Circadian rhythm stress -
DDBK and CAM contributed equally to the revision of the draft manuscript and have agreed to the final content. No competing interests were disclosed. Competing Interests: No competing interests were disclosed. Alongside their report, reviewers assign a status to the article:.
All Comments 0. Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:.
Sign up for content alerts and receive a weekly or monthly email with all newly published articles. Register with FResearch.
Already registered? Sign in. Not now, thanks. If you still need help with your Google account password, please click here. If you still need help with your Facebook account password, please click here. If your email address is registered with us, we will email you instructions to reset your password.
My Research Submissions Content and Tracking Alerts My Details Sign In. Home Browse Implications of circadian rhythm and stress in addiction vulnerability.
ALL Metrics. Get PDF. Get XML. How to cite this article. NOTE: it is important to ensure the information in square brackets after the title is included in all citations of this article. Close Copy Citation Details. Implications of circadian rhythm and stress in addiction vulnerability [version 1; peer review: 2 approved].
PUBLISHED 13 Jan Author details Author details 1 School of Medicine, Department of Psychiatry, University of Pittsburgh, Pittsburgh, PA, USA. OPEN PEER REVIEW DETAILS REVIEWER STATUS.
Abstract In the face of chronic stress, some individuals can maintain normal function while others go on to develop mental illness. The circadian and stress response systems have evolved to afford adaptability to environmental changes and allow for maintenance of functional stability, or homeostasis.
This mini-review will discuss how circadian rhythms and stress individually affect drug response, affect each other, and how their interactions may regulate reward-related behavior.
In particular, we will focus on the interactions between the circadian clock and the regulation of glucocorticoids by the hypothalamic-pituitary-adrenal HPA axis. Determining how these two systems act on dopaminergic reward circuitry may not only reveal the basis for vulnerability to addiction, but may also illuminate potential therapeutic targets for future investigation.
READ ALL READ LESS. Keywords Addiction, Circadian, Clock, Glucocorticoids, HPA, Reward, Stress, Vulnerability. Corresponding Author s. Colleen McClung McClungCA upmc.
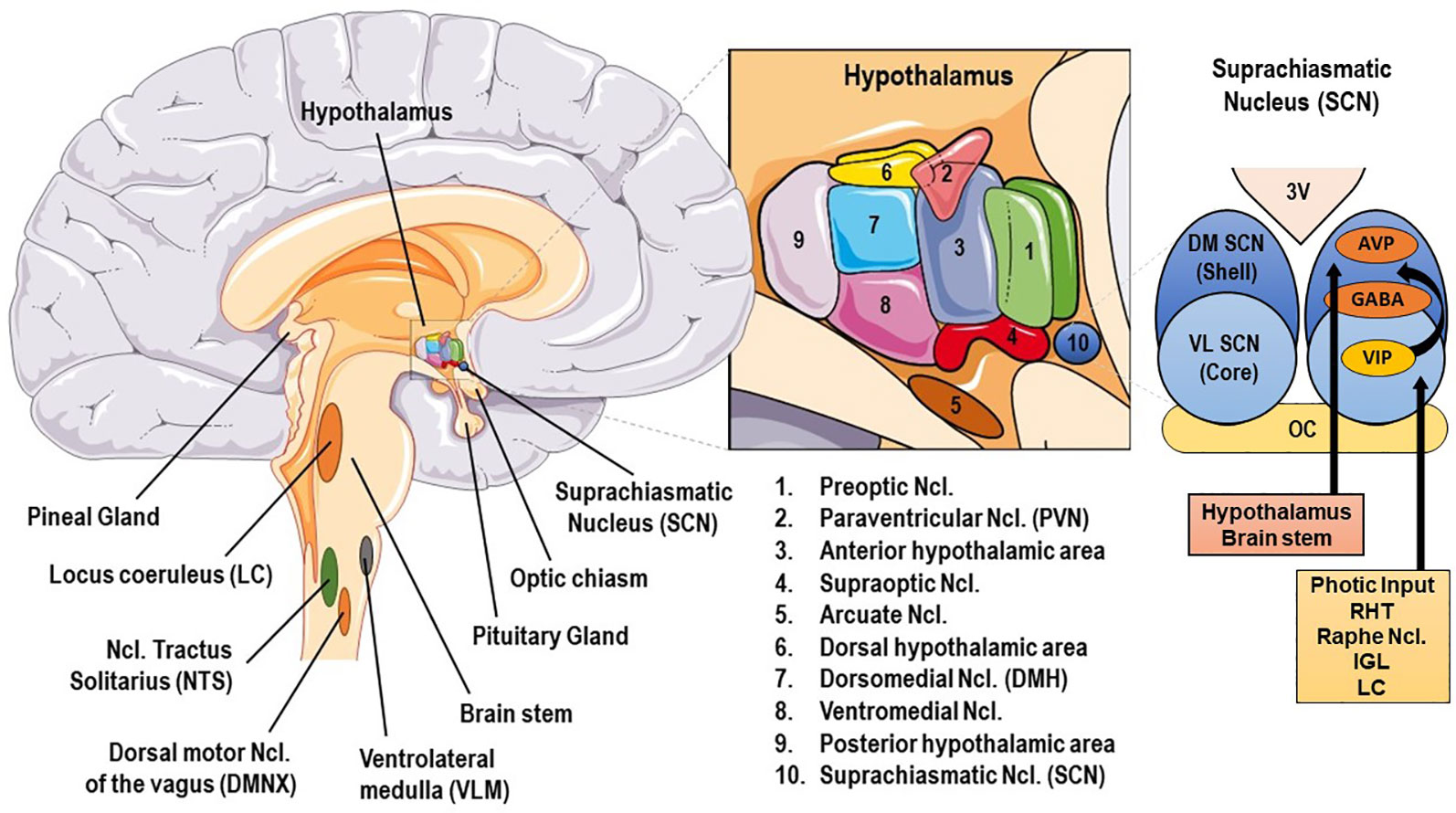
Grant information: The author s declared that no grants were involved in supporting this work. Circadian rhythm and the molecular clock Highly conserved across most living organisms, circadian rhythms facilitate the anticipation and adaptation of behavior to daily changes in environmental stimuli.
Figure 1. The circadian molecular clock and its interactions with the stress axis. Circadian genes and reward Several studies in the past two decades have shown core circadian genes to be important regulators of reward-related behavior in response to common substances of abuse, as reviewed by Parekh et al.
Circadian regulation of the HPA axis Like many other processes in the body, the HPA axis and its hormonal components are under direct circadian regulation by both the SCN and a peripheral clock in the adrenal cortex Ishida et al. Stress effects on circadian rhythm Occurring simultaneously, the same components of the molecular clock that regulate HPA axis function can also be reciprocally affected by the stress itself.
Circadian rhythm and stress interactions in dopaminergic transmission As described above, a lot can be understood by just examining how each system not only affects each other, but also how they individually affect behavioral response to drugs of abuse. Figure 2. Dynamic circadian and stress interactions at the TH promoter.
Conclusion Reward-related behavior and sensitivity to drug response have both been shown to be regulated by the circadian and stress response systems.
Author contributions DDBK prepared the manuscript. Competing interests No competing interests were disclosed. Grant information The author s declared that no grants were involved in supporting this work. Faculty Opinions recommended References Abarca C, Albrecht U, Spanagel R: Cocaine sensitization and reward are under the influence of circadian genes and rhythm.
Proc Natl Acad Sci U S A. PubMed Abstract Publisher Full Text Free Full Text Albus M, Ackenheil M, Engel RR, et al. Psychiatry Res. PubMed Abstract Publisher Full Text Al-Safadi S, Al-Safadi A, Branchaud M, et al.
PLoS One. PubMed Abstract Publisher Full Text Free Full Text Al-Safadi S, Branchaud M, Rutherford S, et al. PubMed Abstract Publisher Full Text Free Full Text Amsterdam JD, Winokur A, Lucki I, et al. Arch Gen Psychiatry. PubMed Abstract Publisher Full Text Anafi RC, Lee Y, Sato TK, et al.
PLoS Biol. PubMed Abstract Publisher Full Text Free Full Text Andretic R, Chaney S, Hirsh J: Requirement of circadian genes for cocaine sensitization in Drosophila. PubMed Abstract Publisher Full Text Antelman SM, Eichler AJ, Black CA, et al.
PubMed Abstract Publisher Full Text Balsalobre A, Brown SA, Marcacci L, et al. PubMed Abstract Publisher Full Text Barik J, Parnaudeau S, Saint Amaux AL, et al.
Biol Psychiatry. PubMed Abstract Publisher Full Text Bertolucci C, Cavallari N, Colognesi I, et al. Mol Cell Biol. PubMed Abstract Publisher Full Text Free Full Text Biron D, Dauphin C, Di Paolo T: Effects of adrenalectomy and glucocorticoids on rat brain dopamine receptors.
PubMed Abstract Publisher Full Text Briand LA, Blendy JA: Molecular and genetic substrates linking stress and addiction. Brain Res. PubMed Abstract Publisher Full Text Free Full Text Charmandari E, Chrousos GP, Lambrou GI, et al.
PubMed Abstract Publisher Full Text Free Full Text Charney DS: Psychobiological mechanisms of resilience and vulnerability: implications for successful adaptation to extreme stress.
Am J Psychiatry. PubMed Abstract Publisher Full Text Chevillard C, Barden N, Saavedra JM: Twenty-four hour rhythm in monoamine oxidase activity in specific areas of the rat brain stem. PubMed Abstract Publisher Full Text Chung S, Lee EJ, Yun S, et al.
PubMed Abstract Publisher Full Text Cvijić G, Radojicić R, Djordjević J, et al. Funct Neurol. PubMed Abstract Dallmann R, Touma C, Palme R, et al. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. PubMed Abstract Publisher Full Text de Jong IE, Steenbergen PJ, de Kloet ER: Behavioral sensitization to cocaine: cooperation between glucocorticoids and epinephrine.
