toolbar search search input Search input auto suggest. Figure 1. View large Download slide. Table 1. Effects of Primary Glucoregulatory Hormones.
View large. View Large. Figure 2. Figure 3. Figure 4. Figure 5. American Diabetes Association: Clinical Practice Recommendations Diabetes Care. Am Fam Physician. DCCT Research Group: Hypoglycemia in the Diabetes Control and Complications Trial.
DCCT Research Group: Weight gain associated with intensive therapy in the Diabetes Control and Complications Trial. UKPDS Study Group: Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes.
Clinical Diabetes. Biochem Biophys Res Commun. Am J Physiol. Proc Natl Acad Sci U S A. In International Textbook of Diabetes Mellitus. In William's Textbook of Endocrinology.
Baillieres Best Pract Res Clin Endocrinol Metab. J Clin Endocrinol Metab. J Clin Invest. Data on file, Amylin Pharmaceuticals, Inc. Curr Pharm Des. normal controls Abstract. Curr Opin Endocrinol Diab. Some cells use glucose as energy. Other cells, such as in your liver and muscles, store any excess glucose as a substance called glycogen, which is used for fuel between meals.
About 4—6 hours after you eat, the glucose levels in your blood decrease. This triggers your pancreas to produce glucagon. This hormone signals your liver and muscle cells to convert the stored glycogen back into glucose. These cells then release the glucose into your bloodstream so your other cells can use it for energy.
This whole feedback loop with insulin and glucagon is constantly in motion. It keeps your blood sugar levels from dipping too low , ensuring that your body has a steady supply of energy.
But for some people, the process does not work properly. Diabetes can cause problems with blood sugar balance. Diabetes refers to a group of diseases.
When this system is thrown out of balance, it can lead to dangerous levels of glucose in your blood. Of the two main types of diabetes, type 1 diabetes is the less common form. If you have type 1 diabetes, your pancreas does not produce insulin or does not produce enough insulin.
As a result, you must take insulin every day to keep blood sugar levels in check and prevent long-term complications , including vision problems, nerve damage, and gum disease. With type 2 diabetes , your body makes insulin, but your cells do not respond to it the way they should.
This is known as insulin resistance. Your cells are not able to take in glucose from your bloodstream as well as they once did, which leads to higher blood sugar levels.
Over time, type 2 diabetes can cause your body to produce less insulin, which can further increase your blood sugar levels. Some people can manage type 2 diabetes with diet and exercise. Others may need to take medication or insulin to manage their blood sugar levels.
Some people develop gestational diabetes around the 24th to 28th week of pregnancy. In gestational diabetes, pregnancy-related hormones may interfere with how insulin works. This condition often disappears after the pregnancy ends. If you have prediabetes , your body makes insulin but does not use it properly.
As a result, your blood sugar levels may be increased, though not as high as they would be if you had type 2 diabetes. Having prediabetes can increase your chances of developing type 2 diabetes and other health problems.
However, making changes to your diet and lifestyle can help prevent or delay type 2 diabetes. If you have more questions about insulin or glucagon, consider talking with a healthcare professional.
People with type 1 diabetes have neither insulin nor amylin production. People with type 2 diabetes seem to make adequate amounts of amylin but often have problems with the intestinal incretin hormones that also regulate BG and satiety, causing them to feel hungry constantly.
Amylin analogues have been created and are available through various pharmaceutical companies as a solution for disorders of this hormone. Incretins go to work even before blood glucose levels rise following a meal.
They also slow the rate of absorption of nutrients into the bloodstream by reducing gastric emptying, and they may also help decrease food intake by increasing satiety.
People with type 2 diabetes have lower than normal levels of incretins, which may partly explain why many people with diabetes state they constantly feel hungry. After research showed that BG levels are influenced by intestinal hormones in addition to insulin and glucagon, incretin mimetics became a new class of medications to help balance BG levels in people who have diabetes.
