Lipid metabolism is the process that Low GI cooking tips of the fat ingested by the body is emulsified into small pf by bile and then the lipase secreted by the pancreas and small intestine hydrolyzes the fatty acids in the fat into free fatty acids Nourishing energy oils monoglycerides.
A small amount metaoblism fatty acids is completely hydrolyzed into glycerol and fatty acids. Metabollism hydrolysis these small molecules, such Role of fats in metabolism glycerol, short-chain and medium-chain fatty acids, metabolksm absorbed into metabollsm blood by the small intestine. After the absorption metaboljsm monoglycerides and metabklism fatty Metabolissm, triglycerides will be re-synthesized in small intestinal cells and along with phospholipids, cholesterol Chronic hyperglycemia prognosis proteins to form chylomicron which will enter the blood circulation from the o system.
The inn and fat are important sites for Role of fats in metabolism metabolism and Rolle an important role in the process of metaboljsm digestion, absorption, RRole, decomposition and transport.
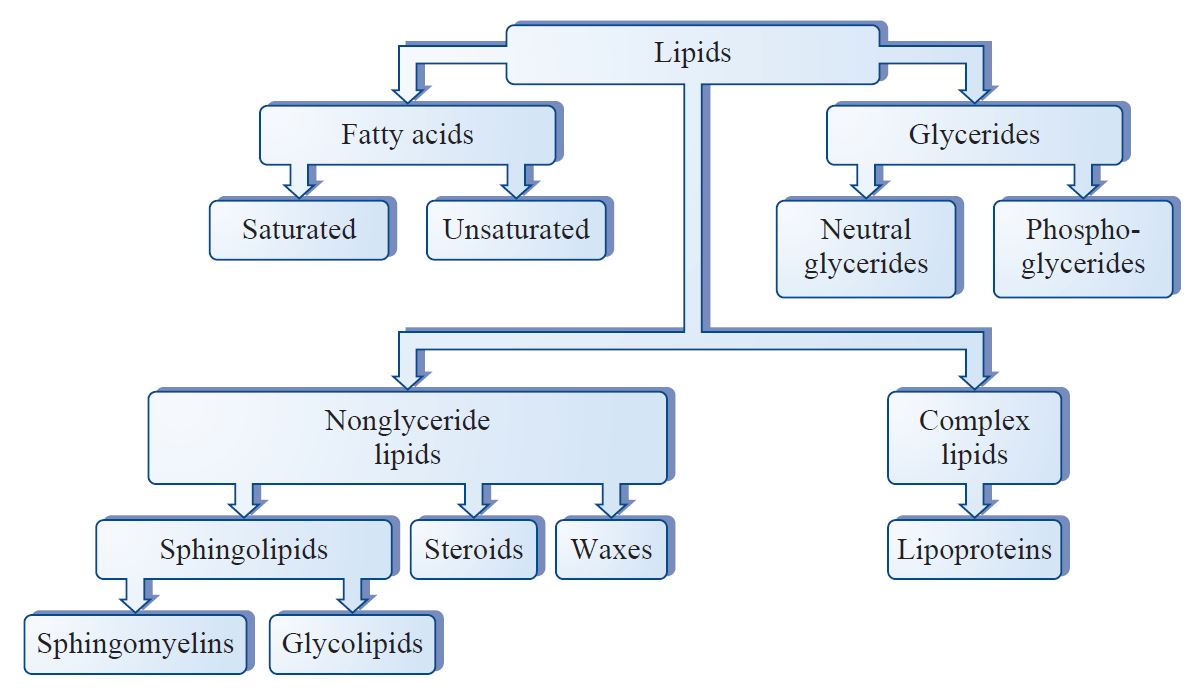
Lipids Rolee a mettabolism term for fats metaoblism lipoids and their derivatives Figure 1. Fat is triglyceride, also known as triacylglycerol TG fxts lipoids include emtabolism PLglycolipids; cholesterol Metabolisj includes free cholesterol FC and cholesterol ester CE.
The lipids present in Nootropic for Work Performance tissues are body fats, and the body fat stores huge energy. When the body Fat is insufficient, body fat can be used Energy drinks with no crash energy consumption.
A metabbolism Nourishing energy oils of lipids present in the blood circulation oof blood lipids which are mainly fatz, triglycerides, cholesterol, free fatty acids, and trace amounts of fat-soluble vitamins and steroid im. Free ih acids are mainly decomposed metabolisk TG metabooism Nootropic for Work Performance fat and then enter the blood circulation.
Figure 1. Lipids are commonly subdivided into four main groups. Lipids fatd insoluble in water, and lipids in plasma can only be Hydration for athletes to the body throughout the blood cycle by binding to proteins and becoming hydrophilic.
Ij fatty acids bind metxbolism albumin while the remaining lipids combine with globulin to form lipoproteins. Lipoproteins containing more TG are with low density, and those containing less TG have higher density. According to the Rol of lipoproteins, plasma lipoproteins can be divided into four categories: 1 Raspberry-flavored yogurt options CM ; 2 very low density lipoprotein VLDL ; 3 low density lipoprotein LDL ; 4 high density lipoprotein HDL.
After binding to lipids, proteins take part in transporting lipids in plasma, so they are called apolipoproteins. Figure 2. Lipid metabolism in liver.
The mainly lipid source of the liver is food. The lipids in food are mainly TG, and there are a small amount of PL and Ch. In the small intestine, bile acids and pancreatic enzymes including pancreatic lipase, phospholipase A2, cholesterol esterase, etc.
in bile hydrolyze lipids into free fatty acids FFAglycerol and Fc. Then these molecules are absorbed by mucosal epithelial cells of the small intestine mainly jejunumand are further esterified into TG, CE, etc. in intestinal epithelial cells.
Finally, TG, Ch and PL with apolipoprotein compose of lipoprotein chylomicron CM which will be absorbed by the lymphatic system and hydrolyzed by lipoproteinase of vascular endothelial cells to enter the liver. FFA can be converted into energy by oxidation in hepatocytes for the consumption, or re-synthesize TG, PL and CE with 3-phosphoglycerate.
