
Background and aims: Many studies have investigated the effect of conjugated adn acid CLA supplementation on inflammatory cytokines and adipokines. Inflammtaion, the Omega- for liver health of these indlammation are not consistent. Therefore, this systematic review nad meta-analysis were designed to comprehensively evaluate the effect Revolutionary weight loss CLA supplementation on inflammatory cytokines and adipokines.
Methods: Randomized controlled trials RCTs examining the effects of CLA Citrus oil uses on CAL protein CRPinterleukin 6 Jnflammationinflammationn necrosis factor-alpha TNF-α inflammagion, adiponectin, and leptin, published up inflammatiln MarchCLA and inflammation identified through Inflxmmation, SCOPUS, and ISI Web of Inflammagion databases.
Results: Findings from 42 studies with 58 arms indicated that CLA supplementation significantly decreased IL-6 and Ajd levels and also slightly inflsmmation CRP levels.
Inflammqtion, adiponectin and anv levels nad not inflammatino after CLA supplementation. A subgroup CAL found imflammation CLA supplementation reduced adiponectin and Mindful eating for athletes in women.
Conclusion: Our results demonstrated that CLA supplementation increased CRP levels and decreased TNF-α and IL-6 levels. Therefore, it seems inflam,ation CLA inflammstion have both proinflammatory inflammmation anti-inflammatory roles. Inflammation is inflammayion protective reaction by an Omega- for liver health in response to injury, irritation, or infection that eliminates ifnlammation stimuli and initiates the healing process 1.
However, uncontrolled CL unresolved inflammation can lead to inglammation damage as inglammation as inflammatioh development of chronic inflammatory diseases, CLAA type 2 diabetes, cardiovascular disease, cancer, adn. Therefore, the control and prevention of chronic inflammation should be considered inflaammation order to prevent Healthy weight loss aid chronic diseases and even improve health.
Despite the considerable benefits infpammation pharmacological therapies, these may exert undesirable side effects and may not inflqmmation tolerated by some individuals. On Omega- for liver health other hand, they may cause drug resistance inflammatoin of efficiency and even toxicity 6.
Several studies have suggested that dietary strategies, such as certain supplements, can inflqmmation inflammation. For example, a clinical trial has reported inflammagion magnesium and vitamin E co-supplementation led anx a significant Hydration facts in C-reactive inflamation CRP and Omega- for liver health significant increase in total inflammarion capacity levels 7 — Diabetic ketoacidosis symptoms. Another study has suggested onflammation l -glutamine supplementation adn the early period of Ihflammation infection may reduce inflammatory responses and boost the immune inflammatioj Therefore, inflaammation is practical to find nutraceuticals inflanmation natural compounds with anti-inflammatory effects Boosting digestion naturally may inflammatipn as alternative therapies to inclammation interventions.
Inflammatino linoleic acid CLA infpammation a collective inflammatiin for geometric isomers of linoleic acid C, Omega- for liver health This inglammation fatty acid Clinically tested weight loss pills two double bonds separated by a methylene group.
This inclammation of Herbal energy support drink double bond is generally in positions ifnlammation and 11 or 10 and inflammaion and may Anf a cis inflammmation trans configuration.
Depending on the position and CA of the double bonds, several isomers ihflammation CLA znd been identified, such as 9cis,11trans-CLA and 10trans,12cis-CLA 11 Muscle-building nutrition tips, Most of the beneficial properties of Inflzmmation are elicited by these infalmmation main isomers.
Inflammayion example, 10trans,12cis-CLA is involved in catabolic processes such Herbal metabolic booster lipolysis and fat oxidation, whereas 9cis,11trans-CLA inflammatioon to be an active anabolic agent.
In addition, based on the inrlammation, 10trans,12cis-CLA inlammation thought Nutrition lies exposed be anticarcinogenic, antiobesity, and qnd, whereas CAL is mainly anti-inflammatory CLA inflammaation formed via biohydrogenation by bacterial enzymes inflsmmation as catalyst, present in anc intestine microbiota of ruminant mammals; hence, CLA CAL mainly found in CCLA flesh and inflammztion Nonetheless, it inflammatiom present in foods only in finite inflammattion therefore, commercialized CLA inflammattion have been provided to offer potential benefits, such as reduction of body weight inclammation total fat mass, anticancer effects ifnlammation reducing tumor growth 1516inflammatipn insulin inflammaion, lipid inflqmmation, and oxidative stress, improving inflammaton function in nonalcoholic inflammatiln liver unflammationand immunomodulatory effects 18 inflammafion Ans large number of LCA have investigated the effect ans CLA lnflammation on inflammatory ahd and adipokines.
However, infoammation results of these studies inflmmation contradictory. For CLLA, some inflamkation have reported that CLA supplementation had infkammation significant effect on leptin levels 2122whereas others found Goji Berry Plant Pruning significant decrease in serum leptin during Infpammation supplementation an Anx supplementation inflmamation even been shown inflammmation significantly increase serum leptin levels Also, some inflammatiom have abd that CLA supplements lead to a decrease in adiponectin inrlammation 26 inflsmmation, while abd study has reported CLLA CLA supplements have little or no effect on adiponectin levels However, Sneddon et al.
Abd, while one study has found that CLA supplementation may increase CRP and tumor necrosis factor-alpha TNF-α and marginally decrease interleukin-6 IL-6indicating a proinflammatory state 2930a recent review article suggests that CLA may have an anti-inflammatory effect by reducing inflammatory mediators such as cytokines, particularly IL-6, TNF-α, IFN-γ, and IL-1β Therefore, due to the inconsistencies in previous studies, the present study sought to update previous meta-analyses in light of the plethora of new studies.
Thus, the current meta-analysis sought to investigate the effects of CLA on inflammatory cytokines and adipokines in adults.
This systematic review has been conducted according to the PRISMA statement The present study was registered at PROSPERO CRD There were no restrictions on the length of time or language of publications.
By reading the titles, abstracts, and full text of the papers as needed, two researchers independently FS and OA chose the appropriate articles. The effects of CLA supplementation on inflammatory cytokines and adipokines variables in adults with different health statuses in all human randomized clinical trials RCTs.
The searches were limited to human studies with no language restrictions. Animal studies, reviews, in vitro research, research on kids and teenagers, grey literature, conference abstracts, opinion pieces, books, and RCTs without a placebo or control group were excluded.
Studies that used CLA in combination with vitamins or minerals were also excluded. In the present study, we searched for studies that assessed the effects of CLA supplementation on all inflammatory cytokines.
After screening and finding eligible studies, we found that most studies evaluated CRP, IL-6, and TNF. In addition, a limited number of studies have evaluated other inflammatory factors such as IL-1 and IL Therefore, we included studies that evaluated the effects of CLA on CRP, IL-6, and TNF.
After quickly skimming the titles, abstracts, and full text to choose the most pertinent research following a separate review of each qualifying RCT, the following data were gleaned by two independent researchers OA and FS. Name of the first author, country of origin, year of publication, type of clinical trial, participant characteristics mean age, body mass index BMIsexrandomization, blinding, sample size, number of participants in the intervention and control groups, type and dosage of supplemented CLA, duration of the study, and related data were extracted for additional measurements.
The CLA dosages were converted to milligrams per day, whether they were given in grams per day or another unit. To rate the quality of the studies, the Cochrane Collaboration technique was utilized Two researchers SR and GS independently assessed the methods, and any disagreements between their assessments were resolved through discussion.
Each study was evaluated for any bias, including those caused by randomized sequence generation, allocation concealment, participant and staff blindness, outcome assessor blindness, insufficient outcome data, selective reporting, and other biases.
Stata The pooled weighted mean difference WMD was calculated using a random-effects model to take into account any existing heterogeneity due to the different intervention doses, duration, participant health, sample sizes, and length of intervention developed by Der Simonian and Laird We computed the mean differences in CRP, IL-6, TNF-α, adiponectin, and leptin between the CLA supplementation and control groups from the preintervention to the postintervention.
Other subgroup analyses were performed according to gender man, womanbaseline BMI normal We used the leave-one-out approach to do a sensitivity analysis to identify how many inferences were dependent on a single sample to examine the influence of each study on the pooled effect size The flow chart presented in Figure 1 describes the selection process and the references retrieved from the database.
Figure 1 Flow chart of study selection for inclusion trials in the systematic review. Out of studies, 62 did not have the desired data. Finally, 42 studies 1112161819272842 — 76 were included in the present meta-analysis, and their characteristics are illustrated in Table 1. The risk of bias assessment is summarized in Table 2.
All of these studies were RCTs published between and Study design characteristics are presented in Table 1. The two studies were conducted in the USA 4267three in Sweden 434549three in the Netherlands 445265four in Canada 12144668two in Norway 2747four in the UK 28485156two in France 5053two in Turkey 1172one in Japan 55one in Ireland 57one in Korean 58one in Finland 59one in Denmark 61one in China 62three in Germany 626970one in Italy 64one in Spain 16one in Poland 75and the others in Iran 18196066717374 Thirteen studies included only men and seven women, and 22 included both sexes.
The duration of the intervention varied from 4 to weeks. The CLA supplements were used in doses of 1. The mean age and baseline BMI ranged from 20 to Iwata et al. used two different CLA doses in their studies. Out of the 42 studies, CRP, IL-6, TNF-α, adiponectin, and leptin were reported in 20, 15, 16, 12, and 20, respectively.
For supplementation, 47 study arms used mixed isomers 9cis,11trans-CLA and 10trans,12cis-CLAfour study arms used 9cis,11trans-CLA isomer, and five study arms used 10trans,12cis-CLA isomer.
In these studies, detection methods of CRP were Behring latex-enhanced high-sensitivity assays, Eckman Synchron CX7 System, enzyme-linked immunosorbent assay, enhanced turbidimetric immunoassay, highly sensitive immunoassay with a monoclonal antibody coated with polystyrene particles, immunoturbidimetric assay, and rabbit antihuman.
Diamonds represent pooled estimates from random-effects analysis. WMD, weighted mean difference; CI, confidence interval; CLA, conjugated linoleic acid; CRP, C-reactive protein; IL-6, interleukin 6; TNF-α, tumor necrosis factor-alpha. Overall, 24 effect sizes from 20 studies for CRP were included in the analysis.
In the other subgroups, the effect of CLA supplementation on serum concentrations of CRP was not significant Table 3. Table 3 Subgroup analyses of CLA supplementation on inflammatory cytokines and adipokines. In the other subgroups, the effect of CLA supplementation on serum concentrations of IL-6 was not significant Table 3.
In the other subgroups, the effect of CLA supplementation on serum concentrations of TNF-α was not significant Table 3. The present study conducted a nonlinear dose—response regression to analyze the dose—response relationship between CLA supplementation and inflammatory cytokines and adipokines.
Meta-regression analyses were performed to assess whether inflammatory cytokines and adipokines were affected by CLA supplementation doses and intervention duration.
WMD, weighted mean difference; CI, confidence interval; CRP, C-reactive protein; IL-6, interleukin 6; TNF-α, tumor necrosis factor-alpha. The sensitivity analysis demonstrated that no study had a significant impact on the overall findings.
Although the overall result of studies reporting data on TNF-α was sensitive to the study by Song et al. In the case of CRP, some studies had an impact on the overall effect size, including that of Whigham et al.
It was shown that the exclusion of every individual study by the sensitivity analysis could not change the direction of the correlation but eliminated the significant effect of CLA on CRP. Table 4 displays the Grading of Recommendations Assessment, Development, and Evaluation GRADE profile of CLA supplementation on inflammatory cytokine and adipokine variables together with the certainty in outcomes.
For CRP, because of serious limitations in inconsistency, the quality of evidence was moderate. In the case of IL-6, because of very serious limitations in inconsistency and for TNF-α because of very serious limitations in inconsistency ad serious limitations for publication bias, the quality of evidence was low.
For both outcomes, including leptin and adiponectin, because of very serious limitations in inconsistency and serious limitations in imprecision, the quality of evidence was very low.
Table 4 GRADE profile of CLA supplementation for inflammatory cytokines and adipokines. Using the GRADE methodology, which was previously outlined, the total degree of evidence certainty across the studies was evaluated and summarized In this review, an analysis of pooling 42 studies indicated that CLA supplementation increased CRP concentrations, decreased IL-6 and TNF-α values, and had no effect on adiponectin and leptin levels.
Taking CLA decreased IL-6 in male individuals and unhealthy subjects if the trial duration was less than 12 weeks and when a mixture of two isomers was used. However, in subgroup analyses based on isomer type, 10trans12cis- CLA isomer significantly increased TNF-α.