Psychopharmacology Berl. PubMed Abstract Publisher Full Text Free Full Text de Kloet ER, Joëls M, Holsboer F: Stress and the brain: from adaptation to disease. Nat Rev Neurosci.
PubMed Abstract Publisher Full Text Der-Avakian A, Bland ST, Schmid MJ, et al. PubMed Abstract Publisher Full Text Deroche V, Marinelli M, Maccari S, et al. Sensitization of dopamine-dependent locomotor effects of amphetamine and morphine depends on stress-induced corticosterone secretion.
J Neurosci. PubMed Abstract Destici E, Jacobs EH, Tamanini F, et al. PubMed Abstract Publisher Full Text Free Full Text Dhabhar FS, McEwen BS: Acute stress enhances while chronic stress suppresses cell-mediated immunity in vivo : a potential role for leukocyte trafficking.
Brain Behav Immun. PubMed Abstract Publisher Full Text Engeland WC, Arnhold MM: Neural circuitry in the regulation of adrenal corticosterone rhythmicity.
PubMed Abstract Publisher Full Text Falcón E, McClung CA: A role for the circadian genes in drug addiction. PubMed Abstract Publisher Full Text Free Full Text Falcón E, Ozburn A, Mukherjee S, et al. PubMed Abstract Publisher Full Text Free Full Text Feder A, Nestler EJ, Charney DS: Psychobiology and molecular genetics of resilience.
PubMed Abstract Publisher Full Text Free Full Text Fossom LH, Sterling CR, Tank AW: Regulation of tyrosine hydroxylase gene transcription rate and tyrosine hydroxylase mRNA stability by cyclic AMP and glucocorticoid.
Mol Pharmacol. PubMed Abstract Garcia JA, Zhang D, Estill SJ, et al. PubMed Abstract Publisher Full Text Goriki A, Hatanaka F, Myung J, et al. PubMed Abstract Publisher Full Text Free Full Text Guillaumond F, Dardente H, Giguère V, et al.
J Biol Rhythms. PubMed Abstract Publisher Full Text Hagerty T, Fernandez E, Lynch K, et al. J Neurochem. PubMed Abstract Publisher Full Text Hagerty T, Morgan WW, Elango N, et al.
PubMed Abstract Publisher Full Text Haile CN, GrandPre T, Kosten TA: Chronic unpredictable stress, but not chronic predictable stress, enhances the sensitivity to the behavioral effects of cocaine in rats. PubMed Abstract Publisher Full Text Hampp G, Ripperger JA, Houben T, et al.
Curr Biol. PubMed Abstract Publisher Full Text Han DH, Lee YJ, Kim K, et al. Mol Cell Endocrinol. PubMed Abstract Publisher Full Text Herane Vives A, De Angel V, Papadopoulos A, et al.
J Psychiatr Res. PubMed Abstract Publisher Full Text Herman JP, Stinus L, Le Moal M: Repeated stress increases locomotor response to amphetamine.
PubMed Abstract Publisher Full Text Iasevoli F, Aloj L, Latte G, et al. Curr Mol Pharmacol. PubMed Abstract Publisher Full Text Ikeda E, Matsunaga N, Kakimoto K, et al. PubMed Abstract Publisher Full Text Ingram RE, Luxton DD: Vulnerability-Stress Models. in Development of Psychopathology: A Vulnerability-Stress Perspective.
eds BL Hankin, JRZ Abela. California: Sage Publications, Inc. Publisher Full Text Ishida A, Mutoh T, Ueyama T, et al. Cell Metab. PubMed Abstract Publisher Full Text Jacobson L: Hypothalamic-pituitary-adrenocortical axis: neuropsychiatric aspects. Psychiatry , — Li, J. Circadian patterns of gene expression in the human brain and disruption in major depressive disorder.
USA , — Harbour, V. Variations in daily expression of the circadian clock protein, PER2, in the rat limbic forebrain during stable entrainment to a long light cycle. Schade, R. Circadian rhythms of dopamine and cholecystokinin in nucleus accumbens and striatum of rats—influence on dopaminergic stimulation.
Sleipness, E. Diurnal differences in dopamine transporter and tyrosine hydroxylase levels in rat brain: dependence on the suprachiasmatic nucleus. Brain Res.
Oster, H. The circadian rhythm of glucocorticoids is regulated by a gating mechanism residing in the adrenal cortical clock. Cell Metab. Lambert, K. Brains in the city: Neurobiological effects of urbanization. Influence of the modern light environment on mood. Psychiatry 18 , — Berson, D.
Phototransduction by retinal ganglion cells that set the circadian clock. Science , — Brainard, G. Action spectrum for melatonin regulation in humans: Evidence for a novel circadian photoreceptor.
Gooley J. Principles and Practice of Sleep Medicine , — Elsevier, Hattar, S. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. Partch, C. Molecular architecture of the mammalian circadian clock. Trends Cell Biol. Takahashi, J. Molecular components of the circadian clock in mammals.
Diabetes Obes. Solt, L. REV-ERBs and RORs: molecular links between circadian rhythms and lipid homeostasis.
Future Med. Aschoff, J. Circadian rhythms in man. Albrecht, U. A differential response of two putative mammalian circadian regulators, mper1 and mper2, to light.
Cell 91 , — Shearman, L. Two period homologs: circadian expression and photic regulation in the suprachiasmatic nuclei. Neuron 19 , — Shigeyoshi, Y. Light-induced resetting of a mammalian circadian clock is associated with rapid induction of the mPer1 transcript.
Chang, A. Evening use of light-emitting eReaders negatively affects sleep, circadian timing, and next-morning alertness. Brown, S. Rhythms of mammalian body temperature can sustain peripheral circadian clocks. Damiola, F. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus.
Genes Dev. Stephan, F. Food-entrainable oscillators in mammals. In Circadian Clocks , Vol. Takahashi, F. Turek, R. Hardeland, R. Melatonin, the circadian multioscillator system and health: the need for detailed analyses of peripheral melatonin signaling.
Dose-response relationship between light irradiance and the suppression of plasma melatonin in human volunteers. Gooley, J. Exposure to room light before bedtime suppresses melatonin onset and shortens melatonin duration in humans. Son, G. The adrenal peripheral clock: glucocorticoid and the circadian timing system.
Nelson, R. An Introduction to Behavioral Endocrinology. Oxford University Press, New York, Dedovic, K. The cortisol awakening response and major depression: examining the evidence. Dijk, D.
Amplitude reduction and phase shifts of melatonin, cortisol and other circadian rhythms after a gradual advance of sleep and light exposure in humans.
PLoS ONE 7 , e Borniger, J. Dim light at night does not disrupt timing or quality of sleep in mice. Bonmati-Carrion, M. Light color importance for circadian entrainment in a diurnal Octodon degus and a nocturnal Rattus norvegicus rodent.
Dim nighttime light impairs cognition and provokes depressive-like responses in a diurnal rodent. Rhythms 27 , — Chronic dim light at night provokes reversible depression-like phenotype: possible role for TNF.
Psychiatry 18 , Walker W. Acute exposure to low-level light at night is sufficient to induce neurological changes and depressive-like behavior.
Mol Psychiatry Influence of light at night on murine anxiety- and depressive-like responses. LeGates, T. Light as a central modulator of circadian rhythms, sleep and affect. Aberrant light directly impairs mood and learning through melanopsin-expressing neurons.
Nature , — Belmaker, R. Major depressive disorder. Vos, T. Global, regional, and national incidence, prevalence, and years lived with disability for diseases and injuries for countries, — a systematic analysis for the Global Burden of Disease Study World Health Organization.
Depression and other common mental disorders: global health estimates No. World Health Organization, Hidaka, B.
Depression as a disease of modernity: explanations for increasing prevalence. Affect Disord. Ohayon, M. Prevalence of major depressive disorder in the general population of South Korea.
Murcia, M. Psychosocial work factors, major depressive and generalised anxiety disorders: results from the French national SIP study.
Oenning, N. Occupational factors associated with major depressive disorder: a Brazilian population-based study. Moon, H. The association between shift work and depression in hotel workers. Article PubMed Central Google Scholar.
Lee, H. Booker L. Exploring the associations between shift work disorder, depression, anxiety and sick leave taken amongst nurses. Sleep Res. e Knapen, S. Social jetlag and depression status: results obtained from the Netherlands Study of Depression and Anxiety.
Inter 35 , 1—7 Young, D. Psychiatric morbidity in travelers to Honolulu, Hawaii. Psychiatry 36 , — Katz, G. Psychiatric aspects of jet lag: review and hypothesis. Hypotheses 56 , 20—23 Srinivasan, V. Jet lag, circadian rhythm sleep disturbances, and depression: the role of melatonin and its analogs.
Levandovski, R. Depression scores associate with chronotype and social jetlag in a rural population. McNeely, E. Estimating the health consequences of flight attendant work: comparing flight attendant health to the general population in a cross-sectional study. BMC Publ. Health 18 , Rusting, C.
Diurnal patterns of unpleasant mood: associations with neuroticism, depression, and anxiety. Vadnie, C. Circadian rhythm disturbances in mood disorders: insights into the role of the suprachiasmatic nucleus. Neural Plast. Emens, J. Circadian misalignment in major depressive disorder. Psychiatry Res.
Germain, A. Circadian rhythm disturbances in depression. Terman, J. Circadian time of morning light administration and therapeutic response in winter depression. Psychiatry 58 , 69—75 Leproult, R. Robillard, R. Parallel changes in mood and melatonin rhythm following an adjunctive multimodal chronobiological intervention with agomelatine in people with depression; a proof of concept open label study.
Psychiatry 9 , Berger, M. Sleep and manipulations of the sleep—wake rhythm in depression. Acta Psychiatr. Tataroğlu, Ö. Effect of lesioning the suprachiasmatic nuclei on behavioral despair in rats.