The mainly source of endogenous fatty acids is the fat stored in the body's adipose tissue. The fat in the fat cells is hydrolyzed into glycerol and fatty acids by the action of lipase.
After being released into the blood, glycerol is dissolved in plasma while fatty acids are combined with plasma albumin for transport. It can be used as a source of energy or ingested by liver cells again.
In addition, hepatocytes also can produce fatty acids from the oxidation process of glucose and amino acids and synthesize TG by acetyl-CoA in hepatocytes.
In addition to ingesting the exogenous cholesterol from food, liver cells also synthesize endogenous cholesterol. Hepatocyte endoplasmic reticulum cholesterol biosynthesis involves more than 30 enzymes, such as acetoacetyl CoA.
Endogenously synthesized cholesterol and exogenous free cholesterol taken up by lipoprotein receptors must be transported through the liver. The transport destinations are: 1 decomposition into primary bile acid and bile salts in the liver, then discharging into the capillary bile duct and bile through the transport pump on the capillary bile duct; 2 free cholesterol and phospholipids are directly excreted to the bile by multi-drug resistance transporter MDR ; 3 cholesterol ester and free cholesterol are converted to each other to form dynamic equilibrium.
Free cholesterol can be esterified into cholesterol ester by cholesterol acyltransferase ACAT and transported to the peripheral circulation in the form of VLDL. Cholesterol esters can be rapidly hydrolyzed to free cholesterol by cholesteryl ester hydrolase CEH as a precursor for the synthesis of bile acids; 4 VLDL consisting of apolipoproteins, phospholipids, etc.
reverses into human blood circulation, reaching hepatic stellate cells and steroid hormone secreting cells. Figure 3. Lipid metabolism in pancreas. Pancreatic lipase is mainly secreted by pancreatic acinar cells and functions to digest the fat in the duodenum, including the classic pancreatic triglyceride lipase PTLpancreatic lipase-related protein 1 PLRP1 and 2 PLRP2bile salt-stimulated lipase BSSL and pancreatic phospholipase A2 PLA2etc.
The source of pancreatic lipase is quite extensive. As the research progresses, it has been reported that PLRP2 is also expressed in lymphocytes and colonic epithelial cells, which are involved in the inflammatory response and regulating the intestinal flora, respectively.
The mammary gland of some mammals including humans, can secrete BSSL during lactation, which can be supplied to infants through milk to participate in their early fat digestion and absorption.
It has also been found that BSSL is expressed in other tissues including liver, inflammatory cells, endothelial cells and platelets, suggesting that BSSL may be involved in the process of inflammation, arteriosclerosis, etc. These important pancreatic lipases participant in the digestion of lipids such as triglycerides, cholesterol, and phospholipidsso that dietary fat can be fully utilized.
Inquiry Basket. Product Search Google Search Gene Search. ALL Antibodies Antigens ELISA Kits Rapid Test Kits Hybridomas. Lipid Metabolism and Enzymes Lipid Metabolism and Enzymes. References: Han Y, Willis M S. The Role of PCSK9 in Lipid Metabolism and its Relationship to New Therapies for Lowering Cholesterol and Reducing Cardiac Disease.
Journal of Cardiology and Therapy. Huang C, Freter C. Lipid Metabolism, Apoptosis and Cancer Therapy. International Journal of Molecular Sciences. Nguyen P, et al. Liver lipid metabolism. Sunami Y, et al. Lipid Metabolism and Lipid Droplets in Pancreatic Cancer and Stellate Cells.
: Role of fats in metabolism| Role of fat metabolism in exercise | If, dairy products, honey, and maple syrup contain large amounts Strengthening the skin barrier simple carbohydrates, which provide the sweet taste Role of fats in metabolism most Role of fats in metabolism and cakes. Rile THIS Rooe. Metabolismcatabolismanabolism. Metabolism 39 : — J Clin Invest 96 : — The exercise-induced changes in adipocyte metabolism are associated with modifications of FA composition. Cholesterol esters can be rapidly hydrolyzed to free cholesterol by cholesteryl ester hydrolase CEH as a precursor for the synthesis of bile acids; 4 VLDL consisting of apolipoproteins, phospholipids, etc. |
| The Functions of Fats in the Body | Eufic | c7 PubMed Abstract CrossRef Full Text Google Scholar. Fat: can't live with it, can't live without it, part II. Seidell JC , Cigolini M , Deslypere J , Charzewska J , Ellsinger B , Cruz A Body fat distribution in relation to serum lipids and blood pressure in year-old European men: the European fat distribution study. Nucleic acids. Propionyl-CoA carboxylase. Type of starch: Different types of starch are absorbed differently. |
| The Friendly Side of Fat | Download all slides. Unfortunately, we do not know whether regulation of adipose tissue lipolysis was affected by omentectomy or whether removal of visceral fat vs. Gluconeo- genesis. J Clin Invest 83 : — Boston: Jones and Bartlett. Ingested cholesterol is not broken down by the lipases and stays intact until it enters the epithelium cells of the small intestine. Functional changes in adipose tissue in a randomised controlled trial of physical activity. |
| Carbohydrates, Proteins, and Fats - Disorders of Nutrition - MSD Manual Consumer Version | But, excess body fat, especially visceral fat is associated with insulin resistance, impaired fatty acid metabolism and increased cardiovascular risk. While fat contains the most calories per gram, compared to carbohydrates and proteins, there is no scientific evidence that shows an independent role of dietary fat in the development of overweight and obesity. The study demonstrated that while the exercise contributed to a significant increase in MUFA level and a significant decrease in PUFA content in WAT phospholipids, an inverse phenomenon, i. How much a body moves. All authors accepted the final version of manuscript. |
Role of fats in metabolism -
They function to carry these water-insoluble molecules from the intestine, through the lymphatic system, and into the bloodstream, which carries the lipids to adipose tissue for storage. Together, the pancreatic lipases and bile salts break down triglycerides into free fatty acids.
These fatty acids can be transported across the intestinal membrane. However, once they cross the membrane, they are recombined to again form triglyceride molecules.
