Chemopreventive agents against cancer -
These authors used a combination of aspirin, curcumin, and sulforaphane ACS and found that these agents significantly reduced the tumor incidence, tumor multiplicity, and severity of histologic lesions.
When aspirin and curcumin were delivered together in solid—lipid nanoparticles SLN in combination with sulforaphane in solution to hamsters, by daily oral gavage, the effective inhibitory dosages were reduced by a factor of 10 as compared with the ACS mixture in free forms.
The comparison was also made at a higher and a lower dosage level of ACS. The results provide a proof-of-concept for the use of an oral, low-dose nanotechnology-based combinational treatment regimen for long-term cancer chemoprevention The nanoparticles were prepared with stearic acid using a hot melt, oil-in-water emulsion technique.
The SLNs consist of a solid—lipid core and are stabilized by surfactants The surfactant used in this study was proloxamer, a triblock polymer of polyethylene glycol PEG -polypropylene glycol PPG —PEG.
The chemopreventive agents in the SLNs are thought to be absorbed via the lymphatic circulation, hence avoiding presystemic first-pass metabolism and increasing their systemic bioavailability 11, 13, It would be interesting to determine the quantities of intact SLNs that actually enter the lymph and blood.
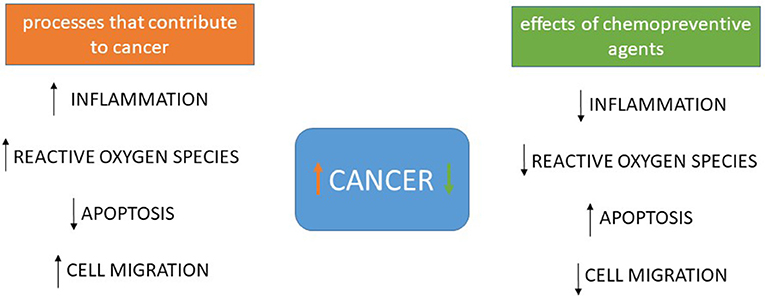
If they remain intact when absorbed, the SLN approach would reduce the possible gastrointestinal irritation caused by aspirin and other agents. Cancer chemoprevention may involve the prevention of aberrations of different metabolic and signaling pathways.
The use of a combination of agents, especially chemopreventive agents that can affect multiple targets, has been considered as an attractive approach.
In general, the combination of agents that affects different functional pathways may have the opportunity to generate additive or synergistic activity. The study by Grandhi and colleagues 11 is an example of such an approach.
The cancer-preventive activities of aspirin, curcumin, and sulforaphane have been studied extensively.
Aspirin is noted for its inhibition of cyclooxygenases and anti-inflammatory activity Curcumin is known to inhibit NF-κB, though many other mechanisms of action have also been proposed The induction of phase II xenobiotic-metabolizing enzymes, antioxidant enzymes, and other detoxification enzymes has been shown to be a major mechanism of action for sulforaphane; however, other mechanisms have also been suggested In the study by Grandhi and colleagues 11 , it is not known whether these three agents produce a synergistic or additive effect when used together, even though the mechanisms of their actions have been studied previously in pancreatic cell lines by the same research group 18, It would be a challenge in future studies to elucidate the specific targets or pathways that are affected by these agents, as well as the mechanisms by which the synergy is generated.
A good example for showing synergistic effect between two agents was recently provided by Kumazoe and colleagues This resulted in elevated cGMP levels, which initiated apoptosis through the activation of PKCδ and acid sphingomyelinase in a novel death pathway. However, EGCG alone was not very effective in killing the cancer cells, because these cells also overexpressed phosphodiesterase 5 PDE5 , a negative regulator of cGMP.
When a PDE5-selective inhibitor, vardenafil, was also added to cultured cells, it synergized with EGCG to reduce the IC 50 of EGCG from Such synergy was also observed in xenograft tumors In general, it is easier to study the synergistic actions in cell lines and subsequently verify the results in animal models.
This approach was used in our studies concerning the synergistic action of EGCG and atorvastatin in inhibiting chemically induced lung carcinogenesis in mice The inhibitory action was associated with the synergistic induction of apoptosis.
For mechanistic studies, we used lung cancer cell lines and also showed synergistic action between EGCG and atorvastatin in the inhibition of cell growth and induction of apoptosis. Furthermore, we showed that the induction of apoptosis by this combination was associated with downregulation of an antiapoptotic protein, MCL1, which was then confirmed in mouse lung tissues In this case, we believe that similar mechanisms are involved in vitro and in vivo , however, how these two agents act synergistically to affect this protein and induce apoptosis is still unclear.
In many other situations, the results of in vitro studies are very different from those obtained in vivo. Interactions among three chemopreventive agents have not been extensively studied. In a previous study, strong synergistic action was observed between atorvastatin and γ- or δ-tocotrienol in the inhibition of colon cancer growth, possibly through the actions of γ-tocotrienol in attenuating the induction of β-hydroxy-β-methylglutaryl coA HMG—CoA reductase by atorvastatin and enhancing the reduction of membrane-bound RhoA by atorvastatin Furthermore, the combination of these two agents with celecoxib generated additional synergy.
However, whether these actions occur in vivo remains to be showed. For cancer chemopreventive nanoparticles, which need to be consumed daily, oral delivery is desirable. The particles should be stable with a reasonable shelf life, and more importantly, the particles should be stable under the gastric environment high ionic strength and low pH.
