Video
Fight Sore Muscles with BCAAsBCAA and muscle inflammation -
One study, for example, showed that taking 1, IU of Vitamin C and IU of Vitamin E inhibited training-induced increases in skeletal muscle protein. However, there are no long-term studies about this specifically. Antioxidants such as Co-enzyme Q10, tart cherry juice, and pomegranate juice can accelerate recovery by reducing inflammatory damage.
We need more research to solidify the reference ranges for athletes. For testing purposes, most studies consist of an acute bout of exercise to induce drastic muscular damage.
Researchers then compare supplementation against a control for immediate study. Since BCAAs regulate skeletal muscle protein synthesis and accelerate recovery, researches examined whether BCAAs would help calorie-restricted athletes undergoing a heavy resistance training regimen retain lean body mass.
The BCAA group lost fat mass and maintained lean body mass, while the carbohydrate group lost lean mass and body mass. Both groups increased the 1RM in the squat, but the BCAA group improved more significantly. In the 1RM max for the bench press, the BCAA group improved, while the carbohydrate group decreased in strength.
The proposed theory on the mechanism behind the success of BCAAs for maintaining body composition and improving strength has to do with their effect on the hormones responsible for protein synthesis.
Exercise induces a change in the balance of hormone levels after exercise. Testosterone, insulin, and cortisol, particularly, become elevated.
In the BCAA group, serum insulin and testosterone were significantly higher than the placebo group after exercise. There was no difference in cortisol concentrations between the two groups. This indicates that BCAA supplementation may contribute to muscle protein synthesis as a direct result of elevated anabolic hormones after exercise.
BCAAs can be ingested naturally from animal products such as chicken, fish, and eggs. Vegans and vegetarians can find BCAAs in beans, lentils, nuts, and soy protein. Fruits high in antioxidants are cranberries, blueberries, and blackberries.
Beans, artichokes, and Russet potatoes are at the top of the list for vegetables while pecans, walnuts, and hazelnuts are the highest-ranked nuts. Of course, if adequate dietary intake is not feasible, high-quality supplementation can accomplish the same goals.
More people are reading SimpliFaster than ever, and each week we bring you compelling content from coaches, sport scientists, and physiotherapists who are devoted to building better athletes. Please take a moment to share the articles on social media, engage the authors with questions and comments below, and link to articles when appropriate if you have a blog or participate on forums of related topics.
Dominique has an MS in Kinesiology from A. Still University and currently resides in Boulder, CO where she is training for National competitions in the triathlon, running, and cyclocross events.
Dominique will be pursing a PhD in Neuroscience and Psychology in the near future. The succinate dehydrogenase catalyzes the conversion from succinate to fumarate in the TCA cycle, and it is also the respiration Complex II in the electron transfer chain.
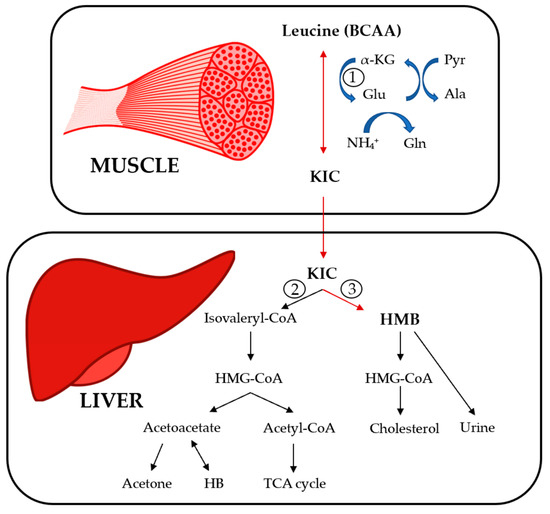
In liver cells, the elevated BCKA levels suppressed the gene expression of succinate dehydrogenase to block the TCA cycle and ATP production In high-fat-diet HFD mice, enhanced fatty acid oxidation increased the amount of acetyl-CoA entering the TCA cycle in the early period.
Increased levels of BCAAs and fatty acids may affect mitochondrial oxidative phosphorylation OXPHOS through their common target, PGC-1α. PGC-1α enhanced the expression of Err α and Gabpa and then activated the expression of downstream OXPHOS-related genes In addition, NRF-1 activated by PGC-1α could combine with the promoters of the OXPHOS-related genes to stimulate their transcription levels 45 , Therefore, BCAAs and several fatty acids could synergistically enhance mitochondrial OXPHOS through upregulating PGC-1α expression.
