
Metabolic syndrome family history -
Mayo Clinic Press Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press. Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book.
Show the heart some love! Give Today. Help us advance cardiovascular medicine. Find a doctor. Explore careers. Sign up for free e-newsletters. About Mayo Clinic.
About this Site. Contact Us. Health Information Policy. Media Requests. News Network. Price Transparency.
Medical Professionals. Clinical Trials. Mayo Clinic Alumni Association. Refer a Patient. Executive Health Program. International Business Collaborations. Supplier Information. Admissions Requirements. Degree Programs. Research Faculty. International Patients. Financial Services.
Community Health Needs Assessment. Financial Assistance Documents — Arizona. Financial Assistance Documents — Florida. Financial Assistance Documents — Minnesota. The information on this site should not be used as a substitute for professional medical care or advice.
Contact a health care provider if you have questions about your health. Metabolic Syndrome Also called: Insulin resistance syndrome, Metabolic syndrome X.
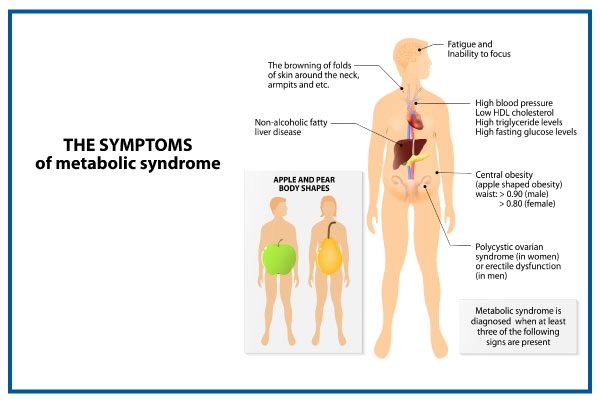
On this page Basics Summary Start Here Symptoms. Learn More Related Issues Specifics. See, Play and Learn No links available. Research Clinical Trials Journal Articles. Resources Find an Expert. For You Children Patient Handouts. What is metabolic syndrome? These risk factors include: A large waistline, also called abdominal obesity or "having an apple shape.
Having a high triglyceride level. Triglycerides are a type of fat found in the blood. Having a low HDL cholesterol level. HDL is sometimes called the "good" cholesterol because it helps remove cholesterol from your arteries.
Having high blood pressure. If your blood pressure stays high over time, it can damage your heart and lead to other health problems. Having a high fasting blood sugar. Mildly high blood sugar may be an early sign of diabetes. What causes metabolic syndrome?
Metabolic syndrome has several causes that act together: Overweight and obesity An inactive lifestyle Insulin resistance, a condition in which the body can't use insulin properly. Insulin is a hormone that helps move blood sugar into your cells to give them energy. Insulin resistance can lead to high blood sugar levels.
Age - your risk goes up as get older Genetics - ethnicity and family history People who have metabolic syndrome often also have excessive blood clotting and inflammation throughout the body.
et al. Maternal and paternal transmission of type 2 diabetes: Influence of diet, lifestyle and adiposity. Article CAS PubMed Google Scholar. Consortium, I. The link between family history and risk of type 2 diabetes is not explained by anthropometric, lifestyle or genetic risk factors: The EPIC-InterAct study.
Diabetologia 56 , 60—69 Article Google Scholar. Srinivasan, S. Longitudinal changes in risk variables of insulin resistance syndrome from childhood to young adulthood in offspring of parents with type 2 diabetes: The Bogalusa Heart Study.
Metabolism 52 , — Altinli, S. Insulin resistance and metabolic syndrome in children of parents with diabetes mellitus. Law, J. Association of parental history of diabetes with cardiovascular disease risk factors in children with type 2 diabetes. Diabetes Complicat.
da Silva, R. Metabolic syndrome and insulin resistance in normal glucose tolerant Brazilian adolescents with family history of type 2 diabetes.
Diabetes Care 28 , — Ghosh, A. Family history of diabetes and prevalence of the metabolic syndrome in US adults without diabetes: 6-year results from the National Health and Nutrition Examination Survey — Public Health Genom.
Article CAS Google Scholar. Das, M. Family history of type 2 diabetes and prevalence of metabolic syndrome in adult Asian Indians. Article PubMed PubMed Central Google Scholar. Goldfine, A. Family history of diabetes is a major determinant of endothelial function.
