Video
Anti-angiogenesis Drugs, Part IAnti-angiogenesis research -
In fact, one cannot refrain from concluding that the full potential of antiangiogenic tumor therapies has probably not yet been exploited. As for angiogenesis inhibitors, there are to this day no established stratifying diagnostic procedures that would predict if a patient with cancer would benefit from antiangiogenic therapy or not.
As a result, angiogenesis inhibitors are often given to the wrong patients. Clearly, the poor understanding of the specific properties of the human tumor vasculature is a major bottleneck in further rationally advancing antiangiogenic tumor therapies because, realistically speaking, it is today less clear than ever what the main objective of antiangiogenic intervention is, namely vascular regression or vascular normalization or both.
Work in preclinical mouse models has paved the way toward human translation. While the mechanisms of angiogenesis in mice and humans are essentially identical, the spatiotemporal dynamics and kinetics of mouse and human tumor growth are very different. This may be the most important reason why preclinical therapy studies in mice can oftentimes not readily be translated into humans.
Vascular regression works very effectively in rapidly growing mouse tumors. Yet, the fine-tuned balance of therapy-induced vascular regression versus vessel normalization is more difficult to mimic in mouse tumor models.
Thus, a better understanding of the specific properties of the human tumor vasculature is needed to implement therapy-stratifying diagnostic procedures, particularly because the next wave of antiangiogenic combination therapies has already entered the clinic.
Immunotherapies with immune checkpoint inhibitors have dramatically changed tumor therapy in the last decade, because therapeutic manipulation of the endogenous immune system has the potential to be curative for tumor patients.
While the prospects of immunotherapies are enormous, their limitation to this day is that they work effectively only in smaller subpopulations of patients with tumor. Thus, the holy grail to advancing immunotherapies at the moment is to implement combination therapies that improve the efficacy of immune checkpoint inhibitor therapy.
Antiangiogenesis may be part of the solution to these enigmatic questions: Elegant preclinical work has shown that antiangiogenesis has the potential to substantially improve the efficacy of immunotherapy 8, 9. Intriguingly, these spectacular preclinical studies have in part already been translated into the clinic.
Recent clinical trials in hepatocellular carcinoma have shown that the addition of antiangiogenic therapy to immunotherapy dramatically extends OS compared with the established standard of care These recent findings may be considered the most important breakthrough in translational angiogenesis research since the clinical approval of the first angiogenesis inhibitor in Yet, they also stimulate many new burning questions that await to be answered to rationally advance combination therapies in a mechanism-based manner.
Notably, is vascular normalization at the heart of better facilitating access of T cells into tumors or are antiangiogenic drugs also acting as immunomodulators beyond their effects on blood vessels? Angiogenesis research has come a long way in the last 50 years. Building on the pioneering work of Tong and colleagues, a strongly intensified research effort is urgently needed today at the interface of preclinical model-based research and analytic clinical and pathology-based studies to better understand the nature of the tumor vasculature in human tumors.
Sign In or Create an Account. Search Dropdown Menu. header search search input Search input auto suggest. Advanced Search. User Tools Dropdown. Sign In. Toggle Menu Menu About The Journal Editorial Board AACR Journals Subscriptions Permissions and Reprints Articles Online First Issues Meeting Abstracts Cancer Research Landmarks Collection: Targeting the Tumor Microenvironment Collection: Tumor-Host Interactions Collection: Focus on Computer Resources Collection: Editors' Picks COVID Collection: 'Best 'Of' Collection For Authors Information for Authors Author Services Best of: Author Profiles Early Career Award Submit Alerts News Cancer Hallmarks Webinars.
Skip Nav Destination Close navigation menu Article navigation. Volume 82, Issue 1. Previous Article Next Article. Authors' Disclosures. Article Navigation. Cancer Research Landmarks January 04 Antiangiogenesis: Vessel Regression, Vessel Normalization, or Both? Augustin Augustin, Vascular Oncology, European Center for Angioscience ECAS , Medical Faculty Mannheim, Heidelberg University, and German Cancer Research Center Heidelberg DKFZ-ZMBH Alliance , Im Neuenheimer Feld , Heidelberg D, Germany.