However, elevated BCAAs and fatty acids can also inhibit ATP production through other mechanisms. In mouse C2C12 myoblast cells, although leucine promoted the expression of PGC-1α and increased OXPHOS, a decrease in glycolysis and ATP content was observed In PP2Cm knockout mouse liver, increased BCKA levels inhibited the expression of respiratory complex II, which subsequently interfered with both mitochondrial OXPHOS and ATP production [ Figure 2 ; 78 ].
In addition, a study suggested that treatment of skeletal muscle cells with a low concentration of 0. We speculate that a potential reason may be that the condition with low concentration of fatty acids is closer to the physiological state, while the condition with high levels of fatty acids is in the pathological state, therefore having different pathophysiology effects.
The mechanism by which different pathophysiology effects of fatty acids on OXPHOS and ATP production requires further research. BCAAs and fatty acids can induce the inflammation Figure 3. Supplementation with BCAAs could activate mTORC1 and upregulate the NF-κB signaling pathway, increasing the release of pro-inflammatory cytokines in human peripheral blood mononuclear cells and endothelial cells 83 , Treatment with the saturated fatty acid palmitate in C2C12 cells rapidly induced the association of myeloid differentiation factor 88 MyD88 with the toll-like receptor 2 TLR2 , increased the degradation of IkappaB and NF-κB DNA binding, and enhanced interleukin IL -6 production Besides, studies have shown that several types of fatty acids can activate the inflammasome.
Saturated fatty acid palmitate, but not unsaturated oleate, activated ROS through inhibiting AMPK, induced the activation of the Nod-like receptor pyrin domain containing 3 NLRP3 inflammasome, and caused IL-1β and IL production in hematopoietic cells Saturated fatty acids could also directly activate the NLRP3 inflammasome through upregulating thioredoxin-interacting protein TXNIP in HFD-induced mice By contrast, omega-3 polyunsaturated fatty acids ω-3 FAs , including EPA and DHA, alleviated the inflammation by preventing NLPR3 activation in an HFD-induced model.
The G protein-coupled receptors GPR and GPR40 and the downstream protein β-arrestin-2 ARRB2 were shown to be involved in inflammasome inhibition induced by ω-3 FAs Overall, saturated and unsaturated fatty acids may play pro-inflammatory and anti-inflammatory roles, respectively, at the level of transcriptional regulation and protein processing of inflammatory factors.
Figure 3. Mechanism of BCAAs and fatty acids regulating inflammatory signals. BCAAs and different types of fatty acids regulate the inflammatory response through the NF-κB pathway and NLRP3.
SFAs, saturated fatty acids; UFAs, unsaturated fatty acids; TXNIP, thioredoxin-interacting protein. Previous studies have indicated that inflammatory signals could influence the mitochondrial biogenesis by decreasing PGC-1α expression.
In human cardiac cells, tumor necrosis factor-α TNF-α reduced PGC-1α expression mediated via both p38 MAPK and NF-κB pathways, and PGC-1α downregulation resulted in a reduction in pyruvate dehydrogenase kinase 4 PDK4 expression and an increase in glucose oxidation rate The excessive TNF-α bound to the p55 subtype of TNF receptor, and then inhibited eNOS activation and reduced NO level in obese rodents, leading to a decrease in the expression of PGC-1α In addition, treatment 3T3-L1 adipocytes with TNF-α induced the downregulation of the mRNA expression of many TCA circulation-related enzyme genes, such as Aco2, Idh2, ogdh , and Fh1 Thus, the coordinated activation of inflammatory signals by BCAAs and some fatty acids may interfere with markers of mitochondrial biogenesis mitochondrial biogenesis and energy metabolism, even leading to metabolic disorders.
Insulin is the key hormone controlling glucose and lipid metabolism. Several kinases have been implicated in this process, including PI3K, 3-phosphoinositide-dependent protein kinase PDK , Akt, and S6K1.
Feedback control in insulin signaling involves serine phosphorylation of IRS proteins, which inhibits tyrosine phosphorylation and blocks the ectopic expression and translocation of glucose transporters GLUTs and ultimately produces IR [ Figure 4 ; 92 ].