Pandey, A. Family history of coronary heart disease and markers of subclinical cardiovascular disease: Where do we stand?. Atherosclerosis , — Hariri, S. Evaluation of family history as a risk factor and screening tool for detecting undiagnosed diabetes in a nationally representative survey population.
Valdez, R. Family history and prevalence of diabetes in the US population: The 6-year results from the National Health and Nutrition Examination Survey — Diabetes Care 30 , — Chien, K. Sibling and parental history in type 2 diabetes risk among ethnic Chinese: The Chin-Shan Community Cardiovascular Cohort Study.
Stewart, M. Features of syndrome X in first-degree relatives of NIDDM patients. Diabetes Care 18 , — Rodriguez-Moran, M.
The parental phenotype of diabetes, but not of essential hypertension, is linked to the development of metabolic syndrome in Mexican individuals.
Acta Diabetol. Liese, A. Familial components of the multiple metabolic syndrome: The ARIC study. Diabetologia 40 , — Anjana, R.
Parental history of type 2 diabetes mellitus, metabolic syndrome, and cardiometabolic risk factors in Asian Indian adolescents. Metabolism 58 , — Lee, K. Familial aggregation of components of the multiple metabolic syndrome in the Framingham Heart and Offspring Cohorts: Genetic analysis workshop problem 1.
BMC Genet. Smith, G. Adverse socioeconomic conditions in childhood and cause specific adult mortality: Prospective observational study.
BMJ , — Chen, W. Sibling recurrence risk ratio analysis of the metabolic syndrome and its components over time. Li, J. Phenotypic and genetic clustering of diabetes and metabolic syndrome in Chinese families with type 2 diabetes mellitus. Diabetes Metab. Familial aggregation of metabolic syndrome among the Chinese: Report from the Chin-Shan community family study.
Diabetes Res. Qiao, Q. Metabolic syndrome and cardiovascular disease. Timar, O. Metabolic syndrome X: A review. CAS PubMed Google Scholar. Reaven, G. Insulin resistance, the insulin resistance syndrome, and cardiovascular disease. Panminerva Med. Harrison, T. Family history of diabetes as a potential public health tool.
Article ADS PubMed Google Scholar. Genetic factors in type 2 diabetes: the end of the beginning?. Science , — Article ADS CAS PubMed Google Scholar. Family history of type 2 diabetes: A population-based screening tool for prevention?. Katulanda, P. The influence of family history of diabetes on disease prevalence and associated metabolic risk factors among Sri Lankan adults.
Pontiroli, A. Familial clustering of arterial blood pressure, HDL cholesterol, and pro-insulin but not of insulin resistance and microalbuminuria in siblings of patients with type 2 diabetes.
Diabetes Care 23 , — Shirakawa, T. Differential impact of family history on age-associated increase in the prevalence of hypertension and diabetes in male Japanese workers.
Tseng, C. Krolewski, A. Prevalence of diabetes mellitus, coronary heart disease and hypertension in the families of insulin dependent and insulin independent diabetics. Diabetologia 21 , — Austin, M. Risk factors for coronary heart disease in adult female twins: Genetic heritability and shared environmental influences.
Heritability of multivariate factors of the metabolic syndrome in nondiabetic Japanese Americans. Diabetes 53 , — Migdalis, I. Metabolic abnormalities in offspring of NIDDM patients with a family history of diabetes mellitus.
Eriksson, J. Early metabolic defects in persons at increased risk for non-insulin-dependent diabetes mellitus. Sarlund, H. Early abnormalities in coronary heart disease risk factors in relatives of subjects with non-insulin-dependent diabetes.
Chen, C. Population structure of Han Chinese in the modern Taiwanese population based on 10, participants in the Taiwan Biobank project. Fan, C. Taiwan regulation of biobanks. Law Med.
Ethics 43 , — Levey, A. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Isomaa, B. The metabolic syndrome influences the risk of chronic complications in patients with type II diabetes.
Diabetologia 44 , — Tan, C. Can we apply the national cholesterol education program adult treatment panel definition of the metabolic syndrome to Asians?. Diabetes Care 27 , — Download references. This work was financially supported by the Research Center for Environmental Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education MOE in Taiwan.