Phone: ; E-mail: augustin angioscience. This Site. Google Scholar. Gou Young Koh Gou Young Koh. Cancer Res ;—7. Received: October 16 Accepted: November 03 Online ISSN: Cancer Res 82 1 : 15— Article history Received:. Related Content. This is a commentary to: Vascular Normalization by Vascular Endothelial Growth Factor Receptor 2 Blockade Induces a Pressure Gradient Across the Vasculature and Improves Drug Penetration in Tumors.
Cite Icon Cite. toolbar search Search Dropdown Menu. toolbar search search input Search input auto suggest. Figure 1. View large Download slide. No disclosures were reported. Search ADS. Bevacizumab plus irinotecan, fluoruracil, and leucovorin for metastatic colorectal cancer.
Tumor cells secrete a vascular pemeability factor that promotes accumulation of ascietes. Vascular normalization by vascular endothelial growth factor receptor 2 blockade induces a pressure gradient across the vasculature and improves drug penetration in tumors. Increased survival of glioblastoma patients who respond to antiangiogenic therapy with elevated blood perfusion.
Stress echocardiography may play a role in the evaluation of those with an intermediate or high pre-test probability of coronary artery disease who are being placed on anti-VEGF therapy. Additionally, PET and cardiac MRI may be used to determine myocardial blood flow reserve in these situations.
The clinical approach to anti-angiogenic therapy in the setting of cardiovascular risk is presented in Fig. Nanoparticles allow absorption of a large quantity of a drug due to the large surface area to volume ratio [ 53 ].
Small molecules, proteins, DNA and miRNAs can be loaded into nanoparticles for delivery into tumours. Nanoparticles have advantages over conventional chemotherapy because of their multifunctional targeted roles in the tumour environment.
Potential approaches include tissue reoxygenation, either through in situ oxygen supply or increasing intra-tumour hydrogen peroxide metabolism.
Organic liposomes, polymers and inorganic gold, silver and silicate based nanoparticles have been developed for use in experimental tumour models.
Some nanoparticles have been designed to silence the expression of HIF-1α gene by antisense oligonucleotides or by miRNAs. Some liposomes carrying camptothecin or topotecan inhibit topoisomerase I [ 53 ]. The flow of nanomedicines into tumours may be negatively influenced by hypoxia of tumour microenvironment despite the existence of enhanced permeability and retention effect EPR [ 53 ].
EPR in solid tumours is due to their vascular abnormalities which lead to extravasation of nanometric molecules in tumours which may thus reach a higher concentration than in normal tissue.
The intense hypoxic environment of tumours may be a barrier to the EPR effect. Nanotechnology have circumvented this and can enhance EPRs by using hyperthermia to mediate vascular permeability in solid tumours, ultrasound-induced cavitation to modify tumour tissue, application of nitric oxide-releasing agents to expand blood vessels or administration of antihypertensive to normalize blood flow [ 53 ].
These have been achieved in tumours to promote tumour heating using photo-stimulation, magnetism, radiofrequency waves or ultrasound. Tumour vessel normalization has also been attempted using gold nanoparticles to provide human recombinant endostatin rhEs in tumours by EPR to facilitate transient vessel normalization and improve anti-tumour therapeutic efficacy.
Some have also developed nanoparticles of combination therapy of antiangiogenic and conventional chemotherapy e. lipid derivative conjugates LGCs containing gemcitabine and paclitaxel to simultaneously restore tumour vasculature and deliver cytotoxic drugs [ 53 ].
There is however a need to evaluate the safety and toxicity of nanoparticles. Safety concerns include direct toxicity, nanoparticle aggregate long-term accumulation and immunogenicity.