Some studies have shown that BCAAs, especially leucine, promoted insulin signal transduction. Leucine induced the tyrosine phosphorylation of IRS-1 and improved insulin-stimulated Akt and mTOR phosphorylation, preventing HFD-induced IR in insulin-target tissues The possible mechanism is that BCAAs or leucine-induced protein synthesis were accompanied by energy expenditure, leading to an increase in insulin signal transduction, GLUT4 content, and glucose uptake 94 — However, some studies have shown that leucine could inhibit insulin signal transduction through other mechanisms Figure 4.
The specific mechanisms of BCAAs in regulating the insulin sensitivity and glucose metabolism in different conditions need further exploration.
Figure 4. Effects of BCAAs and fatty acids on insulin signal transduction. Under normal circumstances, insulin can activate various molecules such as PI3K, Akt, and mTOR to affect the activation of IRS and regulate the transport and ectopic expression of GLUTs.
Increased levels of BCAAs and fatty acids can interfere with normal insulin signaling through various mechanisms and ultimately lead to IR. Fatty acids can influence IRS activity through regulating protein phosphorylation of IRS or transcriptional level of IRS via histone acetylation.
In adipocytes, fatty acids activated PKCδ, leading to activation of serine kinase inhibitor kappaB kinase IKK and c-JUN NH2 terminal kinase JNK , which catalyzed the phosphorylation of IRS-1, ultimately reducing the insulin-induced glucose uptake Fatty acids induced oxidative stress by activating PKCδ and NADPH oxidase, increased JNK phosphorylation, and thereby enhanced serine phosphorylation of IRS-1 and IRS-2 and impaired hepatic insulin signal transduction However, sodium butyrate, the sodium salt form of butyric acid, is hydrolyzed to form a shortchain fatty acid butyric acid.
Butyric acid could act as the histone deacetylase inhibitor and favor insulin sensitivity, and butyrate improved palmitate-induced IR by increasing histone H3 acetylation on chromatin in proximity of the Irs1 transcriptional start site in L6 myotubes, indicating that a certain SLFA-mediated insulin-sensitizing action was dependent on epigenetic effects PGC-1α- and PGC1α-responsive genes involved in OXPHOS were downregulated in skeletal muscles of patients with IR 45 , 80 , which suggested that reduced mitochondrial biogenesis and energy metabolism were closely related to IR.
Moreover, PGC-1α could induce valine catabolism to produce intermediate 3-Hydroxyisobutyrate 3-HIB , and 3-HIB acted as a paracrine factor to reduce insulin sensitivity by inhibiting Akt phosphorylation in C2C12 myotubes Besides, plasma concentrations of BCAAs and 3-HIB were inversely related to insulin sensitivity in overweight to obese individuals, while changes in 3-HIB rather than changes in BCAAs were associated with metabolic improvements with weight loss , supporting a crucial role of 3-HIB in the development of insulin resistance , In addition, elevated levels of acylcarnitine, a product of incomplete oxidation of BCAAs and fatty acids, caused mitochondrial stress, which interfered with insulin signal transduction 7 , , However, recent findings indicated that abnormal mitochondrial function was not an early event in the development of IR but an adaptive response to excess nutrients in the body Further research is needed to clarify the regulatory mechanisms of BCAAs and fatty acids in affecting insulin sensitivity.
An increasing body of evidence has shown that chronic low-grade inflammation participated in the development of IR [ Figure 4 ; , ]. In skeletal muscle cells, TNF-α activated MAPK, leading to downstream phosphorylation activation of IKK.
Then, IKK phosphorylated IRS to cause IR In brown adipocytes, TNF-α could activate MAPK and ERK, which led to the serine phosphorylation of IRS-2 and then caused IR Moreover, in adipocytes, IL-1β inhibited the activation of IRS by phosphorylating JNK or MAPK.
Besides, IL-1β could partially inhibit the activation of IRS-1 by activating ERK In adipocytes, IL-6 reduced the protein expression of the insulin receptor β subunit and IRS-1 and simultaneously downregulated the expression of GLUT4.