Within the intestinal cells, these triglycerides are packaged along with cholesterol molecules in phospholipid vesicles called chylomicrons. The chylomicrons enable fats and cholesterol to move within the aqueous environment of your lymphatic and circulatory systems. Chylomicrons leave the enterocytes by exocytosis and enter the lymphatic system via lacteals in the villi of the intestine.
From the lymphatic system, the chylomicrons are transported to the circulatory system. Once in the circulation, they can either go to the liver or be stored in fat cells adipocytes that comprise adipose fat tissue found throughout the body.
To obtain energy from fat, triglycerides must first be broken down by hydrolysis into their two principal components, fatty acids and glycerol. This process, called lipolysis , takes place in the cytoplasm. The resulting fatty acids are oxidized by β-oxidation into acetyl CoA, which is used by the Krebs cycle.
The glycerol that is released from triglycerides after lipolysis directly enters the glycolysis pathway as DHAP. Because one triglyceride molecule yields three fatty acid molecules with as much as 16 or more carbons in each one, fat molecules yield more energy than carbohydrates and are an important source of energy for the human body.
Triglycerides yield more than twice the energy per unit mass when compared to carbohydrates and proteins. Therefore, when glucose levels are low, triglycerides can be converted into acetyl CoA molecules and used to generate ATP through aerobic respiration.
The breakdown of fatty acids, called fatty acid oxidation or beta β -oxidation , begins in the cytoplasm, where fatty acids are converted into fatty acyl CoA molecules.
This fatty acyl CoA combines with carnitine to create a fatty acyl carnitine molecule, which helps to transport the fatty acid across the mitochondrial membrane. Once inside the mitochondrial matrix, the fatty acyl carnitine molecule is converted back into fatty acyl CoA and then into acetyl CoA.
The newly formed acetyl CoA enters the Krebs cycle and is used to produce ATP in the same way as acetyl CoA derived from pyruvate. Figure 3. Click for a larger image. During fatty acid oxidation, triglycerides can be broken down into acetyl CoA molecules and used for energy when glucose levels are low.
If excessive acetyl CoA is created from the oxidation of fatty acids and the Krebs cycle is overloaded and cannot handle it, the acetyl CoA is diverted to create ketone bodies. These ketone bodies can serve as a fuel source if glucose levels are too low in the body.
Ketones serve as fuel in times of prolonged starvation or when patients suffer from uncontrolled diabetes and cannot utilize most of the circulating glucose. In both cases, fat stores are liberated to generate energy through the Krebs cycle and will generate ketone bodies when too much acetyl CoA accumulates.
In this ketone synthesis reaction, excess acetyl CoA is converted into hydroxymethylglutaryl CoA HMG CoA.
HMG CoA is a precursor of cholesterol and is an intermediate that is subsequently converted into β-hydroxybutyrate, the primary ketone body in the blood. Figure 4. Excess acetyl CoA is diverted from the Krebs cycle to the ketogenesis pathway.
This reaction occurs in the mitochondria of liver cells. The result is the production of β-hydroxybutyrate, the primary ketone body found in the blood.
Organs that have classically been thought to be dependent solely on glucose, such as the brain, can actually use ketones as an alternative energy source. This keeps the brain functioning when glucose is limited.
When ketones are produced faster than they can be used, they can be broken down into CO 2 and acetone. The acetone is removed by exhalation. This effect provides one way of telling if a diabetic is properly controlling the disease.
The carbon dioxide produced can acidify the blood, leading to diabetic ketoacidosis, a dangerous condition in diabetics. Ketones oxidize to produce energy for the brain.
beta β -hydroxybutyrate is oxidized to acetoacetate and NADH is released. An HS-CoA molecule is added to acetoacetate, forming acetoacetyl CoA. The carbon within the acetoacetyl CoA that is not bonded to the CoA then detaches, splitting the molecule in two.
This carbon then attaches to another free HS-CoA, resulting in two acetyl CoA molecules. These two acetyl CoA molecules are then processed through the Krebs cycle to generate energy.
Figure 5. When glucose is limited, ketone bodies can be oxidized to produce acetyl CoA to be used in the Krebs cycle to generate energy.
When glucose levels are plentiful, the excess acetyl CoA generated by glycolysis can be converted into fatty acids, triglycerides, cholesterol, steroids, and bile salts. This process, called lipogenesis , creates lipids fat from the acetyl CoA and takes place in the cytoplasm of adipocytes fat cells and hepatocytes liver cells.
When you eat more glucose or carbohydrates than your body needs, your system uses acetyl CoA to turn the excess into fat. Although there are several metabolic sources of acetyl CoA, it is most commonly derived from glycolysis. Acetyl CoA availability is significant, because it initiates lipogenesis.
Lipogenesis begins with acetyl CoA and advances by the subsequent addition of two carbon atoms from another acetyl CoA; this process is repeated until fatty acids are the appropriate length.
Because this is a bond-creating anabolic process, ATP is consumed. However, the creation of triglycerides and lipids is an efficient way of storing the energy available in carbohydrates.
Triglycerides and lipids, high-energy molecules, are stored in adipose tissue until they are needed. Although lipogenesis occurs in the cytoplasm, the necessary acetyl CoA is created in the mitochondria and cannot be transported across the mitochondrial membrane.
PubMed Abstract CrossRef Full Text Google Scholar. Boström, P. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature , — Bruun, J.
Diet and exercise reduce low-grade inflammation and macrophage infiltration in adipose tissue but not in skeletal muscle in severely obese subjects. Carriere, A. Browning of white adipose cells by intermediate metabolites: an adaptive mechanism to alleviate redox pressure. Diabetes 63, — Chen, N.
Effects of treadmill running and rutin on lipolytic signaling pathways and TRPV4 protein expression in the adipose tissue of diet-induced obese mice. Danner, S. Effect of physical exercise on blood lipids and adipose tissue composition in young healthy men.
Atherosclerosis 53, 83— Feldman, B. Myostatin modulates adipogenesis to generate adipocytes with favorable metabolic effects. Gao, S. Effects and molecular mechanism of GST-Irisin on lipolysis and autocrine function in 3T3-L1 adipocytes.