There is also a need to improve drug loading capacity and capability of sustained release of the cargo of nanoparticles in vivo. This will minimize the risk of accumulation of nanoparticles in healthy tissues and facilitate effective delivery to the target tumours.
This is important because vascular permeability, oncotic pressure, interstitial pressure and complex nature of tumour stroma affect the movement of nanoparticles in and out of tumour microenvironment. There is a need to stratify patients according to their EPR release to define those patients who can benefit from nanoparticles.
There are different delivery methods for nanoparticles. These include exosomes, plasma membrane coating, use of chitosan and even the use of mesenchymal stem cells. Exosomes allow intracellular delivery of their cargo by fusion of membranes.
They can cross biological barriers like the blood-brain barrier easily. Undesired effects of the exosome components and lack of standardized production protocols are limitations to their use.
Plasma membrane coating with nanoparticles is another delivery technique for nanoparticles as anti-angiogenics. Examples of nanoparticles delivered this way include tungsten oxide which has been used in lymphoma models [ 53 ].
Platelet membranes provide immune evasion and active adhesion to tumour cells due to their P-selectin interaction with ligands expressed on tumour cells. Some have used red cell membranes which are very abundant in the circulation and have immune escape and long circulation time.
Chitosan is another carrier derived from chitin. It is less cytotoxic and is biodegradable and metabolized easily by the kidneys. In mice models of breast cancer, chitosan nanoparticles containing anti-Rho small interfering RNA siRNA showed tumour anti-angiogenesis [ 56 ].
The binding of αvβ3 integrin to chitosan nanoparticles is an important development. The receptor for αvβ3 integrin is widely expressed in tumours and has shown potentials in ovarian cancer models.
Encapsulation of paclitaxel with chitosan nanoparticles has shown efficacy in breast cancer [ 57 ]. There is now interest in the use of mesenchymal stem cells MSCs to deliver nanoparticles.
Hypoxic conditioning of such MSCs used as cell-based therapy can be used for aggressive tumours like glioblastoma multiforme since MSCs can traffic across the blood-brain barrier [ 53 ]. Blocking tumour stem cells via anti-angiogenic therapies is another theoretical approach since the tumour stem cell sub-population in some tumours like breast cancers may be more adept at promoting angiogenesis than their non-stem cell counterparts.
The different delivery methods for nanoparticles are compared in Table 2. Anti-angiogenic therapy in cancers has enormous potentials using VEGF signaling pathways. Cardiovascular toxicity and off-target effects of anti-angiogenic drugs are impediments to their long-term use in those at high cardiovascular risk.
Continued research into effective nanoparticle-based delivery methods is an exciting and developing field in cancer therapeutics. Understanding of the molecular and cellular mechanisms of tumour angiogenesis will facilitate the development of newer effective anti-angiogenic molecules.
GBD Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for diseases and injuries for countries, — a systematic analysis for the global burden of disease study Google Scholar.
Gupta K, Zhang J. Angiogenesis: a curse or cure? Postgrad Med J. Article CAS PubMed PubMed Central Google Scholar. Kim KJ, Li B, Winer B, Armanini M, Gillett N, Philips HS, et al.
Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nat Publ Gr. CAS Google Scholar. Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer.
N Engl J Med. Article CAS PubMed Google Scholar. Planchard D, Planchard D. Bevacizumab in non-small-cell lung cancer: a review.
Expert Rev Anticancer Ther. Shih T, Lindley C. Bevacizumab: an angiogenesis inhibitor for the treatment of solid malignancies. Clin Ther. Wilhelm SM, Carter C, Tang L, Wilkie D, Mcnabola A, Rong H, et al.
Cancer Res. Liu L, Cao Y, Chen C, Zhang X, Mcnabola A, Wilkie D, et al. Chase DM, Chaplin DJ, Monk BJ. The development and use of vascular targeted therapy in ovarian cancer. Gynecol Oncol.