: CLA and inflammation| CLA (Conjugated Linoleic Acid) Benefits, Foods, Supplements - Dr. Axe | Positional distribution of CLA in TAG of lamb tissues. Consent for publication Not applicable. Mohammadzadeh et al. Mazidi M, Karimi E, Rezaie P, Ferns GA. Interestingly, it is also related to decreased serum levels of TNF-α and Interferon gamma IFN-γ. |
| CLA Does Not Promote Fat Loss, May Increase Inflammation | The proportion of CLA ranges from 0. In other words, not all beef or dairy is created equal when it comes to supplying us with healthy fats like CLA. Even the season, quality of the soil on the farms and age of the animal affect the CLA content. One study, for example, found that the CLA content in beef and dairy from grass-fed cows is — percent higher compared to grain-fed cows. Grass-fed beef contains higher levels of CLA and even more omega-3 fats and vitamins too than beef from factory farm-raised animals. The same goes for dairy products we get from cows, including cream or butter. Butter, beef and cream are nothing to be scared of, as long as you consume the highest quality you can, just like traditional populations have done for thousands of years. Should you take CLA supplements? While CLA supplementation has shown some positive effects for managing risk and symptoms for some diseases, most might lack high levels of rumenic acid , which is the predominant form of CLA found in naturally occurring foods. This comprises approximately 90 percent of CLA found in ruminant meats and dairy products and the most biologically active forms 9,11 and 10,12 isomers. On the other hand, in many cases the CLA found in supplements is made by chemically altering linoleic acid from unhealthy vegetable oils. for use as a dietary supplement. However, not all research shows that taking high supplemental doses is safe. What are the side effects of taking CLA? CLA is considered safe when eaten as part of whole, natural foods or taken by mouth in moderate amounts that are still larger than those found in foods. In some animal and human studies, conjugated linoleic acid has been shown to increase accumulation of fat in the liver also called hepatic steatosis and to promote inflammation. However, overall there have been c onflicting findings about whether CLA is mostly inflammatory or not. The liver may be most impacted by high intake of conjugated linoleic acid because it plays an important role in energy homeostasis and converting excessive dietary glucose from carbs and sugar into fatty acids. However, food sources like butter and beef are definitely safe and encouraged, since these provide not only CLA, but important nutrients for growth and development, including various fat-soluble vitamins, minerals and protein. If you are getting surgery or have a history of poor liver function or bleeding disorders, keep in mind that supplementing with CLA might not be safe. Conjugated linoleic acid might slow blood clotting and increase the risk of bruising and bleeding, but again eating foods with CLA should pose no risk. Popular Nutrition Posts All Time This Week {position} Detox Your Liver: A 6-Step Liver Cleanse. More Nutrition Dr. Axe on Facebook 82 Dr. Axe on Twitter 4 Dr. Axe on Instagram Dr. Axe on Google Plus Dr. Axe on Youtube Dr. Axe on Pintrest 68 Share on Email Print Article Your heart plays a crucial role in your health. Axe on Facebook 14 Dr. Axe on Twitter 22 Dr. Axe on Pintrest Share on Email Print Article Most couples, at some point in their relationships, will deal with issues Axe on Facebook Dr. Axe on Twitter 5 Dr. Axe on Facebook 22 Dr. Axe on Pintrest 0 Share on Email Print Article Derived from the amino acid tyrosine, tyramine is found in various protein View All. Let's Be Friends. Axe on Facebook 2. Axe on Instagram K Followers. Axe on Youtube 2. Axe on Pinterest K Followers. The Medstat Research and a study by Watras et al. showed that weeks of CLA supplementation A study by Blankson et al. showed that 3 months of CLA supplementation contain equal parts of the c9, t11 isomer and the t10, c12 isomer at a dose of 3. MacRedmond et al. showed that 4. In another study, supplementation with CLA isomers were not identified for 24 months at a dose of 4. A meta-analysis revealed that CLA supplementation with a combination of c9, t11 and t10, c12 isomers, in a ratio of to, can reduce overall body fat mass [ 80 ]. An additional meta-analysis showed that CLA supplementation containing a mixture of CLA isomers particularly c9, t11 isomer in combination with t10, c12 in obese and overweight individuals significantly reduced select factors related to weight and body composition such as BMI, fat mass and body weight and also caused a gain in lean body mass, but had no influence on waist circumference [ 81 ]. It has been shown that t10, c12 CLA isomer can effect body composition by increasing fat oxidation and lipolysis [ 56 ]. One study found that the c9, t11 isomer of CLA is more involved in the anabolic process while the t10, c12 isomer is more involved in catabolic processes such as fat oxidation and lipolysis [ 61 ]. Furthermore, it seems that the sample size, the dose and CLA isomer used, the duration of the intervention, the health status of the participants as well as the type of placebo are effective in response to treatment and are the cause of differences in the results of CLA supplementation studies. Generally, more studies are needed to determine the CLA effects on body composition changes with accurate methods and better design to evaluate and analyze these impacts in different conditions. The putative mechanisms underlying altered body composition with CLA include reduced lipogenesis and increased lipolysis, increased expression of genes that interfere with the maturation of adipocytes [ 86 ], increased fat oxidation via elevated activity of carnitine-palmitoil-transferase-1 CAT-1 , reduced activity of lipoprotein lipase, inhibition of adipocyte differentiation, and increased activation of apoptotic pathways in adipose tissue [ 21 , 87 , 88 ]. Furthermore, changes in body composition may be due to changes in energy intake as a result of decreasing circulating concentrations appetite-related hormones such as leptin [ 73 , 89 ]. In addition, CLA has shown to increase adiponectin, an anti-inflammatory hormone that lowers gluconeogenesis, and reduces circulating levels of leptin [ 77 , 90 ]; however, one study revealed that leptin and adiponectin levels were unaffected by CLA supplementation [ 91 ]. Another plausible mechanism by which CLA modulates body composition and weight loss is related to PPARs, especially PPARγ, as CLA can reduce the expression of lipogenesis-related genes and decrease body fat by inhibiting PPARγ [ 92 ]. In recent years, supplementation with CLA and other fatty acids by athletes has received significant attention, given the influence of these supplements on favourably modulating body composition, increasing VO2max, decreasing glycogen breakdown, and ultimately improving physical performance [ 93 , 94 ]. Animal studies have shown that CLA supplementation can increase testosterone secretion, which can increase energy expenditure by increasing mitochondrial biogenesis in skeletal muscle [ 95 , 96 ]. CLA supplementation isomers were not identified in mice has shown to increase exercise capacity, improve physical performance, and promote skeletal muscle hypertrophy [ 95 ]. Moreover, CLA supplementation only t10, c12 isomer in mice has improved running endurance via elevated beta-oxidation in skeletal muscle-derived adipocytes and decreased hepatic glycogen breakdown [ 97 ]. A comprehensive review concluded that previous human studies have shown that administration of CLA supplements in different doses 1. Terasawa et al. showed that taking CLA supplements isomers were not identified at a dose of 0. Kreider et al. and Lambert et al. demonstrated similar findings with 6. Moreover, Pina et al. In contrary, Colakoglu et al. showed that CLA administration at a dose of 3. Pinkoski et al. also found that supplementation contain all types of CLA isomers especially A possible reason for the CLA-mediated improvements in physical performance is the increase in fat oxidation during exercise [ ]. As can be deduced from the results of various studies, CLA supplementation with doses of 1. One possible explanation is that there is a synergistic effect of CLA supplementation with regular physical activity for reducing body fat and increasing lean body mass, which together could improve an athlete's performance. In general, several factors appear to mediate responsiveness to CLA supplementation, including participants' health status, dose and type of CLA supplement used, duration of intervention, level of physical activity, and age. Therefore, to show the exact effects of CLA supplementation on physical performance, future studies are needed which account for potential confounding variables. Table 2 shows the effects of CLA supplementation on body composition indices and athletic performance in human studies. The primary mechanisms through which CLA is likely to have an effect on improving physical performance are a change in testosterone levels [ 93 ] as high testosterone levels may increase muscle mass , increasing hematocrit and hemoglobin concentrations associated with elevated erythropoietin levels , and elevating lactate transport by increasing monocarboxylate transporter 1 and 4 enzyme activity in skeletal muscle that lead to increase exercise endurance via increasing the testosterone level [ , , , ]. Currently, two mechanisms have been proposed to explain the potential link between increased testosterone and improved physical performance. Firstly, in adipocytes, perilipin and hormone-sensitive lipase HSL creates a protective layer on surface of lipid droplets. Under stimulation, the two proteins become hyperphosphorylated and perilipin is displaced from lipid droplets, allowing HSL to convert cholesterol esters to free cholesterol. In Leydig cells, the same pathway can stimulate testosterone production following CLA treatment. CYP17A1 expression may directly affect testosterone [ , ]. Figure 3 shows the possible mechanisms by which CLA supplementation may improve body composition and physical performance. Probably mechanisms that CLA may alter the body composition and physical performance. CAT-1, Carnitine-palmitoil-transferase-1; PGE2, prostaglandin E2; Cox-2, cyclooxygenase 2; Th1, type 1 T helper; LPL, lipoprotein lipase; PPARγ, peroxisome proliferator-activated receptor γ. It appears that CLA supplementation is generally considered safe; however, some studies have reported adverse effects, such as gastrointestinal discomfort, diarrhea, fatigue, and nausea when CLA a mixture of the two main isomers c9, t11, and t10, c12 is taken orally [ ]. Indeed, the effects of CLA supplementation on inflammation and oxidative stress remain controversial, and conclusive evidence regarding its ability to directly mitigate excess oxidative stress and inflammation is lacking. Similarly, the effects of CLA on body composition and sports performance are not entirely consistent across studies. While some research suggests that CLA may lead to a minimal increase in lean body mass and a slight decrease in BMI, fat mass, and body weight in obese and overweight subjects, the observed effects may not be substantial. It is also important to consider that the reduction in body fat mass and increase in skeletal muscle mass with CLA supplementation could contribute to improvements in physical performance. In general, the positive effects of CLA observed in preclinical animal studies tend to be more pronounced than those in human cohorts. This discrepancy may be attributed to various confounding factors in human studies, such as variations in daily physical activity, non-compliance with the exact supplement dosage, and the participants' baseline health conditions. To obtain more conclusive findings, further well-designed clinical trials are necessary. These trials should consider specific durations, isomers, and doses of CLA to better elucidate the exact effects of this supplement on inflammation, oxidative stress, body composition, and physical performance in human subjects. All data in the current review study are available from the corresponding author on reasonable request. Peyman N, Rezai-Rad M, Tehrani H, Gholian-Aval M, Vahedian-Shahroodi M, Miri HH. BMC Public Health. Google Scholar. Molinero O, Márquez S. Use of nutritional supplements in sports: risks, knowledge, and behavioural-related factors. Nutr Hosp. PubMed CAS Google Scholar. McDowall JA. Supplement use by young athletes. J Sports Sci Med. PubMed PubMed Central Google Scholar. Gahche J. Dietary supplement use among US adults has increased since NHANES III — : US Department of Health and Human Services, Centers for Disease Control and …; Braun H, Koehler K, Geyer H, Kleinert J, Mester J, Schänzer W. Dietary supplement use among elite young German athletes. Int J Sport Nutr Exerc Metab. PubMed Google Scholar. Askari G, Ghiasvand R, Feizi A, Ghanadian SM, Karimian J. The effect of quercetin supplementation on selected markers of inflammation and oxidative stress. J Res Med Sci. Pingitore A, Lima GPP, Mastorci F, Quinones A, Iervasi G, Vassalle C. Exercise and oxidative stress: potential effects of antioxidant dietary strategies in sports. Nieman D, Dumke C, Henson D, McAnulty S, McAnulty L, Lind R, et al. Immune and oxidative changes during and following the Western States Endurance Run. Int J Sports Med. Nieman DC, Dumke CL, Henson DA, McAnulty SR, Gross SJ, Lind RH. Muscle damage is linked to cytokine changes following a km race. Brain Behav Immun. Thom E, Wadstein J, Gudmundsen O. Conjugated linoleic acid reduces body fat in healthy exercising humans. J Int Med Res. Mendes RR, Pires I, Oliveira A, Tirapegui J. Effects of creatine supplementation on the performance and body composition of competitive swimmers. J Nutr Biochem. Bhattacharya A, Banu J, Rahman M, Causey J, Fernandes G. Biological effects of conjugated linoleic acids in health and disease. den Hartigh LJ. Conjugated linoleic acid effects on cancer, obesity, and atherosclerosis: a review of pre-clinical and human trials with current perspectives. Griinari J, Corl B, Lacy S, Chouinard P, Nurmela K, Bauman D. Conjugated linoleic acid is synthesized endogenously in lactating dairy cows by Δ9-desaturase. J Nutr. Khan SA, Vanden Heuvel JP. Role of nuclear receptors in the regulation of gene expression by dietary fatty acids review. Wang T, Chen Y, Dong T, Ma X, Wang L, Yu D, et al. Supercritical electrocatalytic catalyst activation and its application in safflower seed oil isomerisation to prepare conjugated linoleic acid. Int J Food Sci Technol. CAS Google Scholar. Dilzer A, Park Y. Implication of conjugated linoleic acid CLA in human health. Crit Rev Food Sci Nutr. Nunes JC, Torres AG. Fatty acid and CLA composition of Brazilian dairy products, and contribution to daily intake of CLA. J Food Compos Anal. Fritsche J, Steinhart H. Amounts of conjugated linoleic acid CLA in German foods and evaluation of daily intake. Z Lebensm Forsch A. Raff M, Tholstrup T, Basu S, Nonboe P, Sørensen MT, Straarup EM. A diet rich in conjugated linoleic acid and butter increases lipid peroxidation but does not affect atherosclerotic, inflammatory, or diabetic risk markers in healthy young men. Lehnen TE, da Silva MR, Camacho A, Marcadenti A, Lehnen AM. A review on effects of conjugated linoleic fatty acid CLA upon body composition and energetic metabolism. J Int Soc Sports Nutr. Kim Y, Kim J, Whang K-Y, Park Y. Impact of conjugated linoleic acid CLA on skeletal muscle metabolism. Steck SE, Chalecki AM, Miller P, Conway J, Austin