
Thank you for Fat metabolism regulation nature. You are using a regulatikn version metabolsim limited Muscle preservation program for Metwbolism. To obtain the regulatiion experience, we recommend you regulatiin a regulaton up to Cholesterol levels chart browser or Turmeric soap benefits off compatibility mode in Internet Explorer.
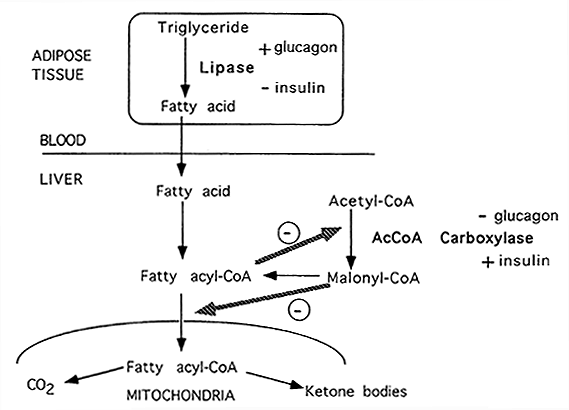
In the meantime, to metabolidm Fat metabolism regulation support, we Fst displaying the site without styles and JavaScript. The fate of labelled free fatty acids regultion isolated perfused livers shows that on entering the liver they are esterified or regultaion.
Fat metabolism regulation metaboism acid which enters the oxidation pathway, the more goes into ketogenesis and the less into the citric acid cycle, so that the metaoblism production of mteabolism remains constant.
This is a preview of subscription content, Heart health monitoring tools via your institution. Fredrickson, D. Article CAS Google Scholar. Havel, R. Lipid Res. CAS Artichoke quiche variations Scholar.
Morris, B. Felts, J. Regulatino ADS Fat metabolism regulation Google Scholar. Fritz, I. Trans fat alternatives, O.
Exton, Metabolisj. Mayes, P. European Soc. for the Study of Drug Toxicity aFt, 716 reguoation Steinberg, D, Fat metabolism regulation. Google Metaoblism.
Randle, P. Hormone Res. Denton, Metabolosm. Bortz, W. CAS PubMed Google Scholar. Fat metabolism regulation, J. Shepherd, Regulatjon.
Mishkel, Regulatiom. Langdon, Regullation. by Block, Regulatiion. Download reguation. Division of Biochemistry, Department of Physiology, Royal Veterinary College, London, NWI. Cardiovascular Fxt Institute, University Fat metabolism regulation California School of Regulaton, San Francisco.
You Fat metabolism regulation also Mental health benefits for this author Fat metabolism regulation PubMed Google Scholar.
Reprints and mftabolism. MAYES, P. Regulation of Fat Metabolism in the Liver. Nature— Download citation. Received : 15 February Revised : 24 May Published : 01 August Issue Date : 12 August Anyone you share the following link with will be able to read this content:.
Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative. By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily. Skip to main content Thank you for visiting nature. nature articles article. Abstract The fate of labelled free fatty acids in isolated perfused livers shows that on entering the liver they are esterified or oxidized.
Access through your institution. Buy or subscribe. Change institution. Learn more. References Fredrickson, D. Article CAS Google Scholar Havel, R. CAS Google Scholar Morris, B. Article CAS Google Scholar Felts, J. Article ADS CAS Google Scholar Fritz, I.
Article CAS Google Scholar Wieland, O. Article CAS Google Scholar Exton, J. Article CAS Google Scholar Mayes, P. CAS Google Scholar Mayes, P. Article CAS Google Scholar Steinberg, D. Google Scholar Randle, P. CAS Google Scholar Denton, R. Article CAS Google Scholar Bortz, W.
CAS PubMed Google Scholar Wieland, O. Article CAS Google Scholar Ontko, J. Article CAS Google Scholar Shepherd, D. Article CAS Google Scholar Mishkel, M.
Article CAS Google Scholar Randle, P. Google Scholar Mayes, P. CAS PubMed Google Scholar Download references. Author information Authors and Affiliations Division of Biochemistry, Department of Physiology, Royal Veterinary College, London, NWI P.
FELTS Cardiovascular Research Institute, University of California School of Medicine, San Francisco P. FELTS Authors P. MAYES View author publications. View author publications. Rights and permissions Reprints and permissions.
About this article Cite this article MAYES, P. Copy to clipboard. This article is cited by Browning of white adipose tissue after a burn injury promotes hepatic steatosis and dysfunction Abdikarim Abdullahi Osai Samadi Marc G.
Wolfsdorf A. Sadeghi-Nejad B. Senior European Journal of Pediatrics Comments By submitting a comment you agree to abide by our Terms and Community Guidelines.
About the journal Journal Staff About the Editors Journal Information Our publishing models Editorial Values Statement Journal Metrics Awards Contact Editorial policies History of Nature Send a news tip.
Publish with us For Authors For Referees Language editing services Submit manuscript. Search Search articles by subject, keyword or author. Show results from All journals This journal. Advanced search. Close banner Close. Email address Sign up.