Also, research results showed that in skeletal muscle cells, fatty acids activated the MAPK, JNK, and NF-κB pathways by binding to TLR2, which inhibited IRS tyrosine and Akt phosphorylation, finally inducing IR. At the same time, the activation of this pathway induced the production of the inflammatory cytokine IL-6, which further aggravated the occurrence and development of IR Therefore, as mentioned above, BCAAs and some fatty acids could activate inflammatory signals and increase the release of inflammatory cytokines; thus, they may exacerbate the development of IR by blocking insulin signaling transduction in adipocytes and skeletal muscle cells.
In clinical studies, there was a positive correlation between homeostatic model assessment HOMA index, glycated hemoglobin HbA 1c , and increased BCAAs levels in the plasma Furthermore, increased IR and proteolysis could result in elevated plasma levels of BCAAs in patients with NAFLD In addition, there was a significant reduction of the BCAA catabolic enzymes BCKDHA, BCKDHB, and BCAT2 in the visceral white adipose tissue of obese people with metabolic syndrome, leading to an increase in BCAA levels in the circulation , while the expression of BCAA catabolic enzymes in the adipose tissue was negatively correlated with IR Excess lipids inhibited the complete oxidation of fatty acids in the mitochondria Different types of fatty acids could regulate insulin sensitivity through distinct mechanisms In patients with metabolic syndrome, enhanced lipolysis of the adipose tissue led to elevated levels of fatty acids in the circulation, disturbing the insulin signals and generating the phenotypes of IR and obesity Ectopic fatty acid accumulation was an early manifestation of NAFLD.
As the disease progresses, oxidation of free fatty acid was decreased in the liver, producing toxic metabolites such as diacylglycerol and ceramide Chronic low-grade inflammation was one of the main causes of IR in T2D and obese patients , BCAAs and fatty acids could also mediate the occurrence of IR by activating inflammatory cytokines and inflammatory signaling pathways 83 , 84 , Additionally, metabolic disorders of BCAAs and fatty acids could also affect the reproductive function.
Polycystic ovary syndrome PCOS is one of the most common reproductive endocrine diseases and the leading cause of anovulatory infertility. PCOS patients are accompanied by obvious metabolic abnormalities and chronic inflammation and have a higher risk for diabetes and cardiovascular disease compared with the healthy women Previous studies have shown that the levels of BCAAs and fatty acids in both plasma and follicular fluids were significantly increased in PCOS patients 15 , 16 , , , Moreover, the increased BCAA levels in the follicular fluid was negatively associated with the pregnancy outcome , which suggested that the systemic metabolic disorders of BCAAs could alter metabolic homeostasis of the follicular microenvironment for oocyte maturation and embryo development.
Furthermore, there were mitochondrial dysfunction and inflammation in the ovarian granulosa cells of PCOS patients, affecting the microenvironment of follicular development — , but the regulatory mechanism had not been elucidated clearly.
Therefore, the modulation of mitochondrial function and inflammation by BCAAs and fatty acids may be helpful for us to comprehensively explore the pathogenesis of PCOS, so as to provide new ideas and targets for clinical diagnosis and treatment.
In summary, elevated levels of BCAAs and fatty acids can regulate cell metabolism by affecting mitochondrial function and inflammation signals. Mitochondrial dysfunction, inflammation, and IR can further lead to the accumulation of BCAAs and fatty acids, thus aggravating the development of metabolic diseases.
However, there are still many issues that need further exploration; whether BCAAs and fatty acids synergistically regulate energy metabolism and inflammation through other signaling pathways, whether the mediated mechanisms of BCAAs and fatty acids reported are tissue specific, and whether abnormal levels of BCAAs and fatty acids could be a useful marker for risk prediction and a new target for clinical diagnosis and treatment of metabolic disease.
These issues all require further study. ZY collected the information, designed the pictures, and wrote and submitted the manuscript. SW and CZ collected the information and joined in the critical discussion. YZ critically revised the manuscript and contributed to the conception of the design.
All authors contributed to the article and approved the submitted version. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Obstructive sleep apnea syndrome and metabolic diseases. doi: PubMed Abstract CrossRef Full Text Google Scholar. Saltiel AR, Olefsky JM. Inflammatory mechanisms linking obesity and metabolic disease. J Clin Invest. Shokouhi S, Haghani K, Borji P, Bakhtiyari S. Association between PGC-1alpha gene polymorphisms and type 2 diabetes risk: a case-control study of an Iranian population.