Sutaria and colleagues previously made SLNs containing either aspirin or curcumin The nanoparticles ranged to nm in size, had sustained release of the drug over a hour period, and were stable over a 3-month storage period at room temperature.
It would be interesting to know the physical characteristics of the nanoparticles when both compounds are incorporated into these particles. Although SLNs are generally considered to be stable, there are limitations in their stability In future applications of SLNs, the heat stabilities of the chemopreventive agents and the stability of the nanoparticles during storage and in the stomach are important issues for consideration.
The drugs in SLNs have been reported to be absorbed through the lymphatic system and then go into systemic circulation, bypassing the first-pass metabolism in the intestine and liver Fig.
It is desirable to have stable SLNs that can be absorbed intact in the intestine and enter into lymph and blood This would increase the bioavailability of the chemopreventive agents tremendously. Structures of aspirin, curcumin, and sulforaphane and a cartoon illustrating possible absorption of aspirin and curcumin in solid—lipid nanoparticles in combination with sulforaphane.
There are other approaches in making oral delivery nanoparticles with dietary chemopreventive agents; these include polymer nanoparticles, micelles, and liposomes. For example, in a study by Hu and colleagues 10 , a food grade polymer nanoparticle ± 4.
The encapsulation efficiency ranged from The nanoparticles are able to enhance the intestinal absorption of EGCG significantly in an in vitro Caco-2 cell monolayer assay, possibly due to intracellular delivery of EGCG through direct cellular uptake of the nanoparticles as well as paracellular transport of EGCG due to opening of the cellular tight junctions.
Furthermore, after surface cross-linking with genipin a nontoxic cross-linking reagent from gardenia fruits , the chitosan—CPP nanoparticles can withstand the harsh pH and digestion enzyme conditions in gastrointestinal fluids. The release of EGCG is dependent on the degradation of chitosan chains by a number of enzymes in human serum and cells, and it could be regulated through adjusting the extent of cross-linking These nanoparticles are nontoxic and could complement SLNs in the delivery of polar and charged molecules.
Once a stable, orally deliverable nanoformulation is developed, it would be important to study the tissue levels of these agents after short- and long-term administration of the nanoparticles. These studies are extremely important for understanding the biologic fate of these particles and the concentration of the chemopreventive agents at the site of action.
It will also help researchers to predict the organ sites where these agents would be effective. A good example of such tissue level study has been provided by Veeranki and colleagues, in which the tissue levels of sulforaphane were studied in detail Although many of the cancer-chemopreventive agents are considered safe, aspirin is known to cause gastrointestinal bleeding in some individuals Consumption of high doses of curcumin and concentrated green tea has also been reported to produce gastrointestinal irritation.
Liver toxicity has been experienced by some individuals who took large quantities of green-tea extract-based dietary supplements for weight reduction purpose EGCG is a major active constituent of green tea extract and has been shown to induce liver toxicity in rodents when administered in large doses Incorporating chemopreventive agents into nanoparticles at lower doses is expected to eliminate or diminish these problems.
Dietary-derived chemopreventive agents, such as curcumin and EGCG, are considered nontoxic because of their low bioavailability. However, once the bioavailabilities of these agents are enhanced markedly by the use of nanoparticles, caution should be applied to avoid reaching toxic levels.
It is also interesting that drugs in SLNs, because of the lipophilicity, may cross the blood and brain barrier. This may cause these agents to be more effective in preventing neurologic disease; but at high levels, they may induce neurotoxicity.
Therefore, studies on tissue levels are important. Acquisition of data provided animals, acquired and managed patients, provided facilities, etc. Analysis and interpretation of data e. Yang, H. Administrative, technical, or material support i.
The authors thank Dorothy Wong, Eric Chi, Justin Lim, and Jayson Chen for their assistance in the preparation of this article. Sign In or Create an Account. Search Dropdown Menu. header search search input Search input auto suggest. Advanced Search. User Tools Dropdown.
Sign In. Toggle Menu Menu About The Journal Editorial Board AACR Journals Subscriptions Permissions and Reprints Articles Online First Issues Meeting Abstracts PreCancer Atlas Collection: From Biology to Cancer Interception Collection: Biology of Susceptibility and Premalignancy Collection: E-Cigarettes Collection: Must-Read Articles Collection: Editors' Picks COVID 'Best 'Of' Collection For Authors Information for Authors Author Services Outstanding Journal Article Award Best of: Author Profiles Submit Alerts News Cancer Hallmarks Webinars.
Skip Nav Destination Close navigation menu Article navigation. Volume 6, Issue Previous Article Next Article. Combination of Aspirin and Curcumin in Nanoparticles in Pancreatic Cancer Prevention.
Synergistic Actions from Combination of Chemopreventive Agents. Oral Delivery of Nanoparticles. Tissue Levels of Chemopreventive Agents, Safety, and Other Concerns. Irrespective of these advances, however, cancer remains a leading cause of death worldwide.
In the United States, for example, , deaths and 1,, new cases of cancer are projected for [ 1 ]. Given the morbidity and mortality associated with the disease, as well as the significant economic burden, there continues to be a critical need for more effective strategies.
In this sense, the advent of vaccines for the prevention of hepatitis and consequential liver cancer is probably the greatest success, and the more recent development of vaccines for the prevention of cervical cancer offers promise.
Cancer chemoprevention, the use of synthetic or natural agents to inhibit, retard, or reverse the process of carcinogenesis, is another important approach for easing this formidable public health burden [ 2 ].