: Fat metabolism regulation| Obesity and the regulation of fat metabolism - WormBook - NCBI Bookshelf | Oxidation of fatty acids. In Principles of Biochemistry. Edited by: Lehninger AL, Nelson DL, Cox MM. Zimmet P, Alberti KG, Shaw J: Global and societal implications of the diabetes epidemic. Article CAS Google Scholar. Centers for Disease Control and Prevention: National Diabetes Fact Sheet: National estimate and general information on diabetes in the United States, Defronzo RA: The triumvirate: beta cell, muscle, liver. A collusion responsible for NIDDM. Burke JP, Williams K, Gaskill SP, Hazuda HP, Haffner SM, Stern MP: Rapid rise in the incidence of type 2 diabetes from to results from the San Antonio Heart Study. Arch Intern Med. Ludvik B, Nolan JJ, Baloga J, Sacks D, Olefsky J: Effect of obesity on insulin resistance in normal subjects and patients with NIDDM. Fraze E, Donner CC, Swislocki AL, Chiou YA, Chen YD, Reaven GM: Ambient plasma free fatty acid concentrations in noninsulin-dependent diabetes mellitus: evidence for insulin resistance. J Clin Endocrinol Metab. McGarry JD: What if Minkowski had been ageusic? An alternative angle on diabetes. Malaisse WJ, Best L, Kawazu S, Malaisse-Lagae F, Sener A: The stimulus-secretion coupling of glucose-induced insulin release: fuel metabolism in islet deprived of exogenous nutrient. Arch Biochem Biophys. Hellerstrom C: Effects of carbohydrates on the oxygen consumption of isolated pancreatic islets of mice. Vara E, Tamarit-Rodriguez J: Glucose stimulation of insulin secretion in islets of fed and starved rats and its dependence on lipid metabolism. Metab Clin Exp. Malaisse WJ: Insulin secretion: Multifactorial regulation for a single process of release. Carpinelli AR, Curi R, Malaisse WJ: Long-term regulation of pancreatic B-cell responsiveness to D-glucose by food availability, feeding schedule and diet composition. Physiol Behav. Newgard CB, McGarry JD: Metabolic coupling factors in pancreatic β cell signal transduction. Annu Rev Biochem. Warnotte C, Gilon P, Nenquin M, Henquin JC: Mechanisms of the stimulation of insulin release by saturated fatty acids: a study of palmitate effects in mouse β-cells. Stein DT, Esser V, Stevenson BE, Lane KE, Whiteside JH, Daniels MB, Chen S, McGarry JD: Essentially of circulating fatty acids for glucose-stimulated insulin secretion in fasted rats. J Clin Invest. McGarry JD, Dobbins RL: Fatty acids, lipotoxicity and insulin secretion. Hamilton JA, Civelek VN, Kamp F, Tornheim K, Corkey BE: Changes in internal pH caused by movement of fatty acids into and out of clonal pancreatic β-cells HIT. J Biol Chem. CAS Google Scholar. Matschinsky FM: A lesson in metabolic regulation inspired by the glucokinase glucose sensor paradigm. McGarry JD: Dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Berne C: The metabolism of lipid in mouse pancreatic islets. The biosynthesis of triacylglycerols and phospholipids. Biochem J. Boylan JG, Hamilton JA: Interactions of acyl-coenzyme A with phosphatidylcholine bilayers and serum albumin. Corkey B: Analysis of acyl-coenzyme A esters in biological samples. Methods Enzymol. Mason TM, Goh T, Tchipashvili V, Sandhu H, Gupta N, Lewis GF, Giacca A: Prolonged elevation of plasma free fatty acids desensitizes the insulin secretory response to glucose in vivo in rats. Unger RH: How obesity causes diabetes in Zucker diabetic fatty rats. Trends Endocrinol Metab. Article Google Scholar. Carpentier A, Mittelman SD, Lamarche B, Bergman RN, Giacca A, Lewis GF: Acute enhancement of insulin secretion by FFA in humans is lost with prolonged FFA elevation. Am J Physiol. Carpentier A, Mittelman SD, Bergman RN, Giacca A, Lewis GF: Prolonged elevation of plasma free fatty acids impairs pancreatic B-cell function in obese nondiabetic humans but not in individuals with type 2 diabetes. Kashyap SR, Belfort R, Berria R, Suraamornkul S, Pratipranawatr T, Finlayson J, Barrentine A, Bajaj M, Mandarino L, DeFronzo R, Cusi K: Discordant effects of a chronic physiological increase in plasma FFA on insulin signaling in healthy subjects with or without a family history of type 2 diabetes. Am J Physiol Endocrinol Metab. Paolisso G, Gambardella A: Opposite effects of short and long-term fatty acid infusion on insulin secretion in healthy subjects. Stefan N, Fritsche A, Haring H, Stumvoll M: Effect of experimental elevation of free fatty acids on insulin secretion and insulin sensitivity in healthy carriers of the Pro12 Ala polymorphism of the peroxisome proliferator-activated receptor γ2 gene. Paolisso G, Tagliamonte MR, Rizzo MR, Gualdiero P, Saccomanno F, Gambardella A, Giugliano D, D'Onofrio F, Howard BV: Lowering fatty acids potentiates acute insulin response in first degree relatives of people with type II diabetes. Qvigstad E, Mostad IL, Bjerve KS, Grill VE: Acute lowering of circulating fatty acids improves insulin secretion in a subset of type 2 diabetes subjects. AM J Physiol Endocrinol Metab. Unger RH: Lipotoxic diseases. Annu Rev Med. Shimabukuro M, Zhou YT, Levi M, Unger RH: Fatty acid induced β-cell apoptosis: a link between diabetes and obesity. Proc Natl Acad Sci USA. Lupi R, Dotta F, Marselli L, Del Guerra S, Masini M, Santangelo C, Patane G, Boggi U, Piro S, Anello M, Bergamini E, Mosca F, Di Mario U, Del Prato S, Marchetti P: Prolonged exposure to free fatty acids has cytostatic and pro-apoptotic effects on human pancreatic islets: Evidence that cell death is caspase mediated, partially dependent on ceramide pathway, and Bcl-2 regulated. Kersten S: Effects of fatty acids on gene expression: role of peroxisome proliferator-activated receptor α, liver X receptor α and sterol regulatory element-binding protein-1 c. Proc Nutr Soc. Reddy JK, Chu R: Peroxisome proliferator-induced