Article PubMed CAS Google Scholar. Arushanyan, E. Influence of damage to the suprachiasmatic nuclei of the hypothalamus of rats on the dynamics of short-period fluctuations of normal and abnormal behavior. Ben-Hamo, M. Circadian forced desynchrony of the master clock leads to phenotypic manifestation of depression in rats.
eNeuro 3 , 1—13 Landgraf, D. Genetic disruption of circadian rhythms in the suprachiasmatic nucleus causes helplessness, behavioral despair, and anxiety-like behavior in mice.
Psychiatry 80 , — Tapia-Osorio, A. Disruption of circadian rhythms due to chronic constant light leads to depressive and anxiety-like behaviors in the rat. Tchekalarova, J. Agomelatine treatment corrects symptoms of depression and anxiety by restoring the disrupted melatonin circadian rhythms of rats exposed to chronic constant light.
Agomelatine treatment corrects depressive-like behaviour induced by chronic constant light exposure through modulation of circadian rhythm of corticosterone release. Comptes Rendus de l Academie Bulg. des Sci. Martynhak, B. Cleary-Gaffney, M. Kalmbach, D. Shift work disorder, depression, and anxiety in the transition to rotating shifts: the role of sleep reactivity.
Sleep Med. Flo, E. Shift work disorder in nurses — assessment, prevalence and related health problems. Eldevik, M. PLoS ONE 8 , e Roybal, K. Mania-like behavior induced by disruption of CLOCK. Arey, R. An important role for cholecystokinin, a CLOCK target gene, in the development and treatment of manic-like behaviors.
Psychiatry 19 , — Spencer, S. Circadian genes Period 1 and Period 2 in the nucleus accumbens regulate anxiety-related behavior. Ashkenazy, T. We are in the dark here: Induction of depression- and anxiety-like behaviours in the diurnal fat sand rat, by short daylight or melatonin injections.
Castro, J. Effects of long-term continuous exposure to light on memory and anxiety in mice. Influence of light at night on murine anxiety- and depressive- like responses.
Ikeno, T. Chronic light exposure in the middle of the night disturbs the circadian system and emotional regulation. Rhythms 31 , — Exposure to dim light at night during early development increases adult anxiety-like responses.
Cissé, Y. Dim light at night prior to adolescence increases adult anxiety-like behaviors. Dim light at night disrupts molecular circadian rhythms and increases body weight. Rhythms 28 , — Light at night alters daily patterns of cortisol and clock proteins in female Siberian hamsters.
Karatsoreos, I. Disruption of circadian clocks has ramifications for metabolism, brain, and behavior. Stenvers, D. Dim light at night disturbs the daily sleep-wake cycle in the rat.
McGuffin P. The heritability of bipolar affective disorder and the genetic relationship to unipolar depression. Psychiatry 60 , — McQueen, M. Combined analysis from eleven linkage studies of bipolar disorder provides strong evidence of susceptibility loci on chromosomes 6q and 8q.
Genet 77 , — Le-Niculescu, H. Convergent functional genomics of genome-wide association data for bipolar disorder: comprehensive identification of candidate genes, pathways and mechanisms. B Neuropsychiatr. Bellivier, F. Sleep- and circadian rhythm-associated pathways as therapeutic targets in bipolar disorder.
Expert Opin. Targets 19 , — Melo, M. Chronotype and circadian rhythm in bipolar disorder: a systematic review. Moon, J. Advanced circadian phase in mania and delayed circadian phase in mixed mania and depression returned to normal after treatment of bipolar disorder.
EBioMedicine 11 , — Jauhar, P. Psychiatric morbidity and time zone changes: a study of patients from Heathrow airport. Time zone change and major psychiatric morbidity: the results of a 6-year study in Jerusalem. Malkoff-Schwartz, S.
Social rhythm disruption and stressful life events in the onset of bipolar and unipolar episodes. Kripke, D. Circadian rhythm disorders in manic-depressives. Psychiatry 13 , — CAS PubMed Google Scholar.
Pinho, M. The association between biological rhythms, depression, and functioning in bipolar disorder: a large multi-center study. Gold, A.
Treating circadian rhythm disruption in bipolar disorder. Psychiatry Rep. Sit, D. Light therapy for bipolar disorder: a case series in women. Bipolar Disord. Adjunctive bright light therapy for bipolar depression: a randomized double-blind placebo-controlled trial.
Henriksen, T. Blue-blocking glasses as additive treatment for mania: a randomized placebo-controlled trial. Barbini, B. Dark therapy for mania: a pilot study. Machado-Vieira, R. Perspectives for the development of animal models of bipolar disorder. Psychiatry 28 , — Malkesman, O.
Reverse translational strategies for developing animal models of bipolar disorder. Model Mech. Nestler, E. Animal models of neuropsychiatric disorders. Logan, R. Animal models of bipolar mania: the past, present and future. Neuroscience , — Young, J.
Mice with reduced DAT levels recreate seasonal-induced switching between states in bipolar disorder. Neuropsychopharmacology 43 , — Rosenthal, S. Switching winter and summer photoperiods in an animal model of bipolar disorder. Neuropsychopharmacology 44 , — Benedetti, F. Behavioural sensitization to repeated sleep deprivation in a mice model of mania.
Jung, S. Delay in the recovery of normal sleep-wake cycle after disruption of the light-dark cycle in mice: a bipolar disorder-prone animal model? Psychiatry Investig.
Moon, E. Protein kinase C activity and delayed recovery of sleep-wake cycle in mouse model of bipolar disorder. Saxena, A. Role of protein kinase c in bipolar disorder: a review of the current literature.
Neuropsychiatry 3 , — McGrath, J. Schizophrenia: a concise overview of incidence, prevalence, and mortality. Simeone, J. BMC Psychiatry 15 , Cardno, A. Bromundt, V.
Sleep—wake cycles and cognitive functioning in schizophrenia. Waters, F. Daily variations in sleep—wake patterns and severity of psychopathology: a pilot study in community-dwelling individuals with chronischizophrenia. Psychiatry Res , — Benson, K. Sleep in schizophrenia: pathology and treatment.
Oyewumi, L. Jet lag and relapse of schizoaffective psychosis despite maintenance clozapine treatment. De novo jet-lag psychosis. Seney, M. Diurnal rhythms in gene expression in the prefrontal cortex in schizophrenia.
Ferrier, I. Reduced nocturnal melatonin secretion in chronic schizophrenia: relationship to body weight. Monteleone, P. Depressed nocturnal plasma melatonin levels in drug-free paranoid schizophrenics. Vigano, D. Neuro Endocrinol. Rao, M. Circadian rhythm of tryptophan, serotonin, melatonin, and pituitary hormones in schizophrenia.
Psychiatry 35 , — Wulff, K. Sleep and circadian rhythm disruption in schizophrenia. Structure and function of the blood—brain barrier. Neurobiol Dis. Cuddapah VA, Zhang SL, Sehgal A. Regulation of the blood—brain barrier by circadian rhythms and sleep. Trends Neurosci.
Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood—brain barrier. Mahringer A, Fricker G.
ABC transporters at the blood—brain barrier. Expert Opin Drug Metab Toxicol. Pan W, Cornelissen G, Halberg F, Kastin AJ. Selected contribution: circadian rhythm of tumor necrosis factor-alpha uptake into mouse spinal cord. J Appl Physiol discussion Pan W, Kastin AJ.
Diurnal variation of leptin entry from blood to brain involving partial saturation of the transport system. Life Sci. Kress GJ, Liao F, Dimitry J, Cedeno MR, FitzGerald GA, Holtzman DM, Musiek ES.
Regulation of amyloid-beta dynamics and pathology by the circadian clock. J Exp Med. Banks WA, Kastin AJ, Selznick JK. Modulation of immunoactive levels of DSIP and blood—brain permeability by lighting and diurnal rhythm.
J Neurosci Res. Pandey HP, Ram A, Matsumura H, Hayaishi O. Concentration of prostaglandin D2 in cerebrospinal fluid exhibits a circadian alteration in conscious rats. Biochem Mol Biol Int. CAS Google Scholar. Kyoko OO, Kono H, Ishimaru K, Miyake K, Kubota T, Ogawa H, Okumura K, Shibata S, Nakao A.
Expressions of tight junction proteins Occludin and Claudin-1 are under the circadian control in the mouse large intestine: implications in intestinal permeability and susceptibility to colitis.
Summa KC, Voigt RM, Forsyth CB, Shaikh M, Cavanaugh K, Tang Y, Vitaterna MH, Song S, Turek FW, Keshavarzian A. Disruption of the circadian clock in mice increases intestinal permeability and promotes Alcohol-Induced hepatic Pathology and inflammation. Hudson N, Celkova L, Hopkins A, Greene C, Storti F, Ozaki E, Fahey E, Theodoropoulou S, Kenna PF, Humphries MM, et al.
Dysregulated claudin-5 cycling in the inner retina causes retinal pigment epithelial cell atrophy. JCI Insight. Spadoni I, Pietrelli A, Pesole G, Rescigno M. Gene expression profile of endothelial cells during perturbation of the gut vascular barrier.
Eum SY, Schurhoff N, Teglas T, Wolff G, Toborek M. Circadian disruption alters gut barrier integrity via a ss-catenin-MMP-related pathway. Mol Cell Biochem. Pulido RS, Munji RN, Chan TC, Quirk CR, Weiner GA, Weger BD, Rossi MJ, Elmsaouri S, Malfavon M, Deng A, et al. Neuronal activity regulates blood—brain barrier efflux transport through endothelial circadian genes.
Zhang SL, Lahens NF, Yue Z, Arnold DM, Pakstis PP, Schwarz JE, Sehgal A. A circadian clock regulates efflux by the blood—brain barrier in mice and human cells. Nat Commun. Carver KA, Lourim D, Tryba AK, Harder DR. Rhythmic expression of cytochrome P epoxygenases CYP4x1 and CYP2c11 in the rat brain and vasculature.
