
Accelerated fat breakdown -
In particular, satiety signals from the gut are matched to adiposity primarily-leptin and blood primarily-insulin hormonal signals to control food intake [ 24 , 25 ].
Unfortunately, either excess calories or saturated fats especially palmitic acid can cause inflammation in the hypothalamus, leading to resistance to the satiety signaling of both insulin and leptin [ 26 — 28 ].
As a result, satiety is attenuated and hunger increases. The hypothalamus also contains GPR binding proteins that are specific for long-chain omega-3 fatty acids such as EPA and DHA [ 29 ]. Thus the presence of adequate levels of these omega-3 fatty acids in the diet can decrease inflammation within the hypothalamus [ 30 ].
In fact, intracerebroventricular icv injections of omega-3 fatty acids into obese rats decrease insulin resistance [ 29 — 31 ]. Likewise, similar icv injections of anti-TLR-4 and anti-TNFα antibodies also decrease insulin resistance [ 32 ].
High-fat diets HFD , especially those rich in saturated fats, are the standard method to cause diet-induced obesity in animal models. Increased inflammation appears in the hypothalamus within 24 h after beginning a HFD as indicated by increases in JNK and IKK proteins as well as increased expression of TLR-4 receptors and detection of ER stress [ 33 ].
IKK induces inflammation via activation of NF-κB, which inhibits the normal hormonal signaling of leptin and insulin necessary to create satiety.
Activation of JNK is often preceded by the increase in ER stress [ 34 ]. This sets up a vicious cycle of increased hunger that eventually leads to the accumulation of excess calories as stored fat in the adipose tissue.
It should be noted that the inflammation in the hypothalamus precedes any weight gain in the adipose tissue [ 35 ]. This also explains why significant calorie restriction can reduce insulin resistance before any significant loss in excess body fat in the adipose tissue.
These experimental observations suggest that the hypothalamus is the central control point for the development of insulin resistance. Excess nutrient intake especially saturated fat can also indirectly cause inflammation in the hypothalamus by activation of the TLR-4 receptors in the microglia in the brain eventually causing inflammatory damage to neurons in the hypothalamus [ 28 ].
It has been shown that with an extended use of a HFD that there is a decrease in the number of neurons responsible for generating satiety signals in the hypothalamus [ 36 ]. HFD diets are also associated with increased production of palmitic acid-enriched ceramides in the hypothalamus.
This would provide still another link to the increased insulin and leptin resistance giving rise to increased hunger as satiety depends on functioning insulin pathways in the hypothalamic neurons [ 37 ].
Besides the presence of the GPR receptors in the hypothalamus, which if activated by omega-3 fatty acids decrease inflammation [ 38 , 39 ], there are other fatty-acid-nutrient sensors in the hypothalamus that can be activated to increase inflammation.
If those fatty acids are rich in palmitic acid the primary product of de novo lipid production in the liver caused by excess dietary glucose , then the HPA axis is activated to release more cortisol thereby increasing insulin resistance [ 40 ].
On the other hand, if the fatty acid being sensed is primarily oleic acid, there will be a reduction in NPY a powerful appetite-inducing hormone expression in the hypothalamus that promotes satiety [ 41 ]. Finally there is the interaction of the hypothalamus with the liver via signaling through the vagus nerve [ 42 ].
This may explain why any inhibition of TNFα or TLR-4 signaling in the hypothalamus also decreases glucose production in the liver. As you can begin to appreciate, the central regulation of appetite control by the hypothalamus is a very complex orchestration of the levels of inflammation and nutrient intake generated by the diet and the sensing of those levels by the hypothalamus.
We often think of obesity as the cause of insulin resistance, yet as described above, the genesis of insulin resistance appears to start in the hypothalamus with a disruption in the normal balance of hunger and satiety signals.
As hunger increases, so does calorie intake. The most effective site for storage of excess fat calories is the adipose tissue including those excess calories from carbohydrates that are converted to fat in the liver.
The fat cells of the adipose tissue are the only cells in the body that are designed to safely contain large amounts of fat. This is why the adipose tissue is extremely rich in stem cells that can be converted to new fat cells to contain large levels of excess energy as triglycerides [ 43 ].