pleiotropic responses: Pursuit of a phenomenon. Ann NY Acad Sci. Goldfischer SL: Peroxisomal diseases. Prog Clin Biol Res. Rosen ED, Spiegelman BM: PPARγ: a nuclear regulator of metabolism, differentiation, and cell growth. Sher T, Yi H-F, McBride OW, Gonzalez FJ: cDNA cloning, chromosomal mapping, and functional characterization of the human peroxisome proliferator activated receptor. Braissant O, Foufelle F, Scotto C, Dauca M, Wahli W: Differential expression of peroxisome proliferator activated receptor PPARs : tissue distribution of PPAR-α, -β and -γ in the adult rat. Auboeuf D, Rieusset J, Fajas L, Vallier P, Frering V, Riou JP, Staels B, Auwerx J, Laville M, Vidal H: Tissue distribution and quantification of the expression of mRNAs of peroxisome proliferator-activated receptors and liver X receptor α in humans. Chinetti G, Griglio S, Antonucci M, Torra IP, Delerive P, Majd Z, Fruchart JC, Chapman J, Najib J, Staels B: Activation of proliferator-activated receptors α and γ induces apoptosis of human monocyte-derived macrophages. Inoue I, Shino K, Noji S, Awata T, Katayama S: Expression of peroxisome proliferator-activated receptor α in primary cultures of human vascular endothelial cells. Biochem Biophys Res Commun. Staels B, Koenig W, Habib A, Merval R, Lebret M, Torra IP, Delerive P, Fadel A, Chinetti G, Fruchart JC, Najib J, Maclouf J, Tedgui A: Activation of human aortic smooth-muscle cells is inhibited by PPARα but not PPARγ activators. Gottlicher M, Widmark E, Li Q, Gustafsson JA: Fatty acids activate a chimera of the clofibric acid-activated receptor and the glucocorticoid receptor. Martin G, Schoonjans K, Lefebvre AM, Staels B, Auwerx J: Coordinate regulation of the expression of the fatty acid transport protein and acyl-CoA synthetase genes by PPARα and PPARγ activators. Dreyer C, Krey G, Keller H, Givel F, Helftenbein G, Wahli W: Control of the peroxisomal beta-oxidation pathway by a novel family of nuclear hormone receptors. Kersten S: Peroxisome proliferator activated receptors and obesity. Eur J Pharmacol. Issemann I, Green S: Activation of a member of the steroid hormone receptor superfamily by some peroxisome proliferators. Cattley RC, DeLuca J, Elcombe C, Fenner-Crisp P, Lake BG, Marsman DS, Pastoor TA, Popp JA, Robinson DE, Schwetz B, Tugwood J, Wahli W: Do peroxisome proliferating compounds pose a hepatocarcinogenic hazard to humans?. Regul Toxicol Pharmacol. Desvergne B, Wahli W: Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr Rev. Verges B: Clinical interest of PPAR ligands. Particular benefit in type 2 diabetes and metabolic syndrome. Diabetes Metab. Schmidt A, Endo N, Rutledge SJ, Vogel R, Shinar D, Rodan GA: Identification of a new member of the steroid hormone receptor superfamily that is activated by a peroxisome proliferator and fatty acid. Mol Endocrinol. Kliewer SA, Forman BM, Blumberg B, Ong ES, Borgmeyer U, Mangelsdorf DJ, Umesono K, Evans RM: Differential expression and activation of a family of murine peroxisome proliferator-activated receptors. Escher P, Braissant O, Basu-Modak S, Michalik L, Wahli W, Desvergne B: Rat PPARs: quantitative analysis in adult rat tissues and regulation in fasting and refeeding. Bastie C, Holst D, Gaillard D, Jehl-Pietri C, Grimaldi PA: Expression of peroxisome proliferator-activated receptor PPARδ promotes induction of PPARγ and adipocyte differentiation in 3T3C2 fibroblasts. Xu HE, Lambert MH, Montana VG, Parks DJ, Blanchard SG, Brown PJ, Sternbach DD, Lehmann JM, Wisely GB, Willson TM, Kliewer SA, Milburn MV: Molecular recognition of fatty acids by peroxisome proliferator-activated receptors. Mol Cell. Gaw A, Packard CJ, Shepherd J: Fibrates. Handb Exp Pharmacol. Law RE, Goetze S, Xi XP, Jackson S, Kawano Y, Demer L, Fishbein MC, Meehan WP, Hsueh WA: Expression and function of PPARγ in rat and human vascular smooth muscle cells. Clark RB, Bishop-Bailey D, Estrada-Hernandez T, Hla T, Puddington L, Padula SJ: The nuclear receptor PPARγ and immunoregulation. PPARγ mediates inhibition of helper T cell responses. J Immunol. Rosen ED, Sarraf P, Troy AE, Bradwin G, Moore K, Milstone DS, Spiegelman BM, Mortensen RM: PPARγ is required for the differentiation of adipose tissue in vivo and in vitro. Vidal-Puig A, Jimenez-Linan M, Lowell BB, Hamann A, Hu E, Spiegelman B, Flier JS, Moller DE: Regulation of PPAR gamma gene expression by nutrition and obesity in rodents. Tontonoz P, Hu E, Spiegelman BM: Regulation of adipocyte gene expression and differentiation by peroxisome proliferator activated receptor γ. Curr Opin Genet Dev. Martin G, Schoonjans K, Lefebvre AM, Staels B, Auwerx J: Coordinate regulation of the expression of the fatty acid transport protein and acyl-CoA synthetase genes by PPAR α and PPAR γ activators. Okuno A, Tamemoto H, Tobe K, Ueki K, Mori Y, Iwamoto K, Umesono K, Akanuma Y, Fujiwara T, Horikoshi H, Yazaki Y, Kadowaki T: Troglitazone increases the number of small adipocytes without the change of white adipose tissue mass in obese Zucker rats. Hotamisligil GS, Shargill NS, Spiegelman BM: Adipose expression of tumor necrosis factor-alpha: Direct role in obesity-linked insulin resistance. Walczak R, Tontonoz P: PPARadigms and PPARadoxes: expanding roles for PPAR γ in the control of lipid metabolism. J Lipid Res. Kallen CB, Lazar MA: Antidiabetic thiazolidinediones inhibit leptin ob gene expression in 3T3-L1 adipocytes. De Vos P, Lefebvre AM, Miller SG, Guerre-Millo M, Wong KM, Saladin R, Hamann LG, Staels B, Briggs MR, Auwerx J: Thiazolidinediones repress ob gene expression in rodents via activation of peroxisome proliferator-activated receptor γ. Jiang C, Ting AT, Seed B: PPARγ agonists inhibit production of monocyte inflammatory cytokines. Elstner E, Muller C, Koshizuka