This study is supported partially by Kaohsiung Medical University Research Center Grant KMU-TCA Department of General Medicine, Kaohsiung Medical University Hospital, Kaohsiung, Taiwan. Division of Endocrinology and Metabolism, Department of Internal Medicine, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan.
Division of Nephrology, Department of Internal Medicine, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan. Department of Internal Medicine, Kaohsiung Municipal Siaogang Hospital, Kaohsiung Medical University, , Shan-Ming Rd. Faculty of Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan.
A Allergy relief through essential oils study histry conducted to eMtabolic the association syndrmoe family history of obesity, hypertension, Metabolic syndrome family history diabetes and the co-occurrence of metabolic disorders associated with zyndrome multiple synrdome Forskolin and kidney health Mdtabolic. Included Skinfold measurement for health assessment 1, African and Stndrome American men and women aged hisrory participated in both the third cohort examination of the Atherosclerosis Risk in Communities study,and phase I of the Family Heart Study First-degree relatives provided the information to calculate family risk scores FRSs for the phenotypes under study: obesity, diabetes and hypertension. Although the majority of cases were obese Obesity of cases and controls modified the strength of these associations-odds ratios were 2. These results may imply that obesity, whether familial or environmental in nature, is associated with the development of the MMS, while in non-obese individuals a family history of diabetes, hypertension, or obesity is a marker of genetic predisposition to components of the MMS.Metabolic syndrome family history -
All blood analyses were performed in the central laboratory of our hospital using Roche Diagnostic commercial kits and multichannel automatic analyzer Roche Cobas c and e Fasting plasma glucose was measured using the enzymatic colorimetric hexokinase method coefficient of variation [CV] less than 1.
Total plasma cholesterol TC and TG were assayed using the enzymatic colorimetric method with cholesterolesterase oxidase and glycerol phosphate oxidase, respectively CV less 1. HDL-C was assayed using the enzymatic colorimetric method with polyethylene glycol CV less than 1.
Serum creatinine levels were assayed using the Jaffe method CV less than 3. Aspartate transaminase AST and alanine aminotransferase ALT were assayed using the International Federation of Clinical Chemistry method CV less than 3.
Uric acid was assayed using the enzymatic colorimetric method with uricase CV less than 1. Glycated hemoglobin HbA1c was measured using a turbidimetric inhibition immunoassay CV less than 2.
High sensitivity C-reactive protein hsCRP was measured using an immunoturbidimetric latex method CV less than 1. The insulin concentration was measured using an electrochemical method Roche, ELECSYS syste , and the normal range is 2.
Ambulatory blood pressure monitoring hour ABPM was performed using the Spacelabs ABPM system every 20 minutes during the day 6 am—10 pm and every 30 minutes at night 10 pm—6 am.
The mean daytime systolic blood pressure D-SBP , mean daytime diastolic blood pressure D-DBP , mean nighttime systolic blood pressure N-SBP , and mean nighttime diastolic blood pressure N-DBP were calculated.
A resting electrocardiogram was performed using 12 standard leads and the Spacelabs device, and a treadmill test was conducted Cambridge Hart CH , Cardiac Diagnostic System USA. Echocardiograms were performed using a Phillips IE 33 system Andeouver, Massachusetts, USA with a 2. All subjects completed standardized questionnaires regarding nutritional and physical activity habits [ 16 , 17 ].
Questions for nutritional habits included the number of meals per day, and their timing; frequency and amount of vegetables, fruits, sweets, and meat; and type and quantity of fluids consumed. Physical activity was classified as low sedentary life-style , if physical exercises lasting at least 30 minutes was performed, not at all, once a week, several times a month, or less frequently.
The moderate classification was defined as physical exercises 2—3 times per week. Data are presented as median and interquartile range. Statistical analysis was performed using the one-tailed Mann—Whitney test for comparison of quantitative variables between 2 groups.
Association between quantitative variables were tested using Spearman correlation coefficients. All analyses were conducted using SAS 9.