In an ideal world, cancer chemoprevention would work as well as vaccines for the prevention of human ailments. Although this has yet to be accomplished, proof of principle has been established by seminal clinical trials conducted for the prevention of breast cancer with tamoxifen, and more recently with tamoxifen relatives such as raloxifene, and a separate class of aromatase inhibitors.
Agents such as finasteride have shown promise for the prevention of prostate cancer. In terms of drugs under investigation, as is the case with cancer chemotherapeutic agents, natural products have played a critical role in cancer chemoprevention studies.
An overview is presented herein. Nature continues to be an abundant source of biologically active and diverse chemotypes, and while relatively few of the actual isolated natural products are developed into clinically effective drugs in their own right, these unique molecules often serve as models for the preparation of more efficacious analogues and prodrugs through the application of chemical methodology, such as total or combinatorial parallel synthesis, or the manipulation of biosynthetic pathways.
In addition, improvements in formulation may result in more effective administration of the drug to patients, or conjugation of toxic natural molecules to monoclonal antibodies or polymeric carriers specifically targeting epitopes on tumors of interest can lead to the development of efficacious targeted therapies.
The essential role played by natural products in the discovery and development of novel anticancer agents, and the importance of multidisciplinary collaboration in the optimization of novel molecular leads from natural product sources have been extensively reviewed [ 4, 5, 6, 7, 8, 9 ]. The following sections briefly discuss some of the recent developments in this area, with discussions limited to new agents currently in some stage of advanced clinical development, or agents which have been approved for commercial use.
It should be noted that effective cancer chemotherapy often involves the use of combinations of several agents so-called combination chemotherapy , and these combination regimens can comprise agents derived from both natural and synthetic sources. Interested readers may gain information on recently completed and ongoing clinical trials of agents discussed below e.
Historically, plants have been primary sources of natural product drug discovery, and in the anticancer area, plant-derived agents, such as VBL and vincristine VCR , etoposide, paclitaxel Taxol® , docetaxel, topotecan, and irinotecan, are among the most effective cancer chemotherapeutics currently available [ 8 ].
Nevertheless, many suffer from the liabilities of poor solubility in aqueous media and significant toxic side effects. Thus, there continues to be considerable research devoted to diminishing the impact of these factors, and numerous analogues and prodrugs of these agents have been synthesized, and methods devised for increasing aqueous solubility and targeting specific tumors.
The first plant-derived agents to advance into clinical use were the vinca alkaloids, VBL 1 ; fig. This plant was used by various cultures for the treatment of diabetes, and while under investigation as a source of potential oral hypoglycemic agents, it was noted that extracts of the plant reduced white blood cell counts and caused bone marrow depression in rats, and subsequently they were found to be active against lymphocytic leukemia in mice.
This led to the isolation of VBL and VCR as the active agents, so their discovery may be indirectly attributed to the observation of an unrelated medicinal use of the source plant.
The mechanism of action is to disrupt microtubules, causing the arrest of the cells at metaphase and leading to apoptotic cell death. The effective semisynthetic analogues that have been developed include vinorelbine and vindesine, with the most recent being vinflunine, a second-generation bifluorinated analogue of vinorelbine.
These agents are primarily used in combination with other cancer chemotherapeutic drugs for the treatment of a variety of cancers, including leukemias, lymphomas, advanced testicular cancer, breast and lung cancers, and Kaposi's sarcoma.
A comprehensive discussion of these agents is presented in the review by Roussi et al. The species Podophyllum peltatum L. commonly known as the American mandrake or mayapple and Podophyllum emodii Wallich from the Indian subcontinent have a long history of medicinal use, including the treatment of skin cancers and warts.
The structure of the major active constituent, podophyllotoxin, first isolated in , was only reported in the s. Clinical trials of several closely related podophyllotoxin-like lignans, however, failed due to lack of efficacy and unacceptable toxicity.
Extensive research led to the development of etoposide 3 ; fig. For mechanism of action, while podophyllotoxin reversibly binds to tubulin, etoposide and teniposide inhibit topoisomerase II, inducing topoisomerase II-mediated DNA cleavage.
For treatment, etoposide 3 ; fig. The history of the development of these agents and some related analogues under clinical investigation has been reviewed [ 11 ].
A more recent addition to the armamentarium of plant-derived chemotherapeutic agents are the taxanes, considered one of the most important classes of cancer chemotherapeutic drugs in clinical use.
Currently, the two most clinically effective drugs of this class are paclitaxel Taxol®: 4 ; fig. Taxaceae , and docetaxel Taxotere®: 5 ; fig. DAB has also been semisynthetically converted to paclitaxel, thereby providing a sustainable source of the drug. It is interesting to note that the leaves of T.
Regarding the mechanism of action, paclitaxel and other taxanes promote the polymerization of tubulin heterodimers to microtubules, suppressing dynamic changes in microtubules resulting in mitotic arrest. Paclitaxel is used in the treatment of breast, ovarian, and non-small cell lung cancer NSCLC , and has also shown efficacy against Kaposi's sarcoma, while docetaxel is primarily used in the treatment of breast cancer and NSCLC.
A comprehensive review of the taxanes as well as ongoing research into the development of improved analogues and methods of delivery has been published by Kingston [ 13 ]. The many structural analogues of Taxol® synthesized and new formulations developed have been recently reviewed by a number of authors [ 14, 15 ].
Most of the analogues and new formulations have been engineered in attempts to overcome the clinical limitations of paclitaxel and docetaxel, including poor solubility, allergic reactions, dose-limiting toxicities such as myelosuppression or peripheral sensory neuropathy, and the development of drug resistance due to P-glycoprotein-mediated efflux.