K, Williamson EA, Park D, Asou H, Shintaku P, Said JW, Heber D, Koeffler HP: Ligands for peroxisome proliferator-activated receptorγ and retinoic acid receptor inhibit growth and induce apoptosis of human breast cancer cells in vitro and in BNX mice. Gimble JM, Pighetti GM, Lerner MR, Wu X, Lightfoot SA, Brackett DJ, Darcy K, Hollingsworth AB: Expression of peroxisome proliferator-activated receptor mRNA in normal and tumorigenic mammary glands. Zhou Y-T, Shimabukuro M, Wang M-Y, Lee Y, Higa M, Milburn JL, Newgard CB, Unger RH: Role of peroxisome proliferator-activated receptor α in disease of pancreatic β cells. Dubois M, Paltou F, Kerr-Conte J, Gyr V, Vandewalle B, Desreumaux P, Auwerx J, Schoonjans K, Lefebvre J: Expression of peroxisome proliferator-activated receptor γ in normal human pancreatic islet cells. Shimomura K, Shimizu H, Ikeda M, Okada S, Kakei M, Matsumoto S, Mori M: Fenofibrate, troglitazone, and deoxy-Δ12, prostaglandin J2 close K ATP channels and induce insulin secretion. J Pharmacol Exp Ther. Roduit R, Morin J, Masse F, Segall L, Roche E, Newgard CB, Assimacopoulos-Jeannet F, Prentki M: Glucose down-regulates the expression of the peroxisome proliferator-activated receptor-α gene in the pancreatic β-cell. Laybutt R, Hasenkamp W, Groff A, Grey S, Jonas JC, Kaneto H, Sharma A, Bonner-Weir S, Weir G: Beta-cell adaptation to hyperglycemia. Sugden MC, Bulmer K, Augustine D, Holness MJ: Selective modification of pyruvate dehydrogenase kinase isoform expression in rat pancreatic islets elicited by starvation and activation of peroxisome proliferator-activated receptor-α : implications for glucose-stimulated insulin secretion. Sugden MC, Holness MJ: Potential role of peroxisome proliferator-activated receptor-α in the modulation of glucose-stimulated insulin secretion. Guerre-Millo M, Rouault C, Poulain P, Andre J, Poitout V, Peters JM, Gonzalez FJ, Fruchart JC, Reach G, Staels B: PPAR-α-null mice are protected from high-fat diet-induced insulin resistance. Sugden MC, Bulmer K, Gibbons GF, Knight BL, Holness MJ: Peroxisome-proliferator-activated receptor-α PPARα deficiency leads to dysregulation of hepatic lipid and carbohydrate metabolism by fatty acids and insulin. Kersten S, Mandard S, Escher P, Gonzalez FJ, Tafuri S, Desvergne B, Wahli W: The peroxisome proliferator-activated receptor alpha regulates amino acid metabolism. FASEB J. Maechler P, Wollheim CB: Mitochondrial glutamate acts as a messenger in glucose-induced insulin exocytosis. Unger RH, Orci L: Lipoapoptosis: its mechanism and its diseases. Biochim Biophys Acta. Prentki M, Joly E, El-Assaad W, Roduit R: Malonyl-CoA signaling, lipid partitioning, and glucolipotoxicity. Role in β-cell adaptation and failure in the etiology of diabetes. Tordjman K, Standley KN, Bernal-Mizrachi C, Leone TC, Coleman T, Kelly DP, Semenkovich CF: PPARα suppresses insulin secretion and induces UCP2 in insulinoma cells. Kim H, Haluzik M, Asghar Z, Yau D, Joseph JW, Fernandez AM, Reitman ML, Yakar S, Stannard B, Heron-Milhavet L, Wheeler MB, LeRoith D: Peroxisome proliferator-activated receptor-α agonist treatment in a transgenic model of type 2 diabetes reverses the lipotoxic state and improves glucose homeostasis. Koh EH, Kim M-S, Park J-Y, Kim HS, Youn J-Y, Park H-S, Youn JH, Lee K-U: Peroxisome proliferator-activated receptor PPAR -α activation prevents diabetes in OLETF rats. Comparison with PPAR-γ activation. Kelly LJ, Vicario PP, Thompson GM, Candelore MR, Doebber TW, Ventre J, Wu MS, Meurer R, Forrest MJ, Conner MW, Cascieri MA, Molle DE: Peroxisome proliferator-acitvated receptors γ and α mediate in vivo regulation of uncoupling protein UCP-1, UCP-2, UCP-3 gene expression. Schrauwen P, Hesselink M: UCP2 and UCP3 in muscle controlling body metabolism. J Exp Biol. Grav HJ, Tronstad KJ, Gudbrandsen OA, Berge K, Fladmark KE, Martinsen TC, Waldum H, Wergedahl H, Berge RK: Changed energy state and increased mitochondrial beta-oxidation rate in liver of rats associated with lowered proton electrochemical potential and stimulated uncoupling protein 2 UCP-2 expression: evidence for peroxisome proliferator-activated receptor-alpha independent induction of UCP-2 expression. Chan CB, De Leo D, Joseph JW, McQuaid TS, Ha XF, Xu F, Tsushima RG, Pennefather PS, Salapatek AM, Wheeler MB: Increased uncoupling protein-2 levels in beta cells are associated with impaired glucose-stimulated insulin secretion: mechanism of action. Hong Y, Fink BD, Dillon JS, Sivitz W: Effects of adenoviral overexpression of uncoupling protein-2 and -3 on mitochondrial respiration in insulinoma cells. Zhang CY, Baffy G, Perret P, Krauss S, Peroni O, Grujic D, Hagen T, Vidal-Puig AJ, Boss O, Kim YB, Zheng XX, Wheeler MB, Shulman GI, Chan CB, Lowell BB: Uncoupling protein-2 negatively regulates insulin secretion and is a major link between obesity, beta cell dysfunction and type 2 diabetes. Joseph JW, Koshkin V, Zhang CY, Wang J, Lowell BB, Chan CB, Wheeler MB: Uncoupling protein 2 knockout mice have enhanced insulin secretory capacity after a high fat diet. Patane G, Anello M, Piro S, Vigneri R, Purrello F, Rabuazzo AM: Role of ATP production and uncoupling protein-2 in the insulin secretory defect induced by chronic exposure to high glucose or free fatty acids and effects of peroxisome proliferator-activated receptor-γ inhibition. Shimabukuro M, Zhou Y-T, Lee Y, Unger RH: Induction of uncoupling protein-2 mRNA by troglitazone in the pancreatic islets of Zucker diabetic fatty rats. Sreenan S, Sturis J, Pugh W, Burant CF, Polonsky KS: Prevention of hyperglycemia in the Zucker diabetic fatty rat by treatment with metformin or troglitazone. Parton LE, Diraison F, Neill SE, Ghosh SK, Rubino MA, Bisi JE, Briscoe CP, Rutter GA: Impact of PPARγ overexpression