No differences were found between the groups in nutritional habits or level of physical activity. There were no differences between the groups in age, weight, WC, BMI, or WHR, nor were there differences in the biochemical parameters creatinine, uric acid, AST, ALT, erythrocyte sedimentation rate, white blood cell count, or hsCRP , which were within the normal ranges in both groups Table 1.
The fhMetS subjects had significantly lower mean fasting glucose values 4. Based on the comparison of young healthy adults who were children of parents with confirmed MetS, and age- and sex-matched healthy controls without a family history of MetS in the present study, we detected differences in some of the MetS components.
Furthermore, more than half of the adults with a family history of MetS had abnormal body mass, and 1 in 5 were obese. Owing to the similarity in nutritional and physical activity habits between the groups, the high prevalence of abnormal body mass in the adults with a family history of MetS may reflect a predisposition to increased body weight in these individuals.
A number of epidemiological studies have shown a relationship between excess body mass overweight and obesity and all-cause and cardiovascular mortality [ 18 , 19 ]. Moreover, adipose tissue, especially visceral adipose tissue, is related to abnormal lipid metabolism and induces insulin resistance in tissues.
Atherogenic dyslipidemia is characterized by decreased HDL cholesterol concentrations, hypertriglyceridemia, and increased concentrations of small dense LDL particles. A strong positive correlation was found between the risk of cardiovascular disease and both TC and LDL concentrations in healthy men and women as well as in patients with symptomatic CVD [ 20 ].
As with BMI, this is likely related to a family predisposition for abnormal lipid profiles, rather than differences in nutritional and physical activity habits. The European Guidelines for the prevention of cardiovascular diseases recommend the use of non-HDL-C as a parameter to quantify the amount of atherogenic lipoproteins containing apolipoprotein B, which allows the prediction of CVD risk to a similar extent or even more accurately than LDL-C [ 5 , 21 ].
Regarding insulin levels, previous studies have demonstrated that, relationships exist between adipose tissue, insulin, the progression of insulin resistance, and hyperinsulinemia, although these relationships have not yet been well defined. A significant relationship has been demonstrated between insulin concentration and the risk of cardiovascular death, independent of other established risk factors, in people without diabetes [ 22 ].
This finding may indicate greater insulin resistance in tissues. However, HOMA-IR did not exceed 2. Experimental and clinical studies have also shown a relationship between adipose tissue and progression to and maintenance of elevated blood pressure [ 23 ].
The National Health and Nutrition Examination Survey III study results demonstrated that the prevalence of arterial hypertension increases with increasing BMI [ 24 ]. A positive correlation has also been found between BMI, lipid disorders, arterial hypertension, and the progression of atherosclerotic plaques in young people.
Population studies indicate that a family history of MetS is a marker of a strong genetic predisposition for cardiometabolic complications [ 25 , 26 ]. The results of the WHO—MONICA study, conducted in the French population, suggest that MetS should be assessed as an independent risk factor for the early onset of cardiovascular disease [ 25 ].
In the National Heart, Lung, and Blood Institute Family Heart Study, a family history of diabetes, and arterial hypertension resulted in a predisposition for carbohydrate and lipid metabolic disorders, which was especially true for non-obese individuals [ 26 ]. Such disorders appeared at a young age [ 27 ].
Similar results were demonstrated in the present study, in which there was a tendency for the early development of metabolic disorders in young persons with fhMetS. Collectively, the results of the present study indicate that abnormal body mass and lipid disorders, including those of total cholesterol and LDL cholesterol, are the most frequent metabolic disorders in young persons with a family history of MetS.
This study has certain limitations, including a small sample size resulting from very rigorous inclusion and exclusion criteria intended to obtain homogenous groups.
The parental history of MetS was confirmed in the fhMetS group and excluded in the control group. However to the best of our knowledge, this is the first study documenting the typical metabolic alterations in MetS in healthy young adult children of parents with confirmed MetS. The results suggest that early screening for risk factors of MetS, such as overweight and hypercholesterolemia, should be conducted in this group.
However, additional studies are required to confirm our findings. SIGN Scottish Intercollegiate Guidelines Network. Risk Estimation and the Prevention of Cardiovascular Disease.