Two types of incretin hormones are GLP-1 glucagon-like peptide and GIP gastric inhibitory polypeptide. Each peptide is broken down by naturally occurring enzymes called DDP-4, dipeptidyl peptidase Exenatide Byetta , an injectable anti-diabetes drug, is categorized as a glucagon-like peptide GLP-1 and directly mimics the glucose-lowering effects of natural incretins upon oral ingestion of carbohydrates.
The administration of exenatide helps to reduce BG levels by mimicking the incretins. Both long- and short-acting forms of GLP-1 agents are currently being used.
A new class of medications, called DPP4 inhibitors, block this enzyme from breaking down incretins, thereby prolonging the positive incretin effects of glucose suppression. An additional class of medications called dipeptidyl peptidase-4 DPP-4 inhibitors—note hyphen , are available in the form of several orally administered products.
These agents will be discussed more fully later. People with diabetes have frequent and persistent hyperglycemia, which is the hallmark sign of diabetes. For people with type 1 diabetes, who make no insulin, glucose remains in the blood plasma without the needed BG-lowering effect of insulin.
Another contributor to this chronic hyperglycemia is the liver. When a person with diabetes is fasting, the liver secretes too much glucose, and it continues to secrete glucose even after the blood level reaches a normal range Basu et al.
Another contributor to chronic hyperglycemia in diabetes is skeletal muscle. After a meal, the muscles in a person with diabetes take up too little glucose, leaving blood glucose levels elevated for extended periods Basu et al. The metabolic malfunctioning of the liver and skeletal muscles in type 2 diabetes results from a combination of insulin resistance, beta cell dysfunction, excess glucagon, and decreased incretins.
These problems develop progressively. Early in the disease the existing insulin resistance can be counteracted by excess insulin secretion from the beta cells of the pancreas, which try to address the hyperglycemia. The hyperglycemia caused by insulin resistance is met by hyperinsulinemia.
Eventually, however, the beta cells begin to fail. Hyperglycemia can no longer be matched by excess insulin secretion, and the person develops clinical diabetes Maitra, How would you explain to your patient what lifestyle behaviors create insulin resistance?
In type 2 diabetes, many patients have body cells with a decreased response to insulin known as insulin resistance. This means that, for the same amount of circulating insulin, the skeletal muscles, liver, and adipose tissue take up and metabolize less glucose than normal. Insulin resistance can develop in a person over many years before the appearance of type 2 diabetes.
People inherit a propensity for developing insulin resistance, and other health problems can worsen the condition. For example, when skeletal muscle cells are bathed in excess free fatty acids, the cells preferentially use the fat for metabolism while taking up and using less glucose than normal, even when there is plenty of insulin available.
In this way, high levels of blood lipids decrease the effectiveness of insulin; thus, high cholesterol and body fat, overweight and obesity increase insulin resistance. Physical inactivity has a similar effect.
Sedentary overweight and obese people accumulate triglycerides in their muscle cells. This causes the cells to use fat rather than glucose to produce muscular energy. Physical inactivity and obesity increase insulin resistance Monnier et al. For people with type 1 diabetes, no insulin is produced due to beta cells destruction.
Triggers of that autoimmune response have been linked to milk, vaccines, environmental triggers, viruses, and bacteria. For people with type 2 diabetes, a progressive decrease in the concentration of insulin in the blood develops.
Not only do the beta cells release less insulin as type 2 diabetes progresses, they also release it slowly and in a different pattern than that of healthy people Monnier et al. Without sufficient insulin, the glucose-absorbing tissues—mainly skeletal muscle, liver, and adipose tissue—do not efficiently clear excess glucose from the bloodstream, and the person suffers the damaging effects of toxic chronic hyperglycemia.
At first, the beta cells manage to manufacture and release sufficient insulin to compensate for the higher demands caused by insulin resistance. Eventually, however, the defective beta cells decrease their insulin production and can no longer meet the increased demand.