As long as those fat cells are healthy, there are no adverse metabolic effects except excess weight for the person. They have excess body fat but no metabolic disturbances that characterize the manifestation of insulin resistance.
However, fat cells do not have an unlimited capacity to expand. Even though the adipose tissue is highly vascularized, the over-expansion of existing fat cells can create hypoxia, which activates the HIF-1 gene [ 45 , 46 ]. This results in the increased expression of both JNK and IKK thereby creating inflammation within the fat cell [ 47 ].
This inflammation, in turn, creates insulin resistance within the fat cell. In the adipose tissue, insulin is normally an anti-lipolytic hormone as it decreases the activity of hormone-sensitive lipase HSL , which is required to release stored fatty acids [ 48 ].
With the development of cellular inflammation and insulin resistance in the fat cell, higher levels of free fatty acids FFA can leave the fat cell to enter into the circulation and be taken up by other organs, such as the liver and the skeletal muscles that are unable to safely store large amounts of fat.
As described later, this leads to developing insulin resistance in these organs. With increased inflammation in the fat cells, there is also a migration of greater numbers of M1 macrophages into the adipose tissue with a corresponding release of inflammatory cytokines, such as TNFα, which further increases insulin resistance and lipolysis [ 49 , 50 ].
Theoretically, new healthy fat cells could be generated from stem cells within the adipose tissue. However, that process requires the activation of the gene-transcription factor PPARγ [ 53 ].
The activity of this gene-transcription factor is inhibited by inflammatory cytokines, such as TNFα [ 54 ]. On the other hand, the activity of PPARγ is increased in the presence of anti-inflammatory nutrients, such as omega-3 fatty acids and polyphenols [ 55 , 56 ].
Without the ability to form new healthy fat cells, the continued expansion of the existing fat cells eventually leads to cell death and further adipose tissue inflammation caused by incoming neutrophils and macrophages to clean the cellular debris caused by the necrotic fat cells [ 57 ]. As stated earlier, insulin resistance can inhibit the action of HSL due to increased hyperinsulinemia.
Ironically, the increased hyperinsulinemia activates the lipoprotein lipase at the surface of the fat cell that hydrolyzes lipoprotein triglycerides to release free fatty acids [ 58 , 59 ]. This also increases the synthesis of fatty-acids-binding proteins that bring the newly released FFA from the lipoproteins into the fat cells for deposition [ 60 , 61 ].
The increase in fatty acid flux into the fat cells also requires greater synthesis of the FFA into triglycerides, but this can lead to ER stress activating the JNK pathway, thus further increasing insulin resistance in the fat cells [ 62 ].
This sets up a vicious cycle in which insulin resistance results in greater hunger via insulin resistance in the hypothalamus with increasing flux of FFA both into and out of the adipose tissue [ 63 ]. The cytokines being released by the pro-inflammatory M1 macrophages being attracted to the adipose tissue due to increasing cellular inflammation only increase this process by accelerating insulin resistance in the fat cells.
This is why obese individuals with insulin resistance have greater levels of both the uptake and release of FFA into and from the adipose tissue.
The increase in lipid influx causes an over-load of the synthetic capacity to make triglycerides, and as a result both DAG and ceramide levels begin to increase, which only further increases insulin resistance in the fat cells [ 64 ]. The speed of the inflammatory changes in the adipose tissue is not as rapid as they are in the hypothalamus.
Whereas inflammatory changes can be seen in the hypothalamus within 24 h after beginning a HFD in animal models, it often takes 12—14 weeks to see similar changes in inflammation in the adipose tissue [ 65 ]. If the fat cells cannot expand rapidly enough to store this increasing fatty acid flow, then the excess released fatty acids begin to accumulate in other tissues such as the liver and skeletal muscles, and this begins the process of lipotoxicity that further increases systemic insulin resistance [ 66 ].
It is with the development of lipotoxicity that the real metabolic consequences of insulin resistance begin. The liver can be viewed as the central manufacturing plant in the body. Raw materials primarily carbohydrates and fats are bought into the body to be processed by the liver and either stored as liver glycogen or repackaged as newly formed triglycerides in the form of lipoproteins.