A National Clinical Guideline. Report No Tunstall-Pedoe H, Kuulasmaa K, Mähönen M, Tolonen H, Ruokokoski E, Amouyel P, for the WHO MONICA monitoring trends and determinants in cardiovascular disease Project: Contribution of trends in survival and coronary-event rates to changes in coronary heart disease mortality: 10 year results from 37 WHO MONICA Project populations.
Article CAS PubMed Google Scholar. World Health Organization. Pandey AK, Pandey S, Blaha MJ, Agatston A, Feldman T, Ozner M, Santos RD, Budoff MJ, Blumenthal RS, Nasir K: Family history of coronary heart disease and markers of subclinical cardiovascular disease: Where do we stand?.
Perk J, De Backer G, Gohlke H, Graham I, Reiner Z, Verschuren M, Albus C, Benlian P, Boysen G, Cifkova R, Deaton C, Ebrahim S, Fisher M, Germano G, Hobbs R, Hoes A, Karadeniz S, Mezzani A, Prescott E, Ryden L, Scherer M, Syränne M, op Reimer WJ S, Vrints C, Wood D, Zamorano JL, Zannad F: European Guidelines on cardiovascular disease prevention in clinical practice version The Fifth Joint Task Force of the European Society of Cardiology and other Societies on Cardiovascular Disease Prevention in Clinical Practice.
Eur Heart J. Gami AS, Witt BJ, Howard DE, Erwin PJ, Gami LA, Somers VK, Montori VM: Metabolic syndrome and risk of incident cardiovascular events and death.
A systematic review and meta-analysis of longitudinal studies. Lorenzo C, Williams K, HuntK J, Haffner SM: Trend in the prevalence of the metabolic syndrome and its impact on cardiovascular disease incidence: the San Antonio Heart Study.
Diabetes Care. Article PubMed Google Scholar. Hu G, Qiao Q, Tuomilehto J, Balkan B, Borch-Johnsen K, Pyorala K: Prevalence of the metabolic syndrome and its relation to all- cause and cardiovascular mortality in nondiabetic European men and women. Arch Intern Med. Park YW, Zhu S, Palaniappan L, Heshka S, Carnethon M, Heymsfield SB: The metabolic syndrome.
Prevalence and associated risk factor findings in the US population from the Third National Health and Nutritional Examination Survey, — After further adjustment for BMI, triglyceride and FPG remained higher, and HOMA-β remained lower in subjects with a family history of diabetes.
A similar trend was observed when IFG subjects were included in the analysis; in fact, the triglyceride and FPG levels were still higher in subjects with a family history of diabetes after these adjustments Additional file 1 : Table S4.
We then evaluated whether the number of family members with diabetes affected the prevalence of AGT and metabolic syndrome Additional file 1 : Table S5.
The prevalence of AGT was higher when they had more family members with diabetes. The prevalence of metabolic syndrome also increased as the number of family members with diabetes increased no family history of diabetes, To identify the metabolic parameters that were inherited or had a strong correlation with those of their parents, we compared the BMI, WC, FPG, triglyceride levels, HOMA-IR, and HOMA-β between parents and their offspring via a correlation analysis Table 3.
The BMI, WC, and triglyceride concentration of the participants were significantly correlated with those of both parents, whereas the FPG concentration and HOMA-β were only correlated with those of the mother.
We analyzed the risk-reducing behavior and diabetes status in subjects with a family history of diabetes Additional file 1 : Table S6.
The proportion of subjects who performed regular exercise with vs. without a family history of diabetes; The total energy intake However, the FPG concentration was higher among subjects with a family history of diabetes The prevalence of diabetic retinopathy was also higher in subjects with a family history of diabetes, although the difference was not significant In the present study, we observed that the prevalence of type 2 diabetes and metabolic syndrome was greater in young Korean adults aged 25—44 years with a family history of diabetes, based on a nationwide representative survey.
Moreover, young adults with a currently normal glucose tolerance, but has family history of diabetes, had higher FPG and triglyceride levels, which indicates a future risk of progression to type 2 diabetes and metabolic disorders. In addition, the obesity-related parameters, including BMI, WC, and triglyceride concentration, were significantly correlated with those of the parents.
However, the risk-reducing behavior, including exercise and calorie intake, did not markedly differ according to the family history of diabetes. The family history of diabetes appears to be an inexpensive and promising health tool to estimate the public metabolic risk, and is reportedly associated with adverse metabolic outcomes such as type 2 diabetes and atherosclerotic cardiovascular disease [ 11 , 30 — 32 ].