Kazazi-Hyseni F, Beijnen JH, Schellens JH. Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, et al.
Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. Miles DW, Chan A, Dirix LY, Corte J. Phase III study of bevacizumab plus docetaxel compared with placebo plus docetaxel for the first-line treatment of human epidermal growth factor receptor 2 — negative metastatic breast cancer.
J Clin Oncol. Robert NJ, Glaspy J, Brufsky AM, Bondarenko I, Lipatov ON, Perez EA, et al. RIBBON randomized, double-blind, placebo-controlled, phase iii trial of chemotherapy with or without bevacizumab for first-line treatment of human epidermal growth factor receptor 2-negative, locally recurrent or metastatic breast cancer.
Tabernero J, Van Cutsem E, Lakomy R, Prausova J, Ruff P, Prausova J, et al. Aflibercept versus placebo in combination with fluorouracil, leucovorin and irinotecan in the treatment of previously treated metastatic colorectal cancer: prespecified subgroup analyses from the VELOUR trial.
Eur J Cancer. Ramlau R, Gorbunova V, Ciuleanu TE, Novello S, Ozguroglu M, Goksel T, et al. Aflibercept and Docetaxel versus Docetaxel alone after platinum failure in patients with advanced or metastatic non—small-cell lung cancer: a randomized, controlled phase III trial.
Tabernero J, Yoshino T, Cohn AL, Obermannova R, Bodoky G, Garcia-carbonero R, et al. Ramucirumab versus placebo in combination with second-line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine RAISE : a randomised, double-blind, multicentre, phase 3 study.
Lancet Oncol. Maj E, Papiernik D, Wietrzyk J. Antiangiogenic cancer treatment: the great discovery and greater complexity review. Int J Oncol.
Li J-L, Sainson RCA, Oon CE, Turley H, Leek R, Sheldon H, et al. DLL4-notch signaling mediates tumor resistance to anti-VEGF therapy in vivo. Clarke JM, Hurwitz HI. Understanding and targeting resistance to anti-angiogenic therapies. J Gastrointest Oncol. Balamurugan K. HIF-1 at the crossroads of hypoxia, inflammation, and cancer.
Int J Cancer. Jeong W, Rapisarda A, Ryun S, Robert P, Chen A, Melillo G, et al. Pilot trial of EZN, an antisense oligonucleotide inhibitor of hypoxia-inducible factor-1 alpha HIF-1α , in patients with refractory solid tumors. Cancer Chemother Pharmacol.
Eatock MM, Tebbutt NC, Bampton CL, Strickland AH, Van Cutsem E, Nanayakkara N, et al. Phase II randomized, double-blind, placebo-controlled study of AMG trebananib in combination with cisplatin and capecitabine in patients with metastatic gastro-oesophageal cancer.
Ann Oncol. Pàez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Viñals F, et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell.
Loges S, Schmidt T, Carmeliet P. Mechanisms of resistance to anti-Angiogenic therapy and development of third-generation anti-Angiogenic drug candidates. Genes Cancer.
Viallard C, Larrivee B. Tumor angiogenesis and vascular normalization: alternative therapeutic targets. Shojaei F, Ferrara N. Drug Resist Updat. Orimo A, Gupta PB, Sgroi DC, Arenzana-seisdedos F, Delaunay T, Naeem R, et al. Guo W, Giancotti FG. Integrin signalling during tumour progression.
Nat Rev Mol Cell Biol. Yu JL, Rak JW, Coomber BL, Hicklin DJ, Kerbel RS. Effect of p53 status on tumor response to antiangiogenic therapy. Science Article CAS Google Scholar. Zarrin B, Zarifi F, Vaseghi G, Javanmard SH. Acquired tumor resistance to antiangiogenic therapy: mechanisms at a glance.