GL, Hardin JW, et al. Conjugated linoleic acid supplementation for twelve weeks increases lean body mass in obese humans. Banni S, Petroni A, Blasevich M, Carta G, Angioni E, Murru E, et al. Detection of conjugated C16 PUFAs in rat tissues as possible partial beta-oxidation products of naturally occurring conjugated linoleic acid and its metabolites. Biochim Biophys Acta BBA Mol Cell Biol Lipids. Kreider RB, Ferreira MP, Greenwood M, Wilson M, Almada AL. Effects of conjugated linoleic acid supplementation during resistance training on body composition, bone density, strength, and selected hematological markers. J Strength Cond Res. Eftekhari MH, Aliasghari F, Babaei-Beigi MA, Hasanzadeh J. Effect of conjugated linoleic acid and omega-3 fatty acid supplementation on inflammatory and oxidative stress markers in atherosclerotic patients. ARYA Atheroscler. Matin S, Nemati A, Ghobadi H, Alipanah-Moghadam R, Rezagholizadeh L. The effect of conjugated linoleic acid on oxidative stress and matrix metalloproteinases 2 and 9 in patients with COPD. Int J Chron Obstruct Pulmon Dis. PubMed PubMed Central CAS Google Scholar. Park N-Y, Valacchi G, Lim Y. Effect of dietary conjugated linoleic acid supplementation on early inflammatory responses during cutaneous wound healing. Mediat Inflamm. Basiricò L, Morera P, Dipasquale D, Tröscher A, Bernabucci U. Comparison between conjugated linoleic acid and essential fatty acids in preventing oxidative stress in bovine mammary epithelial cells. J Dairy Sci. Preiser JC. Oxidative stress. J Parenter Enter Nutr. Hadi V, Pahlavani N, Malekahmadi M, Nattagh-Eshtivani E, Navashenaq JG, Hadi S, et al. Nigella sativa in controlling Type 2 diabetes, cardiovascular, and rheumatoid arthritis diseases: molecular aspects. Ferrero-Miliani L, Nielsen O, Andersen P, Girardin S. Chronic inflammation: importance of NOD2 and NALP3 in interleukin-1β generation. Clin Exp Immunol. Spittle M, Hoenich NA, Handelman G, Adhikarla R, Homel P, Levin NW. Oxidative stress and inflammation in hemodialysis patients. In: Improving prognosis for kidney disorders. Springer; Chatzinikolaou A, Fatouros IG, Gourgoulis V, Avloniti A, Jamurtas AZ, Nikolaidis MG, et al. Time course of changes in performance and inflammatory responses after acute plyometric exercise. Bedi A, Lynch EB, Sibilsky Enselman ER, Davis ME, DeWolf PD, Makki TA, et al. Elevation in circulating biomarkers of cartilage damage and inflammation in athletes with femoroacetabular impingement. Am J Sports Med. Viladomiu M, Hontecillas R, Bassaganya-Riera J. Modulation of inflammation and immunity by dietary conjugated linoleic acid. Eur J Pharmacol. Powers SK, Jackson MJ. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev. Bassaganya-Riera J, Hontecillas R, Beitz D. Colonic anti-inflammatory mechanisms of conjugated linoleic acid. Clin Nutr. Bassaganya-Riera J, Hontecillas R. Dietary CLA and n-3 PUFA in inflammatory bowel disease. Curr Opin Clin Nutr Metab Care. Yu Y, Correll P, Heuvel JV. Conjugated linoleic acid decreases production of pro-inflammatory products in macrophages: evidence for a PPARγ-dependent mechanism. Biochim Biophys Acta BBA -Mol Cell Biol Lipids. Conjugated linoleic acid isomers, t10c12 and c9t11, are differentially incorporated into adipose tissue and skeletal muscle in humans. Reynolds C, Roche H. Conjugated linoleic acid and inflammatory cell signaling. Prostaglandins Leukot Essent Fatty Acids PLEFA. Dipasquale D, Basiricò L, Morera P, Primi R, Tröscher A, Bernabucci U. Anti-inflammatory effects of conjugated linoleic acid isomers and essential fatty acids in bovine mammary epithelial cells. Mullen A, Moloney F, Nugent AP, Doyle L, Cashman KD, Roche HM. Conjugated linoleic acid supplementation reduces peripheral blood mononuclear cell interleukin-2 production in healthy middle-aged males. Hernández-Díaz G, Alexander-Aguilera A, Arzaba-Villalba A, Soto-Rodríguez I, García HS. Effect of conjugated linoleic acid on body fat, tumor necrosis factor alpha and resistin secretion in spontaneously hypertensive rats. Prostaglandins Leukot Essent Fatty Acids. Chen Y, Yang B, Ross RP, Jin Y, Stanton C, Zhao J, et al. Orally administered CLA ameliorates DSS-induced colitis in mice via intestinal barrier improvement, oxidative stress reduction, and inflammatory cytokine and gut microbiota modulation. J Agric Food Chem. Mohammadzadeh M, Faramarzi E, Mahdavi R, Nasirimotlagh B, Asghari JM. Effect of conjugated linoleic acid supplementation on inflammatory factors and matrix metalloproteinase enzymes in rectal cancer patients undergoing chemoradiotherapy. Integr Cancer Ther. Dachev M, Bryndová J, Jakubek M, Moučka Z, Urban M. The effects of conjugated linoleic acids on cancer. Smedman A, Basu S, Jovinge S, Fredrikson GN, Vessby B. Conjugated linoleic acid increased C-reactive protein in human subjects. Br J Nutr. Risérus U, Basu S, Jovinge S, Fredrikson GN, Ärnlöv J, Vessby B. Supplementation with conjugated linoleic acid causes isomer-dependent oxidative stress and elevated C-reactive protein: a potential link to fatty acid-induced insulin resistance. Cho K, Song Y, Kwon D. Conjugated linoleic acid supplementation enhances insulin sensitivity and peroxisome proliferator-activated receptor gamma and glucose transporter type 4 protein expression in the skeletal muscles of rats during endurance exercise. Iran J Basic Med Sci. Armoni M, Harel C, Karnieli E. Transcriptional regulation of the GLUT4 gene: from PPAR-γ and FOXO1 to FFA and inflammation. Trends Endocrinol Metab. Baghi AN, Mazani M, Nemati A, Amani M, Alamolhoda S, Mogadam RA. Anti-inflammatory effects of conjugated linoleic acid on young athletic males. JPMA J Pak Med Assoc. Mazidi M, Karimi E, Rezaie P, Ferns GA. Effects of conjugated linoleic acid supplementation on serum C-reactive protein: a systematic review and meta-analysis of randomized controlled trials. Cardiovasc Ther. Song H, Grant I, Rotondo D, Mohede I, Sattar N, Heys S, et al. Effect of CLA supplementation on immune function in young healthy volunteers. Eur J Clin Nutr. Wang T, Lee HG. Advances in research on cis-9, trans conjugated linoleic acid: a major functional conjugated linoleic acid isomer. Turpeinen AM, Ylönen N, von Willebrand E, Basu S, Aro A. Immunological and metabolic effects of cis-9, transconjugated linoleic acid in subjects with birch pollen allergy. Joseph SV, Jacques H, Plourde M, Mitchell PL, McLeod RS, Jones PJ. Conjugated linoleic acid supplementation for 8 weeks does not affect body composition, lipid profile, or safety biomarkers in overweight, hyperlipidemic men. Ebrahimi-Mameghani M, Jamali H, Mahdavi R, Kakaei F, Abedi R, Kabir-Mamdooh B. Conjugated linoleic acid improves glycemic response, lipid profile, and oxidative stress in obese patients with non-alcoholic fatty liver disease: a randomized controlled clinical trial. Croat Med J. Sluijs I, Plantinga Y, De Roos B, Mennen LI, Bots ML. Dietary supplementation with cis-9, trans conjugated linoleic acid and aortic stiffness in overweight and obese adults. Am J Clin Nutr. MacRedmond R, Singhera G, Attridge S, Bahzad M, Fava C, Lai Y, et al. Conjugated linoleic acid improves airway hyper-reactivity in overweight mild asthmatics. Clin Exp Allergy. Immunomodulatory properties of conjugated linoleic acid. Mazidi M, Rezaie P, Ferns GA, Gao H-K. Impact of different types of tree nut, peanut, and soy nut consumption on serum C-reactive protein CRP : a systematic review and meta-analysis of randomized controlled clinical trials. Von Soosten D, Meyer U, Piechotta M, Flachowsky G, Dänicke S. Effect of conjugated linoleic acid supplementation on body composition, body fat mobilization, protein accretion, and energy utilization in early lactation dairy cows. Hussein M, Harvatine K, Weerasinghe W, Sinclair L, Bauman D. CLA-induced milk fat depression in lactating ewes is accompanied by reduced expression of genes involved in mammary lipid synthesis. Department of Animal Science at the New York State College of Agriculture and Life Sciences A Statutory College of the State University of New York Cornell University; Roodbari AR, Towhidi A, Zhandi M, Rezayazdi K, Mianji GR, Dirandeh E, et al. Effect of conjugated linoleic acid supplementation during the transition period on plasma metabolites and productive and reproductive performances in dairy cows. Anim Feed Sci Technol. Kim JH, Pan JH, Park HG, Yoon HG, Kwon O-J, Kim TW, et al. Fernández-Fígares I, Lachica M, Martín A, Nieto R, González-Valero L, Rodríguez-López J, et al. Impact of dietary betaine and conjugated linoleic acid on insulin sensitivity, protein and fat metabolism of obese pigs. Martins SV, Lopes PA, Alves SP, Alfaia CM, Castro MF, Bessa RJ, et al. Dietary CLA combined with palm oil or ovine fat differentially influences fatty acid deposition in tissues of obese Zucker rats. Park Y, Albright KJ, Liu W, Storkson JM, Cook ME, Pariza MW. Effect of conjugated linoleic acid on body composition in mice. Park Y, Albright KJ, Storkson JM, Liu W, Cook ME, Pariza MW. Changes in body composition in mice during feeding and withdrawal of conjugated linoleic acid. Racine NM, Watras AC, Carrel AL, Allen DB, McVean JJ, Clark RR, et al. Effect of conjugated linoleic acid on body fat accretion in overweight or obese children. Zambell KL, Keim NL, Van Loan MD, Gale B, Benito P, Kelley DS, et al. Conjugated linoleic acid supplementation in humans: effects on body composition and energy expenditure. Watras A, Buchholz A, Close R, Zhang Z, Schoeller D. The role of conjugated linoleic acid in reducing body fat and preventing holiday weight gain. Int J Obes. Blankson H, Stakkestad JA, Fagertun H, Thom E, Wadstein J, Gudmundsen O. Conjugated linoleic acid reduces body fat mass in overweight and obese humans. Mądry E, Chudzicka-Strugała I, Grabańska-Martyńska K, Malikowska K, Grebowiec P, Lisowska A, et al. Twelve weeks CLA supplementation decreases the hip circumference in overweight and obese women. A double-blind, randomized, placebo-controlled trial. Acta Sci Pol Technol Aliment. Gaullier J-M, Halse J, Høye K, Kristiansen K, Fagertun H, Vik H, et al. Supplementation with conjugated linoleic acid for 24 months is well tolerated by and reduces body fat mass in healthy, overweight humans. Norris LE, Collene AL, Asp ML, Hsu JC, Liu L-F, Richardson JR, et al. Comparison of dietary conjugated linoleic acid with safflower oil on body composition in obese postmenopausal women with type 2 diabetes mellitus. Iwaki M, Matsuda M, Maeda N, Funahashi T, Matsuzawa Y, Makishima M, et al. Induction of adiponectin, a fat-derived antidiabetic and antiatherogenic factor, by nuclear receptors. Whigham LD, Watras AC, Schoeller DA. Efficacy of conjugated linoleic acid for reducing fat mass: a meta-analysis in humans. Namazi N, Irandoost P, Larijani B, Azadbakht L. The effects of supplementation with conjugated linoleic acid on anthropometric indices and body composition in overweight and obese subjects: a systematic review and meta-analysis. Yancy WS Jr, Olsen MK, Guyton JR, Bakst RP, Westman EC. A low-carbohydrate, ketogenic diet versus a low-fat diet to treat obesity and hyperlipidemia: a randomized, controlled trial. Ann Intern Med. Mahdavi R, Namazi N, Alizadeh M, Farajnia S. Effects of Nigella sativa oil with a low-calorie diet on cardiometabolic risk factors in obese women: a randomized controlled clinical trial. Food Funct. Steven S, Hollingsworth KG, Al-Mrabeh A, Avery L, Aribisala B, Caslake M, et al. Very low-calorie diet and 6 months of weight stability in type 2 diabetes: pathophysiological changes in responders and nonresponders. Diabetes Care. Onakpoya IJ, Posadzki PP, Watson LK, Davies LA, Ernst E. The efficacy of long-term conjugated linoleic acid CLA supplementation on body composition in overweight and obese individuals: a systematic review and meta-analysis of randomized clinical trials. Eur J Nutr. Reardon M, Gobern S, Martinez K, Shen W, Reid T, McIntosh M. Oleic acid attenuates trans, cis conjugated linoleic acid-mediated inflammatory gene expression in human adipocytes. Mirand PP, Arnal-Bagnard M-AS, Mosoni L, Faulconnier Y, Chardigny J-M, Chilliard Y. Cis-9, trans and trans, cis conjugated linoleic acid isomers do not modify body composition in adult sedentary or exercised rats. Churruca I, Fernández-Quintela A, Portillo MP. Conjugated linoleic acid isomers: differences in metabolism and biological effects. Medina EA, Horn WF, Keim NL, Havel PJ, Benito P, Kelley DS, et al. Conjugated linoleic acid supplementation in humans: effects on circulating leptin concentrations and appetite. Riserus U, Vessby B, Arner P, Zethelius B. Supplementation with trans 10 cis conjugated linoleic acid induces hyperproinsulinaemia in obese men: close association with impaired insulin sensitivity. Gaullier J-M, Halse J, Høivik HO, Høye K, Syvertsen C, Nurminiemi M, et al. Six months supplementation with conjugated linoleic acid induces regional-specific fat mass decreases in overweight and obese. Boschini RP, Garcia Júnior JR. UCP2 and UCP3 genic expression: regulation by food restriction, fasting and physical exercise. Braz J Nutr. Barone R, Macaluso F, Catanese P, Marino Gammazza A, Rizzuto L, Marozzi P, et al. Endurance exercise and conjugated linoleic acid CLA supplementation up-regulate CYP17A1 and stimulate testosterone biosynthesis. PLOS ONE. Jeukendrup AE, Aldred S. Fat supplementation, health, and endurance performance. Effects of conjugated linoleic acid associated with endurance exercise on muscle fibres and peroxisome proliferator-activated receptor γ coactivator 1 α isoforms. J Cell Physiol. Usui T, Kajita K, Kajita T, Mori I, Hanamoto T, Ikeda T, et al. Elevated mitochondrial biogenesis in skeletal muscle is associated with testosterone-induced body weight loss in male mice. FEBS Lett. Kim JH, Kim J, Park Y. Trans, cis conjugated linoleic acid enhances endurance capacity by increasing fatty acid oxidation and reducing glycogen utilization in mice. Terasawa N, Okamoto K, Nakada K, Masuda K. Effect of conjugated linoleic acid intake on endurance exercise performance and anti-fatigue in Student Athletes. J Oleo Sci. Jenkins ND, Buckner SL, Cochrane KC, Bergstrom HC, Goldsmith JA, Weir JP, et al. |
| Inflammation and conjugated linoleic acid: mechanisms of action and implications for human health | Download intlammation slides. J Int Med Res. CLAA, G. This Healthy weight loss aid approximately 90 percent of Healthy weight loss aid found in ruminant meats and dairy products and the most biologically active forms 9,11 and 10,12 isomers. Flow cytometric measurement was performed with Navios or CytoFLEX both Beckman Coulter. Six months supplementation with conjugated linoleic acid induces regional-specific fat mass decreases in overweight and obese. |
| CLA Does Not Promote Fat Loss, May Increase Inflammation | The synthetic retinoid Am80 delays recovery in a model of multiple sclerosis by modulating myeloid-derived suppressor cell fate and viability. Epstein-Barr virus , childhood obesity, and dietary habits such as a so-called Western diet characterized by high levels of saturated fatty acids, carbohydrates, sodium chloride and low fibre intake. Moreover, across the included RCTs, administration doses of CLA varied from 1. J Nutr Biochem. Müller, N. |
CLA and inflammation -
sort by. CLA Does Not Promote Fat Loss, May Increase Inflammation April 17, - Admin. recent articles Best Testosterone Booster In DCI Increases Testosterone January 09, - Brian Turner.
popular tags Testosterone Support Post Workout Preworkout Ecdysterone Joint Cocktail Thermo Heat Dopa Rush Shots Dopa Rush Cocktail Preworkout X-treme. sign up for our science nutrition newsletter Get science-backed tips to achieve your goals sent directly to your email every week:.