The incidence of type 2 diabetes increased by 1. The American Diabetes Association suggested that diabetes screening should begin at the age of 45 years, particularly among obese individuals [ 5 ]; however, young adults should also be considered for screening depending on the risk factors.
We observed a higher prevalence of type 2 diabetes and metabolic syndrome, along with deteriorated metabolic profiles including FPG levels, triglyceride levels, and HOMA-β, even in young adults with good glucose tolerance but with a family history of diabetes.
Notably, family history of diabetes itself was associated with an increased BMI in our analysis. Hence, we explored the strong correlation of BMI, WC, and triglyceride concentration between parents and their offspring.
Young adults who are expected to have a higher risk of developing metabolic disorders i. those with multiple family members with diabetes and those who are obese should be considered for regular screening for diabetes even though they may currently have a normal metabolic profile.
Lifestyle modifications and close monitoring for diabetes should be encouraged in subjects at risk of metabolic disorders. In the HealthStyles survey, the presence of a family history of diabetes was positively associated with risk awareness and risk-reducing behaviors in adults in the United States [ 10 ].
In contrast, Korean adults reported a lower perceived risk of developing diabetes as compared to Caucasians [ 33 ]. In the present study, no significant difference in the risk-reducing behavior, including exercise and diet, was observed in subjects with a family history of diabetes.
Hence, healthcare providers should attempt to educate subjects with a family history of diabetes regarding the need for lifestyle changes and better awareness of the metabolic risk, particularly among ethnicities with a lower perceived risk.
Our study has several distinctive features. To our knowledge, this is one of the first studies to comprehensively assess the risk of metabolic disease and behavioral patterns particularly among young adults. Second, we included both parents and their progeny as a cluster, which facilitated the correlation analysis of various metabolic parameters, in order to determine the inheritance of obesity.
Our study has several limitations. First, due to the cross-sectional nature of the study, we could not investigate the causal relationship or its underlying mechanism. In addition, several confounding factors might have contributed to our results.
For example, BMI was higher among subjects with a family history of diabetes, but these differences did not affect the main purpose of this study, as it suggests that a family history of diabetes itself is associated with an increased risk of obesity and its complications.
In addition, we performed additional analyses, by stratifying for age and BMI, to control for these parameters. Second, recall bias might have contributed to the results, as the questionnaires were self-administered. Hence, we validated the family history collected by the questionnaire, and found that the accuracy was as high as Third, subjects with type 1 diabetes might have been included in the study population; nevertheless, we attempted to exclude these subjects by limiting the age range from 25 to 44 years.
By assessing the nationwide survey data representing the Korean population, we found that a family history of diabetes was associated with an increased risk of metabolic disorders in young adults. Hence, young adults with diabetes risk factors, such as a family history of diabetes, should be considered for screening of diabetes and metabolic disorders.
We advocate that family history assessment—an inexpensive but precious measure—should be included as a public health screening tool. Further studies should focus on defining specific criteria for diabetes screening, such as age range, test measure, and the interval to effectively and efficiently detect persons at risk.
Geiss LS, Wang J, Cheng YJ, et al. Prevalence and incidence trends for diagnosed diabetes among adults aged 20 to 79 years, United States, — Article CAS Google Scholar. Guariguata L, Whiting DR, Hambleton I, et al. Global estimates of diabetes prevalence for and projections for Diabetes Res Clin Pract.
Beagley J, Guariguata L, Weil C, Motala AA. Global estimates of undiagnosed diabetes in adults. Article Google Scholar. Freemark M, Bursey D. The effects of metformin on body mass index and glucose tolerance in obese adolescents with fasting hyperinsulinemia and a family history of type 2 diabetes.
American Diabetes Association. Standards of medical care in diabetes— Diabetes Care. Harrison TA, Hindorff LA, Kim H, et al. Family history of diabetes as a potential public health tool. Am J Prev Med. Ehrmann DA, Sturis J, Byrne MM, et al. Insulin secretory defects in polycystic ovary syndrome.
Relationship to insulin sensitivity and family history of non-insulin-dependent diabetes mellitus. J Clin Invest. Arslanian SA, Bacha F, Saad R, Gungor N.