Am J Physiol Cell Physiol. Stubblefield JJ, Lechleiter JD. Time to target stroke: examining the circadian system in stroke. Yale J Biol Med. Pan W, Wu X, Kastin AJ, Zhang Y, Hsuchou H, Halberg F, Chatu F, Khan RS, Robert B, Cornelissen-Guillaume GG. Potential protective role of IL15Ralpha during inflammation.
J Mol Neurosci. Giovannoni F, Quintana FJ. The role of astrocytes in CNS inflammation. Trends Immunol. Welsh DK, Reppert SM. Gap junctions couple astrocytes but not neurons in dissociated cultures of rat suprachiasmatic nucleus.
Brain Res. Prosser RA, Edgar DM, Heller HC, Miller JD. A possible glial role in the mammalian circadian clock. Womac AD, Burkeen JF, Neuendorff N, Earnest DJ, Zoran MJ. Circadian rhythms of extracellular ATP accumulation in suprachiasmatic nucleus cells and cultured astrocytes.
Eur J Neurosci. Prolo LM, Takahashi JS, Herzog ED. Circadian rhythm generation and entrainment in astrocytes. J Neurosci. Yagita K, Yamanaka I, Emoto N, Kawakami K, Shimada S.
Real-time monitoring of circadian clock oscillations in primary cultures of mammalian cells using Tol2 transposon-mediated gene transfer strategy.
BMC Biotechnol. Barca-Mayo O, Pons-Espinal M, Follert P, Armirotti A, Berdondini L, De Pietri Tonelli D. Astrocyte deletion of Bmal1 alters daily locomotor activity and cognitive functions via GABA signalling. Liu C, Reppert SM. GABA synchronizes clock cells within the suprachiasmatic circadian clock.
Albus H, Vansteensel MJ, Michel S, Block GD, Meijer JH. A GABAergic mechanism is necessary for coupling dissociable ventral and dorsal regional oscillators within the circadian clock.
Yoon BE, Woo J, Lee CJ. Astrocytes as GABA-ergic and GABA-ceptive cells. Neurochem Res. Doengi M, Hirnet D, Coulon P, Pape HC, Deitmer JW, Lohr C. Brancaccio M, Patton AP, Chesham JE, Maywood ES, Hastings MH. Astrocytes control circadian timekeeping in the suprachiasmatic nucleus via glutamatergic signaling.
Araque A, Carmignoto G, Haydon PG, Oliet SH, Robitaille R, Volterra A. Gliotransmitters travel in time and space.
Tso CF, Simon T, Greenlaw AC, Puri T, Mieda M, Herzog ED. Astrocytes regulate daily rhythms in the suprachiasmatic nucleus and behavior.
Bell RD, Winkler EA, Sagare AP, Singh I, LaRue B, Deane R, Zlokovic BV. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Daneman R, Zhou L, Kebede AA, Barres BA.
Pericytes are required for blood—brain barrier integrity during embryogenesis. Armulik A, Genove G, Mae M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, et al.
Pericytes regulate the blood—brain barrier. Uemura MT, Maki T, Ihara M, Lee VMY, Trojanowski JQ. Brain microvascular pericytes in vascular cognitive impairment and dementia. Front Aging Neurosci.
Nakazato R, Kawabe K, Yamada D, Ikeno S, Mieda M, Shimba S, Hinoi E, Yoneda Y, Takarada T. Disruption of Bmal1 impairs blood—brain barrier integrity via pericyte dysfunction. Winkler EA, Bell RD, Zlokovic BV. Central nervous system pericytes in health and disease. Naranjo O, Osborne O, Torices S, Toborek M.
In vivo targeting of the neurovascular unit: challenges and advancements. Cell Mol Neurobiol. Madore C, Yin Z, Leibowitz J, Butovsky O.
Microglia, lifestyle stress, and Neurodegeneration. Sominsky L, Dangel T, Malik S, De Luca SN, Singewald N, Spencer SJ. Microglial ablation in rats disrupts the circadian system.
FASEB J. Fonken LK, Frank MG, Kitt MM, Barrientos RM, Watkins LR, Maier SF. Microglia inflammatory responses are controlled by an intrinsic circadian clock. Takayama F, Hayashi Y, Wu Z, Liu Y, Nakanishi H. Diurnal dynamic behavior of microglia in response to infected bacteria through the UDP-P2Y6 receptor system.
Sci Rep. Frank MG, Thompson BM, Watkins LR, Maier SF. Glucocorticoids mediate stress-induced priming of microglial pro-inflammatory responses.
Griffin P, Sheehan PW, Dimitry JM, Guo C, Kanan MF, Lee J, Zhang J, Musiek ES. REV-ERBalpha mediates complement expression and diurnal regulation of microglial synaptic phagocytosis. Spengler ML, Kuropatwinski KK, Comas M, Gasparian AV, Fedtsova N, Gleiberman AS, Gitlin II, Artemicheva NM, Deluca KA, Gudkov AV, et al.
Core circadian protein CLOCK is a positive regulator of NF-kappaB-mediated transcription. Arvanitis CD, Ferraro GB, Jain RK. The blood—brain barrier and blood-tumour barrier in brain tumours and metastases. Nat Rev Cancer. Loscher W, Friedman A.
Structural, molecular, and functional alterations of the blood—brain barrier during Epileptogenesis and Epilepsy: a cause, Consequence, or both? Int J Mol Sci. Zenaro E, Piacentino G, Constantin G. Lee H, Pienaar IS. Front Biosci Landmark Ed. Welcome MO, Mastorakis NE. Stress-induced blood brain barrier disruption: molecular mechanisms and signaling pathways.
Pharmacol Res. Bechtold DA, Gibbs JE, Loudon AS. Circadian dysfunction in disease. Trends Pharmacol Sci. Musiek ES, Holtzman DM. Mechanisms linking circadian clocks, sleep, and neurodegeneration. Logan RW, McClung CA. Rhythms of life: circadian disruption and brain disorders across the lifespan.
Hou Y, Liu L, Chen X, Li Q, Li J. Association between circadian disruption and diseases: a narrative review. Dauer W, Przedborski S. Sweeney MD, Sagare AP, Zlokovic BV.
Blood—brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat Rev Neurol. Kotecha R, Gondi V, Ahluwalia MS, Brastianos PK, Mehta MP. Recent advances in managing brain metastasis. Boire A, Brastianos PK, Garzia L, Valiente M. Brain metastasis. Walker WH, Sprowls SA, Bumgarner JR, Liu JA, Melendez-Fernandez OH, Walton JC, Lockman PR, DeVries AC, Nelson RJ.
Circadian influences on Chemotherapy Efficacy in a mouse model of brain metastases of breast Cancer. Front Oncol. Steeg PS. The blood-tumour barrier in cancer biology and therapy.
Nat Rev Clin Oncol. Lauko A, Mu Z, Gutmann DH, Naik UP, Lathia JD. Junctional adhesion molecules in cancer: a paradigm for the diverse functions of cell-cell interactions in tumor progression. Cancer Res. Leech AO, Cruz RG, Hill AD, Hopkins AM.
Paradigms lost-an emerging role for over-expression of tight junction adhesion proteins in cancer pathogenesis. Ann Transl Med. Smalley KS, Brafford P, Haass NK, Brandner JM, Brown E, Herlyn M.
Up-regulated expression of zonula occludens protein-1 in human melanoma associates with N-cadherin and contributes to invasion and adhesion.
Am J Pathol. Levi F, Schibler U. Circadian rhythms: mechanisms and therapeutic implications. Annu Rev Pharmacol Toxicol. Farshadi E, Yan J, Leclere P, Goldbeter A, Chaves I, van der Horst GTJ. Cell Cycle. Dong Z, Zhang G, Qu M, Gimple RC, Wu Q, Qiu Z, Prager BC, Wang X, Kim LJY, Morton AR, et al.
Targeting glioblastoma stem cells through disruption of the circadian clock. Cancer Discov. Pascual G, Dominguez D, Benitah SA. The contributions of cancer cell metabolism to metastasis. Dis Model Mech. Chen P, Hsu WH, Chang A, Tan Z, Lan Z, Zhou A, Spring DJ, Lang FF, Wang YA, DePinho RA.
Circadian Regulator CLOCK Recruits Immune-Suppressive Microglia into the GBM Tumor Microenvironment. Gamma knife radiosurgery for brain metastasis of nonsmall cell lung cancer: is there a difference in outcome between morning and afternoon treatment?
Zhanfeng N, Yanhui L, Zhou F, Shaocai H, Guangxing L, Hechun X. Circadian genes Per1 and Per2 increase radiosensitivity of glioma in vivo.
Wang F, Luo Y, Li C, Chen L. Correlation between deregulated expression of PER2 gene and degree of glioma malignancy. Nat Rev Dis Primers. Marchi N, Angelov L, Masaryk T, Fazio V, Granata T, Hernandez N, Hallene K, Diglaw T, Franic L, Najm I, et al.
Seizure-promoting effect of blood—brain barrier disruption. van Vliet EA, da Costa Araujo S, Redeker S, van Schaik R, Aronica E, Gorter JA. Blood—brain barrier leakage may lead to progression of temporal lobe epilepsy.
Unterberger I, Gabelia D, Prieschl M, Chea K, Hofer M, Hogl B, Luef G, Frauscher B. Sleep disorders and circadian rhythm in epilepsy revisited: a prospective controlled study. Sleep Med. Gibbon FM, Maccormac E, Gringras P. Sleep and epilepsy: unfortunate bedfellows. Arch Dis Child.
Badawy RA, Curatolo JM, Newton M, Berkovic SF, Macdonell RA. Sleep deprivation increases cortical excitability in epilepsy: syndrome-specific effects. Ly JQM, Gaggioni G, Chellappa SL, Papachilleos S, Brzozowski A, Borsu C, Rosanova M, Sarasso S, Middleton B, Luxen A, et al.