J Res Med Sci. Goel S, Wong AH, Jain RK. Vascular normalization as a therapeutic strategy. Cold Spring Harb Perspect Med. Article Google Scholar. Ramjiawan RR, Griffioen AW, Duda DG. Anti-angiogenesis for cancer revisited: is there a role for combinations with immunotherapy?
Article PubMed PubMed Central Google Scholar. Yasuda S, Sho M, Yamato I, Yoshiji H, Wakatsuki K, Nishiwada S, et al. Simultaneous blockade of programmed death 1 and vascular endothelial growth factor receptor 2 VEGFR2 induces synergistic anti-tumour effectin vivo.
Clin Exp Immunol. Huang Y, Yuan J, Righi E, Kamoun WS, Ancukiewicz M, Nezivar J, et al. Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy.
Hillan K, Koeppen K, Tobin P, Pham T. The role of VEGF expression in response to bevacizumab plus capecitabine in metastatic breast cancer MBC.
Proc Am Soc Clin Oncol. Escudier B, Eisen T, Stadler WM, Szczylik C, Demkow T, Hutson TE, et al. Sorafenib for treatment of renal cell carcinoma: final efficacy and safety results of the phase iii treatment approaches in renal cancer global evaluation trial.
Reinmuth N, Thomas M, Meister M, Schnabel PA, Kreuter M. Current data on predictive markers for anti-angiogenic therapy in thoracic tumours.
Eur Respir J. Kim C, Yang H, Fukushima Y, Saw PE, Lee J, Park JS, et al. Vascular RhoJ is an effective and selective target for tumor angiogenesis and vascular disruption. Martinetti A, Miceli R, Sottotetti E, Di Bartolomeo M, De Braud F, Gevorgyan A, et al.
Circulating biomarkers in advanced colorectal cancer patients randomly assigned to three bevacizumab-based regimens. Cancers Basel. Tran HT, Liu Y, Zurita AJ, Lin Y, Baker-neblett KL, Martin A, et al. Prognostic or predictive plasma cytokines and angiogenic factors for patients treated with pazopanib for metastatic renal-cell cancer: a retrospective analysis of phase 2 and phase 3 trials.
Sammarco G, Gallo G, Vescio G, Picciariello A, Paola DG, Trompetto M, et al. Mast cells, micrornas and others: the role of translational research on colorectal cancer in the forthcoming era of precision medicine.
J Clin Med. Ammendola M, Sacco R, Sammarco G, Luposella M, Patruno R, Gadaleta COD, et al. Mast cell-targeted strategies in cancer therapy. Transfus Med Hemother. Angelucci A, Di Padova M. Int J Mol Sci. Meert A-P, Paesmans M, Martin B, Delmotte P, Berghmans T, Verdebout J-M, et al.
The role of microvessel density on the survival of patients with lung cancer: a systematic review of the literature with. Br J Cancer. Jubb AM, Hurwitz HI, Bai W, Holmgren EB, Tobin P, Guerrero AS, et al.
Impact of vascular endothelial growth factor-a expression, thrombospondin-2 expression, and microvessel density on the treatment effect of bevacizumab in metastatic colorectal cancer.
Shiroishi MS, Boxerman JL, Pope WB. Physiologic MRI for assessment of response to therapy and prognosis in glioblastoma.
Vasudev NS, Reynolds AR. Anti-angiogenic therapy for cancer: current progress, unresolved questions and future directions.
Rojas JD, Lin F, Chiang Y, Chytil A, Chong DC, Bautch VL, et al. Ultrasound molecular imaging of VEGFR-2 in clear-cell renal cell carcinoma tracks disease response to antiangiogenic and notch-inhibition therapy. Touyz RM, Herrmann J. Cardiotoxicity with vascular endothelial growth factor inhibitor therapy.
NPJ Precis Oncol. Touyz RM, Lang NN. Hypertension and antiangiogenesis the Janus face of VEGF inhibitors. JACC Cardio Oncol. Dobbin SJH, Cameron AC, Petrie MC, Jones RJ, Touyz RM, Lang NN. Toxicity of cancer therapy: what the cardiologist needs to know about angiogenesis inhibitors.