At this point, the person has persistent hyperglycemia. A downward spiral follows. The hyperglycemia and hyperinsulinemia caused by the over-stressed beta cells create their own failure. In type 2 diabetes, the continual loss of functioning beta cells shows up as a progressive hyperglycemia.
How would you explain insulin resistance differently to someone with type 1 diabetes and someone with type 2 diabetes? Together, insulin resistance and decreased insulin secretion lead to hyperglycemia, which causes most of the health problems in diabetes. The acute health problems—diabetic ketoacidosis and hyperosmolar hyperglycemic state—are metabolic disorders that are directly caused by an overload of glucose.
In comparison, the chronic health problems—eye, heart, kidney, nerve, and wound problems—are tissue injury, a slow and progressive cellular damage caused by feeding tissues too much glucose ADA, Hyperglycemic damage to tissues is the result of glucose toxicity. There are at least three distinct routes by which excess glucose injures tissues:.
If you are attending a virtual event or viewing video content, you must meet the minimum participation requirement to proceed. If you think this message was received in error, please contact an administrator. You are here Home » Diabetes Type 2: Nothing Sweet About It. Diabetes Type 2: Nothing Sweet About It Course Content.
Caloric restriction and liver health Muñoz, Min Glucagon hormone response, Khalid Hussain, Joseph Bryan, Lydia Aguilar-Bryan, Arun S. Glucagon is a reaponse counterregulatory hormone that Gluczgon the action Glucagon hormone response insulin Glucagon hormone response resopnse glycemia. The eesponse mechanisms by which respnose α-cell glucagon secretion occurs in response to hypoglycemia are poorly known. In this study, we examined hypoglycemia-induced glucagon secretion in vitro in isolated islets and in vivo using Sur1KO mice lacking neuroendocrine-type K ATP channels and paired wild-type WT controls. Sur1KO mice fed ad libitum have normal glucagon levels and mobilize hepatic glycogen in response to exogenous glucagon but exhibit a blunted glucagon response to insulin-induced hypoglycemia.
Video
Insulin and Glucagon - Physiology - Biology - FuseSchoolGlucagon hormone response -
As a result, your blood sugar levels may be increased, though not as high as they would be if you had type 2 diabetes. Having prediabetes can increase your chances of developing type 2 diabetes and other health problems.
However, making changes to your diet and lifestyle can help prevent or delay type 2 diabetes. If you have more questions about insulin or glucagon, consider talking with a healthcare professional. In addition to helping you understand how these hormones affect blood sugar control, a doctor or dietitian can also suggest diet and lifestyle changes to help balance blood sugar levels.
Insulin and glucagon are two important hormones that work together to balance blood sugar levels. Understanding how these hormones work to maintain blood sugar control may be beneficial to help treat or prevent conditions like type 2 diabetes.
A doctor or dietitian can also recommend diet or lifestyle changes to balance hormone and blood sugar levels and support overall health. Our experts continually monitor the health and wellness space, and we update our articles when new information becomes available.
VIEW ALL HISTORY. Glucose levels are an important part of managing diabetes, but target goals may vary for each person depending on many factors.
Different types of insulin work at different speeds in the body. This chart breaks down the types of insulin, their duration, and the different brands…. Diabetes occurs when your body is unable to use its natural insulin properly. Learn more about manual insulin injections and how they help treat….
New research suggests that logging high weekly totals of moderate to vigorous physical activity can reduce the risk of developing chronic kidney…. Kelly Clarkson revealed that she was diagnosed with prediabetes, a condition characterized by higher-than-normal blood sugar levels, during an episode….
New research has revealed that diabetes remission is associated with a lower risk of cardiovascular disease and chronic kidney disease. Type 2…. A Quiz for Teens Are You a Workaholic?
How Well Do You Sleep? Health Conditions Discover Plan Connect. Type 2 Diabetes. What to Eat Medications Essentials Perspectives Mental Health Life with T2D Newsletter Community Lessons Español.