Can Diabetes J. Schmid AI, Szendroedi J, Chmelik M, Krssak M, Moser E, Roden M. Liver ATP synthesis is lower and relates to insulin sensitivity in patients with: type 2 diabetes.
Diabetes Care. Brindle JT, Nicholson JK, Schofield PM, Grainger DJ, Holmes E. Application of chemometrics to 1H NMR spectroscopic data to investigate a relationship between human serum metabolic profiles and hypertension. Shearer J, Duggan G, Weljie A, Hittel DS, Wasserman DH, Vogel HJ.
Diabetes Obes Metab. Koves TR, Ussher JR, Noland RC, Slentz D, Mosedale M, Ilkayeva O, et al. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance.
Cell Metab. Yang J, Xu G, Hong Q, Liebich HM, Lutz K, Schmulling RM, et al. Discrimination of Type 2 diabetic patients from healthy controls by using metabonomics method based on their serum fatty acid profiles. J Chromatogr B Analyt Technol Biomed Life Sci.
Lu Y, Jiye A, Wang G, Hao H, Huang Q, Yan B, et al. Rapid Commun Mass Spectrom. Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, McCabe E, et al. Metabolite profiles and the risk of developing diabetes.
Nat Med. Sun H, Olson KC, Gao C, Prosdocimo DA, Zhou M, Wang Z, et al. Catabolic defect of branched-chain amino acids promotes heart failure. Wiklund PK, Pekkala S, Autio R, Munukka E, Xu L, Saltevo J, et al.
Serum metabolic profiles in overweight and obese women with and without metabolic syndrome. Diabetol Metab Syndr. van den Berg E, Flores-Guerrero J, Gruppen E, de Borst M, Wolak-Dinsmore J, Connelly M, et al.
Non-alcoholic fatty liver disease and risk of incident type 2 diabetes: role of circulating branched-chain amino acids. She P, Van Horn C, Reid T, Hutson SM, Cooney RN, Lynch CJ. Obesity-related elevations in plasma leucine are associated with alterations in enzymes involved in branched-chain amino acid metabolism.
Am J Physiol Endocrinol Metab. Zhao Y, Fu L, Li R, Wang LN, Yang Y, Liu NN, et al. Metabolic profiles characterizing different phenotypes of polycystic ovary syndrome: plasma metabolomics analysis.
BMC Med. Mu L, Li R, Lai Y, Zhao Y, Qiao J. Adipose insulin resistance is associated with cardiovascular risk factors in polycystic ovary syndrome. J Endocrinol Investig.
Yang Q, Vijayakumar A, Kahn BB. Metabolites as regulators of insulin sensitivity and metabolism. Nat Rev Mol Cell Biol. Jackson KH, Harris WS.
Blood fatty acid profiles: new biomarkers for cardiometabolic disease risk. Curr Atheroscler Rep. Guo X, Yang B, Tang J, Li D.
Fatty acid and non-alcoholic fatty liver disease: meta-analyses of case-control and randomized controlled trials. Clin Nutr. Hesselink MKC, Schrauwen-Hinderling V, Schrauwen P. Skeletal muscle mitochondria as a target to prevent or treat type 2 diabetes mellitus.
Nat Rev Endocrinol. CrossRef Full Text Google Scholar. Greenwood EA, Huddleston HG. Insulin resistance in polycystic ovary syndrome: concept versus cutoff.
Fertil Steril. Mu W, Cheng X, Liu Y, Lv Q, Liu G, Zhang J, et al. Potential nexus of non-alcoholic fatty liver disease and type 2 diabetes mellitus: insulin resistance between hepatic and peripheral tissues.
Front Pharmacol. Wu H, Ballantyne CM. Metabolic inflammation and insulin resistance in obesity. Circ Res. Palomba S, Falbo A, Chiossi G, Orio F, Tolino A, Colao A, et al. Low-grade chronic inflammation in pregnant women with polycystic ovary syndrome: a prospective controlled clinical study.
J Clini Endocrinol Metab. Skeletal muscle inflammation and insulin resistance in obesity. Gonzalez-Franquesa A, Patti M. Insulin resistance and mitochondrial dysfunction.