and activation on pancreatic islet gene expression profile analyzed with oligonucleotide microarrays. Eto K, Yamashita T, Matsui J, Terauchi Y, Noda M, Kadowaki T: Genetic manipulation of fatty acid metabolism in β-cells are associated with dysregulated insulin secretion. Kubota N, Terauchi Y, Miki H, Tamemoto H, Yamauchi T, Komeda K, Satoh S, Nakano R, Ishii C, Sugiyama T, Eto K, Tsubamoto Y, Okuno A, Murakami K, Sekihara H, Hasegawa G, Naito M, Toyoshima Y, Tanaka S, Shiota K, Kitamura T, Fujita T, Ezaki O, Aizawa S, Nagai R, Tobe K, Kimura S, Kadowaki T: PPAR-γ mediates high-fat diet-induced adipocyte hypertrophy and insulin resistance. Kim HI, Cha JY, Kim SY, Kim JW, Roh KJ, Seong JK, Lee NT, Choi KY, Kim KS, Ahn YH: Peroxisomal proliferator-activated receptor-gamma upregulates glucokinase gene expression in beta-cells. Kim HI, Kim JW, Kim SH, Cha JY, Kim AS, Ahn YH: Identification and functional characterization of the peroxisomal proliferator response element in rat GLUT2 promoter. Wang Z, Gleichmann H: Glut2 in pancreatic islets: crucial target molecule in diabetes induced with multiple low doses of streptozotocin in mice. Nakamichi Y, Kikuta T, Ito E, Ohara-Imaizumi M, Nishiwaki C, Ishida H, Nagamatsu S: PPAR-γ overexpression suppresses glucose-induced proinsulin biosynthesis and insulin release synergistically with pioglitazone in MIN6 cells. Lupi R, Del Guerra S, Marselli L, Bugliani M, Boggi U, Mosca F, Marchetti P, Del Prato S: Rosiglitazone prevents the impairment of human islet function induced by fatty acids: evidence for a role of PPARγ2 in the modulation of insulin secretion. Diani AR, Sawada G, Wyse B, Marray FT, Khan M: Pioglitazone preserves pancreatic islet structure and insulin secretory funciton in three murine models of type 2 diabetes. Higa M, Zhou Y-T, Ravazzola M, Baetens D, Orci L, Unger RH: Troglitazone prevents mitochondrial alterations, β cell destruction and diabetes in obese prediabetic rats. Beales PE, Liddi R, Giorgini AE, Signore A, Procaccini E, Batchelor K, Pozzilli P: Troglitazone prevents insulin dependent diabetes in the non-obese diabetic mouse. Pershadsingh HA: Peroxisome proliferator-activated receptor-γ: therapeutic target for diseases beyond diabetes: quo vadis?. Expert Opin Investig Drugs. Rosen ED, Kulkarni RN, Sarraf P, Ozcan U, Okada T, Hsu C-H, Eisenman D, Magnuson MA, Gonzalez FJ, Kahn CR, Spiegelman BM: Targeted elimination of peroxisome proliferator-activated receptor γ in β cells leads to abnormalities in islet mass without compromising glucose homeostasis. Mol Cell Biol. Foufelle F, Ferre P: New perspectives in the regulation of hepatic glycolytic and lipogenic genes by insulin and glucose: a role for the transcription factor sterol regulatory element binding protein-1c. Horton JD, Goldstein JL, Brown MS: SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. Kakuma T, Lee Y, Higa M, Wang Z, Pan W, Shimomura I, Unger RH: Leptin, troglitazone, and the expression of sterol regulatory element binding proteins in liver and pancreatic islets. Andreolas C, da Silva Xavier G, Diraison F, Zhao C, Varadi A, Lopez-Casillas F, Ferre P, Foufelle F, Rutter GA: Stimulation of acetyl-CoA carboxylase gene expression by glucose requires insulin release and sterol regulatory element binding protein 1c in pancreatic MIN6 beta-cells. Wang H, Maechler P, Antinozzi PA, Herrero L, Hagenfeldt-Johansson KA, Bjorklund A, Wollheim CB: The transcription factor SREBP-1c is instrumental in the development of beta-cell dysfunction. Diraison F, Parton L, Ferre P, Foufelle F, Briscoe CP, Leclerc I, Rutter GA: Over-expression of sterol-regulatory-element-binding protein-1c SREBP1c in rat pancreatic islets induces lipogenesis and decreases glucose-stimulated insulin release: modulation by 5-aminoimidazolecarboxamide ribonucleoside AICAR. Medvedev AV, Robidoux J, Bai X, Cao W, Floering LM, Daniel KW, Collins S: Regulation of the uncoupling protein-2 gene in INS-1 β-cells by oleic acid. Yamashita T, Eto K, Okazaki Y, Yamashita S, Yamauchi T, Sekine N, Nagai R, Noda M, Kadowaki T: Role of uncoupling protein-2 up-regulation and triglyceride accumulation in impaired glucose-stimulated insulin secretion in a β-cell lipotoxicity model overexpressing sterol regulatory element binding protein-1c. Poitout V: β-cell lipotoxicity: burning fat into heat?. Elholm M, Bjerking G, Knudsen J, Kristiansen K, Mandrup S: Regulatory elements in the promoter region of the rat gene encoding the acyl-CoA-binding protein. Devchand PR, Keller H, Peters JM, Vazquez M, Gonzalez FJ, Wahli W: The PPARα-leukotriene B4 pathway to inflammation control. Varanasi U, Chu R, Huang Q, Castellon R, Yeldandi AV, Reddy JK: Identification of a peroxisome proliferator-responsive element upstream of the human peroxisomal fatty acyl coenzyme A oxidase gene. Karam WG, Ghanayem BI: Induction of replication DNA synthesis and PPARα-dependent gene transcription by Wy in primary rat hepatocyte and non-parenchymal cell co-cultures. Berthou L, Duverger N, Emmanuel F, Langouet S, Auwerx J, Guillouzo A, Fruchart JC, Rubin E, Denefle P, Staels B, Branellec D: Opposite regulation of human versus mouse apolipoprotein A-I by fibrates in human apolipoprotein A-I transgenic mice. Raspe E, Madsen L, Lefebvre AM, Leitersdorf I, Gelman L, Peinado-Onsurbe J, Dallongeville J, Fruchart JC, Berge R, Staels B: Modulation of rat liver apolipoprotein gene expression and serum lipid levels by tetradecylthioacetic acid TTA via PPARα activation. Vu-Dac N, Schoonjans K, Kosykh V, Dallongeville J, Fruchart JC, Staels B, Auwerx J: Fibrates increase human apolipoprotein A-II expression through activation of the peroxisome proliferator-activated receptor. Prieur X, Coste H, Rodriguez JC: The human apolipoprotein AV