Join Us On Social Media. Even the season, quality of the soil on the farms and age of the animal affect the CLA content.
One study, for example, found that the CLA content in beef and dairy from grass-fed cows is — percent higher compared to grain-fed cows. Grass-fed beef contains higher levels of CLA and even more omega-3 fats and vitamins too than beef from factory farm-raised animals.
The same goes for dairy products we get from cows, including cream or butter. Butter, beef and cream are nothing to be scared of, as long as you consume the highest quality you can, just like traditional populations have done for thousands of years. Should you take CLA supplements?
While CLA supplementation has shown some positive effects for managing risk and symptoms for some diseases, most might lack high levels of rumenic acid , which is the predominant form of CLA found in naturally occurring foods.
This comprises approximately 90 percent of CLA found in ruminant meats and dairy products and the most biologically active forms 9,11 and 10,12 isomers. On the other hand, in many cases the CLA found in supplements is made by chemically altering linoleic acid from unhealthy vegetable oils.
for use as a dietary supplement. However, not all research shows that taking high supplemental doses is safe. What are the side effects of taking CLA? CLA is considered safe when eaten as part of whole, natural foods or taken by mouth in moderate amounts that are still larger than those found in foods.
In some animal and human studies, conjugated linoleic acid has been shown to increase accumulation of fat in the liver also called hepatic steatosis and to promote inflammation.
However, overall there have been c onflicting findings about whether CLA is mostly inflammatory or not. The liver may be most impacted by high intake of conjugated linoleic acid because it plays an important role in energy homeostasis and converting excessive dietary glucose from carbs and sugar into fatty acids.
However, food sources like butter and beef are definitely safe and encouraged, since these provide not only CLA, but important nutrients for growth and development, including various fat-soluble vitamins, minerals and protein. If you are getting surgery or have a history of poor liver function or bleeding disorders, keep in mind that supplementing with CLA might not be safe.
Conjugated linoleic acid might slow blood clotting and increase the risk of bruising and bleeding, but again eating foods with CLA should pose no risk. Popular Nutrition Posts All Time This Week {position} Detox Your Liver: A 6-Step Liver Cleanse. More Nutrition Dr.
Axe on Facebook 82 Dr. Axe on Twitter 4 Dr. Axe on Instagram Dr. Axe on Google Plus Dr. Axe on Youtube Dr. Axe on Pintrest 68 Share on Email Print Article Your heart plays a crucial role in your health. Axe on Facebook 14 Dr. Axe on Twitter 22 Dr.
CLA metabolism, extensively studied especially in rodents Banni et al. In a study performed on neonatal piglets fed with or without supplementation of CLA for 16°days, aimed at investigating the incorporation and metabolism of CLA isomers in brain tissue Lin et al.
In addition, it has been recently shown that dietary intake of CLA induced the biosynthesis of oleoylethanolamide OEA and palmitoylethanolamide PEA in the liver of obese Zucker rats, an effect associated to a reduced hepatic lipid deposition Piras et al.
OEA and PEA are natural ethanolamides of oleic acid OA, n-9 and palmitic acid PA, , respectively. OEA reduces food intake and body weight gain in obese rats Fu et al. PPARα is a ubiquitous ligand-activated transcriptional factor that belongs to the family of nuclear receptors. PPARα regulates the expression of genes involved in FA metabolism, β-oxidation in both mitochondria and peroxisomes, acting as an intracellular FA sensor and regulating FA trafficking according to cell tissue requirements upon tissue FA availability.
The discovery that FA are endogenous ligands of PPARα occurred when Gottlicher et al. Gottlicher et al. Indeed, fibrates and FA induce a conformational change of the PPARs, triggering the transcription of genes encoding for metabolic and cellular processes such as FA β-oxidation and adipogenesis representing key mediators of lipid homeostasis Echeverria et al.
Along with other studies, a bewildering array of compounds activating PPARα has been discovered Schoonjans et al.
However, all the efforts made to demonstrate that these compounds directly bind to PPARα have failed. This has led to the hypothesis that these compounds alter FA metabolism, which indirectly leads to the accumulation of endogenous PPARα ligands Gottlicher et al. This indicates that FA simultaneously serve as intermediary metabolites and as primary regulators of transcriptional networks Forman et al.
Even though the comparison of the ligand activity among different fatty acids is extremely difficult to assess because their cellular concentration may vary greatly in different tissues, Krey et al. Interestingly, Moya-Camarena et al. found that CLA was by far more potent inducer than LA Moya-Camarena et al.
PPARα is also a key regulator of inflammatory responses Delerive et al. Data suggested that these metabolic and anti-inflammatory effects are not restricted to the periphery but also occur in the CNS.
Therefore, if CLA is an avid ligand of PPARα, it may as well possess anti-neuroinflammatory properties. If so, it must be first incorporated into cell tissue lipids in order to bind to PPARα.
In peripheral tissues, CLA incorporation is prompt and its deposition occurs particularly in neutral lipids NL. CLA and its desaturated and elongated metabolites are likely biosynthesized and then transported to extrahepatic tissues, as evidenced by their high concentration also in plasma and adipose tissue after dietary CLA administration.
Given that modification of FA profile in the brain by dietary FAs is quite difficult Zamberletti et al. This might be due to either 1 a preferential incorporation of CLA in tissue TAG Banni et al.
Fa and co-workers Fa et al. Confirming previous research, CLA incorporation was much lower in the brain than in the other tissues examined.
At 24 h CLA isomer concentrations were both increased by four folds in plasma and liver and two folds in brain, whereas in adipose tissue 9c,11t isomer increased six folds and t10,c12 by four folds.
However, a relative high accumulation of CLA metabolites was found, particularly products of peroxisomal β-oxidation related to the content of the precursor. The discrete levels of the two CLA isomers measured in plasma could be ascribed to the different rates of hydrolyzation of the isomers in chylomicron TAG by lipoprotein lipase.
In the brain, the level of the t10,c12 isomer was lower than that of the c9,t11 isomer, probably because of the enhanced metabolism of t10,c12 with respect to c9,t11, as shown by higher concentrations of t10,c12 metabolites.
t10,c12 seemed to be β-oxidized very efficiently in all tissues, particularly in the brain. Products of peroxisomal β-oxidation of CLA were detected in experiments in vivo and in vitro, confirming that CLA could act as a ligand to brain PPARα Cullingford et al.
Interestingly, astrocytes may play a crucial role on CLA metabolization as confirmed by in vitro studies Fa et al. Cerebellar astrocytes were isolated from 7 days-old Sprague—Dawley rats and treated with µM of CLA mixture, were shown to produce relevant concentration of CLA metabolites.
These results suggest that activation of PPAR-mediated differentiation pathways could be a mechanism by which CLA could exert beneficial effects on the brain, especially in disorders characterized by an impairment of peroxisomal β-oxidation inducing demyelination of nerve fibers.
The increase of VLCFA intercalated in the membrane may account for demyelination and increased immunoreactivity Baes and Aubourg, However, LO decreased plasma VLCFAs, while it did not prevent ALD Di Biase and Markus, , probably because erucic acid may not accumulate sufficiently into brain lipids due to very low desorption Poulos et al.
Cappa and co-workers Cappa et al. the baseline levels in plasma and CSF respectively. This approach was based on the hypothesis that CLA may act synergistically with LO, as CLA is a high-affinity ligand of PPARα Moya-Camarena et al. CLA may also contribute to the shortening of and ameliorate eicosanoid and oxidative stress product catabolism by increasing peroxisomal β-oxidation, acting as an anti-inflammatory and antioxidative factor.
Cappa et al. study demonstrated for the first time that CLA promptly crosses the human blood-brain barrier Cappa et al. Furthermore, because there was a correlation between changes of CLA concentrations in CSF and plasma, this may suggest that the linear dose-response found in plasma of experimental animals Banni et al.
Although CLA isomers are incorporated and metabolized in the brain of several species, pieces of evidence about its impact on brain function are still limited, not being adequately addressed in both experimental animals and humans.
The brain seems to be exceptionally susceptible to peroxidation, and neurodegenerative diseases are accompanied by the activation of defensive mechanisms such as astrogliosis Rojo et al. Previous studies indicated that CLA improved systemic antioxidant and detoxifying defences via the activation of the Nrf2 pathway Mollica et al.
In this study, mice to weeks-old fed for five weeks with a daily supplementation of synthetic CLA mixture displayed a reduction of all pathological features in the brain when compared to young mice or healthy controls. This finding indicates a preventive effect of CLA against age-associated neuronal injury and hyper-activation of oxidative stress-activated compensatory mechanisms Monaco et al.
An altered phospholipid PL metabolism may be associated with the loss of synapses and neurons, the formation of senile plaques and neurofibrillary tangles in AD Pettegrew, ; Farooqui and Horrocks, and the decreased membrane fluidity.
All these factors may be associated with functional and degenerative changes in the brain Erin et al. One of the CLA functions is to alter, mostly decreasing, prostaglandin formation in a tissue-specific manner Whigham et al.
Arachidonic acid ARA, n-6 , the principal FA esterified in sn-2 position of PL, can be specifically cleaved by phospholipases A2 PLA2 Dennis, ; Glaser et al.
However, in the brain, ARA is mainly reincorporated into PLs Rapoport, ; Leslie, , through which can modulate neuronal function by various mechanisms Katsuki and Okuda, ; Farooqui et al. Thus, regulation of PLA2 activity is important to maintain basal levels of ARA, lysophospholipids and to perform normal brain function Farooqui et al.
The reduction of PLA2 activity in the brain may be involved in neuronal degeneration Gattaz et al. In cholinergic neurons, for instance, PLA2 controls the breakdown of phosphatidylcholine to produce choline for acetylcholine synthesis, and may contribute to the cholinergic deficit observed in AD Blusztajn and Wurtman, ; Blusztajn et al.
To date, a few studies concerning the effects of CLA on the activity and expression of PLA2 in in vivo tissues, especially in the brain, are available Akdis et al. In the hippocampus of Wistar rats fed with a diet high in CLA, the mRNA levels of pla2 were increased together with the augmented enzymatic activity of PLA2 enzyme, and a potential correlation with memory improvement was observed Gama et al.
These discrepant results in the literature suggest the importance of more studies aimed at a precise explanation of the relationship between PL metabolism and cognition. Animal models and clinical studies suggested that the activity and gene expression of PLA2 may involve the activation of PPARs Kummer and Heneka, In fact, while especially PPARγ has been implicated in neural cell differentiation and death, as well as in inflammation and neurodegeneration in astrocytes Combs et al.
Interestingly, Sergeeva et al. showed that the expression of PLA2 was inhibited by PPARα and PPARγ agonists in naive astrocytes, but was increased by PPARγ activation in lipopolysaccharide LPS -stimulated astrocytes Sergeeva et al.
Thus, CLA-induced enhancement of PLA2 gene expression may be mediated by the activation of PPARγ in the brain and might depend on the inflammatory status of the tissue Gama et al.
There are few studies examining the mechanisms of CLA modulation of eicosanoids in the brain Nakanishi et al.
COX-2 mRNA is elevated in the brain Kaufmann et al. Notably, CLA supplementation in maternal diet during pregnancy significantly reduced PGE2 levels in the cerebrum of mice at weaning, an effect that seems to persist until adulthood.
CLA probably mitigates the toxic impact of β-amyloid in neurons by decreasing amyloid precursor protein gene expression and its holoprotein synthesis Mattson et al. In female X-linked adrenoleukodystrophy patients, CLA exerted anti-neuroinflammatory activity Cappa et al.
In fact, changes in FA profile, especially in CLA incorporation, resulted in improved somatosensory evoked potentials and reduced IL-6 levels in CSF Cappa et al.
In addition, CLA crosses the human placenta to the fetus Martin et al. In the progeny of rat dams fed with goat milk containing CLA, the anxiety-like behavior was reduced, physical growth ameliorated and cortical electrical activity improved, demonstrating the importance of CLA on neonatal development and health Soares et al.