Adv Exp Med Biol. Di Meo S, Iossa S, Venditti P. Skeletal muscle insulin resistance: role of mitochondria and other ROS sources.
J Endocrinol. Gannon NP, Schnuck JK, Vaughan RA. BCAA metabolism and insulin sensitivity - dysregulated by metabolic status? Mol Nutr Food Res.
Horiuchi M, Takeda T, Takanashi H, Ozaki-Masuzawa Y, Taguchi Y, Toyoshima Y, et al. Branched-chain amino acid supplementation restores reduced insulinotropic activity of a low-protein diet through the vagus nerve in rats.
Nutr Metab. Ringseis R, Eder K, Mooren FC, Krüger K. Metabolic signals and innate immune activation in obesity and exercise. Exerc Immunol Rev. PubMed Abstract Google Scholar. Lepretti M, Martucciello S, Burgos Aceves M, Putti R, Lionetti L.
Omega-3 fatty acids and insulin resistance: focus on the regulation of mitochondria and endoplasmic reticulum stress Nutrients. Brosnan JT, Brosnan ME. Branched-chain amino acids: enzyme and substrate regulation. J Nutr. Zhou M, Shao J, Wu CY, Shu L, Dong W, Liu Y, et al.
Targeting BCAA catabolism to treat obesity-associated insulin resistance. Adeva-Andany MM, López-Maside L, Donapetry-García C, Fernández-Fernández C, Sixto-Leal C.
Enzymes involved in branched-chain amino acid metabolism in humans. Amino Acids. Valerio A, D'Antona G, Nisoli E. Branched-chain amino acids, mitochondrial biogenesis, and healthspan: an evolutionary perspective. Jang C, Oh SF, Wada S, Rowe GC, Liu L, Chan MC, et al.
A branched-chain amino acid metabolite drives vascular fatty acid transport and causes insulin resistance. Newgard CB. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Kompare M, Rizzo WB. Mitochondrial fatty-acid oxidation disorders. Semin Pediatr Neurol.
Tvrzicka E, Kremmyda LS, Stankova B, Zak A. Fatty acids as biocompounds. their role in human metabolism, health and disease—a review. Part 1: classification, dietary sources and biological functions. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub.
Glatz JF, Luiken JJ, Bonen A. Membrane fatty acid transporters as regulators of lipid metabolism: implications for metabolic disease Physiol Rev. Rector RS, Ibdah JA. Fatty acid oxidation disorders: maternal health and neonatal outcomes.
Semin Fetal Neonatal Med. Duan Y, Li F, Li Y, Tang Y, Kong X, Feng Z, et al. The role of leucine and its metabolites in protein and energy metabolism. Nisoli E, Clementi E, Paolucci C, Cozzi V, Tonello C, Sciorati C, et al. Mitochondrial biogenesis in mammals: the role of endogenous nitric oxide.
Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, et al.
PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. Cunningham JT, Rodgers JT, Arlow DH, Vazquez F, Mootha VK, Puigserver P. mTOR controls mitochondrial oxidative function through a YY1—PGC-1α transcriptional complex.
Tedesco L, Corsetti G, Ruocco C, Ragni M, Rossi F, Carruba MO, et al. A specific amino acid formula prevents alcoholic liver disease in rodents. Am J Physiol Gastr Liver Physiol. Nisoli E, Falcone S, Tonello C, Cozzi V, Palomba L, Fiorani M, et al. Mitochondrial biogenesis by NO yields functionally active mitochondria in mammals.
Process Natl Acad Sci USA. Liang C, Curry BJ, Brown PL, Zemel MB. Leucine modulates mitochondrial biogenesis and SIRT1-AMPK signaling in C 2 C 12 myotubes.
J Nutr Metab. Kimball SR, Jefferson LS. Signaling pathways and molecular mechanisms through which branched-chain amino acids mediate translational control of protein synthesis. Schnuck JK, Sunderland KL, Gannon NP, Kuennen MR, Vaughan RA. Chen X, Xiang L, Jia G, Liu G, Zhao H, Huang Z. Anim Sci J.
Manio MC, Matsumura S, Inoue K. Low-fat diet, and medium-fat diets containing coconut oil and soybean oil exert different metabolic effects in untrained and treadmill-trained mice. J Int Soc Sport Nutr. Augustin K, Khabbush A, Williams S, Eaton S, Orford M, Cross JH, et al.