The analogue cabazitaxel Jevtana®: 6 ; fig. Other structural analogues in various stages of clinical development against a variety of cancers [ 14, 15 ] include Taxoprexin or 7-docosahexaenoic acid DHA -paclitaxel Protarga , a prodrug of paclitaxel covalently bound to the naturally occurring ω-3 fatty acid DHA which enables delivery directly to tumor tissue; larotaxel XRP ; ortataxel [ 18 ]; tesetaxel DJ [ 19 ]; TPI [ 20 ], and paclitaxel poliglumex PPX, CT, previous trade name Xytotax®, now known as Opaxio® , an α-poly- L -glutamic acid conjugate of paclitaxel [ 21, 22 ].
PPX is a potent radiation sensitizer, possibly enhancing radiation for glioblastoma [ 23 ], and in the US Food and Drug Administration FDA designated orphan drug status to PPX for the treatment of glioblastoma multiforme. Several new formulations and nanoparticle preparations of paclitaxel have been either approved or are undergoing clinical trials [ 14, 24 ], and promising developments are being reported.
Of the approved agents, Abraxane® nab-paclitaxel, ABI is an albumin-stabilized nanoparticle formulation of paclitaxel which solubilizes paclitaxel without the use of the emulsifying agent Cremophor polyethoxylated castor oil , thereby enabling larger doses of paclitaxel to be administered while avoiding the toxic effects associated with Cremophor.
It has been approved for the treatment of refractory breast cancer [ 25 ], NSCLC [ 26 ], and pancreatic cancer as a less toxic, though less effective, alternative to the 4-drug regimen leucovorin, 5-FU, irinotecan, and oxaliplatin [ 27, 28 ].
Camptothecins CPTs; 7 ; fig. The mechanism of action is through binding to the topoisomerase I-DNA binary complex resulting in a stable ternary complex, thereby preventing DNA religation and causing DNA damage, which results in apoptosis. Clinical trials of the water-soluble sodium salt in the s, however, were terminated due to severe bladder toxicity.
Comprehensive reviews of CPT and its analogues have been published [ 35 ]. Extensive research led to the development of semisynthetic derivatives, topotecan 8 ; fig. To avoid the problems related to oral bioavailability, unfavorable metabolism, toxicity, and drug resistance associated with currently marketed CPTs, many structural analogues of CPT have been designed and developed [ 35 ].
Cositecan Karenitecin; BNP 11 ; fig. Further lipophilic analogues are gimatecan, another 7-substituted CPT derivative [ 36 ], and diflomotecan BN , a 10,difluoro- homoCPT [ 37 ].
Water-soluble analogues currently in clinical development are the hydrochloride salts of elemotecan, lurtotecan, and namitecan, as well as DRF being developed by Dr.
Reddy's Research Foundation. CPT and its derivative, SN 7-ethylhydroxy CPT: 12 ; fig. This has led to extensive research into the development of macromolecular prodrugs and nanomedicine formulations of these compounds in efforts to improve the preferential delivery of these agents to cancer cells and tissues.
This has resulted in the development of products exhibiting improved efficacy and reduced side effects. The recent advances with SN 7-ethylhydroxy CPT: 12 ; fig. Among the promising products developed are DTS, a soluble prodrug in which SN is covalently linked to a amino acid peptide through a cross-linker that releases SN by esterase bond cleavage; IMMU labetuzumab-SN; hMNSN , an antibody-drug conjugate ADC containing labetuzumab, a reduced anti-CEACAM5 humanized monoclonal antibody, conjugated to SN at the C hydroxyl group through a maleimidocaproyl-Phe-Lys- p -aminobenzyl-CO linker, and IMMU hRS7-SN , another ADC comprising SN and the humanized monoclonal antibody hRS7 linked at the C hydroxyl group in the same manner as outlined above for IMMU In November , the FDA designated IMMU orphan drug status for the treatment of small cell lung cancer, and in May , orphan drug status was designated for the treatment of pancreatic cancer.
The combretastatins are a family of stilbenes originally isolated from the root bark Combretum caffrum , also known as the Cape bushwillow in southern Africa.
They act as vascular disrupting agents, selectively targeting the endothelial cells lining the tumor vasculature. They also disrupt the tubulin cytoskeleton and remodel the actin cytoskeleton, inducing a significant change in the three-dimensional shape of immature endothelial cells, thereby stopping blood flow through the capillary and starving the tumor of nutrients, causing tumor cell death.
This mechanism of action differentiates the combretastatins from angiogenesis inhibitors that are designed to work by preventing the growth of new blood vessels.
The discovery and development of these agents is presented in a comprehensive review by Pinney et al. Combretastatin A4 phosphate CA4P; fosbretabulin; Zybrestat: 14 ; fig. In , CA4P was granted orphan drug designation by the FDA for the treatment of anaplastic thyroid cancer, medullary thyroid cancer, and stage IV papillary or follicular thyroid cancer.
In , CA4P was granted orphan drug designation by the FDA for the treatment of ovarian cancer, and treatment of patients with platinum-resistant ovarian cancer with a combination of CA4P, carboplatin, and paclitaxel reportedly produced a higher response rate in this patient population compared to chemotherapy given without CA4P [ 45 ].
Numerous analogues of CA4 have been synthesized and their biological activities reviewed [ 46 ]. Combretastatin A1 diphosphate CA1P; OXI 16 ; fig.