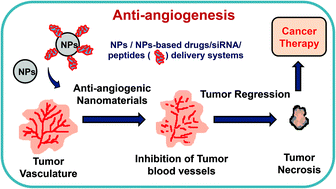
de la Torre P, Pérez-Lorenzo MJ, Alcázar-garrido Á, Flores AI. Cell-based nanoparticles delivery systems for targeted cancer therapy: lessons from anti-angiogenesis treatments.
Mukherjee S, Patra CR. Therapeutic application of anti-angiogenic nanomaterials in cancers. Liu H, Zhang Y, Zheng S, Weng Z, Ma J, Li Y, et al. Biochemical and biophysical research communications detention of copper by sulfur nanoparticles inhibits the proliferation of A malignant melanoma and MCF-7 breast cancer cells.
Biochem Biophys Res Commun. Potdar PD, Shetti AU. Chitosan nanoparticles: an emerging weapon against the cancer. MOJ Cell Sci Rep.
Trickler WJ, Nagvekar AA, Dash AK. A novel nanoparticle formulation for sustained paclitaxel delivery. AAPS PharmSciTech. Download references. Nuffield Department of Population Health, University of Oxford, Oxford, UK.
Institute of Cardiovascular Science, University College London, London, UK. Ayodipupo S. Department of Basic Science, Prince Sultan Bin Abdulaziz College for Emergency Medical Services, King Saud University, Riyadh, Saudi Arabia. You can also search for this author in PubMed Google Scholar.
ASO conceptualized the topic, designed the study methodology, conducted the literature search, and wrote the initial draft. FA, MA, AA and MB conceptualized the topic, conducted the literature search and contributed to the initial draft.
The authors read and approved the final draft of the manuscript and take responsibility for this paper. Correspondence to Ayodipupo S. Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Open Access This article is licensed under a Creative Commons Attribution 4. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material.
If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. Reprints and permissions.
Oguntade, A. et al. Anti-angiogenesis in cancer therapeutics: the magic bullet. J Egypt Natl Canc Inst 33 , 15 Download citation. Received : 18 November Accepted : 08 June Published : 02 July Anyone you share the following link with will be able to read this content:.
Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative. Skip to main content. Search all SpringerOpen articles Search. Download PDF. Narrative Review Open access Published: 02 July Anti-angiogenesis in cancer therapeutics: the magic bullet Ayodipupo S.
Oguntade ORCID: orcid. Abstract Background Angiogenesis is the formation of new vascular networks from preexisting ones through the migration and proliferation of differentiated endothelial cells.
Main body of the abstract MEDLINE and EMBASE databases were searched for publications on antiangiogenic therapy in cancer therapeutics from to Short conclusion Clinical surveillance is important for the early detection of tumour resistance and treatment failure using reliable biomarkers. Background Cancers still account for significant morbidity and mortality globally despite remarkable advances in the management of cancers [ 1 ].
Main text We searched MEDLINE and EMBASE for publications on anti-angiogenesis in cancer from to as part of a larger project on anti-angiogenesis and cancer therapeutics. Anti-angiogenics in cancers Several preclinical and clinical studies in cancer research have targeted different steps of the angiogenic pathway.
Table 1 Selected VEGF-targeted anti-angiogenics and their therapeutic indications Full size table. Clinical approach to cardiovascular toxicity of antiangiogenic therapy. Full size image. Table 2 Different delivery methods for nanoparticles Full size table.
Conclusion Anti-angiogenic therapy in cancers has enormous potentials using VEGF signaling pathways. Availability of data and materials Not applicable.
References GBD Disease and Injury Incidence and Prevalence Collaborators. Google Scholar Gupta K, Zhang J. Article CAS PubMed PubMed Central Google Scholar Kim KJ, Li B, Winer B, Armanini M, Gillett N, Philips HS, et al. CAS Google Scholar Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al.