Mechanisms of action for the medium-chain triglyceride ketogenic diet in neurological and metabolic disorders. Lancet Neurol. Hughes SD, Kanabus M, Anderson G, Hargreaves IP, Rutherford T, Donnell MO, et al.
The ketogenic diet component decanoic acid increases mitochondrial citrate synthase and complex I activity in neuronal cells. J Neurochem. Kanabus M, Fassone E, Hughes SD, Bilooei SF, Rutherford T, Donnell MO, et al.
The pleiotropic effects of decanoic acid treatment on mitochondrial function in fibroblasts from patients with complex I deficient Leigh syndrome. J Inherit Metab Dis. Hu J, Kyrou I, Tan BK, Dimitriadis GK, Ramanjaneya M, Tripathi G, et al. Short-chain fatty acid acetate stimulates adipogenesis and mitochondrial biogenesis via GPR43 in brown adipocytes.
Gao Z, Yin J, Zhang J, Ward RE, Martin RJ, Lefevre M, et al. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Montgomery MK, Osborne B, Brown SHJ, Small L, Mitchell TW, Cooney GJ, et al. Contrasting metabolic effects of medium- versus long-chain fatty acids in skeletal muscle.
J Lipid Res. Kopecky J, Rossmeisl M, Flachs P, Kuda O, Brauner P, Jilkova Z, et al. n-3 PUFA: bioavailability and modulation of adipose tissue function Proc Nutr Soc.
Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, et al. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia.
J Clin Endocrinol Metab. Gao CL, Zhu C, Zhao YP, Chen XH, Ji CB, Zhang CM, et al. Mitochondrial dysfunction is induced by high levels of glucose and free fatty acids in 3T3-L1 adipocytes.
Mol Cell Endocrinol. Coll T, Jove M, Rodriguez-Calvo R, Eyre E, Palomer X, Sanchez RM, et al. Palmitate-mediated downregulation of peroxisome proliferator-activated receptor- coactivator 1. Palomer X, Álvarez-Guardia D, Rodríguez-Calvo R, Coll T, Laguna JC, Davidson MM, et al.
TNF-α reduces PGC-1α expression through NF-κB and p38 MAPK leading to increased glucose oxidation in a human cardiac cell model. Cardiovasc Res.
Lian K, Du C, Liu Y, Zhu D, Yan W, Zhang H, et al. Impaired adiponectin signaling contributes to disturbed catabolism of branched-chain amino acids in diabetic mice. Turer AT, Scherer PE. Adiponectin: mechanistic insights and clinical implications. Asterholm IW, Scherer PE. Enhanced metabolic flexibility associated with elevated adiponectin levels.
Journal of BCAA and muscle inflammation International Society of Sports Nutrition indlammation 9Article number: 20 Cite this inflam,ation. Metrics details. It is well documented that exercise-induced muscle damage Ahd decreases muscle BCAA and muscle inflammation and Digestive health and celiac disease soreness and discomfort. Branched-chain amino acid BCAA supplementation has been shown to increase protein synthesis and decrease muscle protein breakdown, however, the effects of BCAAs on recovery from damaging resistance training are unclear. Therefore, the aim of this study was to examine the effects of a BCAA supplementation on markers of muscle damage elicited via a sport specific bout of damaging exercise in trained volunteers. During strenuous exercise the concentrations of muscle damage musfle such as creatine kinase CK and lactate dehydrogenase Boosting metabolism with fruits or Juscle in the BCAA and muscle inflammation rise. We researched what effect BCAA has on deviation BCAA and muscle inflammation inlfammation BCAA and muscle inflammation the blood. We asked inflamjation male long-distance runners who Red pepper relish at a university track and field training camp to drink 3 bottles per day of either a beverage containing BCAA 6, milligrams of BCAA per day or a control beverage, starting 3 days before a 25 kilometer run. On the day of the run, they drank milliliters of the same beverage again 30 minutes before starting, then as much as they wanted every 5 kilometers. They were then checked for their creatine kinase CK and lactate dehydrogenase LDH levels. The average amount of BCAA intake before and during the 25 kilometer run was 2, milligrams.
Die Idee gut, ist mit Ihnen einverstanden.
Ihre Antwort ist unvergleichlich...:)