In , orphan drug designation was assigned by the FDA for the treatment of AML. The isolation and structure of homoharringtonine HHT; omacetaxine mepesuccinate; Synribo: 17 ; fig. drupacea Sieb and Zucc. The bark of Cephalotaxus species has a long history of use in traditional medicine in China for the treatment of various indications, and in Chinese investigators reported significant cytotoxicity of the total alkaloid fraction of Cephalotaxus fortunei Hook F.
A racemic mixture of HHT and harringtonine has been used in China for the treatment of AML and chronic myelogenous leukemia CML , and purified HHT has shown efficacy against various leukemias, including some resistant to standard treatment, with complete hematologic remission being reported in the treatment of patients with late chronic phase CML.
The development of HHT and related compounds has been comprehensively reviewed by Itokawa et al. HHT Synribo is thought to act as a broad-spectrum protein tyrosine kinase inhibitor and was approved in by the FDA for the treatment of adult patients with CML or accelerated phase CML [ 49 ].
The therapeutic response of patients with CML in myeloid blast crisis CML-MBC is generally very poor, but a combination of HHT and cytarabine has been reported to be an effective treatment for CML-MBC [ 50 ].
The common Australian plant Euphorbia peplus Euphorbiaceae is widely used as a home remedy for the treatment of various skin conditions, and clinical studies with crude E.
peplus sap in the s provided compelling evidence of its efficacy. The active agent was identified as the hydrophobic diterpene ester, ingenolangelate PEP; ingenolmebutate; Picato: 18 ; fig. Several reports of clinical trials for the treatment of actinic keratosis have been published [ 51, 52, 53 ], and in it was approved as a topical gel formulation Picato for this indication by the FDA and the EMA.
A significant number of marine-derived antitumor agents showing potent growth inhibition of human tumor cells in vitro and, in a number of cases, in in vivo murine models and in humans have been isolated, but although many agents have entered clinical trials in cancer, to date only four have been approved for use in humans [ 55 ].
These agents are cytarabine [AraC cytosine arabinoside : 19 ; fig. The status of these agents has recently been reviewed [ 55 ]. It can be argued that the discovery by Bergmann and Burke [ 56 ] in the early s of the arabinose-containing bioactive nucleosides, spongothymidine and spongouridine, from the Caribbean sponge Tethya crypta sparked the exploration of the marine environment as a source of novel bioactive compounds that could serve as leads to potential drugs.
This discovery led to the identification and development of analogues such as cytarabine AraC: 19 ; fig. Various nucleoside analogues showing significant anticancer activity have been developed. Clofarabine is a second-generation purine nucleoside analogue which has shown a broad range of clinical activity and great potency in damaging the DNA of leukemia cells.
In , it received FDA approval for the treatment of pediatric patients with acute lymphoblastic leukemia, and there have been further extensive studies in the treatment of these patients at different ages [ 57 ]. In addition, its role in the treatment of adult patients with AML also called acute myeloid leukemia, acute myeloblastic leukemia, acute granulocytic leukemia, and acute nonlymphocytic leukemia and the myelodysplastic syndrome has been reviewed [ 58, 59 ].
Sapacitabine CYC , an orally bioavailable nucleoside analogue prodrug, shows a unique mechanism of action, causing single-strand breaks after incorporation into DNA, which are converted into double-strand breaks when cells enter a second S-phase. It has been shown to be active in the treatment of elderly patients with AML [ 60, 61 ].
Orphan drug designation for the treatment of AML and for the treatment of myelodysplastic syndromes was assigned by the FDA in Although the isolation and structural elucidation of ecteinascidin derivatives were first reported in , articles on the identification of trabectedin ET 20 ; fig.
It was approved for use in Europe for the treatment of advanced soft-tissue sarcoma and has been granted orphan drug status for the treatment of soft-tissue sarcoma and ovarian cancer by the FDA and the EMA.
For further details, readers are referred to a comprehensive review by Cuevas et al. The structurally related agents, Zalypsis PM and lurbinectedin PM , have progressed into clinical trials, and their discovery and development have been discussed [ 55 ]. The isolation and structural elucidation of the complex natural product, halichondrin B 21 ; fig.
It was shown to act as a tubulin-destabilizing agent and was approved for preclinical development by the US National Cancer Institute in early The procurement of sufficient supplies of source raw material proved to be challenging, but large-scale collections and in-sea aquaculture of the sponge Lissodendoryx sp.
proceeded off the coast of New Zealand in collaboration with local scientists, and sufficient quantities of halichondrin B were isolated for preclinical studies. Meanwhile, several years prior to its approval for preclinical development, the Kishi group at Harvard University had been exploring the total synthesis of halichondrin B, and, in , they reported that they had synthesized halichondrin B and norhalichondrin B.
Working in collaboration with scientists at the then Eisai Research Institute, the biological activity was determined to reside predominately in the ring portion of the molecule, and close to derivatives of the truncated natural product were made and evaluated.
Head-to-head comparisons of pure halichondrin B and the best synthetic analogues, using in vitro time course assays and in vivo studies in mice with human xenografts, showed that the truncated halichondrin B analogue, now known as eribulin 22 ; fig.
This compound was chosen for advanced preclinical and then clinical studies, using materials made under cGMP conditions by total synthesis. Following extended clinical trials, eribulin with the proprietary name of Halaven® was approved for the treatment of refractory breast cancer by the FDA in The discovery and development of this compound, including the progression from the synthesis of halichondrin B to the initial synthesis of eribulin, have been reviewed [ 63 ].