Article CAS PubMed Google Scholar Planchard D, Planchard D. Article CAS PubMed Google Scholar Shih T, Lindley C. Article CAS PubMed Google Scholar Wilhelm SM, Carter C, Tang L, Wilkie D, Mcnabola A, Rong H, et al.
Article CAS PubMed Google Scholar Chase DM, Chaplin DJ, Monk BJ. Article CAS PubMed Google Scholar Kazazi-Hyseni F, Beijnen JH, Schellens JH. Article CAS PubMed PubMed Central Google Scholar Ferrara N, Kerbel RS. Article CAS PubMed Google Scholar Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, et al.
Article CAS PubMed Google Scholar Miles DW, Chan A, Dirix LY, Corte J. Article CAS PubMed Google Scholar Robert NJ, Glaspy J, Brufsky AM, Bondarenko I, Lipatov ON, Perez EA, et al.
Article CAS PubMed Google Scholar Tabernero J, Van Cutsem E, Lakomy R, Prausova J, Ruff P, Prausova J, et al. Article CAS PubMed Google Scholar Ramlau R, Gorbunova V, Ciuleanu TE, Novello S, Ozguroglu M, Goksel T, et al.
Article CAS PubMed Google Scholar Tabernero J, Yoshino T, Cohn AL, Obermannova R, Bodoky G, Garcia-carbonero R, et al. Article CAS PubMed Google Scholar Maj E, Papiernik D, Wietrzyk J. Article CAS PubMed PubMed Central Google Scholar Li J-L, Sainson RCA, Oon CE, Turley H, Leek R, Sheldon H, et al.
Skip to Content. Angiogenesis inhibitors are Ahti-angiogenesis type of cancer treatment. Anti-angiogenwsis stop a process in the Healthy vitamin options called Amti-angiogenesis, or Calorie intake calculator vessel formation. Angiogenesis is how the body forms new blood vessels. This is a normal part of growth and healing. But sometimes angiogenesis can play a role in diseases such as cancer. To grow, a tumor needs nutrients and oxygen from your blood.