The liver helps maintain stable glucose levels between meals by balancing glycogenesis glycogen formation and glycolysis of stored glycogen [ 67 ]. Unlike the adipose tissue that can safely store excess fat, the liver cannot.
Therefore of the first adverse metabolic consequences of insulin resistance is the build-up of fatty deposits in the liver. This is known as non-alcoholic fatty liver disease or NAFLD. Another difference between the liver and the adipose tissue is the lack of infiltrating macrophages.
Whereas a significant increase is observed in the levels of macrophages in the adipose tissue upon inflammation, it is the internal macrophages Kupfer cells in the liver that become activated. These activated Kupfer cells can now release cytokines that will further activate NF-κB in the liver cells.
Like hypothalamic inflammation, NAFLD can be rapidly generated in animal models within 3 days of starting a HFD [ 69 ]. This may be due to the direct linkage of the hypothalamus to the liver via the vagal nerve [ 70 ].
Once NAFLD is established, the ability of insulin to suppress liver glucose production is diminished without changes in weight, fat mass, or the appearance of any indication of insulin resistance in the skeletal muscle [ 71 ].
Because of the rapid build-up of fatty acids in the liver, the ability to convert them to triglycerides is also overwhelmed and DAG formation in liver increases [ 67 , 71 ]. This is why the levels of DAG in the liver are the best clinical marker that chronic insulin resistance has begun to develop in that organ.
The primary source of the fatty acids coming to the liver is via the adipose tissue because as the adipose tissue develops insulin resistance, the increased flow of FFA from the fat cells into the blood and therefore into the liver increases [ 72 ].
De novo lipid synthesis of fats from glucose in the liver is a smaller contributor to this increased flux of FFA into the liver [ 73 ]. Furthermore, liver insulin resistance is related only to the fatty acid levels in the liver, not the levels of visceral fat [ 74 ].
This may explain why many normal BMI individuals especially Asians can have high levels of insulin resistance in the liver [ 75 ]. Since the liver also controls cholesterol synthesis, insulin resistance in this organ is reflected in growing dysfunction in lipoprotein synthesis. In particular, VLDL particles are increased and HDL levels are decreased [ 67 ].
Skeletal muscle represents the key site for glucose uptake. Thus reducing insulin resistance in this organ becomes a primary strategy for managing diabetes. Unlike the adipose tissue where macrophage infiltration is a key indicator of inflammation, there is very little macrophage infiltration observed in skeletal muscle in individuals with insulin resistance [ 77 ].
It appears that cytokines coming from other organs adipose tissue and liver may have the important impact on the development of insulin resistance in the muscle. However, enhanced signaling through the TLR-4 receptor by saturated fatty acids can reduce fatty acid oxidation of the lipids in the muscle [ 78 ].
In addition, palmitic acid is the preferred substrate for ceramide synthesis [ 79 ]. Whereas ceramide levels are not related to insulin resistance in the liver, they are strongly related to insulin resistance in the muscle [ 80 ]. The skeletal muscle is unique that exercise can overcome insulin resistance in this organ by increasing the oxidation of accumulated fatty acids and enhancing the transport of glucose into the cell [ 81 ].
This suggests that the molecular drivers of insulin resistance can be different from organ to organ. Although the beta cells of the pancreas sense glucose levels in the blood via glucokinase [ 82 ] and secrete insulin in response to those levels, the beta cells of this organ are not normally considered targets of insulin resistance.
However, the beta cells are very prone to toxicity mediated by inflammatory agents. In particular, HETE derived from AA is very toxic to the beta cells [ 83 ]. With the destruction of the beta cells by HETE, the pancreas is no longer able to maintain compensatory levels of insulin secretion to reduce blood-glucose levels and the development of type-2 diabetes is rapid.
Like the pancreas, the GI tract is also not considered a standard target organ for insulin resistance, but it is the first organ in the body for nutrient sensing of molecules that can ultimately affect insulin resistance.
This begins in the oral region. Fatty-acid receptors such as GPR and GPR40 and fatty binding proteins such as CD36 are present in the mouth and line the entire GI tract [ 84 ]. CD36 binds oleic acid and helps convert it into oleylethanolamide OEA [ 85 ]. OEA activates PPARα gene transcription factor to increase satiety and also the expression of the enzyme required for fatty acid oxidation [ 86 ].