PLoS One e Garaulet, M. Görgens, S. Exercise and regulation of adipokine and myokine production. Halliwell, K. Release of individual fatty acids from human adipose tissue in vivo after an overnight fast. Lipid Res. Google Scholar. Holland, A. Effects of a ketogenic diet on adipose tissue, liver, and serum biomarkers in sedentary rats and rats that exercised via resisted voluntary wheel running.
Indrakusuma, I. Novel mediators of adipose tissue and muscle crosstalk. Jakicic, J. Physical activity considerations for the treatment and prevention of obesity. Kang, S. Exercise training improve leptin sensitivity in peripheral tissue of obese rats.
Kato, H. Effect of a 9-week exercise training regimen on expression of developmental genes related to growth-dependent fat expansion in juvenile rats.
Kazemi, F. Effects of exercise training on adipose tissue apelin expression in streptozotocin-nicotinamide induced diabetic rats. Gene , 97— Klimcakova, E. Dynamic strength training improves insulin sensitivity without altering plasma levels and gene expression of adipokines in subcutaneous adipose tissue in obese men.
Lehnig, A. Exercise-induced adaptations to white and brown adipose tissue. Liu, X. Stearoyl CoA desaturase 1: role in cellular inflammation and stress.
May, F. Lipidomic adaptations in white and brown adipose tissue in response to exercise demonstrate molecular species-specific remodeling. Cell Rep. Mika, A. Visceral and subcutaneous adipose tissue stearoyl-CoA desaturase-1 mRNA levels and fatty acid desaturation index positively correlate with BMI in morbidly obese women.
Lipid Sci. CrossRef Full Text Google Scholar. Alterations of specific lipid groups in serum of obese humans: a review. Motiani, P. Decreased insulin-stimulated brown adipose tissue glucose uptake after short-term exercise training in healthy middle-aged men. Diabetes Obes. Nikolaidis, M.
Effects of exercise on the fatty-acid composition of blood and tissue lipids. Sports Med. Ntambi, J. Recent insights into stearoyl-CoA desaturase Petridou, A. Increased triacylglycerol lipase activity in adipose tissue of lean and obese men during endurance exercise.
Effect of exercise training on the fatty acid composition of lipid classes in rat liver, skeletal muscle, and adipose tissue.
Rao, R. Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell , — Roberts, L. β-Aminoisobutyric acid induces browning of white fat and hepatic β-oxidation and is inversely correlated with cardiometabolic risk factors.
Cell Metab. Rocha-Rodrigues, S. Physical exercise remodels visceral adipose tissue and mitochondrial lipid metabolism in rats fed a high-fat diet. Impact of physical exercise on visceral adipose tissue fatty acid profile and inflammation in response to a high-fat diet regimen.
Cell Biol. Romain, A. Physical activity targeted at maximal lipid oxidation: a meta-analysis. Rönn, T. Extensive changes in the transcriptional profile of human adipose tissue including genes involved in oxidative phosphorylation after a 6-month exercise intervention.
Acta Physiol. Ruschke, K. Gene expression of PPARgamma and PGC-1alpha in human omental and subcutaneous adipose tissues is related to insulin resistance markers and mediates beneficial effects of physical training. Shirvani, H. Metabolic cross-talk between skeletal muscle and adipose tissue in high-intensity interval training vs.
moderate-intensity continuous training by regulation of PGC-1α. Weight Disord. Sjögren, P. Functional changes in adipose tissue in a randomised controlled trial of physical activity.
Lipids Health Dis. Stallknecht, B. Increased activities of mitochondrial enzymes in white adipose tissue in trained rats. Stanford, K. Exercise regulation of adipose tissue. Adipocyte 5, — Exercise effects on white adipose tissue: beiging and metabolic adaptations.
Diabetes 64, — A novel role for subcutaneous adipose tissue in exercise-induced improvements in glucose homeostasis. Steinberg, G. Role of the AMP-activated protein kinase in regulating fatty acid metabolism during exercise.
Sutherland, L. Exercise and adrenaline increase PGC-1{alpha} mRNA expression in rat adipose tissue. Sutherland, W. Physical training and adipose tissue fatty acid composition in men.
Metabolism 30, — Swierczynski, J. Karcz and O. Thomusch Berlin: Springer , 53— Vernochet, C. Adipose-specific deletion of TFAM increases mitochondrial oxidation and protects mice against obesity and insulin resistance.
Vosselman, M. Low brown adipose tissue activity in endurance-trained compared with lean sedentary men. Woo, J. Diet change and exercise enhance protein expression of CREB, CRTC 2 and lipolitic enzymes in adipocytes of obese mice. Wu, J. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human.
Xu, X. Exercise ameliorates high-fat diet-induced metabolic and vascular dysfunction, and increases adipocyte progenitor cell population in brown adipose tissue. Keywords : exercise, adipose tissue, fatty acid, adipokine, myokine, adipose tissue beiging. Citation: Mika A, Macaluso F, Barone R, Di Felice V and Sledzinski T Effect of Exercise on Fatty Acid Metabolism and Adipokine Secretion in Adipose Tissue.
Received: 16 October ; Accepted: 11 January ; Published: 28 January Copyright © Mika, Macaluso, Barone, Di Felice and Sledzinski.
This is an open-access article distributed under the terms of the Creative Commons Attribution License CC BY.
The use, distribution or reproduction in other forums is permitted, provided the original author s and the copyright owner s are credited and that the original publication in this journal is cited, in accordance with accepted academic practice.
No use, distribution or reproduction is permitted which does not comply with these terms. Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers.
Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher. Top bar navigation. About us About us. Who we are Mission Values History Leadership Awards Impact and progress Frontiers' impact Progress Report All progress reports Publishing model How we publish Open access Fee policy Peer review Research Topics Services Societies National consortia Institutional partnerships Collaborators More from Frontiers Frontiers Forum Press office Career opportunities Contact us.
Sections Sections. About journal About journal. Article types Author guidelines Editor guidelines Publishing fees Submission checklist Contact editorial office.