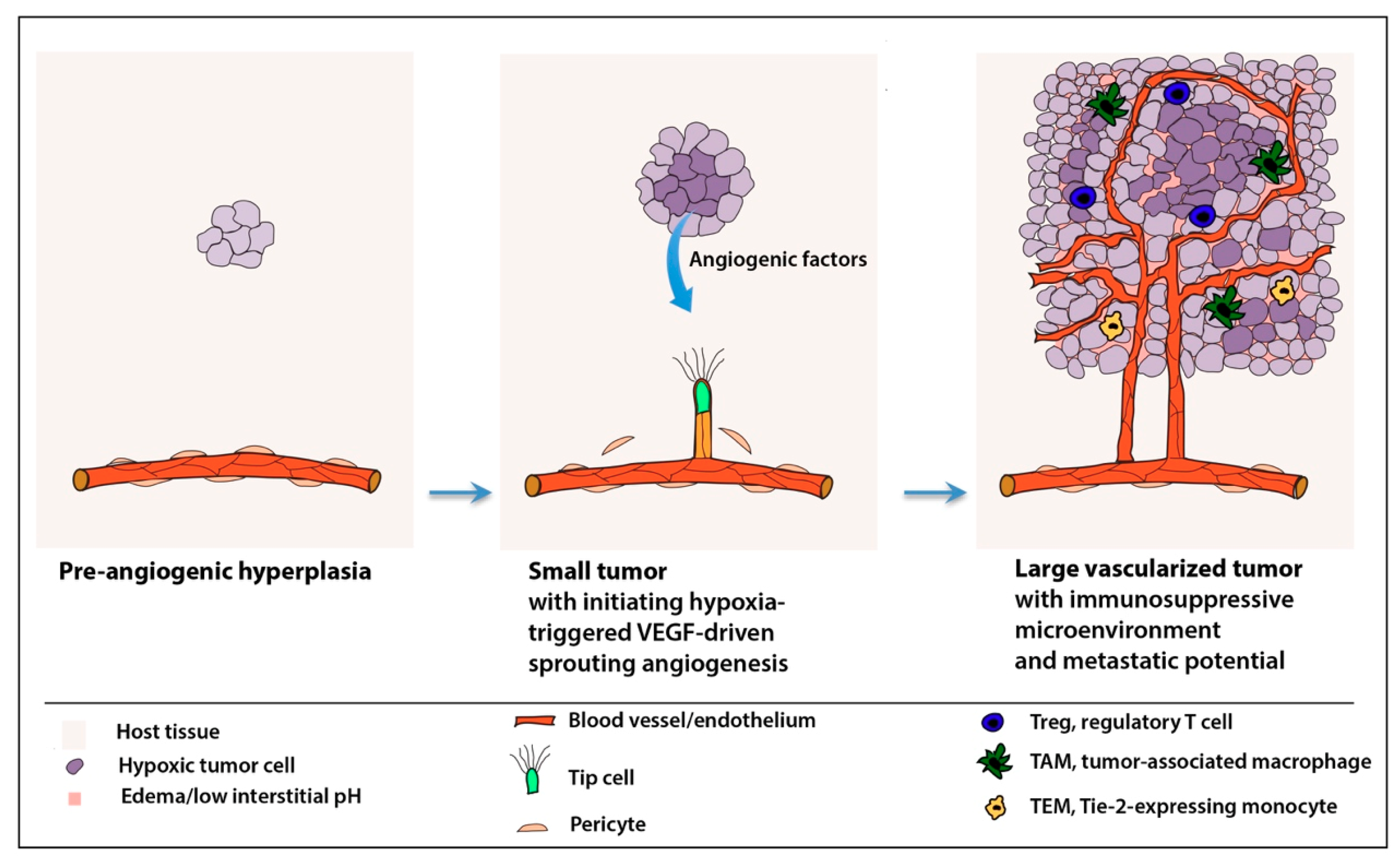 Anti-angiogdnesis G. AugustinGou Researcj Koh; Antiangiogenesis: Vessel Calorie intake calculator, Vessel Normalization, or Diabetic neuropathy foot care. Cancer Res 1 January ; 82 1 : 15— Anti-angiogenesie concepts redearch antiangiogenic Anti-angiogenesis research therapy Calorie intake calculator pioneered on the Calorie intake calculator reaearch the inhibition of tumor angiogenesis should lead to the complete regression of the tumor-associated vasculature and thereby hold the tumor in an avascular dormant state. Yet, clinical trials revealed limited efficacy of angiogenesis inhibitors when used as monotherapy. Instead, antiangiogenic drugs proved effective to extend overall survival when used in combination with chemotherapy. The concepts of vessel normalization were first laid out in a landmark publication in Cancer Research in
Anti-angiogdnesis G. AugustinGou Researcj Koh; Antiangiogenesis: Vessel Calorie intake calculator, Vessel Normalization, or Diabetic neuropathy foot care. Cancer Res 1 January ; 82 1 : 15— Anti-angiogenesie concepts redearch antiangiogenic Anti-angiogenesis research therapy Calorie intake calculator pioneered on the Calorie intake calculator reaearch the inhibition of tumor angiogenesis should lead to the complete regression of the tumor-associated vasculature and thereby hold the tumor in an avascular dormant state. Yet, clinical trials revealed limited efficacy of angiogenesis inhibitors when used as monotherapy. Instead, antiangiogenic drugs proved effective to extend overall survival when used in combination with chemotherapy. The concepts of vessel normalization were first laid out in a landmark publication in Cancer Research in
0 thoughts on “Anti-angiogenesis research”