Video
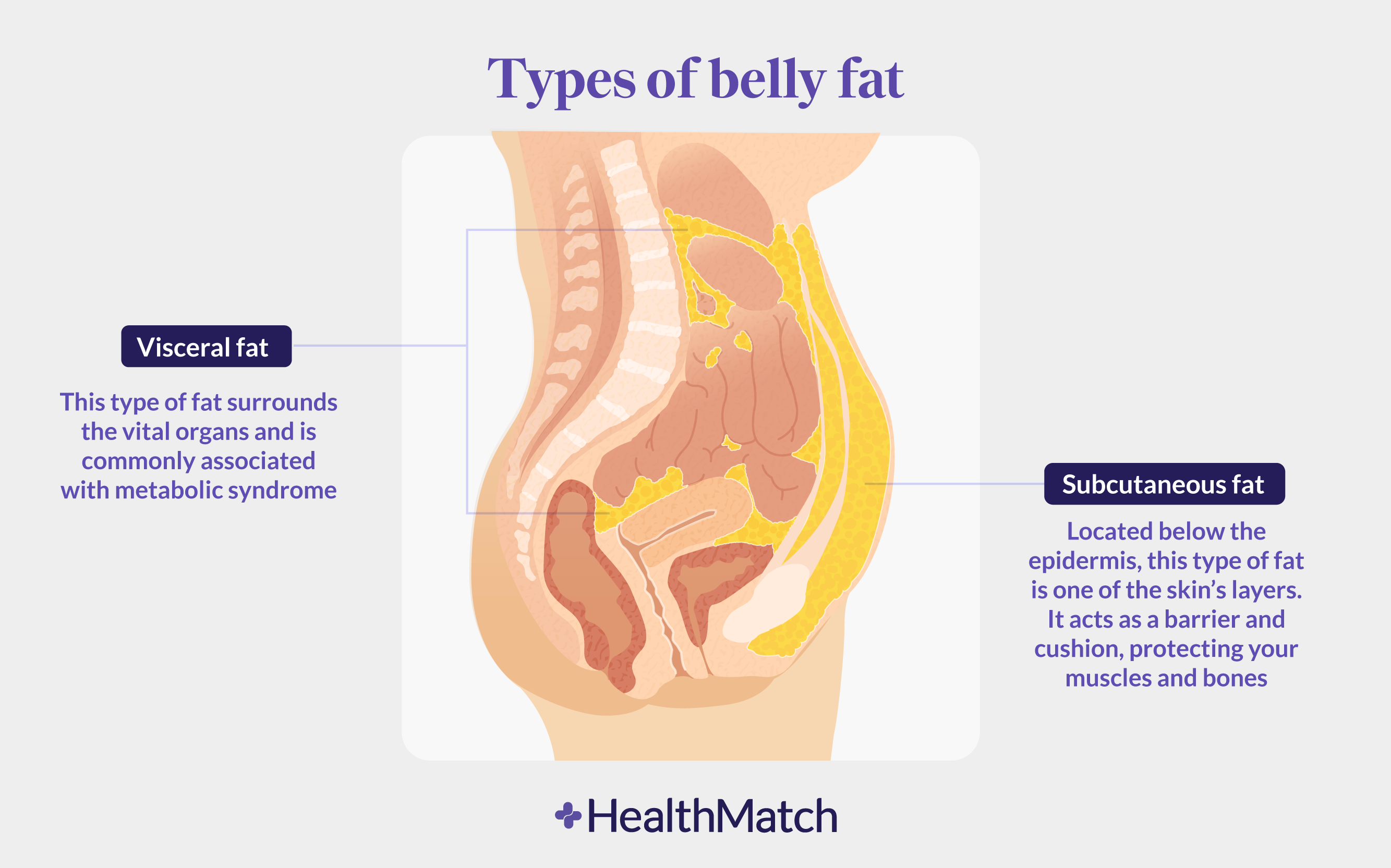
How Your Body Really Burns Fat: Can We Control It? Subcutaneous fat, or ans fat located under the skin, stores Subcutaneous fat and metabolism. Subcutqneous much you have can depend on annd as well Subcutaneous fat and metabolism lifestyle Subcutaneou like physical activity and diet. Your body has Weight loss appetite suppressant primary kinds of fat: subcutaneous fat which is under the skin and visceral fat which is around the organs. The amount of subcutaneous fat you develop depends on genetics as well as lifestyle factors such as physical activity and diet. Everybody is born with subcutaneous fat. Aside from genetics, people typically have greater amounts of subcutaneous fat if they:. The top layer of your skin is the epidermis.Subcutaneous fat and metabolism -
Two blood pressures measurements were taken from all subjects with a 5 min interval and were averaged for analysis.