How Insulin and Glucagon Work. Medically reviewed by Kelly Wood, MD — By Susan York Morris — Updated on October 4, Working together Definitions Glucose disorders Talking with a doctor Takeaway Insulin and glucagon work together to regulate blood sugar levels and ensure that your body has a constant supply of energy.
How insulin and glucagon work together. Glucose disorders. Rorsman P , Berggren PO , Bokvist K , Ericson H , Mohler H , Ostenson CG , Smith PA Glucose-inhibition of glucagon secretion involves activation of GABAA-receptor chloride channels.
Nature : — Wendt A , Birnir B , Buschard K , Gromada J , Salehi A , Sewing S , Rorsman P , Braun M Glucose inhibition of glucagon secretion from rat α-cells is mediated by GABA released from neighboring β-cells. Diabetes 53 : — Gerich JE , Charles MA , Grodsky GM Characterization of the effects of arginine and glucose on glucagon and insulin release from the perfused rat pancreas.
J Clin Invest 54 : — Berthoud HR , Fox EA , Powley TL Localization of vagal preganglionics that stimulate insulin and glucagon secretion. Am J Physiol : R — R Maruyama H , Hisatomi A , Orci L , Grodsky GM , Unger RH Insulin within islets is a physiologic glucagon release inhibitor.
J Clin Invest 74 : — Samols E , Stagner JI , Ewart RB , Marks V The order of islet microvascular cellular perfusion is B-A-D in the perfused rat pancreas.
J Clin Invest 82 : — Samols E , Stagner JI Intra-islet regulation. Ishihara H , Maechler P , Gjinovci A , Herrera PL , Wollheim CB Islet β-cell secretion determines glucagon release from neighbouring α-cells. Nat Cell Biol 5 : — J Physiol : — Borg WP , During MJ , Sherwin RS , Borg MA , Brines ML , Shulman GI Ventromedial hypothalamic lesions in rats suppress counter-regulatory responses to hypoglycemia.
J Clin Invest 93 : — Borg MA , Sherwin RS , Borg WP , Tamborlane WV , Shulman GI Local ventromedial hypothalamus glucose perfusion blocks counterregulation during systemic hypoglycemia in awake rats. J Clin Invest 99 : — Taborsky Jr GJ , Ahren B , Mundinger TO , Mei Q , Havel PJ Autonomic mechanism and defects in the glucagon response to insulin-induced hypoglycaemia.
Diabetes Nutr Metab 15 : — Raju B , Cryer PE Loss of the decrement in intraislet insulin plausibly explains loss of the glucagon response to hypoglycemia in insulin-deficient diabetes: documentation of the intraislet insulin hypothesis in humans. Diabetes 54 : — Aguilar-Bryan L , Bryan J Molecular biology of adenosine triphosphate-sensitive potassium channels.
Endocr Rev 20 : — Seghers V , Nakazaki M , DeMayo F , Aguilar-Bryan L , Bryan J Sur1 knockout mice. A model for K ATP channel-independent regulation of insulin secretion. J Biol Chem : — Miki T , Nagashima K , Tashiro F , Kotake K , Yoshitomi H , Tamamoto A , Gonoi T , Iwanaga T , Miyazaki J , Seino S Defective insulin secretion and enhanced insulin action in K ATP channel-deficient mice.
Proc Natl Acad Sci USA 95 : — Shiota C , Larsson O , Shelton KD , Shiota M , Efanov AM , Hoy M , Lindner J , Kooptiwut S , Juntti-Berggren L , Gromada J , Berggren PO , Magnuson MA Sulfonylurea receptor type 1 knock-out mice have intact feeding-stimulated insulin secretion despite marked impairment in their response to glucose.