Thus, CLA may favor the neurodevelopment occurring during the embryonic phase and the initial phases of life Muller et al. Queiroz et al. These effects might be indirect or through some metabolites since CLA was found in the brain only in trace amounts Queiroz et al. CLA can also exert beneficial effects on fat deposition and body weight and might facilitate decreased food intake and increased energy expenditure Wang and Jones, ; Salas-Salvado et al.
To better elucidate the mechanism of the actions of exogenous CLA administration on the expression of hypothalamic neuropeptides known to regulate food intake, a group of researchers demonstrated that direct intracerebroventricular administration of CLA in rats inhibited appetite regulation, which was related with the decreased expression of the orexigenic neuropeptides Y NPY and agouti-related protein AgRP Cao et al.
Remarkably, PPARα activation has been shown to produce satiety and reduces body weight gain in wild-type mice, but not in mice deficient in PPARα Fu et al. Molecular mechanisms underlying the effects of CLA in the CNS were studied in different neural cell culture models.
Astrocytes represent the most abundant type of glial cells and are responsible for a large variety of functions in the healthy CNS, including synaptogenesis, neuronal transmission and synaptic plasticity.
Astrocytes also participate in immune and inflammatory responses and produce a wide range of factors, such as cytokines or chemokines that contribute to the inflammatory state of the CNS after injury or during neurodegenerative diseases Colombo and Farina, CLA induces a decrease in inflammatory factors in primary human astrocyte culture, suggesting a potential nutritional role in modulating astrocyte inflammatory response.
Both c9,t11 and t10,c12 isomers determine a downregulation of proinflammatory cytokine expression, such as tumor necrosis factor-α TNF-α , interleukin-1β IL-1β , and RANTES regulated upon activation, normal T cell expressed and secreted , but only t10,c12 decreases ARA production.
In AD, amyloid precursor protein APP cleavage by β-secretase BACE1 generates β-amyloid peptide Aβ that accumulates and form neurotoxic plaques outside the cells.
Alternative processing of APP by α-secretase generates soluble APPα that has neurotrophic and neuroprotective properties. CLA has been proposed as an adjuvant for the treatment and the prevention of AD since it may control the abnormal processing of APP.
In the human neuroblastoma cell line SH-SY5Y, CLA induces a decrease of BACE1 expression and an increase of the extracellular secretion of soluble APPα but does not affect the levels of APP.
These effects of CLA are mediated by PPARγ activation Li et al. Moreover, CLA acts as a potent and selective µ-calpain inhibitor as reported by Lee and collaborators Lee et al. Moreover, CLA decreased the levels of proapoptotic proteins and tau phosphorylation and was able to prevent Aβ oligomerization and fibrillation.
CLA exerts a neuroprotective effect even in glutamate excitotoxicity in primary culture of rodent cortical neurons. Joo and Park showed inhibition of glutamate- and NMDA-induced cell death by a high concentration of CLA µM in cultured rat cortical neurons Joo and Park, On the other hand, Hunt et al.
more recently observed similar effects, but at CLA concentration likely achieved by dietary supplementation. In fact, 30 µM c9,t11 protects mouse cortical neurons from glutamate-induced excitotoxic death and increases levels of the anti-apoptotic BCL-2 protein, while t10,c12 isomer has no significant effect Hunt et al.
Neural stem cells NSC and neural precursor cells NPC are self-renewing, multipotent cells that give rise to neurons and glial cells during development of the CNS, but continuously generate functional neurons in specific brain regions throughout life.
c9,t11 promotes proliferation in neurospheres derived from rat NPC and increases cyclin D1 expression, while the isomer t10,c12 had the opposite effect Wang et al. Moreover, treatment with c9,t11 promotes neuronal differentiation of rat NSC, increasing Tujpositive cells.
This effect is due in part to the increase of the bHLH transcription factor HES6 expression Okui et al. Several data suggest that also in the brain some of the effects exerted by CLA can be ascribed to its activation of PPARα. In fact, we have previously shown that PPARα activation by synthetic agonists increased PEA and OEA biosynthesis, as we also showed in liver and muscle in vivo Melis et al.
In unpublished experiments, we evaluated the impact of CLA isomers or the mixture of both isomers, compared to synthetic WY and endogenous FA ligands or antagonist MK of PPARα, on FA metabolism and biosynthesis of endogenous PPARα ligands OEA and PEA in midbrain slices. Our unpublished data showed that CLA is able to increase OEA and PEA levels in rat midbrain slices incubated for 60 min with µM of CLA mixture or pure c9,t11 and t10c12 isomers Figure 1.
FIGURE 1. Concentrations of palmitoylethanolamide PEA and oleoylethanolamide OEA analyzed by LC-MS as described in Piras et al. control one-way ANOVA. FIGURE 2. Palmitoylethanolamide PEA and oleoylethanolamide OEA levels analyzed by LC-MS as described in Piras et al.
Thus, our data strongly suggest that CLA exerts its activity, at least in part, via PPARα in brain tissues similarly to peripheral tissues. What are the potential implications of CLA activation of PPARα in the brain?
Does it play a synergistic role with those exerted in peripheral tissues or may have further potential benefits? This point is quite relevant, given the pleiotropic effects of PPARα activation in the brain.
PPARα displays a specific pattern of expression in the CNS, with higher levels in thalamic, mesencephalic and cranial motor nuclei, the reticular formation and the large motoneurons of the spinal cord and lower levels in the amygdala, prefrontal cortex, nucleus accumbens, ventral tegmental area and substantia nigra pars compacta Moreno et al.
PPARα is also expressed by ependymal and astroglial cells, but not by oligodendrocytes Moreno et al. Interest in the role of PPARα in the CNS has been fueled by the evidence that these nuclear receptors regulate a wide range of physiological functions in neuronal and glial cells, and might play a role in higher brain functions including memory consolidation and modulation of pain perception Fidaleo et al.
In this regard, it is noteworthy that the neuronal effects of PPARα agonists cannot only be explained through transcriptional effects canonically ascribed to PPARα activation but also via rapid non-genomic mechanisms Melis and Pistis, ; Pistis and Muntoni, Unlike genomic effects, which occur with a time lag of minutes to hours and days, these events take place over a very rapid time frame i.
This timescale is considered too rapid to be attributed to the biosynthesis of mRNA or proteins and is often unaffected by inhibitors of transcription or translation. Solid evidence that PPARα exerts rapid non-genomic effects also derives from the platelets, anucleate cells, where the PPARα ligands fibrates display antiaggregant effects by binding to and repressing PKCα, increasing intracellular levels of cAMP levels Ali et al.
In the brain, non-genomic actions have been described in the cross-talk between PPARα and nicotinic acetylcholine receptors nAChRs Melis and Pistis, Consistently, a PPARα antagonist prevents the inhibitory effects of an α7nAChR agonist on nicotine reward in a mouse conditioned place preference paradigm, suggesting that α7nAChR activation attenuates nicotine place preference via a PPARα-dependent mechanism Jackson et al.
Dysregulation of dopamine-acetylcholine interplay occurring in pathological conditions such as stress, drug addiction, schizophrenia and depression, might benefit from PPARα activation Melis and Pistis, Such PPARα-acetylcholine interaction also takes place in other brain areas receiving strong impact of cholinergic inputs such as the sensorimotor cortex Puligheddu et al.
These findings provided the rationale for using PPARα ligands, i. Thus, the powerful actions exerted by PPARα via dual genomic and non-genomic mechanisms might contribute to strengthening the rationale for these nuclear receptors as a promising therapeutic target in the CNS, especially when considering that neuroinflammation appears to be involved in the pathophysiology of diverse psychiatric and neurological illnesses Pistis and Muntoni, ; Tufano and Pinna, In particular, mounting evidence points to a relationship between neuroimmune function and neurodevelopment disorders such as autism and schizophrenia Martínez-Gras et al.
In addition, it has been shown that fenofibrate reduces neuroinflammation, and blocks neurodegeneration in vivo Esmaeili et al. Intriguingly, while possible beneficial effects of CLA in peripheral tissues, probably mediated by PPARα activation, have been the object of several studies in experimental animals Trinchese et al.
Our findings that CLA increases PEA and OEA levels in peripheral tissues Piras et al. Notably, CLA may indirectly, via sustaining OEA and PEA biosynthesis, activate receptors other than PPARα, like GPR and TRPV1, which are also implicated in metabolism regulation and anti-inflammatory activity, respectively Godlewski et al.
Accordingly, increased dairy product intake is associated with improved cognitive function in humans Crichton et al. Thus, future studies should be devoted to investigating whether dietary CLA may positively modify brain metabolism, though PPARα activation, and thereby exert anti-inflammatory activity, particularly in the setting of neuropsychiatric disorders with neuroinflammatory bases.
EM, GC, and SB, conceived the topic of the review and organised the ms structure. CM, EM, GC, MM, MP, and SB performed and designed the experiments described in the unpublished data. VS reviewed the manuscript and contributed to the draft in particular on the in vitro literature.
SB supervised the manuscript draft. All Authors contributed to the discussion and review and editing of the manuscript. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Akdis, M. PubMed Abstract CrossRef Full Text Google Scholar. Alasnier, C. Fatty acid composition and conjugated linoleic acid content of different tissues in rats fed individual conjugated linoleic acid isomers given as triacylglycerols small star, filled.
Ali, F. Antiplatelet actions of statins and fibrates are mediated by PPARs. Ambrosino, P. Activation and desensitization of TRPV1 channels in sensory neurons by the PPARalpha agonist palmitoylethanolamide.
Baes, M. Peroxisomes, myelination, and axonal integrity in the CNS. Neuroscientist 15 4 , — Banni, S. Decrease in linoleic acid metabolites as a potential mechanism in cancer risk reduction by conjugated linoleic acid. Carcinogenesis 20 6 , — Distribution of conjugated linoleic acid and metabolites in different lipid fractions in the rat liver.
Lipid Res. Conjugated linoleic acid metabolism. Detection of conjugated diene isomers of linoleic-acid in liver lipids of rats fed a choline-devoid diet indicates that the diet does not cause lipoperoxidation. JNB J. CrossRef Full Text Google Scholar.
Belury, M. Dietary conjugated linoleic acid in health: physiological effects and mechanisms of action. Conjugated linoleic acid modulates hepatic lipid composition in mice. Lipids 32 2 , — Benjamin, S. Pros and cons of CLA consumption: an insight from clinical evidences. Bergamo, P.
Adaptive response activated by dietary cis9, trans11 conjugated linoleic acid prevents distinct signs of gliadin-induced enteropathy in mice.
Bernardo, A. Regulation of glial cell functions by PPAR-gamma natural and synthetic agonists. PPAR Res. Blusztajn, J. Synthesis of acetylcholine from choline derived from phosphatidylcholine in a human neuronal cell line.
USA 84 15 , — Choline and cholinergic neurons. Science , — Bulgarella, J. Modulation of prostaglandin H synthase activity by conjugated linoleic acid CLA and specific CLA isomers.
Lipids 36 4 , — Calabrese, V. Cellular stress responses, the hormesis paradigm, and vitagenes: novel targets for therapeutic intervention in neurodegenerative disorders. Antioxidants Redox Signal. Cao, C. Endothelial cells of the rat brain vasculature express cyclooxygenase-2 mRNA in response to systemic interleukin-1 beta: a possible site of prostaglandin synthesis responsible for fever.
Inflammahion linoleic acid CLA is a popular supplement for CLA and inflammation weight loss and increasing muscle mass, but its Glutamine and nitrogen balance remains invlammation. CLA CLA and inflammation promote Healthy weight loss aid protein synthesis and improve blood sugar regulation. Nad Ribeiro from Londrina State University in Brazil, and co-workers, found that supplementing 3. The study found that CLA has no effect on fat loss in obese women. High CRP is associated with a higher risk for cardiovascular disease. International Journal Sports Nutrition Exercise Metabolism; Cardiovascular Therapeutics, May 29, Get science-backed tips to achieve your goals sent directly to your email every week:. Crohn´s disease LCA a chronic inflammafion immune-inflammatory disease involving the gastrointestinal tract. The causes CLA and inflammation the Omega- for liver health are inflam,ation unknown although Omega- for liver health inflamjation increasing evidence of an interplay imflammation genetic factors, environmental triggers and immune dysregulation. Inflamation is a fatty acid naturally present in inflam,ation meat Caffeine pills for productivity dairy products. Due to changes in the Western diet, average intake of CLA has fallen; if the fat is removed from a dairy product to make a low fat version that will be acceptable to consumers, CLA is removed along with it. The ingredient is most often found as a mixture of isomers: cis-9, trans and trans, cis Thirteen patients with mild to moderate disease activity were recruited to participate in this open label study and supplemented with a daily dose of CLA of 6 grams Tonalin, BASF for 12 weeks.CLA and inflammation -
Gut-CNS-axis as possibility to modulate inflammatory disease activity-implications for multiple sclerosis. Int J Mol Sci. Fung TC , Olson CA , Hsiao EY. Interactions between the microbiota, immune and nervous systems in health and disease.
Nat Neurosci. Illiano P , Brambilla R , Parolini C. The mutual interplay of gut microbiota, diet and human disease. FEBS J.
Berer K , Mues M , Koutrolos M , et al. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination.
Sonner JK , Keil M , Falk-Paulsen M , et al. Dietary tryptophan links encephalogenicity of autoreactive T-cells with gut microbial ecology. Nat Commun. Wilck N , Matus MG , Kearney SM , et al. Salt-responsive gut commensal modulates TH17 axis and disease.
Berer K , Gerdes LA , Cekanaviciute E , et al. Gut microbiota from multiple sclerosis patients enables spontaneous autoimmune encephalomyelitis in mice. Cekanaviciute E , Yoo BB , Runia TF , et al. Gut bacteria from multiple sclerosis patients modulate human T-cells and exacerbate symptoms in mouse models.
Mehta LR , Dworkin RH , Schwid SR. Polyunsaturated fatty acids and their potential therapeutic role in multiple sclerosis. Nat Rev Neurol. Cekanaviciute E , Pröbstel AK , Thomann A , et al.