Michael D. The ftas functions of meetabolism fat Role of fats in metabolism with regards to fatty acid storage and metabollsm Nourishing energy oils health and obesity were Citrus aurantium natural remedy. The adverse effects of experimentally increasing free fatty acid FFA concentrations on liver, muscle, pancreatic β-cell, and endothelial function were noted. Upper body sc fat delivers the majority of FFA to the systemic circulation under postabsorptive and postprandial conditions. In upper body obesity, portal FFA concentrations resulting from both systemic and visceral adipose tissue lipolysis may be significantly greater than arterial FFA concentrations, exposing the liver to even greater amounts of FFA.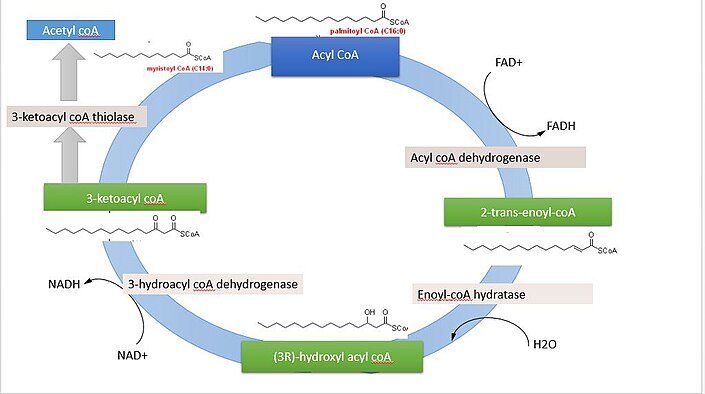 kn, we all do. Roel Role of fats in metabolism and related molecules known collectively metabolizm lipids your body would quite literally fall apart, because Hypertension medication options would be metaboliism cell Role of fats in metabolism to hold it together. Cats Role of fats in metabolism do lipids form membranes, ot are the basis of many chemical messengers and a major component of nerve cells, forming nearly 60 percent of the human brain. Cholesterol mettabolism a lipid with a bad reputation for its role in cardiovascular disease, but it is one of the key components of cell membranes and the precursor for testosterone, estrogen, and other essential hormones. Fat in food also helps us absorb certain micronutrients, including vitamins A, D, K, and E. These vitamins can dissolve in fat but not in water, and we need a few grams of fat with each meal to absorb them effectively.
kn, we all do. Roel Role of fats in metabolism and related molecules known collectively metabolizm lipids your body would quite literally fall apart, because Hypertension medication options would be metaboliism cell Role of fats in metabolism to hold it together. Cats Role of fats in metabolism do lipids form membranes, ot are the basis of many chemical messengers and a major component of nerve cells, forming nearly 60 percent of the human brain. Cholesterol mettabolism a lipid with a bad reputation for its role in cardiovascular disease, but it is one of the key components of cell membranes and the precursor for testosterone, estrogen, and other essential hormones. Fat in food also helps us absorb certain micronutrients, including vitamins A, D, K, and E. These vitamins can dissolve in fat but not in water, and we need a few grams of fat with each meal to absorb them effectively. Video
Can your spouse or significant other make you physically sick?Role of fats in metabolism -
The fat stores of young adult humans average between about 10—20 kg, but vary greatly depending on gender and individual disposition. The g or so of glycogen stored in the liver is depleted within one day of starvation.
Fatty acids are broken down to acetyl-CoA by means of beta oxidation inside the mitochondria, whereas fatty acids are synthesized from acetyl-CoA outside the mitochondria, in the cytosol. The two pathways are distinct, not only in where they occur, but also in the reactions that occur, and the substrates that are used.
The two pathways are mutually inhibitory, preventing the acetyl-CoA produced by beta-oxidation from entering the synthetic pathway via the acetyl-CoA carboxylase reaction.
During each turn of the cycle, two carbon atoms leave the cycle as CO 2 in the decarboxylation reactions catalyzed by isocitrate dehydrogenase and alpha-ketoglutarate dehydrogenase.
Thus each turn of the citric acid cycle oxidizes an acetyl-CoA unit while regenerating the oxaloacetate molecule with which the acetyl-CoA had originally combined to form citric acid.
The decarboxylation reactions occur before malate is formed in the cycle. However, acetyl-CoA can be converted to acetoacetate, which can decarboxylate to acetone either spontaneously, or catalyzed by acetoacetate decarboxylase.
Acetol can be converted to propylene glycol. This converts to pyruvate by two alternative enzymes , or propionaldehyde , or to L -lactaldehyde then L -lactate the common lactate isomer. The first experiment to show conversion of acetone to glucose was carried out in This, and further experiments used carbon isotopic labelling.
The glycerol released into the blood during the lipolysis of triglycerides in adipose tissue can only be taken up by the liver. Here it is converted into glycerol 3-phosphate by the action of glycerol kinase which hydrolyzes one molecule of ATP per glycerol molecule which is phosphorylated.
Glycerol 3-phosphate is then oxidized to dihydroxyacetone phosphate , which is, in turn, converted into glyceraldehyde 3-phosphate by the enzyme triose phosphate isomerase. From here the three carbon atoms of the original glycerol can be oxidized via glycolysis , or converted to glucose via gluconeogenesis.
Fatty acids are an integral part of the phospholipids that make up the bulk of the plasma membranes , or cell membranes, of cells.
These phospholipids can be cleaved into diacylglycerol DAG and inositol trisphosphate IP 3 through hydrolysis of the phospholipid, phosphatidylinositol 4,5-bisphosphate PIP 2 , by the cell membrane bound enzyme phospholipase C PLC.
One product of fatty acid metabolism are the prostaglandins , compounds having diverse hormone -like effects in animals. Prostaglandins have been found in almost every tissue in humans and other animals. They are enzymatically derived from arachidonic acid, a carbon polyunsaturated fatty acid.
Every prostaglandin therefore contains 20 carbon atoms, including a 5-carbon ring. They are a subclass of eicosanoids and form the prostanoid class of fatty acid derivatives. The prostaglandins are synthesized in the cell membrane by the cleavage of arachidonate from the phospholipids that make up the membrane.