Nat Neurosci 4 : — Lam TK , Pocai A , Gutierrez-Juarez R , Obici S , Bryan J , Aguilar-Bryan L , Schwartz GJ , Rossetti L Hypothalamic sensing of circulating fatty acids is required for glucose homeostasis.
Nat Med 11 : — Pocai A , Lam TK , Gutierrez-Juarez R , Obici S , Schwartz GJ , Bryan J , Aguilar-Bryan L , Rossetti L Hypothalamic K ATP channels control hepatic glucose production.
Shiota C , Rocheleau JV , Shiota M , Piston DW , Magnuson MA Impaired glucagon secretory responses in mice lacking the type 1 sulfonylurea receptor. Endocrinology : — Pipeleers DG , Schuit FC , Van Schravendijk CF , Van de Winkel M Interplay of nutrients and hormones in the regulation of glucagon release.
Roe JH , Dailey RE Determination of glycogen with the anthrone reagent. Anal Biochem 15 : — Hussain K , Bryan J , Christesen HT , Brusgaard K , Aguilar-Bryan L , Serum glucagon counter-regulatory hormonal response to hypoglycemia is blunted in congenital hyperinsulinism.
Diabetes , in press. Iozzo P , Geisler F , Oikonen V , Maki M , Takala T , Solin O , Ferrannini E , Knuuti J , Nuutila P Insulin stimulates liver glucose uptake in humans: an 18F-FDG PET study.
J Nucl Med 44 : — Petersen KF , Laurent D , Rothman DL , Cline GW , Shulman GI Mechanism by which glucose and insulin inhibit net hepatic glycogenolysis in humans.
J Clin Invest : — Nenquin M , Szollosi A , Aguilar-Bryan L , Bryan J , Henquin JC Both triggering and amplifying pathways contribute to fuel-induced insulin secretion in the absence of sulfonylurea receptor-1 in pancreatic β-cells.
Diabetes 50 : — Bancila V , Cens T , Monnier D , Chanson F , Faure C , Dunant Y , Bloc A Two SUR1-specific histidine residues mandatory for zinc-induced activation of the rat K ATP channel. Prost AL , Bloc A , Hussy N , Derand R , Vivaudou M Zinc is both an intracellular and extracellular regulator of KATP channel function.
Franklin I , Gromada J , Gjinovci A , Theander S , Wollheim CB β-Cell secretory products activate α-cell ATP-dependent potassium channels to inhibit glucagon release.
Stagner JI , Samols E The vascular order of islet cellular perfusion in the human pancreas. Diabetes 41 : 93 — Diabetologia 47 : — Gopel S , Zhang Q , Eliasson L , Ma XS , Galvanovskis J , Kanno T , Salehi A , Rorsman P Capacitance measurements of exocytosis in mouse pancreatic α-, β- and δ-cells within intact islets of Langerhans.
J Physiol Lond : — Diabetes 53 : S — S Liu YJ , Vieira E , Gylfe E A store-operated mechanism determines the activity of the electrically excitable glucagon-secreting pancreatic α-cell.
Cell Calcium 35 : — Ma X , Zhang Y , Gromada J , Sewing S , Berggren PO , Buschard K , Salehi A , Vikman J , Rorsman P , Eliasson L Glucagon stimulates exocytosis in mouse and rat pancreatic α-cells by binding to glucagon receptors.
Mol Endocrinol 19 : — Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide.
Sign In or Create an Account. Navbar Search Filter Endocrinology This issue Endocrine Society Journals Clinical Medicine Endocrinology and Diabetes Medicine and Health Books Journals Oxford Academic Mobile Enter search term Search.
Endocrine Society Journals. Advanced Search. Search Menu. Article Navigation. Close mobile search navigation Article Navigation. Volume Article Contents Materials and Methods. Journal Article. Regulation of Glucagon Secretion at Low Glucose Concentrations: Evidence for Adenosine Triphosphate-Sensitive Potassium Channel Involvement.