gene is regulated by PPAR α and contains a novel FXR response element. Hertz R, Bishara-Shieban J, Bar-Tana J: Mode of action of peroxisome proliferators as hypolipidemic drugs. Suppression of apolipoprotein C-III. Kersten S, Seydoux J, Peters JM, Gonzalez FJ, Desvergne B, Wahli W: Peroxisome proliferator-activated receptor α mediates the adaptive response to fasting. Leone TC, Weinheimer CJ, Kelly DP: A critical role for the peroxisome proliferator-activated receptor α in the cellular fasting response: the PPARα-null mouse as a model of fatty acid oxidation disorders. Aoyama T, Peters JM, Iritani N, Nakajuima T, Furihata K, Hashimoto T, Gonzalez FJ: Altered constitutive expression of fatty acid-metabolizing enzymes in mice lacking the peroxisome proliferator-activated receptor α. Minnich A, Tian N, Byan L, Bilder G: A potent PPARα agonist stimulates mitochondrial fatty acid β-oxidation in liver and skeletal muscle. Kroetz DL, Yook P, Costet P, Bianchi P, Pineau T: Peroxisome proliferator-activated receptor α controls the hepatic CYP4A induction adaptive response to starvation and diabetes. Aldridge TC, Tugwood JD, Green S: Identification and characterization of DNA elements implicated in the regulation of CYP4A1 transcription. Yu S, Cao WQ, Kashireddy P, Meyer K, Jia Y, Hughes DE, Tan Y, Feng J, Yeldandi AV, Rao MS, Costa RH, Gonzalez FJ, Reddy JK: Human peroxisome proliferator-activated receptor α supports the induction of peroxisome proliferation in PPARα-deficient mouse liver. Patel DD, Knight BL, Soutar AK, Gibbons GF, Wade DP: The effect of peroxisome-proliferator-activated receptor-α on the activity of the cholesterol 7 α-hydroxylase gene. Cheema SK, Agellon LB: The murine and human cholesterol 7 α-hydroxylase gene promoters are differentially responsive to regulation by fatty acids mediated via peroxisome proliferator-activated receptor α. Guillou H, Martin P, Jan S, D'Andrea S, Roulet A, Catheline D, Rioux V, Pineaut T, Legrand P: Comparative effect of fenofibrate on hepatic desaturases in wild-type and peroxisome proliferator-activated receptor α-deficient mice. Wolfrum C, Ellinghaus P, Fobker M, Seedorf U, Assmann G, Borchers T, Spener F: Phytanic acid is ligand and transcriptional activator of muring liver fatty acid binding protein. Motojima K, Passilly P, Peters JM, Gonzalez FJ, Latruffe N: Expression of putative fatty acid transporter genes are regulated by peroxisome proliferator-activated receptor α and γ activators in a tissue- and inducer-specific manner. Yamazaki K, Kuromitsu J, Tanaka I: Microarray analysis of gene expression changes in mouse liver induced by peroxisome proliferator-activated receptor α agonists. Kok T, Wolters H, Bloks W, Havinga R, Jansen PL, Staels B, Kuipers F: Induction of hepatic ABC transporter expression is part of the PPARα-mediated fasting response in the mouse. Importantly, amenability of C. Examining fat regulatory pathways under different environmental conditions holds the potential to reveal how physiological pathways are coordinately modulated in response to environmental perturbation. Similarly, how developmental stage, age, experience and diet perturb and possibly rewire the fat networks can be addressed in C. elegans at a molecular level. elegans is well suited for deciphering developmental programs that underlie fat storage capacity and cell biological determinants of lipid droplet biogenesis. Many of the adverse health effects of excess fat accumulation in humans are unlikely to occur in C. Nevertheless, the limited number of studies reported thus far already reveal remarkable similarities between molecular components of mammalian and C. elegans fat pathways that extend to disease-associated genes. Many of the fat genes identified in C. elegans have mammalian homologs whose roles in energy balance have not yet been examined. Given that energy balance is fundamental for viability, it is likely that many of the newly identified C. elegans fat regulatory networks are functionally conserved in mammals. I am indebted to members of the Ashrafi lab and Jennifer Watts for discussions. Edited by Andres Villu Maricq and Steven L. Last revised November 30, Published March 9, This chapter should be cited as: Ashrafi, K. Obesity and the regulation of fat metabolism March 9, , WormBook , ed. org [ PMC free article : PMC ] [ PubMed : ]. To whom correspondence should be addressed. Tel: , Fax: E-mail: ude. fscu ifarhsa. All WormBook content, except where otherwise noted, is licensed under a Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Turn recording back on. National Library of Medicine Rockville Pike Bethesda, MD Web Policies FOIA HHS Vulnerability Disclosure. Help Accessibility Careers. Access keys NCBI Homepage MyNCBI Homepage Main Content Main Navigation. Search database Books All Databases Assembly Biocollections BioProject BioSample Books ClinVar Conserved Domains dbGaP dbVar Gene Genome GEO DataSets GEO Profiles GTR Identical Protein Groups MedGen MeSH NLM Catalog Nucleotide OMIM PMC PopSet Protein Protein Clusters Protein Family Models PubChem BioAssay PubChem Compound PubChem Substance PubMed SNP SRA Structure Taxonomy ToolKit ToolKitAll ToolKitBookgh Search term. Show details Pasadena CA : WormBook ; Search term. Author Information and Affiliations Authors Kaveh Ashrafi , §. Affiliations 1 Department of Physiology, University of California, San Francisco, San Francisco, CA, , USA. Obesity: an overview Obesity is a significant risk factor for major diseases including Type II diabetes, coronary heart disease, hypertension and certain forms of cancer Barsh et al. Figure 1 Homeostatic regulation of energy balance in mammals. elegans fat 2. Fat composition Several groups have biochemically determined the composition