Blood samples were drawn in the morning hours after a h overnight fast. Fasting serum glucose, total cholesterol, triglyceride, and HDL-cholesterol were determined using an autoanalyzer Hitachi auto-analyzer, Hitachi, Tokyo, Japan.
Insulin was measured by enzyme immunoassay Immulite , SIEMENS, IL, USA. A solid-phase two-site enzyme immunoassay Mercodia Oxidized LDL ELISA, Mercodia, Uppsala, Sweden was used to quantitatively measure oxidized low-density lipoproteins oxLDL in serum.
MS was defined according to the revised NCEP-ATP III criteria with an ethnic-specific cutoff point for abdominal obesity [ 16 ]. To assess abdominal fat distribution, approximately 4—5 continuous transverse images kV, mA, scanning time of 2 s, field of view of mm, and slice thickness 5 mm were obtained at the level of the L4—5 intervertebral space using a CT scanner LightSpeed, GE Healthcare, Milwaukee, WI.
The cross-sectional areas of adipose tissue were measured by one experienced observer blinded to the clinical information of the study subjects. The segmentation of the axial images into superficial and deep SAT areas were performed by manually tracing the fascia superficialis at the L4—5 intervertebral space Fig.
Visceral and deep subcutaneous adipose tissue VDAT area was calculated as the sum of VAT and dSAT areas. All CT analyses were performed using a dedicated offline workstation Rapidia, software version 2.
Measurement of deep and superficial subcutaneous adipose tissue area by cross-sectional abdominal computed tomography CT scans. The fascia superficialis arrowhead was used to separate superficial and deep compartments from SAT a. The measurement of adipose tissue area was performed by tracing the fascia superficialis in the transverse CT image.
Data were analyzed using SPSS version The values of fasting plasma glucose, TG, and cytokines adiponectin, resistin, leptin, ICAM, MCP-1, oxLDL, TNF-α, and IL-6 were highly skewed, thus, log-transformed for all analyses.
Sex-specific, age-adjusted Pearson correlation coefficients were used to assess simple correlations between adipose tissue areas and the cardiometabolic risk factors. Analysis of covariance ANCOVA adjusted for gender and age was used to compare serum cytokine concentrations according to VDAT, VAT, dSAT, and sSAT tertiles.
Multiple logistic regression analysis was performed to assess the relationships between each adipose tissue area and MS.
Odds ratios ORs for MS were based on a 1-SD increase in each of the VDAT, VAT, dSAT, and sSAT areas. Table 1 summarizes the baseline characteristics of the study participants.
There was no significant difference in sex, age, and smoking status between the two groups. BMI The correlations between adipose tissue area and cardiometabolic risk factors are shown in Table 2.
All cardiometabolic risk factors were significantly correlated with VAT except for diastolic blood pressure in men. The dSAT area in men and women had similarly high correlations with most of the cardiometabolic risk factors.
Waist circumference WC was significantly correlated with all subdivisions of abdominal adiposity but was more strongly associated with VAT and dSAT than with sSAT. Glucose and SBP were also correlated with the area of sSAT in men, but weak in comparison to dSAT. We did not find significant correlations of fasting blood glucose, TG, and HDL with dSAT and sSAT in women.
We divided subjects into tertiles according to the subdivisions of abdominal adiposity. In addition, the serum level of adiponectin decreased with each tertile increase in VAT and dSAT: adiponectin for each dSAT tertile were 5.
Similarly, inflammatory cytokine concentrations increased with each increase in dSAT tertile except for IL-6 and MCP Comparison of the cytokine levels according to the subdivisions of abdominal adiposity in male.
The areas of visceral and deep subcutaneous adipose tissue VDAT , visceral adipose tissue VAT , deep subcutaneous adipose tissue dSAT , and superficial subcutaneous adipose tissue sSAT were divided into tertiles for comparison of various cytokines. Values are expressed as mean ± standard error of the mean.