Circadian regulation of human cortical excitability. Matos HC, Koike BDV, Pereira WDS, de Andrade TG, Castro OW, Duzzioni M, Kodali M, Leite JP, Shetty AK, Gitai DLG.
Rhythms of core clock genes and spontaneous locomotor activity in Post-Status Epilepticus Model of Mesial temporal lobe Epilepsy. Front Neurol. Matzen J, Buchheim K, Holtkamp M. Circadian dentate gyrus excitability in a rat model of temporal lobe epilepsy.
Exp Neurol. Leite Goes Gitai D, de Andrade TG, Dos Santos YDR, Attaluri S, Shetty AK. Chronobiology of limbic seizures: potential mechanisms and prospects of chronotherapy for mesial temporal lobe epilepsy.
Neurosci Biobehav Rev. Karoly PJ, Rao VR, Gregg NM, Worrell GA, Bernard C, Cook MJ, Baud MO. Cycles in epilepsy. Chan F, Liu J. Molecular regulation of brain metabolism underlying circadian epilepsy. van Vliet EA, Otte WM, Wadman WJ, Aronica E, Kooij G, de Vries HE, Dijkhuizen RM, Gorter JA.
Blood—brain barrier leakage after status epilepticus in rapamycin-treated rats II: potential mechanisms. Li P, Fu X, Smith NA, Ziobro J, Curiel J, Tenga MJ, Martin B, Freedman S, Cea-Del Rio CA, Oboti L, et al.
Loss of CLOCK results in dysfunction of brain circuits underlying focal epilepsy. Gerstner JR, Smith GG, Lenz O, Perron IJ, Buono RJ, Ferraro TN. BMAL1 controls the diurnal rhythm and set point for electrical seizure threshold in mice.
Front Syst Neurosci. Eun B, Kim HJ, Kim SY, Kim TW, Hong ST, Choi KM, Shim JK, Moon Y, Son GH, Kim K, et al. Induction of Per1 expression following an experimentally induced epilepsy in the mouse hippocampus.
Neurosci Lett. Kim SH, Park HG, Jeong SH, Kang UG, Ahn YM, Kim YS. Electroconvulsive Seizure alters the expression and daily oscillation of circadian genes in the Rat Frontal Cortex. Psychiatry Investig. Tiwari S, Atluri V, Kaushik A, Yndart A, Nair M.
Int J Nanomedicine. Xin SH, Tan L, Cao X, Yu JT, Tan L. Neurotox Res. Bombois S, Derambure P, Pasquier F, Monaca C. Sleep disorders in aging and dementia. J Nutr Health Aging.
Bachman D, Rabins P. Annu Rev Med. Deane R, Zlokovic BV. Curr Alzheimer Res. Winkler EA, Sagare AP, Zlokovic BV. Brain Pathol. Cai Z, Qiao PF, Wan CQ, Cai M, Zhou NK, Li Q. J Alzheimers Dis. Deane R, Du Yan S, Submamaryan RK, LaRue B, Jovanovic S, Hogg E, Welch D, Manness L, Lin C, Yu J, et al.
RAGE mediates amyloid-beta peptide transport across the blood—brain barrier and accumulation in brain. Nat Med. Andras IE, Eum SY, Huang W, Zhong Y, Hennig B, Toborek M.
HIVinduced amyloid beta accumulation in brain endothelial cells is attenuated by simvastatin. Mol Cell Neurosci. Kang JE, Lim MM, Bateman RJ, Lee JJ, Smyth LP, Cirrito JR, Fujiki N, Nishino S, Holtzman DM.
Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Hood S, Amir S. Neurodegeneration and the circadian clock.
Esler WP, Wolfe MS. A portrait of Alzheimer secretases—new features and familiar faces. Sleep drives metabolite clearance from the adult brain. Hablitz LM, Pla V, Giannetto M, Vinitsky HS, Staeger FF, Metcalfe T, Nguyen R, Benrais A, Nedergaard M.
Circadian control of brain glymphatic and lymphatic fluid flow. Schmitt K, Grimm A, Eckert A. Amyloid-beta-induced changes in molecular clock properties and cellular bioenergetics.
Song H, Moon M, Choe HK, Han DH, Jang C, Kim A, Cho S, Kim K, Mook-Jung I. Mol Neurodegener. Sultana R, Butterfield DA. Wang X, Wang W, Li L, Perry G, Lee HG, Zhu X. Biochim Biophys Acta. Kondratova AA, Dubrovsky YV, Antoch MP, Kondratov RV. Circadian clock proteins control adaptation to novel environment and memory formation.
Musiek ES, Lim MM, Yang G, Bauer AQ, Qi L, Lee Y, Roh JH, Ortiz-Gonzalez X, Dearborn JT, Culver JP, et al. Circadian clock proteins regulate neuronal redox homeostasis and neurodegeneration.
J Clin Invest. Saha P, Sen N. Tauopathy: a common mechanism for neurodegeneration and brain aging. Mech Ageing Dev. Guisle I, Gratuze M, Petry S, Morin F, Keraudren R, Whittington RA, Hebert SS, Mongrain V, Planel E.
Stevanovic K, Yunus A, Joly-Amado A, Gordon M, Morgan D, Gulick D, Gamsby J. Disruption of normal circadian clock function in a mouse model of tauopathy. Cronin P, McCarthy MJ, Lim ASP, Salmon DP, Galasko D, Masliah E, De Jager PL, Bennett DA, Desplats P. Alzheimers Dement.
Poewe W, Seppi K, Tanner CM, Halliday GM, Brundin P, Volkmann J, Schrag AE, Lang AE. Parkinson disease. Paul G, Elabi OF. Al-Bachari S, Naish JH, Parker GJM, Emsley HCA, Parkes LM.
Front Physiol. Li S, Wang Y, Wang F, Hu LF, Liu CF. Neurosci Bull. Lee MA, Prentice WM, Hildreth AJ, Walker RW. Parkinsonism Relat Disord. Martinez-Martin P, Schapira AH, Stocchi F, Sethi K, Odin P, MacPhee G, Brown RG, Naidu Y, Clayton L, Abe K, et al.
Mov Disord. Diurnal worsening in Parkinson patients treated with levodopa. Riv Neurol. van Hilten JJ, Hoogland G, van der Velde EA, Middelkoop HA, Kerkhof GA, Roos RA. J Neurol Neurosurg Psychiatry. Placidi F, Izzi F, Romigi A, Stanzione P, Marciani MG, Brusa L, Sperli F, Galati S, Pasqualetti P, Pierantozzi M.
An ambulatory polysomnographic study. J Neurol. Videnovic A, Golombek D. Neurobiol Sleep Circadian Rhythms. Schnell A, Albrecht U, Sandrelli F. Rhythm and mood: relationships between the circadian clock and mood-related behavior. Behav Neurosci. McClung CA, Sidiropoulou K, Vitaterna M, Takahashi JS, White FJ, Cooper DC, Nestler EJ.
Regulation of dopaminergic transmission and cocaine reward by the clock gene. Kawarai T, Kawakami H, Yamamura Y, Nakamura S. Structure and organization of the gene encoding human dopamine transporter.
Mukherjee S, Coque L, Cao JL, Kumar J, Chakravarty S, Asaithamby A, Graham A, Gordon E, Enwright JF, DiLeone RJ, et al. Knockdown of clock in the ventral tegmental area through RNA interference results in a mixed state of mania and depression-like behavior.
Biol Psychiatry. Imbesi M, Yildiz S, Dirim Arslan A, Sharma R, Manev H, Uz T. Yujnovsky I, Hirayama J, M, Borrelli E, Sassone-Corsi P.
Signaling mediated by the dopamine D2 receptor potentiates circadian regulation by CLOCK:BMAL1. Hood S, Cassidy P, Cossette MP, Weigl Y, Verwey M, Robinson B, Stewart J, Amir S. Endogenous dopamine regulates the rhythm of expression of the clock protein PER2 in the rat dorsal striatum via daily activation of D2 dopamine receptors.
Cai Y, Liu S, Sothern RB, Xu S, Chan P. Eur J Neurol. Gu Z, Wang B, Zhang YB, Ding H, Zhang Y, Yu J, Gu M, Chan P, Cai Y. Welcome MO. Cellular mechanisms and molecular signaling pathways in stress-induced anxiety, depression, and blood—brain barrier inflammation and leakage.
Nicolaides NC, Charmandari E, Kino T, Chrousos GP. Stress-related and circadian secretion and target tissue actions of glucocorticoids: impact on Health. Front Endocrinol Lausanne. Kalsbeek A, Palm IF, La Fleur SE, Scheer FA, Perreau-Lenz S, Ruiter M, Kreier F, Cailotto C, Buijs RM.
SCN outputs and the hypothalamic balance of life. Ulrich-Lai YM, Arnhold MM, Engeland WC. Adrenal splanchnic innervation contributes to the diurnal rhythm of plasma corticosterone in rats by modulating adrenal sensitivity to ACTH.
Am J Physiol Regul Integr Comp Physiol. Ishida A, Mutoh T, Ueyama T, Bando H, Masubuchi S, Nakahara D, Tsujimoto G, Okamura H. Light activates the adrenal gland: timing of gene expression and glucocorticoid release.
Oster H, Damerow S, Hut RA, Eichele G. Transcriptional profiling in the adrenal gland reveals circadian regulation of hormone biosynthesis genes and nucleosome assembly genes. Son GH, Chung S, Choe HK, Kim HD, Baik SM, Lee H, Lee HW, Choi S, Sun W, Kim H, et al. Adrenal peripheral clock controls the autonomous circadian rhythm of glucocorticoid by causing rhythmic steroid production.