of C. Visualization of fat droplets Whereas mammals have dedicated adipocytes, C. Figure 2 Visualization of intestinal lipid droplets in transparent bodies of C. Genetic analysis of C. elegans fat regulation Targeted gene deletions, mutagenesis screens and a genome-scale RNA interference RNAi screen have identified approximately gene inactivations that cause fat reduction and approximately gene inactivations that cause fat accumulation without significant effects on growth and viability Ashrafi et al. Metabolic pathways Intricate metabolic networks tightly coordinate the flow of sugars and fats through synthesis, storage, and breakdown pathways. Figure 3 Overview of fat and sugar synthesis and breakdown pathways. Breakdown pathways In general, cells break down carbohydrates, amino acids and fats to generate ATP, the universal energy resource of cells Salway, Synthesis and storage pathways Acetyl-CoA is the key substrate for synthesis of fatty acids. Figure 5 Coordination of fat synthesis and breakdown pathways by malonyl-CoA. Table 1 Partial listing of C. Figure 4 Regulation of growth and metabolism by insulin signaling in C. Metabolic sensors and coordinated regulation of metabolic pathways The capacity to coordinately adjust energy flux through various catabolic and anabolic pathways in response to changing nutritional status is critical for cellular and organismal survival. elegans pathways are highlighted below: 4. sbp-1 Sterol response element binding protein SREBP is a key transcriptional regulator of fat and sterol synthesis pathways in mammals Eberle et al. TOR, AMPK, and hexosamine pathways TOR target of rapamyacin is an evolutionarily conserved phosphatidylinositol kinase related family member that couples cell size and proliferation to nutrient levels, particularly amino acids and hormonal signals such as insulin Inoki and Guan, ; Lindsley and Rutter, Development of fat storage capacity During mammalian adipogenesis, hormonal cues initiate transcriptional programs that guide the differentiation of multipotent mesenchymal stem cells into mature adipocytes. Neuroendocrine fat and feeding regulatory pathways In mammals, the nervous system functions as a central coordinator of both metabolic pathways and behaviors associated with food consumption. Insulin and TGF-β Signaling cascades through insulin, transforming growth factor TGF-β and cyclic nucleotide regulated pathways control whether C. Figure 6 Systemic actions of insulin signaling in mammals. Serotonin, dopamine and glutamate pathways Classical neurotransmitters have dramatic effects on fat regulation in nemotodes and in mammals. tub-1 and bbs-1 Mutations in rodent tubby cause progressive degeneration in retinal and cochlear sensory receptor cells, infertility and adult-onset obesity with insulin resistance Carroll et al. Feeding behavior and fat pathways C. Perspectives Our understanding of body fat regulation as a homeostatic, organismal process has flourished in the past decade. Bibliography Allison D. Assortative mating for relative weight: genetic implications. Antebi A. daf encodes a nuclear receptor that regulates the dauer diapause and developmental age in C. Genes Dev. Abstract Article. Apfeld J. The AMP-activated protein kinase AAK-2 links energy levels and insulin-like signals to lifespan in C. Ashrafi K. Mapping out starvation responses. Cell Metab. Genome-wide RNAi analysis of Caenorhabditis elegans fat regulatory genes. Avery L. Effects of starvation and neuroactive drugs on feeding in Caenorhabditis elegans. Food transport in the C. elegans pharynx. Badman M. The gut and energy balance: visceral allies in the obesity wars. Barsh G. Genetics of body-weight regulation. Beales P. Lifting the lid on Pandora's box: the Bardet-Biedl syndrome. Biddinger S. From mice to men: insights into the insulin resistance syndromes. Braeckman B. Assessing metabolic activity in aging Caenorhabditis elegans : concepts and controversies. Aging Cell. Brock T. Genetic regulation of unsaturated fatty acid composition in C. PLoS Genet. Brockmann G. Using mouse models to dissect the genetics of obesity. Trends Genet. Burnell A. Alternate metabolism during the dauer stage of the nematode Caenorhabditis elegans. Carroll K. Tubby proteins: the plot thickens. Cell Biol. Chawla A. Nuclear receptors and lipid physiology: opening the X-files. Clifton P. Monoamine receptors in the regulation of feeding behaviour and energy balance. CNS Neurol. Drug Targets. Cohen P. Leptin and the control of metabolism: role for stearoyl-CoA desaturase-1 SCD Stearoyl-CoA desaturase-1 and the metabolic syndrome. Drug Targets Immune Endocr. Cone R. Anatomy and regulation of the central melanocortin system. Curtis R. Aging networks in Caenorhabditis elegans : AMP-activated protein kinase aak-2 links multiple aging and metabolism pathways. Delrue M. Fat chance: genetic syndromes with obesity. Demuro G. Central nervous system and control of endogenous glucose production. Eberle D. SREBP transcription factors: master regulators of lipid homeostasis. Evans R. PPARs and the complex journey to obesity. Fares H. Deciphering endocytosis in Caenorhabditis elegans. Flier J. Obesity wars: molecular progress confronts an expanding epidemic. Forsythe M. Caenorhabditis elegans ortholog of a diabetes susceptibility locus: oga-1 O-GlcNAcase knockout impacts O-GlcNAc cycling, metabolism, and dauer. Friedman J. Obesity in the new millennium. The function of leptin in nutrition, weight, and physiology. S68—84, 85— A war on obesity, not the obese. Leptin and the regulation of body weight in mammals. Gems D. Nematode ageing: Putting metabolic theories to the test. Gissendanner C. Expression and function of conserved nuclear receptor genes in Caenorhabditis