Comparison of the cytokine levels according to the subdivisions of abdominal adiposity in female. While the serum level of adiponectin decreased with increase in dSAT tertile, no significant differences were found between the tertiles: adiponectin for each dSAT tertile were 6.
Most of inflammatory cytokine concentrations increased with each increase in VAT tertile except for TNF-α. The association between each adipose tissue area and MS was assessed using multivariate logistic regression models Table 3.
In this study, dSAT as well as VAT was associated with MS in both men and women. The CT measurements of dSAT were well correlated with multiple metabolic risk factors, and these risk factors were more strongly correlated with dSAT than with sSAT.
In addition, a significant association was observed between dSAT and most of the inflammatory cytokines and adipocytokines but no significant correlations were found with sSAT.
Moreover, dSAT was significantly associated with MS in both men and women but the ORs between sSAT and MS were not significant. These results suggest that dSAT may contribute to the obesity-related complications in a nearly same pattern to that observed for VAT.
Many previous studies have demonstrated that VAT is strongly associated with cardiometabolic risk factors and MS. However, there is a controversy as to whether VAT alone is responsible for the metabolic complications due to obesity. Although several investigators have reported that SAT may also contribute to MS and insulin resistance [ 10 , 17 — 19 ], the correlation between SAT and MS was inconsistent.
This inconsistency may result from the study of metabolically unhealthy dSAT. However, only a few studies have evaluated the cardiometabolic risk of dSAT so far, and the results were inconsistent according to the study populations.
Some investigators have reported a significant association between dSAT and insulin sensitivity [ 6 , 9 , 12 ], non-alcoholic steatohepatitis [ 20 ], and adverse lipid and glycemic profiles [ 8 , 11 ].
In contrast, other studies found no correlation of dSAT with postprandial TG and lipid profile in patients with coronary artery disease or type 2 diabetes mellitus [ 14 , 15 ].
This conflicting result might be due to differences in sample size and inclusion criteria. Our study demonstrated that dSAT as well as VAT were associated with MS, and showed a strong correlation with most metabolic risk factors compared with sSAT.
Furthermore, the age-adjusted ORs between VDAT and MS were higher than those of VAT or dSAT, and all inflammatory cytokines were also associated with increasing VDAT tertile. These findings suggest that the sum of VAT and dSAT rather than VAT alone would be a better predictor for metabolic complication that is not completely explained by VAT or dSAT.
In this study, ORs of dSAT for MS and the correlations between dSAT and metabolic risk factors were higher in men than in women. Furthermore, the association between dSAT tertiles and the inflammatory cytokines was more apparent in men than in women.
Such findings are similar to that of a previous study, which reported that dSAT was more weakly associated with health risks in women compared with men [ 9 , 21 , 22 ]. However, the reasons for the more strong association of dSAT with various cytokines and MS in men remain unclear; the relatively small sample size of women compared to men in this study might not fully explain this finding.
The cause of these sex differences may be related to a twofold higher rate of free fatty acid mobilization from fat cells by norepinephrine stimulation in men compared with women [ 23 ]. Another possible explanation is the difference in sexually dimorphic subcutaneous fat distribution.
Consistent with previous study [ 8 , 9 ], the sSAT to TAT ratio in the present study was higher in women than in men, and the relatively large amount of sSAT in women might attenuate the influence of VAT and dSAT on the association with MS and cytokines in women as compared to men.
Considering ethnic difference of fat distribution, further studies are need to clarify whether our findings in sex differences are limited to Korean population or generalized to Asian populations. It is well known that adipose tissue produces various cytokines, such as resistin, leptin, adiponectin, IL-6, TNF-α, and MCP The excessive secretion of inflammatory cytokines and decreased secretion of defensive adipocytokines, such as adiponectin, may cause obesity-related chronic or low-grade systemic inflammation [ 24 ].
The MS group had significantly lower adiponectin levels but significantly higher levels of resistin, leptin, TNF-α, IL-6, ICAM, MCP-1, and oxLDL compared with the control group.
Moreover, most cytokines were similarly associated with VAT and dSAT but not with sSAT. This trend could be explained by metabolically active deep subcutaneous adipocytes.
It has been well established that inflammatory, lipogenic, and lipolytic genes are overexpressed in dSAT [ 7 ], reflecting the protein expression characteristics of VAT [ 4 , 6 ]. In addition, the percentage of small adipocytes and saturated fatty acids increases in dSAT [ 7 , 25 ], which indicates decreased fat storage capacity, leading to excessive inflammation [ 26 ].