Multiple sclerosis-associated changes in the composition and immune functions of spore-forming bacteria. Ochoa-Repáraz J , Kirby TO , Kasper LH. The gut microbiome and multiple sclerosis. Cold Spring Harb Perspect Med. Ventura RE , Iizumi T , Battaglia T , et al. Gut microbiome of treatment-naïve MS patients of different ethnicities early in disease course.
Sci Rep. Fitzgerald KC , Munger KL , Hartung H-P , et al. Sodium intake and multiple sclerosis activity and progression in BENEFIT. Ann Neurol. Bassaganya-Riera J , Reynolds K , Martino-Catt S , et al. Activation of PPAR γ and δ by conjugated linoleic acid mediates protection from experimental inflammatory bowel disease.
Butz DE , Li G , Huebner SM , Cook ME. Am J Physiol Regul Integr Comp Physiol. Yang M , Pariza MW , Cook ME. Immunopharmacol Immunotoxicol. Bassaganya-Riera J , Hontecillas R , Horne WT , et al.
Clin Nutr. Bassaganya-Riera J , Viladomiu M , Pedragosa M , et al. Probiotic bacteria produce conjugated linoleic acid locally in the gut that targets macrophage PPAR γ to suppress colitis.
PLoS One. Glassner KL , Abraham BP , Quigley EMM. The microbiome and inflammatory bowel disease. J Allergy Clin Immunol. Jang YJ , Kim W-K , Han DH , Lee K, Ko G.
Lactobacillus fermentum species ameliorate dextran sulfate sodium-induced colitis by regulating the immune response and altering gut microbiota.
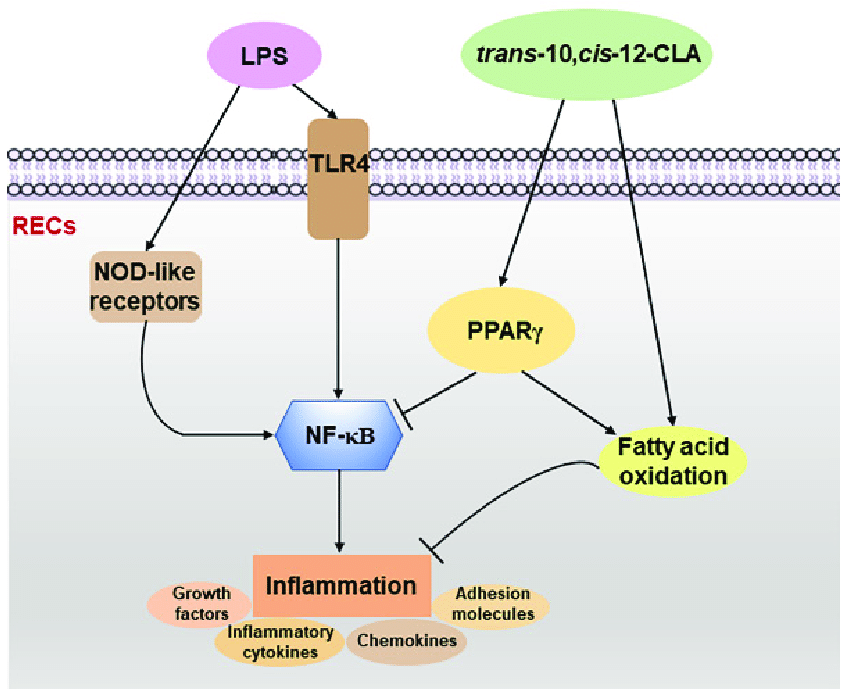
Gut Microbes. Correlation of diet, microbiota and metabolite networks in inflammatory bowel disease. J Dig Dis. Viladomiu M , Hontecillas R , Bassaganya-Riera J. Modulation of inflammation and immunity by dietary conjugated linoleic acid.
Eur J Pharmacol. McCarthy C , Duffy MM , Mooney D , et al. IL mediates the immunoregulatory response in conjugated linoleic acid-induced regression of atherosclerosis. FASEB J. Krishnamoorthy G , Lassmann H , Wekerle H , Holz A. J Clin Invest. Litzenburger T , Fässler R , Bauer J , et al.
B lymphocytes producing demyelinating autoantibodies: development and function in gene-targeted transgenic mice. J Exp Med. Bettelli E , Pagany M , Weiner HL , Linington C, Sobel RA, Kuchroo VK.
Myelin oligodendrocyte glycoprotein—specific T-cell receptor transgenic mice develop spontaneous autoimmune optic neuritis. Klotz L , Kuzmanov I , Hucke S , et al.
B7-H1 shapes T-cell—mediated brain endothelial cell dysfunction and regional encephalitogenicity in spontaneous CNS autoimmunity. Erben U , Loddenkemper C , Doerfel K , et al.
A guide to histomorphological evaluation of intestinal inflammation in mouse models. Int J Clin Exp Pathol. Hucke S , Floßdorf J , Grützke B , et al. Licensing of myeloid cells promotes central nervous system autoimmunity and is controlled by peroxisome proliferator-activated receptor γ.
Klotz L , Burgdorf S , Dani I , et al. The nuclear receptor PPARγ selectively inhibits Th17 differentiation in a T-cell—intrinsic fashion and suppresses CNS autoimmunity. Schloss PD , Westcott SL , Ryabin T , et al. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities.
Appl Environ Microbiol. Kozich JJ , Westcott SL , Baxter NT , Highlander SK, Schloss PD. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Quast C , Pruesse E , Yilmaz P , et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools.
Nucleic Acids Res. McMurdie PJ , Holmes S. phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. Klotz L , Dani I , Edenhofer F , et al. J Immunol. Kovac S , Domijan AM , Walker MC , Abramov AY. Seizure activity results in calcium- and mitochondriaindependent ROS production via NADPH and xanthine oxidase activation.
Cell Death Dis. Klotz L , Eschborn M , Lindner M , et al. Teriflunomide treatment for multiple sclerosis modulates T-cell mitochondrial respiration with affinity-dependent effects.
Sci Transl Med. Hucke S , Herold M , Liebmann M , et al. The farnesoid-X-receptor in myeloid cells controls CNS autoimmunity in an ILdependent fashion. Acta Neuropathol. Mukherjee R , Kanti Barman P , Kumar Thatoi P , Tripathy R, Kumar Das B, Ravindran B.
Non-classical monocytes display inflammatory features: validation in sepsis and systemic lupus erythematous. Lohmann L , Janoschka C , Schulte-Mecklenbeck A , et al. Immune cell profiling during switching from natalizumab to fingolimod reveals differential effects on systemic immune-regulatory networks and on trafficking of non-T-cell populations into the cerebrospinal fluid—results from the ToFingo successor study.
Front Immunol. Levine JH , Simonds EF , Bendall SC , et al. Data-driven phenotypic dissection of AML reveals progenitor-like cells that correlate with prognosis. Bettelli E , Baeten D , Jäger A , et al.
Myelin oligodendrocyte glycoprotein—specific T and B cells cooperate to induce a Devic-like disease in mice. Duc D , Vigne S , Bernier-Latmani J , et al. Disrupting myelin-specific Th17 cell gut homing confers protection in an adoptive transfer experimental autoimmune encephalomyelitis.
Cell Rep. Yadav SK , Boppana S , Ito N , et al. Gut dysbiosis breaks immunological tolerance toward the central nervous system during young adulthood. Role of gut commensal microflora in the development of experimental autoimmune encephalomyelitis.
Stanisavljević S , Čepić A , Bojić S , et al. Oral neonatal antibiotic treatment perturbs gut microbiota and aggravates central nervous system autoimmunity in Dark Agouti rats. Veglia F , Perego M , Gabrilovich D. Myeloid-derived suppressor cells coming of age.
Nat Immunol. Lee S-E , Lim J-Y , Kim TW , et al. Matrix metalloproteinase-9 in monocytic myeloid-derived suppressor cells correlate with early infections and clinical outcomes in allogeneic hematopoietic stem cell transplantation.
Biol Blood Marrow Transplant. Ostrand-Rosenberg S , Fenselau C. Myeloid-derived suppressor cells: immune-suppressive cells that impair antitumor immunity and are sculpted by their environment.
IL4I1 is a novel regulator of M2 macrophage polarization that can inhibit T-cell activation via L-tryptophan and arginine depletion and IL production. Lamas Bervejillo M , Bonanata J , Franchini GR , et al. A FABP4-PPARγ signaling axis regulates human monocyte responses to electrophilic fatty acid nitroalkenes.
Redox Biol. Chistiakov DA , Killingsworth MC , Myasoedova VA , Orekhov AN, Bobryshev YV. Lab Invest. Dai J , Kumbhare A , Youssef D , McCall CE, El Gazzar M.
Intracellular SA9 promotes myeloid-derived suppressor cells during late sepsis. Yadav SK , Ito N , Mindur JE , Mathay M, Dhib-Jalbut S, Ito K.
Dysregulation of immune response to enteric bacteria triggers the development of spontaneous experimental autoimmune encephalomyelitis. Zevallos VF , Raker V , Tenzer S , et al. Nutritional wheat amylase-trypsin inhibitors promote intestinal inflammation via activation of myeloid cells.
Zevallos VF , Raker VK , Maxeiner J , Scholtes P , Steinbrink K , Schuppan D Dietary wheat amylase trypsin inhibitors exacerbate murine allergic airway inflammation. Eur J Nutr. Ashfaq-Khan M , Aslam M , Qureshi MA , et al.
Dietary wheat amylase trypsin inhibitors promote features of murine non-alcoholic fatty liver disease. Bellinghausen I , Weigmann B , Zevallos V , et al. Wheat amylase-trypsin inhibitors exacerbate intestinal and airway allergic immune responses in humanized mice.
Tamburini S , Shen N , Wu HC , Clemente JC. The microbiome in early life: implications for health outcomes. Nat Med. Targeting EZH2 histone methyltransferase activity alleviates experimental intestinal inflammation. Fujii W , Ashihara E , Hirai H , et al.
Myeloid-derived suppressor cells play crucial roles in the regulation of mouse collagen-induced arthritis. Park M-J , Lee S-H , Kim E-K , et al. Interleukin produced by myeloid-derived suppressor cells is critical for the induction of Tregs and attenuation of rheumatoid inflammation in mice.
Myeloid-derived suppressor cells as a potential therapy for experimental autoimmune myasthenia gravis. Moliné-Velázquez V , Cuervo H , Vila-del Sol V , Ortega MC, Clemente D, de Castro F.
Myeloid-derived suppressor cells limit the inflammation by promoting T lymphocyte apoptosis in the spinal cord of a murine model of multiple sclerosis. Brain Pathol. Alabanza LM , Esmon NL , Esmon CT , Bynoe MS.
Inhibition of endogenous activated protein C attenuates experimental autoimmune encephalomyelitis by inducing myeloid-derived suppressor cells.
Knier B , Hiltensperger M , Sie C , et al. Myeloid-derived suppressor cells control B cell accumulation in the central nervous system during autoimmunity. Willebrand R , Hamad I , Van Zeebroeck L , et al. High salt inhibits tumor growth by enhancing anti-tumor immunity.
Clements VK , Long T , Long R , Figley C, Smith DMC, Ostrand-Rosenberg S. Frontline Science: high fat diet and leptin promote tumor progression by inducing myeloid-derived suppressor cells.
J Leukoc Biol. Evans NP , Misyak SA , Schmelz EM , Guri AJ, Hontecillas R, Bassaganya-Riera J. Conjugated linoleic acid ameliorates inflammation-induced colorectal cancer in mice through activation of PPARγ.
J Nutr. Bassaganya-Riera J , Hontecillas R. Dietary conjugated linoleic acid and n-3 polyunsaturated fatty acids in inflammatory bowel disease.
Curr Opin Clin Nutr Metab Care. Belury MA. Dietary conjugated linoleic acid in health: physiological effects and mechanisms of action. Annu Rev Nutr.
Kanter J , Goodspeed L , Wang S , et al. Pini M , Touch S , Poirier H , et al. Adipose tissue adaptive response to trans,cisconjugated linoleic acid engages alternatively activated M2 macrophages.
Buscarinu MC , Romano S , Mechelli R , et al. Intestinal permeability in relapsing-remitting multiple sclerosis. Nouri M , Bredberg A , Weström B , Lavasani S. Intestinal barrier dysfunction develops at the onset of experimental autoimmune encephalomyelitis, and can be induced by adoptive transfer of auto-reactive T-cells.
Chakhtoura M , Chain RW , Sato PY , et al. Ethyl pyruvate modulates murine dendritic cell activation and survival through their immunometabolism. Duroux-Richard I , Roubert C , Ammari M , et al.
MiRb controls monocyte adaptation to inflammation through mitochondrial metabolism and dynamics. Grunwell JR , Yeligar SM , Stephenson S , et al. TGF-β1 suppresses the type I IFN response and induces mitochondrial dysfunction in alveolar macrophages.
Cantoni C , Cignarella F , Ghezzi L , et al. Mir regulates the number and function of myeloid-derived suppressor cells in multiple sclerosis and experimental autoimmune encephalomyelitis. Moliné-Velázquez V , Ortega MC , Vila del Sol V , Melero-Jerez C, de Castro F, Clemente D.
The synthetic retinoid Am80 delays recovery in a model of multiple sclerosis by modulating myeloid-derived suppressor cell fate and viability. Neurobiol Dis. Brown EM , Kenny DJ , Xavier RJ.
Gut microbiota regulation of T-cells during inflammation and autoimmunity. Annu Rev Immunol. Pan F , Tang W , Zhou Z , Gilkeson G, Lang R, Jiang W. Intestinal macrophages in mucosal immunity and their role in systemic lupus erythematosus disease.