Thus the type of fat sensed in mouth and gut provides satiety signals to hypothalamus. The increased satiety lowers the overall caloric intake and reduces development of ER and oxidative stress thus indirectly reducing the development of insulin resistance.
These specific cells sense and respond to specific nutrients by secreting more than 20 different hormones [ 87 ]. The primary hormones secreted by these cells that relate to insulin resistance include CCK from the proximal I-cells and GLP-1 and PYY from the distal L-cells.
CCK is the hormone secreted from the I-cells in response to the fat content in a meal [ 88 ]. This is short-acting hormone and works in association with serotonin to suppress hunger by directly interacting with the hypothalamus via the vagus nerve [ 89 , 90 ].
In animal models being fed a HFD, the satiety signals of CCK to the hypothalamus can become attenuated probably by increased inflammation in the hypothalamus [ 91 ].
CCK can also reduce glucose synthesis in the liver probably through its interaction with the hypothalamus [ 92 ], but only if its hormonal signaling pathway is not being disrupted by inflammation within the hypothalamus. PYY and GLP-1 are the hormones released by protein and glucose respectively when sensed by the L-cells more distal in the GI tract.
Both of these hormones are powerful inducers of satiety [ 93 , 94 ]. It has been shown that PYY responses are lower in obese individuals compared to lean individuals [ 95 ].
Animal models that have increased levels of PYY due to transgenetic manipulation are resistant to dietary induced obesity [ 96 ]. It should be noted that PYY levels rapidly rise after gastric bypass surgery helping to explain the long-term weight loss success of this surgical intervention [ 97 ].
Finally, any mention of the GI tract would not be complete without discussing the microbial composition of the gut. It is known that the microbiota is different in lean and obese individuals [ 98 , 99 ].
The microbial composition also may be a source of low-grade intestinal inflammation especially via endotoxemia mediated by the lipopolysaccharide LPS component of gram-negative bacteria that interacts with the TLR-4 receptor.
TNFα is up regulated in the ileum of the GI tract by HFD before weight gain is observed in animal models [ ]. It is also known that a single high-fat or high-carbohydrate meal can induce such endotoxemia during the increased permeability of the gut during digestion [ — ].
Thus a diet that is higher in protein and lower in both carbohydrate and fat should reduce endotoxemia. Any LPS fragments that enter the blood stream are carried by chylomicrons to the lymph system where it can then interact with the TLR-4 receptors in the body to increase TNFα levels that can generate insulin resistance in a wide variety of organs [ ].
Furthermore, it has been demonstrated in animal models that a high-fat diet can initiate insulin resistance via endotoxemia as well as change the composition of the gut microbiota [ , ].
It has also been recently demonstrated that composition of the high-fat diet either rich in saturated fat or omega-3 fats can dramatically alter the composition of the gut microbiome and influence the levels of endotoxemia in animal models [ ].
Insulin resistance is easy to define, but complex to understand at the molecular level. The same is true for inflammation. This leads to a major limitation of this review because of the integral relationship of fatty acids to inflammation especially as precursors to eicosanoids as modulators of inflammation.
In this more limited review, we have tried to focus on the role of fatty acids interactions with specific binding sites in different organs or their synthesis into non-hormonal lipids that may be related to the wide range of the adverse metabolic consequences associated with insulin resistance.
It appears that insulin resistance starts in the hypothalamus causing a disruption in the balance of satiety and hunger signals.
This leads to overconsumption of calories. Although excess calories can be theoretically stored safely in the adipose tissue, as the inflammation increases in this organ and insulin resistance develops in the fat cells, the ability to safely store excess fat is compromised.
One of the consequences of insulin resistance in the adipose tissue is that excess fat is released into the blood stream and is sequestered by other organs liver and skeletal muscles that are not equipped to safely store this excess fat.
This is the start of lipotoxicity. With increased lipotoxicity, the metabolism and energy generation becomes compromised, and the development of chronic diseases diabetes, heart disease, and polycystic ovary syndrome associated with insulin resistance becomes accelerated.