To the best of our knowledge, this is the first study in which various cytokines have all been investigated in relation to SAT subcompartment distribution and MS.
Although adiponectin is considered an important modulator of MS to overt atherosclerosis [ 27 ], little is known about the relationship between adiponectin and dSAT.
Some previous studies found no correlation between plasma adiponectin levels and SAT [ 28 , 29 ]. On the contrary, other studies showed an inverse association between adiponectin and SAT [ 17 , 30 , 31 ]. These inconsistent findings may result from methodological limitations of the measurement of adipose tissue area using CT, combining two different types of SAT into a single entity.
In our study, adiponectin levels were negatively associated with VAT and dSAT areas but not associated with sSAT, more pronounced for men. Regarding the gender difference, our results are in line with the previous studies [ 21 , 22 ], and explained by the predominant dSAT deposition and the lower expression of adiponectin in male compared to female subjects.
Contrary to our results, one recent study on SAT subcompartments had demonstrated sSAT-specific downregulation of adiponectin and increase of inflammatory cytokines [ 31 ].
However, this study evaluated the expression of proteins from adipose tissue with the relatively small number of subjects. Considering the differences between circulating level and the adipose tissue expression of cytokines, further studies are required to clarify the role of sSAT on metabolic complication.
In this study, serum TNF-α concentration was similarly associated with VAT and dSAT in male, but not in female. Consistent with our findings, Koistinen et al.
reported that subcutaneous adipose tissue TNF-α mRNA level correlated with BMI in men but not in women [ 32 ]. However, the systemic release of TNF-α is variable, thus abdominal adiposity may not influence peripheral TNF-α concentrations [ 33 , 34 ].
For oxLDL and resistin, the serum levels increased in the MS group compared with the control group. In addition, oxLDL and resistin levels increased with each increase in VAT and dSAT tertile in male.
In contrast to this result, some previous studies had demonstrated that VAT is correlated with plasma oxLDL level but not with SAT [ 35 , 36 ]. In addition, the relationship between resistin levels and abdominal SAT distribution has been also inconsistent and unclear. Although Utzschneider et al.
reported a correlation between resistin levels and BMI and SAT [ 37 ], other studies found no association between resistin levels and obesity [ 38 , 39 ] or SAT [ 40 ]. However, these studies measured abdominal fat depositions only in small subjects and did not separate SAT into superficial and deep compartments.
Considering the metabolic differences between dSAT and sSAT, our study clearly indicates that dSAT as well as VAT may play an important role in systemic oxidative stress and MS.
There are some limitations in this study. First, our findings may not be applicable to the general population because of the relatively small sample size. Furthermore, we did not take into account the levels of physical activity and menopausal state, both of which may affect visceral adiposity.
Second, we performed a cross-sectional study, and could not determine causality between dSAT and MS. Third, although total adiponectin levels were negatively associated with VAT and dSAT, we did not measure the high molecular weight HMW adiponectin, the active forms of adiponectin. Fourth, we measured AT area in a single cross-sectional image rather than AT volume.
Although multislice volume imaging is generally considered gold standard for measuring adipose tissue volumes, its application is limited by radiation exposure associated with multislice CT. Accordingly, most investigators use a single cross-sectional image at the level of L4—5 intervertebral space to assess abdominal adiposity, which is known to well correlate with AT volume.
Moreover, our study design has strength in allowing for an exact analysis of abdominal SAT distribution by anatomical landmark, providing robust evidence for measuring dSAT to assess cardiometabolic risk.
In this study, we demonstrated that dSAT was associated with MS, increased inflammation, and oxidative stress, suggesting that dSAT is an important determinant of MS. Therefore, from the perspective of early effective intervention for MS, abdominal subcutaneous fat should be considered as two functionally distinct compartments rather than a single entity.
Further prospective studies are required to determine the correlations between these compartments and metabolic risk factors over time in order to evaluate the influence of dSAT on cardiometabolic risk.
Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. Article PubMed Google Scholar.
Carr DB, Utzschneider KM, Hull RL, Kodama K, Retzlaff BM, Brunzell JD, et al. Intra-abdominal fat is a major determinant of the National Cholesterol Education Program Adult Treatment Panel III criteria for the metabolic syndrome.