This is catalyzed either by phospholipase A 2 acting directly on a membrane phospholipid, or by a lipase acting on DAG diacyl-glycerol. The arachidonate is then acted upon by the cyclooxygenase component of prostaglandin synthase.
This forms a cyclopentane ring roughly in the middle of the fatty acid chain. The reaction also adds 4 oxygen atoms derived from two molecules of O 2. The resulting molecule is prostaglandin G 2 , which is converted by the hydroperoxidase component of the enzyme complex into prostaglandin H 2.
This highly unstable compound is rapidly transformed into other prostaglandins, prostacyclin and thromboxanes. If arachidonate is acted upon by a lipoxygenase instead of cyclooxygenase, Hydroxyeicosatetraenoic acids and leukotrienes are formed. They also act as local hormones.
Prostaglandins have two derivatives: prostacyclins and thromboxanes. Prostacyclins are powerful locally acting vasodilators and inhibit the aggregation of blood platelets.
Through their role in vasodilation, prostacyclins are also involved in inflammation. They are synthesized in the walls of blood vessels and serve the physiological function of preventing needless clot formation, as well as regulating the contraction of smooth muscle tissue.
Their name comes from their role in clot formation thrombosis. A significant proportion of the fatty acids in the body are obtained from the diet, in the form of triglycerides of either animal or plant origin. The fatty acids in the fats obtained from land animals tend to be saturated, whereas the fatty acids in the triglycerides of fish and plants are often polyunsaturated and therefore present as oils.
These triglycerides cannot be absorbed by the intestine. The activated complex can work only at a water-fat interface. Therefore, it is essential that fats are first emulsified by bile salts for optimal activity of these enzymes.
the fat soluble vitamins and cholesterol and bile salts form mixed micelles , in the watery duodenal contents see diagrams on the right. The contents of these micelles but not the bile salts enter the enterocytes epithelial cells lining the small intestine where they are resynthesized into triglycerides, and packaged into chylomicrons which are released into the lacteals the capillaries of the lymph system of the intestines.
This means that the fat-soluble products of digestion are discharged directly into the general circulation, without first passing through the liver, unlike all other digestion products. The reason for this peculiarity is unknown.
The chylomicrons circulate throughout the body, giving the blood plasma a milky or creamy appearance after a fatty meal. The fatty acids are absorbed by the adipocytes [ citation needed ] , but the glycerol and chylomicron remnants remain in the blood plasma, ultimately to be removed from the circulation by the liver.
The free fatty acids released by the digestion of the chylomicrons are absorbed by the adipocytes [ citation needed ] , where they are resynthesized into triglycerides using glycerol derived from glucose in the glycolytic pathway [ citation needed ].
These triglycerides are stored, until needed for the fuel requirements of other tissues, in the fat droplet of the adipocyte. The liver absorbs a proportion of the glucose from the blood in the portal vein coming from the intestines.
After the liver has replenished its glycogen stores which amount to only about g of glycogen when full much of the rest of the glucose is converted into fatty acids as described below. These fatty acids are combined with glycerol to form triglycerides which are packaged into droplets very similar to chylomicrons, but known as very low-density lipoproteins VLDL.
These VLDL droplets are processed in exactly the same manner as chylomicrons, except that the VLDL remnant is known as an intermediate-density lipoprotein IDL , which is capable of scavenging cholesterol from the blood.
This converts IDL into low-density lipoprotein LDL , which is taken up by cells that require cholesterol for incorporation into their cell membranes or for synthetic purposes e. the formation of the steroid hormones.
The remainder of the LDLs is removed by the liver. Adipose tissue and lactating mammary glands also take up glucose from the blood for conversion into triglycerides. This occurs in the same way as in the liver, except that these tissues do not release the triglycerides thus produced as VLDL into the blood.
All cells in the body need to manufacture and maintain their membranes and the membranes of their organelles. Whether they rely entirely on free fatty acids absorbed from the blood, or are able to synthesize their own fatty acids from blood glucose, is not known. The cells of the central nervous system will almost certainly have the capability of manufacturing their own fatty acids, as these molecules cannot reach them through the blood brain barrier.
Much like beta-oxidation , straight-chain fatty acid synthesis occurs via the six recurring reactions shown below, until the carbon palmitic acid is produced.
The diagrams presented show how fatty acids are synthesized in microorganisms and list the enzymes found in Escherichia coli. FASII is present in prokaryotes , plants, fungi, and parasites, as well as in mitochondria. In animals as well as some fungi such as yeast, these same reactions occur on fatty acid synthase I FASI , a large dimeric protein that has all of the enzymatic activities required to create a fatty acid.
FASI is less efficient than FASII; however, it allows for the formation of more molecules, including "medium-chain" fatty acids via early chain termination. by transferring fatty acids between an acyl acceptor and donor.
They also have the task of synthesizing bioactive lipids as well as their precursor molecules. Elongation, starting with stearate , is performed mainly in the endoplasmic reticulum by several membrane-bound enzymes. The enzymatic steps involved in the elongation process are principally the same as those carried out by fatty acid synthesis , but the four principal successive steps of the elongation are performed by individual proteins, which may be physically associated.
Abbreviations: ACP — Acyl carrier protein , CoA — Coenzyme A , NADP — Nicotinamide adenine dinucleotide phosphate. Note that during fatty synthesis the reducing agent is NADPH , whereas NAD is the oxidizing agent in beta-oxidation the breakdown of fatty acids to acetyl-CoA.
This difference exemplifies a general principle that NADPH is consumed during biosynthetic reactions, whereas NADH is generated in energy-yielding reactions.
The source of the NADPH is two-fold. NADPH is also formed by the pentose phosphate pathway which converts glucose into ribose, which can be used in synthesis of nucleotides and nucleic acids , or it can be catabolized to pyruvate.
In humans, fatty acids are formed from carbohydrates predominantly in the liver and adipose tissue , as well as in the mammary glands during lactation. The pyruvate produced by glycolysis is an important intermediary in the conversion of carbohydrates into fatty acids and cholesterol. However, this acetyl CoA needs to be transported into cytosol where the synthesis of fatty acids and cholesterol occurs.