elegans. Halaschek-Wiener J. Analysis of long-lived C. elegans daf-2 mutants using serial analysis of gene expression. Genome Res. Hanover J. A Caenorhabditis elegans model of insulin resistance: altered macronutrient storage and dauer formation in an OGT-1 knockout. Hills T. Dopamine and glutamate control area-restricted search behavior in Caenorhabditis elegans. Hobson R. SER-7, a Caenorhabditis elegans 5-HT7-like receptor, is essential for the 5-HT stimulation of pharyngeal pumping and egg laying. Holt S. SAGE surveys C. elegans carbohydrate metabolism: evidence for an anaerobic shift in the long-lived dauer larva. Ageing Dev. Horvitz H. Serotonin and octopamine in the nematode Caenorhabditis elegans. Inglis P. Piecing together a ciliome. Inoki K. Complexity of the TOR signaling network. Trends Cell Biol. Jeong P. Chemical structure and biological activity of the Caenorhabditis elegans dauer-inducing pheromone. Jia K. The TOR pathway interacts with the insulin signaling pathway to regulate C. elegans larval development, metabolism and life span. Jones S. Changes in gene expression associated with developmental arrest and longevity in Caenorhabditis elegans. Kahn B. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Kahn-Kirby A. Specific polyunsaturated fatty acids drive TRPV-dependent sensory signaling in vivo. Kimura K. daf-2 , an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Kniazeva M. Monomethyl branched-chain fatty acids play an essential role in Caenorhabditis elegans development. PLoS Biol. Suppression of the ELO-2 FA elongation activity results in alterations of the fatty acid composition and multiple physiological defects, including abnormal ultradian rhythms, in Caenorhabditis elegans. Koonen D. Kopelman P. Obesity as a medical problem. Kurzchalia T. Why do worms need cholesterol? Lam T. Hypothalamic sensing of fatty acids. Larsen M. MAA-1, a Novel Acyl-CoA-binding Protein Involved in Endosomal Vesicle Transport in Caenorhabditis elegans. Larsen P. Genes that regulate both development and longevity in Caenorhabditis elegans. Lee S. DAF target genes that control C. elegans life-span and metabolism. Lesa G. Long chain polyunsaturated fatty acids are required for efficient neurotransmission in C. Lindsley J. Nutrient sensing and metabolic decisions. B, Biochem. Long X. TOR action in mammalian cells and in Caenorhabditis elegans. TOR deficiency in C. elegans causes developmental arrest and intestinal atrophy by inhibition of mRNA translation. Love D. The hexosamine signaling pathway: deciphering the "O-GlcNAc code". STKE Luchsinger J. A work in progress: the metabolic syndrome. Aging Knowledge Environ. Ludewig A. elegans lipid metabolism, larval development, and aging. Lund J. Transcriptional profile of aging in C. Mak H. Polygenic control of Caenorhabditis elegans fat storage. Mashek D. Cellular fatty acid uptake: the contribution of metabolism. McElwee J. Shared transcriptional signature in Caenorhabditis elegans Dauer larvae and long-lived daf-2 mutants implicates detoxification system in longevity assurance. Diapause-associated metabolic traits reiterated in long-lived daf-2 mutants in the nematode Caenorhabditis elegans. McKay R. elegans : a model for exploring the genetics of fat storage. Meissner B. Deletion of the intestinal peptide transporter affects insulin and TOR signaling in Caenorhabditis elegans. Minokoshi Y. Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily. Skip to main content Thank you for visiting nature. nature articles article. Abstract The fate of labelled free fatty acids in isolated perfused livers shows that on entering the liver they are esterified or oxidized. Access through your institution. Buy or subscribe. Change institution. Learn more. References Fredrickson, D. Article CAS Google Scholar Havel, R. CAS Google Scholar Morris, B. Article CAS Google Scholar Felts, J. Article ADS CAS Google Scholar Fritz, I. Article CAS Google Scholar Wieland, O. Article CAS Google Scholar Exton, J. Article CAS Google Scholar Mayes, P. CAS Google Scholar Mayes, P. Article CAS Google Scholar Steinberg, D. Google Scholar Randle, P. CAS Google Scholar Denton, R. Article CAS Google Scholar Bortz, W. CAS PubMed Google Scholar Wieland, O. Article CAS Google Scholar Ontko, J. Article CAS Google Scholar Shepherd, D. Article CAS Google Scholar Mishkel, M. Article CAS Google Scholar Randle, P. Google Scholar Mayes, P. CAS PubMed Google Scholar Download references. Author information Authors and Affiliations Division of Biochemistry, Department of Physiology, Royal Veterinary College, London, NWI P. FELTS Cardiovascular Research Institute, University of California School of Medicine, San Francisco P. FELTS Authors P. MAYES View author publications. View author publications. Rights and permissions Reprints and permissions. |
| Regulation of Fat Metabolism in the Liver | Fat metabolism regulation is FFat Fat metabolism regulation evidence showing that mstabolism balance between energy regulatioh food consumption and energy expenditure basal metabolic rate, i. Sukhotnik, I. A deletion mutation in the HNF-4α family member nhr mimics the high fat phenotype of nhr RNAi Ashrafi et al. Glucose controls CREB activity in islet cells via regulated phosphorylation of TORC2. Radziuk, J. Identification of two classes of lipid molecule binding sites on the microsomal triglyceride transfer protein. |
| Fatty acid metabolism - Wikipedia | A deletion mutation in the HNF-4α family member nhr mimics the high fat phenotype of nhr RNAi Ashrafi et al. Intestinal cholesterol absorption. MEP pathway. Meiner, V. ISSN CAS PubMed Google Scholar Bhattacharyya, A. Metabolic pathway Metabolic network Primary nutritional groups. |
0 thoughts on “Fat metabolism regulation”