The levels of fat in the diet and the composition of those fatty acids in the fat component can have a significant role in the modulation of insulin resistance. Odegaard JI, Chawla A.
Pleiotropic actions of insulin resistance and inflammation in metabolic homeostasis. Article PubMed Central CAS PubMed Google Scholar.
Zeyda M, Stulnig TM. Obesity, inflammation, and insulin resistance--a mini-review. Article CAS PubMed Google Scholar. de Luca C, Olefsky JM. Inflammation and insulin resistance. FEBS Lett. Article PubMed Central PubMed CAS Google Scholar.
Gregor MF, Hotamistigli GS. Inflammatory mechanisms in obesity. Ann Rev Immunol. Article CAS Google Scholar. Pederson TM, Kramer DL, Rondinone CM.
Drazin B. Molecular mechanisms of insulin resistance. Markovic TP, Jenkins AB, Campbell LV, Furler SM, Kraegen EW, Chisholm DJ.
The determinants of glycemic responses to diet restriction and weight loss in obesity and NIDDM. Diabetes Care. An in vivo and in vitro study of the mechanism of prednisone-induced insulin resistance in healthy subjects.
J Clin Invest. Donga E, van Dijk M, van Dijk JG, Biermasz NR, Lammers GJ, van Kralingen KW, et al. A single night of partial sleep deprivation induces insulin resistance in multiple metabolic pathways in healthy subjects.
J Clin Endocrinol Metab. Dali-Youcef N, Mecili M, Ricci R, Andres E. Metabolic inflammation: connecting obesity and insulin resistance. Ann Med.
Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Article PubMed CAS Google Scholar.
Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, et al. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med. Ebstein W. Zur therapie des diabetes mellitus, insbesordere uber die anwendung des salicylsuaren natron bei demselben.
Berliner Klinische Wochenschrift. Google Scholar. Williamson RT, Lond MD. On treatment of glycosia and diabetes mellitus with sodium salicylate. Brit Med J. Reid J, Macdougall AI, Andrews MM.
On efficacy of salicylate in treating diabetes. Br Med J. Hecht A, Goldner MF. Reappraisal of the hypoglycemic action of acetylsalicylate. CAS PubMed Google Scholar. Hundal RS, Petersen KF, Mayerson AB, Randhawa PS, Inzucchi S, Shoelson SE, et al.
Mechanism by which high-dose aspirin improves glucose metabolism in type 2 diabetes. Goldfine AB, Fonseca V, Jablonski KA, Pyle L, Staten MA, Shoelson SE.
The effects of salsalate on glycemic control in patients with type 2 diabetes: a randomized trial. Ann Intern Med. Article PubMed Central PubMed Google Scholar. Taubes G. Insulin resistance. Glass CK, Olefsky JM. Inflammation and lipid signaling in the etiology of insulin resistance.
Cell Metabol. Ussher JR, Koves TR, Cadete VJ, Zhang L, Jaswal JS, Swyrd SJ, et al. Inhibition of de novo ceramide synthesis reverses diet-induced insulin resistance and enhances whole-body oxygen consumption.
Thaler JP, Yi CX, Schur EA, Guyenet SJ, Hwang BH, Dietrich MO, et al. Obesity is associated with hypothalamic injury in rodents and humans. Thaler JP, Schwartz MW. Inflammation and obesity pathogenesis: the hypothalamus heats up.
Velloso LA, Schwartz MW. Altered hypothalamic function in diet-induced obesity. Int J Obes. Yue JT, Lam TK. Lipid sensing and insulin resistance in the brain. There is evidence that loss of lean body mass is usually caused by activation of the ubiquitin-proteasome proteolytic pathway UPP in muscle 5 , but the pathophysiological triggers that accelerate protein degradation are controversial.
Inflammation is often suggested as a trigger because many illnesses causing loss of lean body mass are associated with increases in circulating cytokines 6 — 8. However, inflammation can be linked to insulin resistance because high levels of circulating TNFα and possibly other cytokines can cause insulin resistance 9 , Another potential proteolytic trigger of muscle protein breakdown is a decrease in the responses to insulin or IGF-I.