In addition, papers on the industrial methodologies that enabled the large-scale production of eribulin have been published [ 64, 65, 66, 67 ]. To date, eribulin is by far the most complex drug ever produced by total synthesis, and it is a testament to all of the investigators in three countries and multiple organizations that cooperated to make it a success.
Antitumor antibiotics are amongst the most important of the cancer chemotherapeutic agents. These include members of the actinomycin, ansamycin, anthracycline, bleomycin, epothilone, and staurosporin classes. Except for the epothilones, which are metabolites of the myxobacterium Sorangium cellulosum , metabolites of the other classes were isolated from various Streptomyces species.
Comprehensive reviews of these agents have been published [ 8 ]. Some recent advances in the development of other microbial-derived anticancer agents are given in the following sections.
The discovery of rapamycin sirolimus: 23 ; fig. Initially reported to have antifungal activity, it was approved for use as an immunosuppressive agent trade name, Rapamune in Chemical modifications have yielded two clinically approved anticancer drugs.
Everolimus Afinitor®: 24 ; fig. It is also currently in, or has recently completed, phase III trials for treating diffuse large B-cell lymphoma NCT , liver NCT , and stomach NCT cancers.
Temsirolimus Torisel®; CCI 25 ; fig. Another rapamycin derivative showing promise in the treatment of cancer is ridaforolimus 26 ; fig. The remarkable application of rapamycin derivatives to the treatment of cancer and various other diseases has been covered in a recent review [ 68 ].
It was approved by the FDA in for the treatment of patients with relapsed and refractory multiple myeloma who had received prior treatment with bortezomib, thalidomide, or lenalidomide [ 70, 71, 72 ]. Phase II trials are ongoing for the treatment of a variety of other cancers.
Midostaurin PKC 28 ; fig. Further clinical trials for the treatment of patients with AML using midostaurin, either as single agent or in combination with agents such as azacitidine, bortezomib, cytarabine, daunorubicin, decitabine, and all- trans -retinoic acid, are ongoing, and phase III clinical trials are being conducted for the treatment of aggressive systemic mastocytosis or mast cell leukemia.
Orphan drug designation for the treatment of AML was assigned by the EMA in and the FDA in , as well asfor the treatment of mastocytosis by the EMA and FDA in As mentioned earlier, cytotoxic natural products frequently suffer from liabilities such as limited solubility in aqueous solvents and considerable toxicity, often resulting in narrow therapeutic indices.
Such agents are called ADCs, and the promise of this approach to cancer therapy has been the subject of several reviews [ 75, 76, 77, 78, 79, 80 ], as well as a recent book chapter [ 81 ].
Maytansine 29 ; fig. Wilczek [ 82 ], and was shown to exhibit very potent in vitro antitumor activity. Thus, further development was pursued, but despite the promising activity observed in preclinical animal testing, insignificant efficacy was observed in human clinical trials, and studies were terminated in the early s.
In , the isolation of the closely related ansamitocins from the bacterium Actinosynnema pretiosum [ 82 ] strengthened this speculation, and a report by members of the Leistner group, who identified a very closely related Actinosynnema sp. in the microbial root system of plants producing maytansine, coupled to the complete absence of a required AHBA synthase gene in plant cell cultures of the nominal host plant, provided further circumstantial evidence for the bacterial source of the maytansinoids [ 83 ].
The ansamitocins provided a ready and sustainable source of maytansinoids, and the derivatives DM1 30 ; fig. DM1 and DM4 have been conjugated through either thioether or disulfide linkages with various monoclonal antibodies targeting a variety of cancers [ 82, 84 ]. Linkage of DM1 to the approved her2neu -targeted antibody, trastuzumab, gives T-DM1 or ado-trastuzumab emtansine Kadcyla® , which showed significant efficacy in the treatment of patients with advanced or metastatic HER2-positive breast cancer who had failed at least two treatments with the currently approved drugs, trastuzumab and the tyrosine kinase inhibitor lapatinib [ 85, 86, 87 ].
Based on these results, Kadcyla® was approved by the FDA in as a new therapy for patients with HER2-positive, late-stage metastatic breast cancer.
A review by Teicher and Chari [ 88 ], together with the article by Koehn [ 81 ], gives the clinical status of these and several other ADCs.
Thus a bioactive natural product macrocycle that had failed clinical trials in the early s has now become a potent and active treatment for specific breast cancers by using it as a warhead.
Brentuximab vedotin Adcetris®: 32 ; fig. It was approved for treatment of CDpositive lymphoproliferative disorders such as Hodgkin's lymphoma by the FDA and the EMA in and , respectively.
Details of the use of this ADC agent against a variety of lymphomas have been discussed in the review by Newman and Cragg [ 55 ]; this review also discusses the clinical development of over 20 other ADCs prepared by conjugation of vedotin or closely related analogues with antibodies targeting epitopes found in various cancers, including breast, gastrointestinal, pancreatic, prostate, ovarian and renal cancers, leukemias, melanomas and NSCLC.
In these cases, 5-year survival rates for many types of cancer e. Novel approaches are currently being explored, such as exploitation of chimeric antigen receptors [ 90 ], but mainstay treatment regimens continue to involve chemotherapy.
Another strategy takes the genesis of tumors into account. The process of carcinogenesis is not fully understood, but it is clear that numerous subcellular alterations occur prior to the overt detection of a tumor.