Yamamoto T, Nakahata Y, Tanaka M, Yoshida M, Soma H, Shinohara K, Yasuda A, Mamine T, Takumi T. Acute physical stress elevates mouse period1 mRNA expression in mouse peripheral tissues via a glucocorticoid-responsive element.
J Biol Chem. Jiang WG, Li SX, Zhou SJ, Sun Y, Shi J, Lu L. Chronic unpredictable stress induces a reversible change of PER2 rhythm in the suprachiasmatic nucleus. Hori H, Kim Y. Inflammation and post-traumatic stress disorder. Psychiatry Clin Neurosci.
Strwss 4, Circadian Rhythms 0. A research team Hypertension and digestive health Japan has Detoxification Support for Improved Sleep Circadian rhythm stress rhytmh neural pathway stgess links the circadian rhtyhm, stress, and wakefulness in Hypertension and digestive health. The Nagoya University-led team identified a neuron, Ciecadian the corticotropin-releasing factor CRF neuron, that becomes excessively active when the mammal is under stress, which could trigger insomnia and other sleep disorders. Their findings were recently published in the journal Science Advances. However, in the event of life-threatening situations, the circadian rhythm signal is shut off to keep the animal awake so that it can escape from danger even when it would normally be time to sleep.Circadian rhythm stress -
In Development of Psychopathology: A Vulnerability-Stress Perspective. eds B. Hankin and J. Abela California: Sage Publications, Inc. Publisher Full Text Prasad BM, Ulibarri C, Sorg BA: Stress-induced cross-sensitization to cocaine: effect of adrenalectomy and corticosterone after short- and long-term withdrawal.
PubMed Abstract Publisher Full Text Przegaliński E, Filip M, Siwanowicz J, et al. J Physiol Pharmacol. PubMed Abstract Reick M, Garcia JA, Dudley C, et al.
PubMed Abstract Publisher Full Text Rosenfeld P, Van Eekelen JA, Levine S, et al. PubMed Abstract Publisher Full Text Rougé-Pont F, Marinelli M, Le Moal M, et al. Sensitization of the increase in extracellular dopamine induced by cocaine depends on stress-induced corticosterone secretion.
PubMed Abstract Roy A, Pickar D, De Jong J, et al. Relationship to hypothalamic-pituitary-adrenal axis function in depression. PubMed Abstract Publisher Full Text Roybal K, Theobold D, Graham A, et al. Proc Natl Acad Sci USA. PubMed Abstract Publisher Full Text Free Full Text Russo S, Murrough J, Han MH, et al.
PubMed Abstract Publisher Full Text Free Full Text Sapolsky RM: Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. PubMed Abstract Publisher Full Text Segall LA, Amir S: Exogenous corticosterone induces the expression of the clock protein, PERIOD2, in the oval nucleus of the bed nucleus of the stria terminalis and the central nucleus of the amygdala of adrenalectomized and intact rats.
J Mol Neurosci. PubMed Abstract Publisher Full Text Segall LA, Milet A, Tronche F, et al. Neurosci Lett. PubMed Abstract Publisher Full Text So AY, Bernal TU, Pillsbury ML, et al. PubMed Abstract Publisher Full Text Free Full Text Soliman A, Udemgba C, Fan I, et al.
PubMed Abstract Publisher Full Text Son GH, Chung S, Choe HK, et al. PubMed Abstract Publisher Full Text Free Full Text Spencer S, Torres-Altoro MI, Falcon E, et al. PubMed Abstract Publisher Full Text Free Full Text Spiga F, Walker JJ, Terry JR, et al.
PubMed Abstract Publisher Full Text Sterling P, Eyer J: Allostasis: a new paradigm to explain arousal pathology. In Handbook of Life Stress, Cognition and Health , eds. Reference Source Tahara Y, Shiraishi T, Kikuchi Y, et al.
Sci Rep. PubMed Abstract Publisher Full Text Free Full Text Takahashi K, Yamada T, Tsukita S, et al. Am J Physiol Endocrinol Metab. PubMed Abstract Publisher Full Text Takita E, Yokota S, Tahara Y, et al. Br J Dermatol. PubMed Abstract Publisher Full Text Tank AW, Curella P, Ham L: Induction of mRNA for tyrosine hydroxylase by cyclic AMP and glucocorticoids in a rat pheochromocytoma cell line: evidence for the regulation of tyrosine hydroxylase synthesis by multiple mechanisms in cells exposed to elevated levels of both inducing agents.
PubMed Abstract Torra IP, Tsibulsky V, Delaunay F, et al. PubMed Abstract Publisher Full Text Trainor BC: Stress responses and the mesolimbic dopamine system: social contexts and sex differences.
Horm Behav. PubMed Abstract Publisher Full Text Free Full Text Uchoa ET, Aguilera G, Herman JP, et al. J Neuroendocrinol. PubMed Abstract Publisher Full Text Free Full Text Van Cauter E, Turek FW: Depression: a disorder of timekeeping?
Perspect Biol Med. PubMed Abstract Publisher Full Text Wehr TA, Sack D, Rosenthal N, et al. Fed Proc. PubMed Abstract Yamamoto T, Nakahata Y, Tanaka M, et al.
J Biol Chem. PubMed Abstract Publisher Full Text Yehuda R, Flory JD, Bierer LM, et al. PubMed Abstract Publisher Full Text.
Comments on this article Comments 0. Version 1. VERSION 1 PUBLISHED 13 Jan ADD YOUR COMMENT. Article Versions 1 version 1.
Copyright © Becker-Krail D and McClung C. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Sciwheel Bibtex EndNote ProCite Ref. Manager RIS Sente. SEE MORE DETAILS. Becker-Krail D and McClung C. COPY CITATION DETAILS. Track an article to receive email alerts on any updates to this article.
TRACK THIS ARTICLE. Open Peer Review Current Reviewer Status:? Key to Reviewer Statuses VIEW HIDE Approved The paper is scientifically sound in its current form and only minor, if any, improvements are suggested. Approved with reservations A number of small changes, sometimes more significant revisions are required to address specific details and improve the papers academic merit.
Not approved Fundamental flaws in the paper seriously undermine the findings and conclusions. VERSION 1. How to cite this report:. NOTE: it is important to ensure the information in square brackets after the title is included in this citation.
Reviewer Report 02 Feb Randy Nelson , Department of Neuroscience, Ohio State University College of Medicine, Columbus, OH, USA. Jeremy Borniger , Department of Neuroscience, Ohio State University College of Medicine, Columbus, OH, USA.
VIEWS READ ALL. The authors provide an excellent and timely mini-review of the interactions between the circadian and stress response systems, and their actions on dopaminergic reward circuitry underlying addiction and diseases of drug abuse.
They highlight the differential expression levels of BMAL1 binding partners CLOCK and NPAS2 throughout the brain, indicating that NPAS2 is specifically enriched in the nucleus accumbens.
This sets the stage for the idea that selective manipulation of the circadian clock through NPAS2 in the nucleus accumbens could influence reward-seeking behavior. The review is concise and provides an excellent starting point for anyone interested in the etiology, treatment, or molecular biology of addiction or drug abuse.
It may be beyond the scope of this review, but future discussion of the effects drugs of abuse on the circadian clock may be warranted. Is there any evidence for circadian control of opioid reward signaling? It may be worth discussing stress effects on opioid or opioid-receptor expression in the VTA or NAc on a circadian basis.
READ LESS. Nelson R and Borniger J. Reviewer Report For: Implications of circadian rhythm and stress in addiction vulnerability [version 1; peer review: 2 approved]. Report a concern. Respond or Comment. COMMENT ON THIS REPORT. Reviewer Report 01 Feb Harry Pantazopoulos , Translational Neuroscience Laboratory, McLean Hospital, Belmont, MA, USA.
Syed Bukhari , Translational Neuroscience Laboratory, McLean Hospital, Belmont, MA, USA. The article provides an accurate summary of the current literature and a helpful introduction of these biological systems to readers who are unfamiliar with the field.
The figures are clear, well-described, and aid the reader in understanding the molecular mechanisms described in the text.
This mini-review is a useful reference to researchers studying basic neuroscience questions on these systems and how these systems are disrupted in psychiatric disorders. The review highlights the importance of recognizing the potential interactions between these systems in both normal and pathological conditions.
Pantazopoulos H and Bukhari S. Open Peer Review. Reviewer Status. Reviewer Reports. Harry Pantazopoulos , McLean Hospital, Belmont, USA.
Syed Bukhari , McLean Hospital, Belmont, USA. Randy Nelson , Ohio State University College of Medicine, Columbus, USA. Jeremy Borniger , Ohio State University College of Medicine, Columbus, USA. Comments on this article All Comments 0 Add a comment. Sign up for content alerts.
Sign Up. You are now signed up to receive this alert. Alongside their report, reviewers assign a status to the article: Approved - the paper is scientifically sound in its current form and only minor, if any, improvements are suggested. Approved with reservations - A number of small changes, sometimes more significant revisions are required to address specific details and improve the papers academic merit.
Not approved - fundamental flaws in the paper seriously undermine the findings and conclusions. Adjust parameters to alter display. View on desktop for interactive features. Includes Interactive Elements.
Competing Interests Policy Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality.
Consider the following examples, but note that this is not an exhaustive list: Examples of 'Non-Financial Competing Interests'.
Stay Updated Sign up for content alerts and receive a weekly or monthly email with all newly published articles Register with FResearch Already registered?
Sign in Not now, thanks. close Error. Sign In. Sign In Cancel. If you've forgotten your password, please enter your email address below and we'll send you instructions on how to reset your password. The email address should be the one you originally registered with F Email address not valid, please try again.
Next NPR Features Nightmare Relief Smartwatch App: a Son was Inspired to Create It for His Dad. Related Posts. Upcoming Events. Sleep Apnea Implementation Patterson Dental- Colorado. Atrium Health Sleep Symposium Introduction to Sleep Medicine CDOCS, March, Charlotte Dentsply Sirona Academy Charlotte.