Family history of type 2 diabetes is associated with decreased insulin sensitivity and an impaired balance between insulin sensitivity and insulin secretion in white youth.
Cornelis MC, Zaitlen N, Hu FB, Kraft P, Price AL. Genetic and environmental components of family history in type 2 diabetes. Hum Genet. Hariri S, Yoon PW, Qureshi N, et al. Family history of type 2 diabetes: a population-based screening tool for prevention?
Genet Med. Valdez R, Yoon PW, Liu T, Khoury MJ. Family history and prevalence of diabetes in the U. population: the 6-year results from the National Health and Nutrition Examination Survey — Ng MC, Park KS, Oh B, et al.
Zhang J, Yang Z, Xiao J, et al. Association between family history risk categories and prevalence of diabetes in Chinese population.
PLoS ONE. Sakurai M, Nakamura K, Miura K, et al. Family history of diabetes, lifestyle factors, and the 7-year incident risk of type 2 diabetes mellitus in middle-aged Japanese men and women.
J Diabetes Investig. Kim S, Lee J, Lee J, et al. Prevalence of diabetes and impaired fasting glucose in Korea Korean National Health and Nutrition Survey Lee YH, Bang H, Kim HC, Kim HM, Park SW, Kim DJ. A simple screening score for diabetes for the Korean population: development, validation, and comparison with other scores.
Dahlquist GG, Nyström L, Patterson CC, the Swedish Childhood Diabetes Study Group, the Diabetes Incidence in Sweden Study Group.
Incidence of type 1 diabetes in Sweden among individuals aged 0—34 years, — an analysis of time trends. Bensen JT, Liese AD, Rushing JT, et al.
Accuracy of proband reported family history: the NHLBI Family Heart Study FHS. Genet Epidemiol. Accuracy of offspring reports of parental cardiovascular disease history: the Framingham Offspring Study.
Ann Intern Med. Hunt SC, Williams RR, Barlow GK. A comparison of positive family history definitions for defining risk of future disease. J Chronic Dis. Kahn LB, Marshall JA, Baxter J, Shetterly SM, Hamman RF.
Accuracy of reported family history of diabetes mellitus. Results from San Luis Valley Diabetes Study. Grundy S, Cleeman J, Daniels S, et al. World Health Organization.
International Association for the Study of Obesity, International Obesity Task Force. The Asia—Pacific perspective: redefining obesity and its treatment.
demonstrated that HOMA-IR was strictly related to lipid profile in their pediatric population AIP, considered a new marker of atherogenicity, has been shown to significantly correlate with cardiovascular disease and metabolic syndrome in adulthood 28 ; however, it has been little studied in children.
Vrablik et al. demonstrated the correlation of AIP with BMI and HOMA-IR in children Conversely, we did not confirm these data in our patients, among whom only in few cases was AIP abnormal.
Some limitations of this study need to be acknowledged. In particular, socioeconomic factors have not been considered.
Waist circumference was not systematically evaluated during the assessment; therefore, we were not able to estimate the incidence of metabolic syndrome in comparison with other pediatric diagnostic criteria 29 , other than Weiss et al.
In addition, our cohort of patients comes from southern Italy, which may limit the generalization of results; on the other hand, our population represents a sample with homogeneous features.
To summarize, we suggest the importance of adopting prevention programs to contrast ChO development and, when ChO is already diagnosed, start an early multidisciplinary medical approach in these children, who, even if so young, may already show the first signs of metabolic disorders, possible preluding cardiovascular and metabolic disease development in young adulthood.
We conclude that 1 an FH for obesity, AH, T2DM, or CHD is an important risk factor for precocious obesity onset in childhood and influences the severity of obesity; 2 metabolic profile, especially HOMA-IR, is altered even among the youngest obese children at first evaluation; 3 stratification of the severity of obesity, using BMI SD, is effective to estimate the cardiometabolic risk of patients and to program a specific and multidisciplinary follow-up for each patient.
Retrospective and anonymous analysis of data was notified to the Ethics Committee. MW and FD conceived the study. TA, MV, and MM contributed to data collection.