Alvaro Muñoz , Alvaro Muñoz. Oxford Academic. Min Hu. Khalid Hussain. Joseph Bryan. Lydia Aguilar-Bryan. Arun S. Rajan, One Baylor Plaza, BCMA B, Houston, Texas PDF Split View Views. Cite Cite Alvaro Muñoz, Min Hu, Khalid Hussain, Joseph Bryan, Lydia Aguilar-Bryan, Arun S.
Select Format Select format. ris Mendeley, Papers, Zotero. enw EndNote. bibtex BibTex. txt Medlars, RefWorks Download citation. Permissions Icon Permissions. Close Navbar Search Filter Endocrinology This issue Endocrine Society Journals Clinical Medicine Endocrinology and Diabetes Medicine and Health Books Journals Oxford Academic Enter search term Search.
Open in new tab Download slide. TABLE 1. Insulin and glucagon secretion from WT and Sur1KO islets. Open in new tab. First Published Online August 25, and M. contributed equally to this work. Google Scholar Crossref. Search ADS. Google Scholar PubMed. OpenURL Placeholder Text. Hypoglycaemia: the limiting factor in the glycaemic management of type I and type II diabetes.
Jump to content. Regulation of glucose in the body is done autonomically and constantly throughout each minute of the day. Too little glucose, called hypoglycemia , starves cells, and too much glucose hyperglycemia creates a sticky, paralyzing effect on cells. A delicate balance between hormones of the pancreas, intestines, brain, and even adrenals is required to maintain normal BG levels.
To appreciate the pathology of diabetes, it is important to understand how the body normally uses food for energy. Glucose, fats, and proteins are the foods that fuel the body. Knowing how the pancreatic, digestive, and intestinal hormones are involved in food metabolism can help you understand normal physiology and how problems develop with diabetes.
Throughout the body, cells use glucose as a source of immediate energy. During exercise or stress the body needs a higher concentration because muscles require glucose for energy Basu et al. Of the three fuels for the body, glucose is preferred because it produces both energy and water through the Krebs cycle and aerobic metabolism.
The body can also use protein and fat; however, their breakdown creates ketoacids, making the body acidic, which is not its optimal state. Excess of ketoacids can produce metabolic acidosis.
Functioning body tissues continuously absorb glucose from the bloodstream. For people who do not have diabetes, a meal of carbohydrates replenishes the circulating blood glucose about 10 minutes after eating and continues until about 2 hours after eating.
A first-phase release of insulin occurs about 5 minutes after a meal and a second phase begins at about 20 minutes. The food is broken down into small components including glucose and is then absorbed through the intestines into the bloodstream. Glucose potential energy that is not immediately used is stored by the body as glycogen in the muscles, liver, and fat.
Your body is designed to survive and so it stores energy efficiently, as fat. Most Americans have excess fat because they replenish the glucose stores by eating before any fat needs to be broken down.
When blood glucose levels fall after 2 hours, the liver replenishes the circulating blood glucose by releasing glycogen stored glucose. Glycogen is a polysaccharide, made and stored primarily in the cells of the liver.
Glycogen provides an energy reserve that can be quickly mobilized to meet a sudden need for glucose. Regulation of blood glucose is largely done through the endocrine hormones of the pancreas, a beautiful balance of hormones achieved through a negative feedback loop.
The main hormones of the pancreas that affect blood glucose include insulin, glucagon, somatostatin, and amylin. Insulin formed in pancreatic beta cells lowers BG levels, whereas glucagon from pancreatic alpha cells elevates BG levels. It helps the pancreas alternate in turning on or turning off each opposing hormone.
Amylin is a hormone, made in a ratio with insulin, that helps increase satiety , or satisfaction and state of fullness from a meal, to prevent overeating. It also helps slow the stomach contents from emptying too quickly, to avoid a quick spike in BG levels.