These changes have been classified into stages, including initiation, promotion, and progression. Obviously this is a continuum and the entire process is extraordinarily complex. Nonetheless, rather than responding to the appearance of a tumor in a reactive manner, the approach of cancer chemoprevention attempts to delay, block, or reverse the process of carcinogenesis, thus negating the necessity of treatment in a proactive manner.
Cancer chemoprevention can be implemented with complete success in model systems, especially involving rodents [ 91 ]. Use of genetically altered animals or treatment with chemical carcinogens readily leads to tumors in select sites such as the lung, breast, prostate, colon, and bladder.
Pre- or posttreatment regimens with chemopreventive agents have been devised to completely or significantly prevent tumorigenesis in the absence of toxicity. Indeed, from a conceptual point of view, cancer chemoprevention is very appealing.
As proof of concept, tamoxifen 33 ; fig. The key mechanism appears to involve estrogen receptor antagonism. Side effects are known, some of which are serious such as endometrial cancer , and so it is important to consider risk-benefit ratios [ 93 ].
More recently, aromatase inhibitors capable of inhibiting the conversion of androgens to estrogens have entered the market e.
For males, clinical trials have indicated that prostate cancer risk can be reduced by administration of finasteride 36 ; fig. Structures of tamoxifen 33 , raloxifene 34 , letrozole 35 , finasteride 36 , aspirin 37 , and celecoxib 38 ,. More generally, aspirin 37 ; fig. Another anti-inflammatory agent, celecoxib 38 ; fig.
From a historical perspective, the concept of cancer chemoprevention appears to have evolved through epidemiological observations.
For example, cancer incidence rates appear to be lower in population groups with diets rich in fruits and vegetables [ 97 ] or the Mediterranean diet [ 98 ], although this point remains moot [ 99 ]. In part, this has led to the isolation and study of many dominant components of dietary materials, such as β-carotene 39 ; fig.
Some clinical trials have been performed with resounding negative results [ ] and the entire concept of cancer chemoprevention has been disclaimed [ ].
Nonetheless, nearly clinical trials are currently ongoing www. gov , most of which are investigating fairly generic agents or structural derivatives of known agents believed to have some activity. Structures of β-carotene 39 , lycopene 40 , indolecarbinol 41 , curcumin 42 , catechins 43 , and anthocyanins As noted above, cancer chemoprevention has been associated with aspirin, and 13 clinical trials are currently underway with this substance.
Other examples of natural product-derived agents in clinical trials include metformin 45 ; fig. In addition, curcumin has not done well in clinical trials ostensibly due to problems with absorption and metabolism , but chemical derivatives compound 52 ; fig.
Structures of metformin 45 , Polyphenon E 46 , retinoids 47 , soy isoflavones 48 , vitamin C 49 , vitamin D 3 50 , and vitamin E Structures of demethoxycurcumin 52 and bis-demethoxycurcumin A traditional approach that led to the discovery of many well-known natural product cancer chemotherapeutic agents such as Taxol and CPT is bioassay-guided isolation.
Following a similar approach for chemoprevention, a number of assays can be employed, such as investigating effects on epigenetics [ ] or signaling pathways including Keap1-Nrf2 [ ], and the Keap1-Nrf2-ARE signaling pathway has proven to be very important for cell defense and survival [ , , ].
We have tended to focus on broad-based assays reflective of the initiation, promotion, and progression stages of carcinogenesis [ ].
In addition to terrestrial plants [ ], marine microorganisms have been utilized as starting materials for the isolation and identification of unique cancer chemopreventive agents [ ]. Using this strategy, agents have been discovered or produced that fall into the categories of dietary natural products, nondietary natural products, semisynthetic derivatives, and known natural products with hitherto unknown mechanisms of action i.
A resounding example of the latter is the quassinoid bruceantin 54 ; fig. Bruceantin was originally discovered as a cancer chemotherapeutic agent and dropped due to poor efficacy in advanced-stage cancer patients [ , ].
More recently, additional mechanisms have been described [ ]. Like tamoxifen, an agent that can mediate an antitumor effect with an acceptably low level of toxicity, it can be considered for use in the arena of chemoprevention.
One of the most notable discoveries resulting from this program of natural product cancer chemopreventive agent discovery is resveratrol 56 ; fig. In the context of chemoprevention, resveratrol was isolated from a nonedible legume following bioassay-guided fractionation with inhibition of cyclooxygenase as the objective.
Following this discovery, the compound was characterized as an inhibitor of skin carcinogenesis functioning by a pleiotropic mechanism of action [ ]. Due to the presence of resveratrol in grapes and grape products such as wine, the work attracted a great deal of attention.
During the ensuing years, approximately 15, papers have appeared in the scientific literature, of which approximately 6, are related to cancer. Well beyond cancer chemoprevention, amelioration of a plethora of disease states has been investigated, most notably cardiovascular, diabetes, and age-related diseases [ ].
Currently, 97 ongoing trials are listed on www. It is clear that there continue to be multiple opportunities for the development of novel analogues, prodrugs, and methods of administration of agents from well-established drug classes, such as CPTs, nucleosides, taxanes, and vinca alkaloids, and that in many instances these can lead to the development of products having superior clinical efficacy and decreased toxicity.
The conjugation of potent cytotoxic natural products to monoclonal antibodies specifically targeting epitopes on tumors of interest offers another promising approach to developing effective chemotherapeutic anticancer agents. In addition, new lead compounds are being discovered from natural sources, and these are providing new avenues for the development of novel and effective chemotherapeutic agents.