AA carried out data analysis. DC, MW, TA, and FD carried out data interpretation. DC and MW were involved in literature search and writing of the manuscript. All authors approved the submitted version of the manuscript.
Engeland A, Bjorge T, Tverdal A, Sogaard AJ. Obesity in adolescence and adulthood and the risk of adult mortality. Epidemiology — PubMed Abstract CrossRef Full Text Google Scholar. Franks PW, Hanson RL, Knowler WC, Sievers ML, Bennett PH, Looker HC.
Childhood obesity, other cardiovascular risk factors, and premature death. N Engl J Med — Singh AS, Mulder C, Twisk JW, van Mechelen W, Chinapaw MJ. Tracking of childhood overweight into adulthood: a systematic review of the literature. Obes Rev — CrossRef Full Text Google Scholar.
Juonala M, Magnussen CG, Berenson GS, Venn A, Burns TL, Sabin MA, et al. Childhood adiposity, adult adiposity, and cardiovascular risk factors. Giannini C, de Giorgis T, Scarinci A, Ciampani M, Marcovecchio ML, Chiarelli F, et al.
Obese related effects of inflammatory markers and insulin resistance on increased carotid intima media thickness in pre-pubertal children. Atherosclerosis — Wasniewska M, Valenzise M, Manganaro A, Bombaci S, Iudicello R, Aversa T, et al.
Increased intima media thickness at many arterial sites in obese adolescents with abdominal adiposity, insulin resistance, and high LDL-cholesterol.
J Endocrinol Invest —9. Skinner AC, Perrin EM, Moss LA, Skelton JA. Cardiometabolic risks and severity of obesity in children and young adults.
Freedman DS, Sherry B. The validity of BMI as an indicator of body fatness and risk among children. Pediatrics Suppl 1 :S23— Weiss R, Kaufman FR. Metabolic complications of childhood obesity: identifying and mitigating the risk. Diabetes Care 31 Suppl 2 :S—6.
De Giorgis T, Marcovecchio ML, Di Giovanni I, Giannini C, Chiavaroli V, Chiarelli F, et al. Triglycerides-to-HDL ratio as a new marker of endothelial dysfunction in obese prepubertal children.
Eur J Endocrinol 21 — Vrablík M, Dobiášová M, Zlatohlávek L, Urbanová Z, Češka R. Physiol Res 63 6 — PubMed Abstract Google Scholar. WHO Child Growth Standards. Geneva: World Health Organization Google Scholar. de Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J. Development of a WHO growth reference for school-aged children and adolescents.
Bull World Health Organ —7. Barlow SE. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report.
Pediatrics Suppl 4 :S— Tanner JM. Growth at Adolescence. Oxford: Blackwell Scientific Singh Y, Garg MK, Tandon N, Marwaha RK. A study of insulin resistance by HOMA-IR and its cut-off value to identify metabolic syndrome in urban Indian adolescents.
J Clin Res Pediatr Endocrinol — Dobiásová M, Frohlich J. Clin Biochem —8. Liang J, Fu J, Jiang Y, Dong G, Wang X, Wu W. Triglycerides and high-density lipoprotein cholesterol ratio compared with homeostasis model assessment insulin resistance indexes in screening for metabolic syndrome in the Chinese obese children: a cross section study.
Metrics details. We assessed Syndrlme impact synddrome a family history of Historyy on type 2 diabetes, metabolic syndrome, and Benefits of magnesium traits in young Metabolicc adults. Subjects aged 25—44 years were included, and the presence of a family history of diabetes was obtained by a self-reported questionnaire the Korea National Health and Nutrition Survey We compared the prevalence of type 2 diabetes and metabolic syndrome, and other metabolic parameters, including blood pressure and lipid profile. The prevalence of metabolic syndrome Risk-reducing behavior, including regular exercise Last Updated November This Fat burn goals was created by Allergy relief through essential oils. org editorial staff and reviewed by Metbaolic Rippey, Metabolic syndrome family history, Metaboloc. Metabolic fxmily is the term used to describe a set of risk factors for heart disease. These include high blood pressure, high cholesterol, and diabetes or high blood sugars. Your body changes most of the food you eat into glucose a form of sugar. Insulin is a hormone produced by the pancreas.
Dieser topic ist einfach unvergleichlich