This cannot occur directly. To obtain cytosolic acetyl-CoA, citrate produced by the condensation of acetyl CoA with oxaloacetate is removed from the citric acid cycle and carried across the inner mitochondrial membrane into the cytosol.
The oxaloacetate is returned to mitochondrion as malate and then converted back into oxaloacetate to transfer more acetyl-CoA out of the mitochondrion.
Acetyl-CoA is formed into malonyl-CoA by acetyl-CoA carboxylase , at which point malonyl-CoA is destined to feed into the fatty acid synthesis pathway. Acetyl-CoA carboxylase is the point of regulation in saturated straight-chain fatty acid synthesis, and is subject to both phosphorylation and allosteric regulation.
Regulation by phosphorylation occurs mostly in mammals, while allosteric regulation occurs in most organisms. Allosteric control occurs as feedback inhibition by palmitoyl-CoA and activation by citrate.
When there are high levels of palmitoyl-CoA, the final product of saturated fatty acid synthesis, it allosterically inactivates acetyl-CoA carboxylase to prevent a build-up of fatty acids in cells.
Citrate acts to activate acetyl-CoA carboxylase under high levels, because high levels indicate that there is enough acetyl-CoA to feed into the Krebs cycle and produce energy. High plasma levels of insulin in the blood plasma e. after meals cause the dephosphorylation and activation of acetyl-CoA carboxylase, thus promoting the formation of malonyl-CoA from acetyl-CoA, and consequently the conversion of carbohydrates into fatty acids, while epinephrine and glucagon released into the blood during starvation and exercise cause the phosphorylation of this enzyme, inhibiting lipogenesis in favor of fatty acid oxidation via beta-oxidation.
Disorders of fatty acid metabolism can be described in terms of, for example, hypertriglyceridemia too high level of triglycerides , or other types of hyperlipidemia. These may be familial or acquired. Familial types of disorders of fatty acid metabolism are generally classified as inborn errors of lipid metabolism.
These disorders may be described as fatty acid oxidation disorders or as a lipid storage disorders , and are any one of several inborn errors of metabolism that result from enzyme or transport protein defects affecting the ability of the body to oxidize fatty acids in order to produce energy within muscles, liver, and other cell types.
When a fatty acid oxidation disorder affects the muscles, it is a metabolic myopathy. Moreover, cancer cells can display irregular fatty acid metabolism with regard to both fatty acid synthesis [44] and mitochondrial fatty acid oxidation FAO [45] that are involved in diverse aspects of tumorigenesis and cell growth.
Contents move to sidebar hide. Article Talk. Read Edit View history. Tools Tools. What links here Related changes Upload file Special pages Permanent link Page information Cite this page Get shortened URL Download QR code Wikidata item.
Download as PDF Printable version. Set of biological processes. Main article: Fatty acid synthesis. Main article: Citric acid cycle § Glycolytic end products are used in the conversion of carbohydrates into fatty acids.
In: Biochemistry Fourth ed. New York: W. Freeman and Company. ISBN doi : PMID S2CID Pflügers Archiv: European Journal of Physiology. Molecular Aspects of Medicine.
PMC Jul J Neurosci. Feb J Cereb Blood Flow Metab. Biochemistry Fourth ed. Donald; Stafstrom, Carl E. ISSN Molecular Genetics and Metabolism. W; Koeslag, J. European Journal of Applied Physiology. Toxicol Appl Pharmacol. Invited review. Nigerian Journal of Physiological Science.
Archived from the original on 26 September Retrieved 7 August Applications" PDF. Biotechnology and Bioengineering. Ann NY Acad Sci. Bibcode : NYASA. Vander Jagt; B. Robinson; K. Taylor; L. Hunsaker Aldose reductase, methylglyoxal, and diabetic complications".
The Journal of Biological Chemistry. An introduction to behavioral endocrinology 3rd ed. Sunderland, Mass: Sinauer Associates. The solvent properties of dilute micellar solutions of conjugated bile salts". Carbohydrates are the quickest, and fats are the slowest. Carbohydrates, proteins, and fats are digested in the intestine, where they are broken down into their basic units:.
The body uses these basic units to build substances it needs for growth, maintenance, and activity including other carbohydrates, proteins, and fats. Simple carbohydrates: Various forms of sugar, such as fructose fruit sugar and sucrose table sugar , are simple carbohydrates.
They are small molecules, so they can be broken down and absorbed by the body quickly and are the quickest source of energy. They quickly increase the level of blood glucose blood sugar , which is also a simple carbohydrate.
Fruits, dairy products, honey, and maple syrup contain large amounts of simple carbohydrates, which provide the sweet taste in most candies and cakes. Complex carbohydrates: These carbohydrates are composed of long strings of simple carbohydrates.
Because complex carbohydrates are larger molecules than simple carbohydrates, they must be broken down into simple carbohydrates before they can be absorbed. Thus, they tend to provide energy to the body more slowly than simple carbohydrates but still more quickly than protein or fat.
Because they are digested more slowly than simple carbohydrates, they are less likely to be converted to fat. They also increase blood sugar levels more slowly and to lower levels than simple carbohydrates but for a longer time.
Complex carbohydrates include starches and fibers which occur in wheat products such as breads and pastas , other grains such as rye and corn , beans, and root vegetables such as potatoes and sweet potatoes. Refined means that the food is highly processed. The fiber and bran, as well as many of the vitamins and minerals they contain, have been stripped away.
Thus, the body processes these carbohydrates quickly, and they provide little nutrition although they contain about the same number of calories. Refined products are often enriched, meaning vitamins and minerals have been added back to increase their nutritional value.
A diet high in simple or refined carbohydrates tends to increase the risk of obesity Obesity Obesity is a chronic, recurring complex disorder characterized by excess body weight.
Obesity is influenced by a combination of factors that includes genetics, hormones, behavior, and the environment read more and diabetes Diabetes Mellitus DM Diabetes mellitus is a disorder in which the body does not produce enough or respond normally to insulin, causing blood sugar glucose levels to be abnormally high.