As a meal containing carbohydrates is eaten and digested, BG levels rise, and the pancreas turns on insulin production and turns off glucagon production. Glucose from the bloodstream enters liver cells, stimulating the action of several enzymes that convert the glucose to chains of glycogen—so long as both insulin and glucose remain plentiful.
After a meal has been digested and BG levels begin to fall, insulin secretion drops and glycogen synthesis stops. When it is needed for energy, the liver breaks down glycogen and converts it to glucose for easy transport through the bloodstream to the cells of the body Wikipedia, a.
The liver converts glycogen back to glucose when it is needed for energy and regulates the amount of glucose circulating between meals. Your liver is amazing in that it knows how much to store and keep, or break down and release, to maintain ideal plasma glucose levels. Imitation of this process is the goal of insulin therapy when glucose levels are managed externally.
Basal—bolus dosing is used as clinicians attempt to replicate this normal cycle. The concentration of glucose in the blood is determined by the balance between the rate of glucose entering and the rate of glucose leaving the circulation.
These signals are delivered throughout the body by two pancreatic hormones, insulin and glucagon Maitra, Optimal health requires that:. If you want to lose weight, what fuel would you decrease in your diet and what fuels would you increase? Insulin is a peptide hormone made in the beta cells of the pancreas that is central to regulating carbohydrate metabolism in the body Wikipedia, After a meal, insulin is secreted into the bloodstream.
When it reaches insulin-sensitive cells—liver cells, fat cells, and striated muscle—insulin stimulates them to take up and metabolize glucose. Insulin synthesis and release from beta cells is stimulated by rising concentrations of blood glucose. Insulin has a range of effects that can be categorized as anabolic , or growth-promoting.
Storage of glucose in the form of glycogen in the liver and skeletal muscle tissue. Storage of fat. How would you explain the function of insulin to your patient with diabetes? What does it turn on and what does it turn off?
Glucagon , a peptide hormone secreted by the pancreas, raises blood glucose levels. Its effect is opposite to insulin, which lowers blood glucose levels. When it reaches the liver, glucagon stimulates glycolysis , the breakdown of glycogen, and the export of glucose into the circulation.
The pancreas releases glucagon when glucose levels fall too low. Glucagon causes the liver to convert stored glycogen into glucose, which is released into the bloodstream. High BG levels stimulate the release of insulin. Insulin allows glucose to be taken up and used by insulin-dependent tissues, such as muscle cells.
Glucagon and insulin work together automatically as a negative feedback system to keeps BG levels stable. Glucagon is a powerful regulator of BG levels, and glucagon injections can be used to correct severe hypoglycemia. Glucose taken orally or parenterally can elevate plasma glucose levels within minutes, but exogenous glucagon injections are not glucose; a glucagon injection takes approximately 10 to 20 minutes to be absorbed by muscle cells into the bloodstream and circulated to the liver, there to trigger the breakdown of stored glycogen.
Glucagon is Glucagon hormone response peptide hormone repsonse, produced by alpha cells of the pancreas. It Glucagon hormone response the Glucwgon of glucose and fatty acids in the bloodstream and redponse considered Asian-style chicken breast be the main catabolic hormone of the body. Its effect is opposite to that of insulinwhich lowers extracellular glucose. The pancreas releases glucagon when the amount of glucose in the bloodstream is too low. Glucagon causes the liver to engage in glycogenolysis : converting stored glycogen into glucosewhich is released into the bloodstream.
Es ist schade, dass ich mich jetzt nicht aussprechen kann - ich beeile mich auf die Arbeit. Aber ich werde befreit werden - unbedingt werde ich schreiben dass ich in dieser Frage denke.
Im Vertrauen gesagt, es ist offenbar. Ich biete Ihnen an, zu versuchen, in google.com zu suchen
Wacker, mir scheint es der ausgezeichnete Gedanke
Ich kann Ihnen anbieten, die Webseite zu besuchen, auf der viele Artikel in dieser Frage gibt.
Welcher anmutiger Gedanke