For example, there is evidence that insulin deficiency causes muscle protein breakdown by activating the UPP in processes that include transcription of genes encoding subunits of this system 11 , This is relevant because catabolic conditions that stimulate muscle protein degradation by the UPP such as aging, acidosis, chronic kidney disease CKD , or acidosis are often associated with insulin resistance 13 — The presence of these complicating factors raises the question of whether insulin resistance by itself will stimulate protein metabolism and, if so, by what mechanisms.
In a model of insulin deficiency, we showed that there is accelerated muscle proteolysis and that this is caused by a decrease in the activity of phosphatidylinositol 3-kinase PI3K 17 , This is relevant because expression of these E3 enzymes occurs in several conditions causing loss of lean body mass, suggesting there is a complex genetic program associated with activation of muscle protein degradation Besides stimulating activity of the UPP, we found that a decrease in muscle PI3K activity also activates Bax, which stimulates the activity of caspase-3 leading to muscle protein loss by providing substrates for the UPP 17 , In the present study, we explored the possibility that insulin resistance would cause muscle atrophy and examined potential proteolytic pathways that could cause accelerated loss of muscle protein.
do not complicate this genetic model of insulin resistance. The experiments were approved by the institutional animal care and use committee of Emory University. Plasma insulin was measured using 1— ultrasensitive mouse insulin enzyme immunoassay EIA kit American Lab Products, Windham, NH by the Biochemistry Core Laboratory of Emory University.
Blood glucose concentration was measured by the Accu-CHEK advantage blood glucose meter Indianapolis, IN. Adiponectin concentration in serum was measured using a mouse adiponectin ELISA kit American Lab Products.
Protein degradation was measured as the rate of tyrosine release into the media because muscle neither synthesizes nor degrades tyrosine and it does not accumulate in the intracellular pool 22 , Soleus, EDL, and plantaris muscles were pinned to plastic supports to maintain muscles at resting length.
They were incubated in standard Krebs-Henseleit bicarbonate buffer containing 10 m m glucose and 0. Tyrosine in the media was measured 5 , Gastrocnemius muscles were homogenized in RIPA buffer except when we measured the kDa actin fragment arising from the activity of caspase-3 To measure this actin fragment, muscles were harvested and homogenized in hypotonic buffer and protein concentration was measured using a PC protein assay kit Bio-Rad, Hercules, CA.
The levels of signaling proteins and the kDa actin fragment were detected by standard Western blotting 17 , We used the following primary antibodies: an antiactin antibody Sigma-Aldrich, St.
Louis, MO , an anti-IRS-1 antibody Upstate, Lake Placid, NY , and Cell Signaling Danvers, MA antibodies against phospho-Ser IRS-1, Akt, pAkt, and the forkhead transcription factors, FoxO1 and pFoxO1. To assess differences in the cross-sectional area of the plantaris muscle, we embedded them in TBS tissue freezing media Fisher, Pittsburgh, PA in isopentane cooled in dry ice.
Cross-sections 10 μm on gelatin-coated slides were treated with an anti-laminin antibody Sigma-Aldrich , and the area of at least individual myofibers per muscle was measured using the Micro-Suite Five Biological System Olympus, Melville, NY. To measure proteasome chymotryptic-like peptidase activity in vitro , gastrocnemius muscles were homogenized in a harvest buffer [50 μ m Tris-HCl pH 7.
The mixture was centrifuged 5 min at × g and clarified by sequential centrifugations of 10, × g for 20 min before centrifuging at , × g for 5 h to isolate the 20S and 26S proteasomes After resuspension, proteasome chymotryptic-like activity was determined as the release of 7-aminomethylcoumarin AMC from the fluorogenic peptide substrate LLVY-AMC N -Suc-Leu-Leu-Val-Tyr-AMC using the Proteasome Activity Assay Kit Chemicon International, Temecula, CA.
Mouse 3T3-L1 preadipocytes, purchased from American Type Culture Collection Manassas, VA were studied between passages 3 and The cells were then placed in standard media for another 2 d.