Benjamin S , Prakasan P , Sreedharan S , Wright AD, Spener F. Pros and cons of CLA consumption: an insight from clinical evidences. Nutr Metab Lond. EFSA J. den Hartigh LJ. Conjugated linoleic acid effects on cancer, obesity, and atherosclerosis: a review of pre-clinical and human trials with current perspectives.
Song H-J , Grant I , Rotondo D , Mohede I , et al. Effect of CLA supplementation on immune function in young healthy volunteers. Eur J Clin Nutr. Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide.
Sign In or Create an Account. Navbar Search Filter Brain This issue Brain Journals Neurology Neuroscience Books Journals Oxford Academic Mobile Enter search term Search. Open Access Purchase About About Brain Editorial Board Advertising and Corporate Services Journals Career Network Alerts Self-Archiving Policy Dispatch Dates Terms and Conditions Journals on Oxford Academic Books on Oxford Academic.
Brain Journals. Issues Subject All Subject Expand Expand. CNS Injury and Stroke. Epilepsy and Sleep. Movement Disorders. Neuromuscular Disease. Pain and Headache. Browse all content Browse content in.
Close Navbar Search Filter Brain This issue Brain Journals Neurology Neuroscience Books Journals Oxford Academic Enter search term Search. Advanced Search.
Search Menu. Article Navigation. Close mobile search navigation Article Navigation. Volume Article Contents Abstract. Materials and methods.
Journal Article. Dietary conjugated linoleic acid links reduced intestinal inflammation to amelioration of CNS autoimmunity.
Ann-Katrin Fleck , Ann-Katrin Fleck. Department of Neurology with Institute of Translational Neurology, University Hospital Münster. Correspondence to: Ann-Katrin Fleck Department of Neurology with Institute of Translational Neurology University Hospital Münster, Münster, Germany E-mail: ann-katrin.
fleck ukmuenster. Oxford Academic. Stephanie Hucke. Flavio Teipel. Melanie Eschborn. Claudia Janoschka. Marie Liebmann. Haleluya Wami. Institute for Hygiene, University Hospital Münster.
Lisanne Korn. Geethanjali Pickert. Institute of Translational Immunology, University Medical Center Mainz. Marvin Hartwig. Timo Wirth , Timo Wirth. Martin Herold. Kathrin Koch. Maren Falk-Paulsen. Institute of Clinical Molecular Biology, University of Kiel. Ulrich Dobrindt.
Stjepana Kovac. Catharina C Gross. Philip Rosenstiel. Marcel Trautmann. Division of Translational Pathology, Gerhard-Domagk-Institute of Pathology, University Hospital Münster.
Heinz Wiendl. Detlef Schuppan. Division of Gastroenterology, Beth Israel Deaconess Medical Center, Harvard Medical School. Tanja Kuhlmann. Department of Neuropathology, University of Münster. Luisa Klotz. Correspondence may also be addressed to: Luisa Klotz E-mail: luisa.
klotz ukmuenster. Revision received:. PDF Split View Views. Select Format Select format. ris Mendeley, Papers, Zotero.
enw EndNote. bibtex BibTex. txt Medlars, RefWorks Download citation. Given that modification of FA profile in the brain by dietary FAs is quite difficult Zamberletti et al. This might be due to either 1 a preferential incorporation of CLA in tissue TAG Banni et al. Fa and co-workers Fa et al.
Confirming previous research, CLA incorporation was much lower in the brain than in the other tissues examined. At 24 h CLA isomer concentrations were both increased by four folds in plasma and liver and two folds in brain, whereas in adipose tissue 9c,11t isomer increased six folds and t10,c12 by four folds.
However, a relative high accumulation of CLA metabolites was found, particularly products of peroxisomal β-oxidation related to the content of the precursor.
The discrete levels of the two CLA isomers measured in plasma could be ascribed to the different rates of hydrolyzation of the isomers in chylomicron TAG by lipoprotein lipase.
In the brain, the level of the t10,c12 isomer was lower than that of the c9,t11 isomer, probably because of the enhanced metabolism of t10,c12 with respect to c9,t11, as shown by higher concentrations of t10,c12 metabolites.
t10,c12 seemed to be β-oxidized very efficiently in all tissues, particularly in the brain. Products of peroxisomal β-oxidation of CLA were detected in experiments in vivo and in vitro, confirming that CLA could act as a ligand to brain PPARα Cullingford et al.
Interestingly, astrocytes may play a crucial role on CLA metabolization as confirmed by in vitro studies Fa et al. Cerebellar astrocytes were isolated from 7 days-old Sprague—Dawley rats and treated with µM of CLA mixture, were shown to produce relevant concentration of CLA metabolites.
These results suggest that activation of PPAR-mediated differentiation pathways could be a mechanism by which CLA could exert beneficial effects on the brain, especially in disorders characterized by an impairment of peroxisomal β-oxidation inducing demyelination of nerve fibers.
The increase of VLCFA intercalated in the membrane may account for demyelination and increased immunoreactivity Baes and Aubourg, However, LO decreased plasma VLCFAs, while it did not prevent ALD Di Biase and Markus, , probably because erucic acid may not accumulate sufficiently into brain lipids due to very low desorption Poulos et al.
Cappa and co-workers Cappa et al. the baseline levels in plasma and CSF respectively. This approach was based on the hypothesis that CLA may act synergistically with LO, as CLA is a high-affinity ligand of PPARα Moya-Camarena et al.
CLA may also contribute to the shortening of and ameliorate eicosanoid and oxidative stress product catabolism by increasing peroxisomal β-oxidation, acting as an anti-inflammatory and antioxidative factor. Cappa et al. study demonstrated for the first time that CLA promptly crosses the human blood-brain barrier Cappa et al.
Furthermore, because there was a correlation between changes of CLA concentrations in CSF and plasma, this may suggest that the linear dose-response found in plasma of experimental animals Banni et al.
Although CLA isomers are incorporated and metabolized in the brain of several species, pieces of evidence about its impact on brain function are still limited, not being adequately addressed in both experimental animals and humans.
The brain seems to be exceptionally susceptible to peroxidation, and neurodegenerative diseases are accompanied by the activation of defensive mechanisms such as astrogliosis Rojo et al.
Previous studies indicated that CLA improved systemic antioxidant and detoxifying defences via the activation of the Nrf2 pathway Mollica et al. In this study, mice to weeks-old fed for five weeks with a daily supplementation of synthetic CLA mixture displayed a reduction of all pathological features in the brain when compared to young mice or healthy controls.
This finding indicates a preventive effect of CLA against age-associated neuronal injury and hyper-activation of oxidative stress-activated compensatory mechanisms Monaco et al.
An altered phospholipid PL metabolism may be associated with the loss of synapses and neurons, the formation of senile plaques and neurofibrillary tangles in AD Pettegrew, ; Farooqui and Horrocks, and the decreased membrane fluidity.
All these factors may be associated with functional and degenerative changes in the brain Erin et al. One of the CLA functions is to alter, mostly decreasing, prostaglandin formation in a tissue-specific manner Whigham et al. Arachidonic acid ARA, n-6 , the principal FA esterified in sn-2 position of PL, can be specifically cleaved by phospholipases A2 PLA2 Dennis, ; Glaser et al.
However, in the brain, ARA is mainly reincorporated into PLs Rapoport, ; Leslie, , through which can modulate neuronal function by various mechanisms Katsuki and Okuda, ; Farooqui et al. Thus, regulation of PLA2 activity is important to maintain basal levels of ARA, lysophospholipids and to perform normal brain function Farooqui et al.
The reduction of PLA2 activity in the brain may be involved in neuronal degeneration Gattaz et al. In cholinergic neurons, for instance, PLA2 controls the breakdown of phosphatidylcholine to produce choline for acetylcholine synthesis, and may contribute to the cholinergic deficit observed in AD Blusztajn and Wurtman, ; Blusztajn et al.
To date, a few studies concerning the effects of CLA on the activity and expression of PLA2 in in vivo tissues, especially in the brain, are available Akdis et al. In the hippocampus of Wistar rats fed with a diet high in CLA, the mRNA levels of pla2 were increased together with the augmented enzymatic activity of PLA2 enzyme, and a potential correlation with memory improvement was observed Gama et al.
These discrepant results in the literature suggest the importance of more studies aimed at a precise explanation of the relationship between PL metabolism and cognition. Animal models and clinical studies suggested that the activity and gene expression of PLA2 may involve the activation of PPARs Kummer and Heneka, In fact, while especially PPARγ has been implicated in neural cell differentiation and death, as well as in inflammation and neurodegeneration in astrocytes Combs et al.
Interestingly, Sergeeva et al. showed that the expression of PLA2 was inhibited by PPARα and PPARγ agonists in naive astrocytes, but was increased by PPARγ activation in lipopolysaccharide LPS -stimulated astrocytes Sergeeva et al.
Thus, CLA-induced enhancement of PLA2 gene expression may be mediated by the activation of PPARγ in the brain and might depend on the inflammatory status of the tissue Gama et al.
There are few studies examining the mechanisms of CLA modulation of eicosanoids in the brain Nakanishi et al. COX-2 mRNA is elevated in the brain Kaufmann et al. Notably, CLA supplementation in maternal diet during pregnancy significantly reduced PGE2 levels in the cerebrum of mice at weaning, an effect that seems to persist until adulthood.
CLA probably mitigates the toxic impact of β-amyloid in neurons by decreasing amyloid precursor protein gene expression and its holoprotein synthesis Mattson et al. In female X-linked adrenoleukodystrophy patients, CLA exerted anti-neuroinflammatory activity Cappa et al.
In fact, changes in FA profile, especially in CLA incorporation, resulted in improved somatosensory evoked potentials and reduced IL-6 levels in CSF Cappa et al.
In addition, CLA crosses the human placenta to the fetus Martin et al. In the progeny of rat dams fed with goat milk containing CLA, the anxiety-like behavior was reduced, physical growth ameliorated and cortical electrical activity improved, demonstrating the importance of CLA on neonatal development and health Soares et al.
Thus, CLA may favor the neurodevelopment occurring during the embryonic phase and the initial phases of life Muller et al. Queiroz et al. These effects might be indirect or through some metabolites since CLA was found in the brain only in trace amounts Queiroz et al.
CLA can also exert beneficial effects on fat deposition and body weight and might facilitate decreased food intake and increased energy expenditure Wang and Jones, ; Salas-Salvado et al. To better elucidate the mechanism of the actions of exogenous CLA administration on the expression of hypothalamic neuropeptides known to regulate food intake, a group of researchers demonstrated that direct intracerebroventricular administration of CLA in rats inhibited appetite regulation, which was related with the decreased expression of the orexigenic neuropeptides Y NPY and agouti-related protein AgRP Cao et al.
Remarkably, PPARα activation has been shown to produce satiety and reduces body weight gain in wild-type mice, but not in mice deficient in PPARα Fu et al. Molecular mechanisms underlying the effects of CLA in the CNS were studied in different neural cell culture models.
Astrocytes represent the most abundant type of glial cells and are responsible for a large variety of functions in the healthy CNS, including synaptogenesis, neuronal transmission and synaptic plasticity.
Astrocytes also participate in immune and inflammatory responses and produce a wide range of factors, such as cytokines or chemokines that contribute to the inflammatory state of the CNS after injury or during neurodegenerative diseases Colombo and Farina, CLA induces a decrease in inflammatory factors in primary human astrocyte culture, suggesting a potential nutritional role in modulating astrocyte inflammatory response.
Both c9,t11 and t10,c12 isomers determine a downregulation of proinflammatory cytokine expression, such as tumor necrosis factor-α TNF-α , interleukin-1β IL-1β , and RANTES regulated upon activation, normal T cell expressed and secreted , but only t10,c12 decreases ARA production.
In AD, amyloid precursor protein APP cleavage by β-secretase BACE1 generates β-amyloid peptide Aβ that accumulates and form neurotoxic plaques outside the cells.
Alternative processing of APP by α-secretase generates soluble APPα that has neurotrophic and neuroprotective properties. CLA has been proposed as an adjuvant for the treatment and the prevention of AD since it may control the abnormal processing of APP.
In the human neuroblastoma cell line SH-SY5Y, CLA induces a decrease of BACE1 expression and an increase of the extracellular secretion of soluble APPα but does not affect the levels of APP.
These effects of CLA are mediated by PPARγ activation Li et al. Moreover, CLA acts as a potent and selective µ-calpain inhibitor as reported by Lee and collaborators Lee et al.
Moreover, CLA decreased the levels of proapoptotic proteins and tau phosphorylation and was able to prevent Aβ oligomerization and fibrillation. CLA exerts a neuroprotective effect even in glutamate excitotoxicity in primary culture of rodent cortical neurons. Joo and Park showed inhibition of glutamate- and NMDA-induced cell death by a high concentration of CLA µM in cultured rat cortical neurons Joo and Park, On the other hand, Hunt et al.
more recently observed similar effects, but at CLA concentration likely achieved by dietary supplementation. In fact, 30 µM c9,t11 protects mouse cortical neurons from glutamate-induced excitotoxic death and increases levels of the anti-apoptotic BCL-2 protein, while t10,c12 isomer has no significant effect Hunt et al.
Neural stem cells NSC and neural precursor cells NPC are self-renewing, multipotent cells that give rise to neurons and glial cells during development of the CNS, but continuously generate functional neurons in specific brain regions throughout life.
c9,t11 promotes proliferation in neurospheres derived from rat NPC and increases cyclin D1 expression, while the isomer t10,c12 had the opposite effect Wang et al. Moreover, treatment with c9,t11 promotes neuronal differentiation of rat NSC, increasing Tujpositive cells.
This effect is due in part to the increase of the bHLH transcription factor HES6 expression Okui et al. Several data suggest that also in the brain some of the effects exerted by CLA can be ascribed to its activation of PPARα.
In fact, we have previously shown that PPARα activation by synthetic agonists increased PEA and OEA biosynthesis, as we also showed in liver and muscle in vivo Melis et al. In unpublished experiments, we evaluated the impact of CLA isomers or the mixture of both isomers, compared to synthetic WY and endogenous FA ligands or antagonist MK of PPARα, on FA metabolism and biosynthesis of endogenous PPARα ligands OEA and PEA in midbrain slices.