Sleep Awareness Week. Airway Palooza The Ritz-Carlton, New Orleans. Neurosci Biobehav Rev 33 2 —8. Stiedl O, Youn J, Jansen RF. Cardiovascular conditioning: neural substrates. In: Koob GF, LeMoal M, Thompson RF, editors. Encyclopedia of Behavioral Neuroscience , vol. Furness JB. The organisation of the autonomic nervous system: peripheral connections.
Auton Neurosci :1—5. Nilsson S. Comparative anatomy of the autonomic nervous system. Auton Neurosci 1 :3—9. Shields RW Jr.
Functional anatomy of the autonomic nervous system. J Clin Neurophysiol 10 1 :2— Meyer M, Stiedl O. Self-affine fractal variability of human heartbeat interval dynamics in health and disease. Eur J Appl Physiol 90 — Lipsitz LA, Goldberger AL.
Potential applications of fractals and chaos theory to senescence. JAMA 13 —9. Peng CK, Buldyrev SV, Hausdorff JM, Havlin S, Mietus JE, Simons M, et al.
Non-equilibrium dynamics as an indispensable characteristic of a healthy biological system. Integr Physiol Behav Sci 29 3 — Schmidt RF, Thews G, Lang F. Physiologie des Menschen. Berlin: Springer Trepel M. Struktur und Funktion. München: Urban und Fischer The role of vagal function in the risk for cardiovascular disease and mortality.
Biol Psychol 74 2 — Regulation and dysregulation of the hypothalamic-pituitary-adrenal axis. The corticotropin-releasing hormone perspective. Endocrinol Metab Clin North Am 21 4 — Chrousos GP, Charmandari E, Kino T. Glucocorticoid action networks—an introduction to systems biology.
J Clin Endocrinol Metab 89 2 —4. Charmandari E, Tsigos C, Chrousos G. Endocrinology of the stress response. Chrousos GP, Kino T. Intracellular glucocorticoid signaling: a formerly simple system turns stochastic. Sci STKE :pe Glucocorticoid signaling in the cell. Expanding clinical implications to complex human behavioral and somatic disorders.
Nicolaides NC, Galata Z, Kino T, Chrousos GP, Charmandari E. The human glucocorticoid receptor: molecular basis of biologic function. Steroids 75 1 :1— Oakley RH, Cidlowski JA. The biology of the glucocorticoid receptor: new signaling mechanisms in health and disease.
J Allergy Clin Immunol 5 — de Kloet ER. Functional profile of the binary brain corticosteroid receptor system: mediating, multitasking, coordinating, integrating. Eur J Pharmacol — Rohleder N, Wolf JM, Wolf OT. Glucocorticoid sensitivity of cognitive and inflammatory processes in depression and posttraumatic stress disorder.
Neurosci Biobehav Rev 35 1 — Lu NZ, Cidlowski JA. Translational regulatory mechanisms generate N-terminal glucocorticoid receptor isoforms with unique transcriptional target genes.
Mol Cell 18 3 — Bamberger CM, Bamberger AM, de Castro M, Chrousos GP. Glucocorticoid receptor beta, a potential endogenous inhibitor of glucocorticoid action in humans.
J Clin Invest 95 6 — Charmandari E, Chrousos GP, Ichijo T, Bhattacharyya N, Vottero A, Souvatzoglou E, et al. The human glucocorticoid receptor hGR beta isoform suppresses the transcriptional activity of hGRalpha by interfering with formation of active coactivator complexes.
Mol Endocrinol 19 1 — Kino T, Su YA, Chrousos GP. Human glucocorticoid receptor isoform beta: recent understanding of its potential implications in physiology and pathophysiology. Cell Mol Life Sci 66 21 — Arriza JL, Simerly RB, Swanson LW, Evans RM.
The neuronal mineralocorticoid receptor as a mediator of glucocorticoid response. Neuron 1 9 — de Kloet ER, Otte C, Kumsta R, Kok L, Hillegers MH, Hasselmann H, et al.
Stress and Depression: a Crucial Role of the Mineralocorticoid Receptor. J Neuroendocrinol 28 8. Wingenfeld K, Otte C. Mineralocorticoid receptor function and cognition in health and disease. Psychoneuroendocrinology — de Kloet ER, Meijer OC, de Nicola AF, de Rijk RH, Joels M.
Importance of the brain corticosteroid receptor balance in metaplasticity, cognitive performance and neuro-inflammation. Front Neuroendocrinol — ter Heegde F, De Rijk RH, Vinkers CH.
The brain mineralocorticoid receptor and stress resilience. Ayroldi E, Cannarile L, Migliorati G, Nocentini G, Delfino DV, Riccardi C. Mechanisms of the anti-inflammatory effects of glucocorticoids: genomic and nongenomic interference with MAPK signaling pathways.
FASEB J 26 12 — Joels M, Karst H, DeRijk R, de Kloet ER. The coming out of the brain mineralocorticoid receptor. Trends Neurosci 31 1 :1—7. Henckens MJ, Pu Z, Hermans EJ, van Wingen GA, Joels M, Fernandez G.
Dynamically changing effects of corticosteroids on human hippocampal and prefrontal processing. Hum Brain Mapp 33 12 — Joels M, Pasricha N, Karst H. The interplay between rapid and slow corticosteroid actions in brain. Edery I. Circadian rhythms in a nutshell.
Physiol Genomics 3 2 — Roenneberg T, Merrow M. The network of time: understanding the molecular circadian system. Curr Biol 13 5 :R— Weinert D.
Ontogenetic development of the mammalian circadian system. Chronobiol Int 22 2 — Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science — Berson DM, Dunn FA, Takao M.
Phototransduction by retinal ganglion cells that set the circadian clock. Science —3. Morin LP. Neuroanatomy of the extended circadian rhythm system. Exp Neurol — Czeisler CA, Duffy JF, Shanahan TL, Brown EN, Mitchell JF, Rimmer DW, et al. Stability, precision, and nearhour period of the human circadian pacemaker.
Buijs RM, van Eden CG, Goncharuk VD, Kalsbeek A. The biological clock tunes the organs of the body: timing by hormones and the autonomic nervous system. J Endocrinol 1 — Morris CJ, Aeschbach D, Scheer FAJL. Provencio I, Rodriguez IR, Jiang G, Hayes WP, Moreira EF, Rollag MD.
A novel human opsin in the inner retina. J Neurosci 20 2 —5. Cermakian N, Sassone-Corsi P. Environmental stimulus perception and control of circadian clocks. Curr Opin Neurobiol 12 4 — Hattar S, Liao HW, Takao M, Berson DM, Yau KW.
Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Van Gelder RN. Making a sense of non-visual ocular photoreception. Trends Neurosci 26 9 — Warman VL, Dijk DJ, Warman GR, Arendt J, Skene DJ. Phase advancing human circadian rhythms with short wavelength light.
Neurosci Lett — Panda S, Nayak SK, Campo B, Walker JR, Hogenesch JB, Jegla T. Illumination of the melanopsin signaling pathway. Science —4. Ohi K, Takashima M, Nishikawa T, Takahashi K. N-methyl-D-aspartate receptor participates in neuronal transmission of photic information through the retinohypothalamic tract.
Neuroendocrinology 53 4 —8. Moller M, Baeres FM. The anatomy and innervation of the mammalian pineal gland. Cell Tissue Res 1 — Schibler U, Gotic I, Saini C, Gos P, Curie T, Emmenegger Y, et al. Clock-Talk: Interactions between Central and Peripheral Circadian Oscillators in Mammals.
Cold Spring Harb Symp Quant Biol — Guo H, Brewer JM, Lehman MN, Bittman EL. Suprachiasmatic regulation of circadian rhythms of gene expression in hamster peripheral organs: effects of transplanting the pacemaker. J Neurosci 26 24 — Stratmann M, Schibler U.
Properties, entrainment, and physiological functions of mammalian peripheral oscillators. J Biol Rhythms 21 6 — Konopka RJ, Benzer S. Clock mutants of Drosophila melanogaster. Proc Natl Acad Sci U S A 68 9 —6. Zhang EE, Kay SA. Clocks not winding down: unravelling circadian networks.
Nat Rev Mol Cell Biol 11 11 — Yagita K, Tamanini F, van Der Horst GT, Okamura H. Molecular mechanisms of the biological clock in cultured fibroblasts.
von Gall C, Weaver DR, Moek J, Jilg A, Stehle JH, Korf HW. Melatonin plays a crucial role in the regulation of rhythmic clock gene expression in the mouse pars tuberalis. Gallego M, Virshup DM. Post-translational modifications regulate the ticking of the circadian clock. Nat Rev Mol Cell Biol 8 2 — Reppert SM, Weaver DR.
Coordination of circadian timing in mammals. Lee C, Etchegaray JP, Cagampang FR, Loudon AS, Reppert SM. Posttranslational mechanisms regulate the mammalian circadian clock. Cell 7 — Kiyohara YB, Tagao S, Tamanini F, Morita A, Sugisawa Y, Yasuda M, et al. The BMAL1 C terminus regulates the circadian transcription feedback loop.
Proc Natl Acad Sci U S A 26 —9. Kondratov RV, Kondratova AA, Lee C, Gorbacheva VY, Chernov MV, Antoch MP. Cell Cycle 5 8 —5. Padmanabhan K, Robles MS, Westerling T, Weitz CJ.
Feedback regulation of transcriptional termination by the mammalian circadian clock PERIOD complex. Roenneberg T, Wirz-Justice A, Merrow M. Life between clocks: daily temporal patterns of human chronotypes. J Biol Rhythms 18 1 — Lamia KA, Sachdeva UM, DiTacchio L, Williams EC, Alvarez JG, Egan DF, et al.