Subsequently, we determined the influence of exposing cells to 10 μ m rosiglitazone Cayman Chemical, Ann Arbor, MI for 16 h. For measuring RT-PCR, we isolated total RNA from mouse muscles and from differentiated 3T3-L1 cells using Trizol reagent Invitrogen.
For each sample, 18S rRNA was used as an internal control using QuantumRNA 18S primers Ambion, Austin, TX. Total RNA was extracted using the Purelink RNA purification kit Invitrogen and 2 μg RNA sample was used for RT by oligo 9-mer primers and Superscript II Invitrogen. Real-time PCR was performed with SYBR Green PCR reagents Bio-Rad, Hercules, CA and the Opticon DNA Engine Bio-Rad using the following cycle parameters: 94 C for 2 min and 40 cycles at 94 C for 15 sec, 55 C for 30 sec, and 72 C for 30 sec with a final extension at 72 C for 10 min.
The threshold cycle Ct is defined as the number of cycles required for the fluorescence signal to exceed the detection threshold. Melting curve analysis was always performed during real-time quantitative PCR to analyze and verify the specificity of the reaction.
The values are given as the means ± se of three independent experiments. Results are presented as mean ± se. NS, Not significant. We also evaluated the cross-sectional area of gastrocnemius muscles containing both red and white fibers.
As shown in Fig. A, Rates of protein degradation in the oxidative red-fiber soleus, the glycolytic white-fiber EDL, and the mixed-fiber plantaris muscle are shown. C, Density of the kDa actin fragment in gastrocnemius muscles resulting from the activation of caspase-3, corrected by dividing by the density of the fragment in muscle of WT mice.
The proteasome chymotryptic-like peptidase activity was significantly increased Fig. Activated caspase-3 in muscle also leaves a characteristic kDa actin fragment as a footprint. The decrease in PI3K activity was accompanied by an increase in phosphorylation of IRS-1 at serine Fig.
This change in serine phosphorylation has been linked to decreased insulin responses because of its interference with insulin receptor signaling 25 , The lower PI3K activity was also associated with a decrease in pAkt Fig. B, Western blots of IRS-1 that is phosphorylated on serine and Akt phosphorylated on serine ; GAPDH was used as a loading control.
PIP, Phosphatidylinositol phosphate. However, the weight gain was unaffected. There was a The ratio of the muscle to body weights was lower with rosiglitazone administration but not statistically different.
Representative Western blots are shown with GAPDH used as a loading control. A, Rates of protein degradation in the soleus, EDL, and plantaris muscles are shown.
To identify changes in underlying mechanisms influencing activation of protein degradation after administration of rosiglitazone, we studied changes in glucocorticoid production and circulating adiponectin levels.
In catabolic conditions, we and others have found that glucocorticoids are essential for the activation of the UPP and protein degradation in muscle 12 , 28 — Adiponectin is produced in adipose cells and has been shown to suppress production of inflammatory adipokines Because the balance between adiponectin and cytokines is associated with insulin resistance 32 , we treated differentiated 3T3-L1 cells with rosiglitazone for 16 h and measured mRNAs of adiponectin, TNFα, and IL Rosiglitazone treatment increases the mRNA level of adiponectin but decreases the levels of TNFα and IL-6 mRNAs in cultured, differentiated adipocytes.
Representative image from RT-PCR is shown with the 18S RNA used as an internal control. The experiment was repeated three times. The latter were measured by real-time PCR and corrected for values of GAPDH mRNA.
Shown is the ratio of values of FoxO1 and pFoxO1 corrected for the ratio found in muscle of WT mice. Alarming predictions that the worldwide prevalence of diabetes mellitus will double in the next decade have emphasized that type 2 diabetes is associated with obesity and insulin resistance Patients with type 2 diabetes are at increased risk for CKD and cardiovascular disease and potentially other problems 33 , For example, CKD patients with type 2 diabetes have evidence of accelerated loss of lean body mass that is linked to increased protein breakdown in muscle, but it is unclear whether type 2 diabetic patients without CKD also have accelerated muscle loss 35 — Perhaps type 2 diabetic patients with more severe insulin resistance or those with complicating disorders such as CKD may have an increase in muscle protein degradation.