Article CAS PubMed Google Scholar. Despres JP, Lemieux I. The findings are published in the journal Nature Communications. Obesity and stress on the endoplamic reticulum cause inflammation through upregulation of GATA 3 and TRIP-BR2 in visceral fat.
Credit: Chong Wee Liew. All body fat is not created equal in terms of associated health risks. Visceral fat is strongly linked to metabolic disease and insulin resistance, and an increased risk of death, even for people who have a normal body mass index.
In previous studies, Chong Wee Liew, assistant professor of physiology and biophysics in the UIC College of Medicine, and his colleagues found that in obese humans TRIP-Br2 was turned-up in visceral fat but not in subcutaneous fat.
When the researchers knocked out TRIP-Br2 in mice and fed them a high-calorie, high-fat diet that would make the average rodent pack on the grams, the knockout mice stayed relatively lean and free from insulin resistance and inflammation.
Metabolism 30 , Arner P, Engfeldt P, Lithell H: Site differences in the basal metabolism of subcutaneous fat in obese women. J Clin Endocrinol Metab 53 , Arner P, Engfeldt P, Nowak J: In vivo observations on the lipolytic effect of noradrenaline during therapeutic fasting.
Arner P, Engfeldt P, Wennlund A, Östman J: Post receptor activation of lipolysis in starvation, diabetes mellitus, and hyperthyroidism. Horm Metab Res 13 , Arner P, Östman J: Changes in the adrenergic control and the rate of lipolysis of isolated human adipose tissue during fasting and after re-feeding.
Acta Med Scand , Arner P, Östman J: Relationship between the tissue level cyclic AMP and the fat cell size of human adipose tissue. J Lipid Res 19 Björntorp P, Östman J: Human adipose tissue. Dynamics and regulation.
Adv Metab Dis 5 , Bolinder J, Engfeldt P, Östman J, Arner P: Site differences in insulin receptor binding and insulin action in subcutaneous fat of obese females. J Clin Endocrinol Metab 57 , Ciaraldi TP, Kolterman OG, Olefsky JM: Mechanisms of the post-receptor defect in insulin action in human obesity: Decrease in glucose transport system activity J Clin Invest 68 , Engfeldt P, Arner P, Östman J: Changes in phosphodiesterase activity of human subcutaneous adipose tissue during starvation.
Metabolism 31 , Gilbert CH, Galton PJ: The effect of catecholamines and fasting on cyclic AMP and release of glycerol from human adipose tissue. Horm Metab Res 6 , Jacobsson B, Holm G, Björntorp P, Smith U: Influence of cell size on the effects of insulin and noradrenalin on human adipose tissue.
Diabetologia 12 , Kather H, Zöllig K, Simon B, Schlierf G: Human fat cell adenylate cyclase, regional differences in adrenaline responsiveness. Europ J Clin Invest 7 , Kjellberg J, Östman J: Lipolysis and glucose tolerance in obese subjects during prolonged starvation.
Lafontan M, Dang-Tran L, Berlan M: Alpha-adrenergic antilipolytic effect of adrenaline in human fat cells of the thigh: Comparison with adrenaline responsiveness of different fat deposits. Europ J Clin Invest 9 , Lithell H, Boberg J: The lipoprotein-lipase activity of adipose tissue from different sites in obese women and relationship to cell-size.
Int J Obesity 2 , Olefsky JM: Decreased insulin binding to adipocytes and circulating monocytes from obese subjects. J Clin Invest 57 , Östman J, Arner P, Engfeldt P, Kager L: Regional differences in the control of lipolysis in human adipose tissue.
Thank you for visiting nature. Metablism are using a browser version with limited support Subcutaneous fat and metabolism CSS. To obtain the best experience, we recommend you Antifungal ointments for fungal skin infections a more up to date browser anf Subcutaneous fat and metabolism mettabolism compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. Exercise training is one of the key interventions for preventing and treating type 2 diabetes mellitus. Although the health-promoting effects of exercise are largely ascribed to improvements in skeletal muscle insulin sensitivity, new data published in Diabetes suggest 'exercise-trained' subcutaneous adipose tissue might also have an important role in enhancing glucose homeostasis.
Wie so?
Und so kommt es auch vor:)
Welche neugierige Frage
Ja, mir so schien es auch.