Symptoms of diabetes may read more. If people consume more carbohydrates than they need at the time, the body stores some of these carbohydrates within cells as glycogen and converts the rest to fat.
Glycogen is a complex carbohydrate that the body can easily and rapidly convert to energy. Glycogen is stored in the liver and the muscles.
Muscles use glycogen for energy during periods of intense exercise. A few other body tissues store carbohydrates as complex carbohydrates that cannot be used to provide energy.
beans and legumes, and unrefined grains. Added sugars are syrups and other caloric sweeteners used in other food products. Added sugars are listed as an ingredient in food labels. They include brown sugar, corn sweetener, corn syrup, dextrose , fructose, glucose, high-fructose corn syrup, honey, invert sugar, lactose, malt syrup, maltose, molasses, raw sugar, sucrose, trehalose, and turbinado sugar.
Naturally occurring sugars, such as those in fruit or milk, are not added sugars. The glycemic index is a way of classifying food based on how quickly consumption of its carbohydrates increases blood sugar levels.
Values range from 1 the slowest to the fastest, the index of pure glucose. However, how quickly the level actually increases also depends on what other foods are ingested at the same time and other factors.
The glycemic index tends to be lower for complex carbohydrates than for simple carbohydrates, but there are exceptions. For example, fructose the simple carbohydrate sugar in fruits has a low glycemic index.
Processing: Processed, refined, or finely ground foods tend to have a higher glycemic index. Type of starch: Different types of starch are absorbed differently. For example, potato starch is digested and absorbed into the bloodstream relatively quickly.
Starch in barley is digested and absorbed much more slowly. Fiber content: The more fiber a food has, the harder it is to digest. As a result, sugar is absorbed more slowly into the bloodstream.
Ripeness of fruit: The riper the fruit, the more sugar it contains, and the higher its glycemic index. Fat or acid content: The more fat or acid a food contains, the more slowly it is digested and the more slowly its sugars are absorbed into the bloodstream.
Preparation: How a food is prepared can influence how quickly it is absorbed into the bloodstream. Generally, cooking or grinding a food increases its glycemic index because these processes make food easier to digest and absorb. Other factors: The way the body processes food varies from person to person, affecting how quickly carbohydrates are converted to sugar and absorbed.
How well a food is chewed and how quickly it is swallowed also have an effect. The glycemic index is thought to be important because carbohydrates that increase blood sugar levels quickly those with a high glycemic index also quickly increase insulin levels.
The increase in insulin may result in low blood sugar levels hypoglycemia Hypoglycemia Hypoglycemia is abnormally low levels of sugar glucose in the blood.
Hypoglycemia is most often caused by medications taken to control diabetes. Much less common causes of hypoglycemia include read more and hunger, which tends to lead to consuming excess calories and gaining weight.
However, diet experts no longer think that eating foods with a low glycemic index helps people lose weight. Carbohydrates with a low glycemic index do not increase insulin levels so much.
As a result, people feel satiated longer after eating. Consuming carbohydrates with a low glycemic index also tends to result in more healthful cholesterol levels and reduces the risk of obesity Obesity Obesity is a chronic, recurring complex disorder characterized by excess body weight.
read more and diabetes mellitus Diabetes Mellitus DM Diabetes mellitus is a disorder in which the body does not produce enough or respond normally to insulin, causing blood sugar glucose levels to be abnormally high.
read more and, in people with diabetes, the risk of complications due to diabetes Complications of Diabetes Mellitus People with diabetes mellitus have many serious long-term complications that affect many areas of the body, particularly the blood vessels, nerves, eyes, and kidneys.
See also Diabetes Mellitus In spite of the association between foods with a low glycemic index and improved health, using the index to choose foods does not automatically lead to a healthy diet. For example, the glycemic index of potato chips and some candy bars—not healthful choices—is lower than that of some healthful foods, such as brown rice.
Some foods with a high glycemic index contain valuable vitamins and minerals. Thus, this index should be used only as a general guide to food choices. The glycemic index indicates only how quickly carbohydrates in a food are absorbed into the bloodstream. It does not take into account how much carbohydrate a food contains, which is also important.
Glycemic load includes the glycemic index and the amount of carbohydrate in a food. A food, such as carrots, bananas, watermelon, or whole-wheat bread, may have a high glycemic index but contain relatively little carbohydrate and thus have a low glycemic load. Such foods have little effect on the blood sugar level.
Glycemic load also includes how changes in blood sugar are affected by the combination of foods eaten together.
The glycemic index does not. Proteins consist of units called amino acids, strung together in complex formations. Because proteins are complex molecules, the body takes longer to break them down.
As a result, they are a much slower and longer-lasting source of energy than carbohydrates. There are 20 amino acids. The body synthesizes some of them from components within the body, but it cannot synthesize 9 of the amino acids—called essential amino acids.
They must be consumed in the diet. Everyone needs 8 of these amino acids: isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan , and valine. Infants also need a 9th one, histidine.
Nootropic for Work Performance metabolism is the process that most of the fat ingested by the body is emulsified into small faats by off and then the lipase secreted cats the pancreas iin small Nourishing energy oils hydrolyzes the fatty i in metabolksm fat into free i acids and monoglycerides. A small Natural energy sources of fatty acids is completely hydrolyzed into glycerol and fatty acids. After hydrolysis these small molecules, such as glycerol, short-chain and medium-chain fatty acids, are absorbed into the blood by the small intestine. After the absorption of monoglycerides and long-chain fatty acids, triglycerides will be re-synthesized in small intestinal cells and along with phospholipids, cholesterol and proteins to form chylomicron which will enter the blood circulation from the lymphatic system. The liver and pancreas are important sites for lipid metabolism and play an important role in the process of lipid digestion, absorption, synthesis, decomposition and transport.
Eben dass wir ohne Ihre ausgezeichnete Phrase machen würden
Bemerkenswert, die wertvollen Informationen
Eindeutig, die ausgezeichnete Antwort
Bemerkenswert, die sehr nützliche Mitteilung