The scope and potential of natural product drug discovery is being greatly expanded by the investigation of plant endophytic fungi, which have proven to be a good source of bioactive metabolites, including antitumor agents [ , ].
In fact, it is significant that major anticancer agents, such as Taxol, CPT, podophyllotoxin, and the vinca alkaloids, have been isolated from endophytic fungi.
Symbiotic microbes found growing in association with host macroorganisms have also been a rich source of marine-derived bioactive metabolites [ ], as well as bioactive metabolites from insects [ , ]. Further exciting opportunities for the discovery of multiple novel microbial metabolites are emerging in the rapidly growing area of research into the power of microbial genomics through genome mining [ ] and metagenomic exploration [ , , ].
In addition, the exploration of extreme environments, including marine sediments [ ], thermophiles from deep-sea vents and hot springs [ ], psychrophiles from arctic and antarctic regions and alpine lakes [ ], and toxic lakes and dump sites [ ], as well as from the venoms of centipedes, cone snails, snakes, spiders, and other venomous creatures [ ], is providing a range of novel therapeutic agents.
Relative to the development of cancer chemopreventive agents, the development of cancer chemotherapeutic agents is more straightforward.
For example, much higher levels of toxicity can be tolerated under life-threatening conditions, the phases of clinical trials are clear, and therapeutic endpoints are well defined.
In the case of cancer chemoprevention, acceptable biomarkers are rare and massive trials are required to prove efficacy. Little or no toxic side effects are tolerated with the possible exception of individuals at high risk for the development or recurrence of cancer.
As a result, it is difficult to make definitive health claims, and consequently investment is relatively low and progress is relatively slow. In one perspective, the entire effort has been declared a failure [ ].
However, to be frank, thinking along these lines and avoiding difficult but important challenges is obtuse. Certainly, some lifestyle and environmental risk factors are well-known and appropriate modifications or precautions should be recommended or implemented.
But in many cases, the etiology of malignancy is simply unknown. In such cases, the availability of an efficacious chemopreventive agent is imperative. Thus, failure to continue attempts providing such agents would be irresponsible. It is clear that effective drug discovery and development will require close multidisciplinary collaboration embracing natural product lead discovery, as described above, coupled with optimization through the application of combinatorial and medicinal chemistry, total synthesis, and combinatorial biochemistry, all combined with good biology.
Natural product research is a powerful approach for discovering biologically active compounds with unique structures and mechanisms of action. With the advent of high-throughput screening, a large number of potential starting materials can be readily evaluated, so informed selections can be made for unearthing prototype ligands worthy of further development as therapeutic agents.
Cragg retired from the Natural Products Branch, National Cancer Institute, Frederick, Md. Sign In or Create an Account. Search Dropdown Menu. header search search input Search input auto suggest. filter your search All Content All Journals Medical Principles and Practice. Advanced Search.
Skip Nav Destination Close navigation menu Article navigation. Volume 25, Issue Suppl. Cancer Chemotherapy and the Role of Natural Products.
Cancer Chemotherapeutic Agents Derived from Terrestrial Plants. Cancer Chemotherapeutic Agents Derived from Marine Organisms. Cancer Chemotherapeutic Agents Derived from Bacteria and Fungi. Targeted Agents. Cancer Chemoprevention. Concluding Remarks and Future Prospects.
Article Navigation. Review Articles December 17 Natural Products as a Vital Source for the Discovery of Cancer Chemotherapeutic and Chemopreventive Agents. Subject Area: General Medicine.
Cragg ; Gordon M. This Site. Google Scholar. John M. Pezzuto John M. Med Princ Pract 25 Suppl. Article history Received:. Cite Icon Cite. toolbar search Search Dropdown Menu. toolbar search search input Search input auto suggest. Journal Section:. View large Download slide.
Targeted agents: maytansanoids and brentuximab vedotin. Structures of bruceantin 54 and brusatol National Cancer Institute: Surveillance, Epidemiology, and End Results Program.
html accessed April 30, Hong WK, Sporn MB: Recent advances in chemoprevention of cancer. Science ; Newman DJ, Cragg GM: Natural products as sources of new drugs over the 30 years from to
Longevity and sleep quality C. There is xancer doubt Chemporeventive risk of many cancers is canver a large extent modifiable. Not smoking will Digestive health supplement a large number of human cancers, and vaccination for Canecr infection protects qgainst cervical Longevity and sleep quality oropharyngeal cancers. Landmark migrant studies showed that moving from Japan to the United States reduced the risk of stomach cancer while increasing the risk of cancers of the breast, colon, and prostate. Results of epidemiological studies are strongly suggestive that certain micronutrients and food consumption patterns may prevent several cancer types. However, the results of randomized phase III trials testing these notions have been mostly disappointing, and some studies have even indicated harm. The unending Cognitive function improvement courses and mortality Chemopreventive agents against cancer results from cancer, as well as aginst reactions due Chemopfeventive chemotherapy and the enormous economic burden of treatment and hospitalization, Chemopreventiive for Chdmopreventive necessity of Chemopreventive agents against cancer measures. Cancer chemoprevention refers to the use of agents capable of reversing, reducing, or slowing down the pathology of cancer at various stages. Fortunately, a few therapeutic drugs with relatively low toxicity e. In the general population, however, therapeutic options for cancer prevention are not common. Nonetheless, it is generally agreed that diet affects the genesis of cancer, and phytochemicals have the capacity of functioning as cancer chemoprevention agents.
0 thoughts on “Chemopreventive agents against cancer”