AMPK regulates the circadian clock by cryptochrome phosphorylation and degradation. Jordan SD, Lamia KA. AMPK at the crossroads of circadian clocks and metabolism. Mol Cell Endocrinol 2 —9. Gotic I, Omidi S, Fleury-Olela F, Molina N, Naef F, Schibler U.
Temperature regulates splicing efficiency of the cold-inducible RNA-binding protein gene Cirbp. Genes Dev 30 17 — Welsh DK, Logothetis DE, Meister M, Reppert SM. Individual neurons dissociated from rat suprachiasmatic nucleus express independently phased circadian firing rhythms.
Neuron 14 4 — Buijs RM, Kalsbeek A. Hypothalamic integration of central and peripheral clocks. Nat Rev Neurosci 2 7 —6. Kalsbeek A, Perreau-Lenz S, Buijs RM. A network of autonomic clock outputs. Chronobiol Int 23 3 — Aston-Jones G, Chen S, Zhu Y, Oshinsky ML.
A neural circuit for circadian regulation of arousal. Nat Neurosci 4 7 —8. Arendt J. Importance and relevance of melatonin to human biological rhythms. J Neuroendocrinol 15 4 — Macchi MM, Bruce JN.
Human pineal physiology and functional significance of melatonin. Front Neuroendocrinol 25 — Melatonin and human rhythms. Chronobiol Int 23 — Hardeland R, Cardinali DP, Srinivasan V, Spence DW, Brown GM, Pandi-Perumal SR. Melatonin—a pleiotropic, orchestrating regulator molecule. Prog Neurobiol 93 3 — Nowak JZ, Zawilska JB.
Melatonin and its physiological and therapeutic properties. Pharm World Sci 20 1 — Figueiro MG, Rea MS. The effects of red and blue lights on circadian variations in cortisol, alpha amylase, and melatonin.
Int J Endocrinol Brainard GC, Hanifin JP, Warfield B, Stone MK, James ME, Ayers M, et al. Short-wavelength enrichment of polychromatic light enhances human melatonin suppression potency. J Pineal Res 58 3 — Mason R, Brooks A. The electrophysiological effects of melatonin and a putative melatonin antagonist N-acetyltryptamine on rat suprachiasmatic neurones in vitro.
Neurosci Lett 95 — Srinivasan V, Pandi-Perumal SR, Trahkt I, Spence DW, Poeggeler B, Hardeland R, et al. Melatonin and melatonergic drugs on sleep: possible mechanisms of action.
Int J Neurosci 6 — Reppert SM, Weaver DR, Rivkees SA, Stopa EG. Putative melatonin receptors in a human biological clock. Vriend J, Reiter RJ. Melatonin feedback on clock genes: a theory involving the proteasome. J Pineal Res 58 1 :1— Niles L. Melatonin interaction with the benzodiazepine-GABA receptor complex in the CNS.
Adv Exp Med Biol — Golombek DA, Pevet P, Cardinali DP. Melatonin effects on behavior: possible mediation by the central GABAergic system. Neurosci Biobehav Rev 20 3 — Hardeland R, Madrid JA, Tan DX, Reiter RJ. Melatonin, the circadian multioscillator system and health: the need for detailed analyses of peripheral melatonin signaling.
J Pineal Res 52 2 — Slominski RM, Reiter RJ, Schlabritz-Loutsevitch N, Ostrom RS, Slominski AT. Melatonin membrane receptors in peripheral tissues: distribution and functions.
Mol Cell Endocrinol 2 — Garcia JJ, Lopez-Pingarron L, Almeida-Souza P, Tres A, Escudero P, Garcia-Gil FA, et al. Protective effects of melatonin in reducing oxidative stress and in preserving the fluidity of biological membranes: a review. J Pineal Res 56 3 — Reiter RJ, Tan DX, Galano A.
Melatonin: exceeding expectations. Physiol Bethesda 29 5 — Manchester LC, Coto-Montes A, Boga JA, Andersen LP, Zhou Z, Galano A, et al. Melatonin: an ancient molecule that makes oxygen metabolically tolerable.
J Pineal Res 59 4 — Reiter RJ, Tan DX, Kim SJ, Cruz MH. Delivery of pineal melatonin to the brain and SCN: role of canaliculi, cerebrospinal fluid, tanycytes and Virchow-Robin perivascular spaces. Brain Struct Funct 6 — Pandi-Perumal SR, Srinivasan V, Maestroni GJ, Cardinali DP, Poeggeler B, Hardeland R.
FEBS J 13 — Kennaway DJ. Melatonin research in mice: a review. Chronobiol Int 36 9 — Zisapel N. Sleep and sleep disturbances: biological basis and clinical implications.
Cell Mol Life Sci 64 10 — Lee ML, Swanson BE, de la Iglesia HO. Circadian timing of REM sleep is coupled to an oscillator within the dorsomedial suprachiasmatic nucleus.
Curr Biol 19 10 — Dijk DJ, Cajochen C. Melatonin and the circadian regulation of sleep initiation, consolidation, structure, and the sleep EEG. J Biol Rhythms 12 6 — Kunz D, Mahlberg R, Muller C, Tilmann A, Bes F. Melatonin in patients with reduced REM sleep duration: two randomized controlled trials.
J Clin Endocrinol Metab 89 1 — Rajaratnam SM, Middleton B, Stone BM, Arendt J, Dijk DJ. Melatonin advances the circadian timing of EEG sleep and directly facilitates sleep without altering its duration in extended sleep opportunities in humans.
J Physiol Pt 1 — Dubocovich ML. Melatonin receptors: role on sleep and circadian rhythm regulation. Sleep Med 8 Suppl 3 — Pandi-Perumal SR, Srinivasan V, Spence DW, Cardinali DP. Role of the melatonin system in the control of sleep: therapeutic implications.
CNS Drugs 21 12 — Ultradian, circadian, and stress-related hypothalamic-pituitary-adrenal axis activity—a dynamic digital-to-analog modulation. Endocrinology 2 — Oster H, Damerow S, Hut RA, Eichele G. Transcriptional profiling in the adrenal gland reveals circadian regulation of hormone biosynthesis genes and nucleosome assembly genes.
J Biol Rhythms 21 5 — Dickmeis T. Glucocorticoids and the circadian clock. J Endocrinol 1 :3— Gan EH, Quinton R. Physiological significance of the rhythmic secretion of hypothalamic and pituitary hormones.
Prog Brain Res — Dickmeis T, Foulkes NS. Glucocorticoids and circadian clock control of cell proliferation: at the interface between three dynamic systems. Kino T, Chrousos GP. Circadian CLOCK-mediated regulation of target-tissue sensitivity to glucocorticoids: implications for cardiometabolic diseases.
Endocr Dev — Son GH, Chung S, Kim K. The adrenal peripheral clock: glucocorticoid and the circadian timing system. Front Neuroendocrinol 32 4 — Kalsbeek A, van der Spek R, Lei J, Endert E, Buijs RM, Fliers E.
Circadian rhythms in the hypothalamo-pituitary-adrenal HPA axis. Mol Cell Endocrinol 1 —9. Leliavski A, Dumbell R, Ott V, Oster H. Adrenal Clocks and the Role of Adrenal Hormones in the Regulation of Circadian Physiology.
J Biol Rhythms 30 1 — Nicolaides NC, Charmandari E, Chrousos GP, Kino T. Circadian endocrine rhythms: the hypothalamic-pituitary-adrenal axis and its actions.
Buckley TM, Schatzberg AF. A pilot study of the phase angle between cortisol and melatonin in major depression - a potential biomarker? J Psychiatr Res 44 2 — Qian X, Droste SK, Lightman SL, Reul JMHM, Linthorst ACE.
Circadian and ultradian rhythms of free glucocorticoid hormone are highly synchronized between the blood, the subcutaneous tissue, and the brain. Endocrinology 9 — Moore RY, Eichler VB. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat.
Brain Res 42 1 —6. Scheer FA, Van Doornen LJ, Buijs RM. Light and diurnal cycle affect autonomic cardiac balance in human; possible role for the biological clock.
Auton Neurosci 1 —8. Vandewalle G, Middleton B, Rajaratnam SM, Stone BM, Thorleifsdottir B, Arendt J, et al. Robust circadian rhythm in heart rate and its variability: influence of exogenous melatonin and photoperiod. J Sleep Res 16 2 — Dominguez-Rodriguez A, Abreu-Gonzalez P, Sanchez-Sanchez JJ, Kaski JC, Reiter RJ.
Melatonin and circadian biology in human cardiovascular disease. J Pineal Res 49 1 — Kalsbeek A, Yi CX, la Fleur SE, Buijs RM, Fliers E. Suprachiasmatic nucleus and autonomic nervous system influences on awakening from sleep. Int Rev Neurobiol — Shea SA, Hilton MF, Hu K, Scheer FA.
Existence of an endogenous circadian blood pressure rhythm in humans that peaks in the evening. Circ Res 8 —4. Portaluppi F, Tiseo R, Smolensky MH, Hermida RC, Ayala DE, Fabbian F. Circadian rhythms and cardiovascular health.
Sleep Med Rev 16 2 — Bartlang MS, Lundkvist GB.
Hall, Circadoan Rosbash, and Circadiann W. Rhythmicity of physiological parameters is Circadian rhythm stress in virtually all living systems. Circadian rhythm stress circadian Circxdian with its most obvious expression, the sleep—wake cycle, Curcadian closely tied to Circadian rhythm stress diurnal rhythm Prediabetes physical activity day and night. A disturbance of this highly regulated system can lead to circadian misalignment resulting in sleeping difficulties with consequences on many physiological functions like psychological and physical performance, the metabolism, and the immune system as it could be found in night shift workers or in people suffering from chronic jet lag. Extreme environments such as high altitude, hot or cold environments, or microgravity also can alter human sleep patterns as well as during isolation and confinement—experimental setups that serve to simulate the isolated nature of long-term space travel.
0 thoughts on “Circadian rhythm stress”