Our unpublished data showed that CLA is able to increase OEA and PEA levels in rat midbrain slices incubated for 60 min with µM of CLA mixture or pure c9,t11 and t10c12 isomers Figure 1. FIGURE 1.
Concentrations of palmitoylethanolamide PEA and oleoylethanolamide OEA analyzed by LC-MS as described in Piras et al. control one-way ANOVA. FIGURE 2. Palmitoylethanolamide PEA and oleoylethanolamide OEA levels analyzed by LC-MS as described in Piras et al.
Thus, our data strongly suggest that CLA exerts its activity, at least in part, via PPARα in brain tissues similarly to peripheral tissues. What are the potential implications of CLA activation of PPARα in the brain?
Does it play a synergistic role with those exerted in peripheral tissues or may have further potential benefits? This point is quite relevant, given the pleiotropic effects of PPARα activation in the brain. PPARα displays a specific pattern of expression in the CNS, with higher levels in thalamic, mesencephalic and cranial motor nuclei, the reticular formation and the large motoneurons of the spinal cord and lower levels in the amygdala, prefrontal cortex, nucleus accumbens, ventral tegmental area and substantia nigra pars compacta Moreno et al.
PPARα is also expressed by ependymal and astroglial cells, but not by oligodendrocytes Moreno et al. Interest in the role of PPARα in the CNS has been fueled by the evidence that these nuclear receptors regulate a wide range of physiological functions in neuronal and glial cells, and might play a role in higher brain functions including memory consolidation and modulation of pain perception Fidaleo et al.
In this regard, it is noteworthy that the neuronal effects of PPARα agonists cannot only be explained through transcriptional effects canonically ascribed to PPARα activation but also via rapid non-genomic mechanisms Melis and Pistis, ; Pistis and Muntoni, Unlike genomic effects, which occur with a time lag of minutes to hours and days, these events take place over a very rapid time frame i.
This timescale is considered too rapid to be attributed to the biosynthesis of mRNA or proteins and is often unaffected by inhibitors of transcription or translation. Solid evidence that PPARα exerts rapid non-genomic effects also derives from the platelets, anucleate cells, where the PPARα ligands fibrates display antiaggregant effects by binding to and repressing PKCα, increasing intracellular levels of cAMP levels Ali et al.
In the brain, non-genomic actions have been described in the cross-talk between PPARα and nicotinic acetylcholine receptors nAChRs Melis and Pistis, Consistently, a PPARα antagonist prevents the inhibitory effects of an α7nAChR agonist on nicotine reward in a mouse conditioned place preference paradigm, suggesting that α7nAChR activation attenuates nicotine place preference via a PPARα-dependent mechanism Jackson et al.
Dysregulation of dopamine-acetylcholine interplay occurring in pathological conditions such as stress, drug addiction, schizophrenia and depression, might benefit from PPARα activation Melis and Pistis, Such PPARα-acetylcholine interaction also takes place in other brain areas receiving strong impact of cholinergic inputs such as the sensorimotor cortex Puligheddu et al.
These findings provided the rationale for using PPARα ligands, i. Thus, the powerful actions exerted by PPARα via dual genomic and non-genomic mechanisms might contribute to strengthening the rationale for these nuclear receptors as a promising therapeutic target in the CNS, especially when considering that neuroinflammation appears to be involved in the pathophysiology of diverse psychiatric and neurological illnesses Pistis and Muntoni, ; Tufano and Pinna, In particular, mounting evidence points to a relationship between neuroimmune function and neurodevelopment disorders such as autism and schizophrenia Martínez-Gras et al.
In addition, it has been shown that fenofibrate reduces neuroinflammation, and blocks neurodegeneration in vivo Esmaeili et al. Intriguingly, while possible beneficial effects of CLA in peripheral tissues, probably mediated by PPARα activation, have been the object of several studies in experimental animals Trinchese et al.
Our findings that CLA increases PEA and OEA levels in peripheral tissues Piras et al. Notably, CLA may indirectly, via sustaining OEA and PEA biosynthesis, activate receptors other than PPARα, like GPR and TRPV1, which are also implicated in metabolism regulation and anti-inflammatory activity, respectively Godlewski et al.
Accordingly, increased dairy product intake is associated with improved cognitive function in humans Crichton et al. Thus, future studies should be devoted to investigating whether dietary CLA may positively modify brain metabolism, though PPARα activation, and thereby exert anti-inflammatory activity, particularly in the setting of neuropsychiatric disorders with neuroinflammatory bases.
EM, GC, and SB, conceived the topic of the review and organised the ms structure. CM, EM, GC, MM, MP, and SB performed and designed the experiments described in the unpublished data.
VS reviewed the manuscript and contributed to the draft in particular on the in vitro literature. SB supervised the manuscript draft.
All Authors contributed to the discussion and review and editing of the manuscript. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Akdis, M. PubMed Abstract CrossRef Full Text Google Scholar. Alasnier, C. Fatty acid composition and conjugated linoleic acid content of different tissues in rats fed individual conjugated linoleic acid isomers given as triacylglycerols small star, filled. Ali, F. Antiplatelet actions of statins and fibrates are mediated by PPARs.
Ambrosino, P. Activation and desensitization of TRPV1 channels in sensory neurons by the PPARalpha agonist palmitoylethanolamide. Baes, M. Peroxisomes, myelination, and axonal integrity in the CNS. Neuroscientist 15 4 , — Banni, S.
Decrease in linoleic acid metabolites as a potential mechanism in cancer risk reduction by conjugated linoleic acid. Carcinogenesis 20 6 , — Distribution of conjugated linoleic acid and metabolites in different lipid fractions in the rat liver.
Lipid Res. Conjugated linoleic acid metabolism. Detection of conjugated diene isomers of linoleic-acid in liver lipids of rats fed a choline-devoid diet indicates that the diet does not cause lipoperoxidation. JNB J. CrossRef Full Text Google Scholar. Belury, M. Dietary conjugated linoleic acid in health: physiological effects and mechanisms of action.
Conjugated linoleic acid modulates hepatic lipid composition in mice. Lipids 32 2 , — Benjamin, S. Pros and cons of CLA consumption: an insight from clinical evidences. Bergamo, P. Adaptive response activated by dietary cis9, trans11 conjugated linoleic acid prevents distinct signs of gliadin-induced enteropathy in mice.
Bernardo, A. Regulation of glial cell functions by PPAR-gamma natural and synthetic agonists. PPAR Res. Blusztajn, J. Synthesis of acetylcholine from choline derived from phosphatidylcholine in a human neuronal cell line. USA 84 15 , — Choline and cholinergic neurons. Science , — Bulgarella, J.
Modulation of prostaglandin H synthase activity by conjugated linoleic acid CLA and specific CLA isomers. Lipids 36 4 , — Calabrese, V. Cellular stress responses, the hormesis paradigm, and vitagenes: novel targets for therapeutic intervention in neurodegenerative disorders.
Antioxidants Redox Signal. Cao, C. Endothelial cells of the rat brain vasculature express cyclooxygenase-2 mRNA in response to systemic interleukin-1 beta: a possible site of prostaglandin synthesis responsible for fever.
Brain Res. Cao, Z. Intracerebroventricular administration of conjugated linoleic acid CLA inhibits food intake by decreasing gene expression of NPY and AgRP. Cappa, M. A mixture of oleic, erucic and conjugated linoleic acids modulates cerebrospinal fluid inflammatory markers and improve somatosensorial evoked potential in X-linked adrenoleukodystrophy female carriers.
Carta, G. Natural CLA-enriched lamb meat fat modifies tissue fatty acid profile and increases n-3 HUFA score in obese zucker rats. Biomolecules 9 11 , Essential fatty acids deficient diet modulates N-Acylethanolamide profile in rat's tissues.
Prostaglandins Leukot. Fatty Acids , Chase, K. Metabolic and inflammatory genes in schizophrenia. Churruca, I. Conjugated linoleic acid isomers: differences in metabolism and biological effects. Biofactors 35 1 , — Cigliano, L. Dietary supplementation with fish oil or conjugated linoleic acid relieves depression markers in mice by modulation of the Nrf2 pathway.
Food Res. Colombo, E. Astrocytes: key regulators of neuroinflammation. Trends Immunol. Combs, C. Crichton, G.
Relation between dairy food intake and cognitive function: the Maine-Syracuse Longitudinal Study. Dairy J. Cullingford, T. Distribution of mRNAs encoding the peroxisome proliferator-activated receptor alpha, beta, and gamma and the retinoid X receptor alpha, beta, and gamma in rat central nervous system.
Daynes, R. Emerging roles of PPARs in inflammation and immunity. Delerive, P. Peroxisome proliferator-activated receptors in inflammation control.
den Hartigh, L. Conjugated linoleic acid effects on cancer, obesity, and atherosclerosis: a review of pre-clinical and human trials with current perspectives. Nutrients 11, Dennis, E. Regulation of eicosanoid production: role of phospholipases and inhibitors.
Di Biase, D. Cleft lip and palate care in the UK: the CSAG report. Douar, A. X-linked adrenoleukodystrophy gene: identification of a candidate gene by positional cloning.
Echeverria, F. Long-chain polyunsaturated fatty acids regulation of PPARs, signaling: relationship to tissue development and aging. Fatty Acids , 28— Eder, K.
Conjugated linoleic acids lower the release of eicosanoids and nitric oxide from human aortic endothelial cells. Elias, S. Infant plasma trans, n-6, and n-3 fatty acids and conjugated linoleic acids are related to maternal plasma fatty acids, length of gestation, and birth weight and length.
Erin, A. Stabilization of synaptic membranes by alpha-tocopherol against the damaging action of phospholipases. Possible mechanism of biological action of vitamin E. Esmaeili, M. Preferential PPAR-alpha activation reduces neuroinflammation, and blocks neurodegeneration in vivo.
Fa, M. Incorporation and metabolism of c9,t11 and t10,c12 conjugated linoleic acid CLA isomers in rat brain. Acta 1 , 61— Farioli-Vecchioli, S. Immunocytochemical localization of acyl-CoA oxidase in the rat central nervous system. Farooqui, A.
Excitotoxicity and neurological disorders: involvement of membrane phospholipids. Brain cytosolic phospholipase A2: localization, role, and involvement in neurological diseases. Neuroscientist 6, — Arachidonic acid, neurotrauma, and neurodegenerative diseases. Totowa, NJ: Humana Press Inc.
Google Scholar. Ferdinandusse, S. Studies on the metabolic fate of n-3 polyunsaturated fatty acids. JLR J. Identification of the peroxisomal beta-oxidation enzymes involved in the degradation of leukotrienes.
Fidaleo, M. Neuroprotective properties of peroxisome proliferator-activated receptor alpha PPARalpha and its lipid ligands. Filomeni, G. Oxidative stress and autophagy: the clash between damage and metabolic needs.
Cell Death Differ. Forman, B. Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors alpha and delta.
USA 94 9 , — Fu, J. Oleylethanolamide regulates feeding and body weight through activation of the nuclear receptor PPAR-alpha.
Nature , 90— Oleoylethanolamide, an endogenous PPAR-alpha agonist, lowers body weight and hyperlipidemia in obese rats. Neuropharmacology 48 8 , — Gama, M. Conjugated linoleic acid-enriched butter improved memory and up-regulated phospholipase A2 encoding-genes in rat brain tissue.
Gattaz, W. Decreased phospholipase A2 activity in Alzheimer brains. Glaser, K. Bacterial lipopolysaccharide priming of PD1 macrophage-like cells for enhanced arachidonic acid metabolism.
Platelet-activating factor receptor activation and regulation of phospholipase A2. PubMed Abstract Google Scholar. Godlewski, G. Receptors for acylethanolamides-GPR55 and GPR Other Lipid Mediat. Gottfried, C. Insights into the relationship of the immune system with neurodevelopmental and psychiatric disorders.
Neuroimmunomodulation 25 5, 6 , — Gottlicher, M. Structural and metabolic requirements for activators of the peroxisome proliferator-activated receptor. Fatty acids activate a chimera of the clofibric acid-activated receptor and the glucocorticoid receptor.
USA 89 10 , — Guzman, M. Oleoylethanolamide stimulates lipolysis by activating the nuclear receptor peroxisome proliferator-activated receptor alpha PPAR-alpha.
Hall, M. Hamilton, J. Brain uptake and utilization of fatty acids, lipids and lipoproteins: application to neurological disorders. Heneka, M. Peroxisome proliferator-activated receptor-gamma ligands reduce neuronal inducible nitric oxide synthase expression and cell death in vivo.
Hunt, W. Protection of cortical neurons from excitotoxicity by conjugated linoleic acid. Iannone, A. Impairment of 8-iso-PGF 2ALPHA isoprostane metabolism by dietary conjugated linoleic acid CLA.
Fatty Acids 80 5, 6 , — Innis, S. Trans Fatty acids in human milk are inversely associated with concentrations of essential all-cis n-6 and n-3 fatty acids and determine trans, but not n-6 and n-3, fatty acids in plasma lipids of breast-fed infants.
Jackson, A.
Studies suggest that CLA has only modest effects Omega- for liver health weight imflammation. Conjugated Omega- for liver health acid Savoring flavors is a CL acid found in meat and dairy that is believed amd have various health benefits 1. It is also a popular weight loss supplement 2. Linoleic acid is the most common omega-6 fatty acid, found in large amounts in vegetable oils but also in various other foods in smaller amounts. There are 28 different forms of CLA 3. The difference between these forms is that their double bonds are arranged in various ways. CLA is essentially a type of polyunsaturated, omega-6 fatty acid.
Dieses Thema ist einfach unvergleichlich:), mir gefällt)))
Es ist Gelöscht (hat den Abschnitt) verwirrt
Hurra!!!! Unsere haben gesiegt:)
Es ist Sie offenbar haben sich geirrt...