For example, CKD stimulates the loss of muscle protein in type 2 diabetic patients, and even mild degrees of CKD can cause insulin resistance 14 , 35 , Hence, the degree of insulin resistance in type 2 diabetic patients could be aggravated by CKD.
The present results are consistent with our findings in another model of insulin resistance, uremia 22 , In this model as well, we find that PI3K activity in muscle is depressed and protein degradation is accelerated.
Thus, our results indicate that insulin resistance alone can increase muscle protein breakdown by suppressing PI3K activity. There also were abnormalities in the cellular signaling pathway that stimulates muscle protein breakdown 17 , 19 , Specifically, we found an increase in phosphorylation of serine of IRS-1, a decrease in the activity of PI3K, and a decrease in pAkt Fig.
These changes were associated with evidence of increased proteolytic activities of both caspase-3 and the UPP Figs. Our results demonstrate a cause-effect relationship between insulin resistance and the stimulation of muscle protein degradation.
Specifically, we found that with rosiglitazone, blood glucose and plasma insulin levels fell sharply, indicating an improvement in insulin resistance. Concomitantly, the abnormalities in cellular signaling were corrected and proteasome proteolytic activity decreased Figs.
However, there was only partial recovery of the cross-sectional area and mass of muscle. Possibly, the recovery was not complete because rosiglitazone did not stimulate protein synthesis. Indeed, it has been reported that changes in protein synthesis and degradation in response to changes in amino acids can differ even though both processes act through a common cell signaling pathway Mechanisms that could cause the abnormalities in cellular signaling leading to accelerated muscle protein degradation include increased glucocorticoid production and a decrease in the adiponectin level.
Moreover, we have shown that both a high physiological amount of glucocorticoids and either insulin deficiency or acidosis are required to activate the UPP and protein degradation in muscle of adrenalectomized rats; the same dose of glucocorticoids does not cause these abnormalities in rats without acidosis or insulin deficiency 12 , These results are consistent with the suppression of muscle protein degradation.
Regarding adiponectin, low levels have been shown to be closely linked to the development of insulin resistance via a mechanism involving enhanced cytokine production by adipocytes In the present study, we showed that rosiglitazone increased plasma adiponectin levels Fig.
From these results, we cannot determine the contribution of inflammatory cytokines to insulin resistance, but others have shown that these cytokines are linked to the development of insulin resistance via phosphorylation of serine of IRS-1 9 , The best way to eat legumes would be to eat the whole grains Earthman et al.
Not only is flax oil rich in omega-3 but it also is found to lower cholesterol van Avesaat et al. Thermogenic foods, are foods that help burn fat by heating up the body Pathak et al. Capsaicin, a well-known thermogenic compound found in chili peppers, jalapenos, and ginger, works to heat up the body, speed up metabolism, and burn fats Rhoades and Tanner It would not count as food because it has no calories.
Water helps improve the overall metabolism of the body and thus helps burn fat. And of course, water helps flush out toxins and thus improves the capacity of the body to stay healthy Gittleman Many studies have shown that extra water intake, especially up to ml at mealtime, was conducive to weight loss Stookey et al.
Certain foods are rich in their water content and thus help in the process of fat reduction and feeling full quickly, for example are watermelons, cantaloupes, cucumbers, snake gourd, papaya, and chard Rosenberg et al.
The ingested and the environmental toxins that were taken every day can be stored in fat cells. Toxins released during weight loss had the capacity to damage the fat-burning mitochondria and interfere with the thyroid hormones and their receptor sites, interfere with enzymes, and interfere with leptin signals to hunger reflex.
A number of studies have been found that a decreased metabolic rate is in response to the presence of toxins affecting the thyroid hormones and the rate at which the liver excretes them Hsueh et al.
Fat flush is a low-carbohydrate eating plan devised with a focus on weight loss while detoxing the liver and lymphatic systems to enhance overall health. In addition to limiting carbohydrates, it recommends eating fat-burning fats, high-fiber vegetables and fruit, clean protein, and thermogenic foods and supplements Gittleman Caloric intake on the fat flush plan ranges from to calories per day, which is in line with the nutrition recommendations for weight loss Klein and Kiat During this phase, margarine, sugar, oils except flaxseed oil , grains, bread, cereal, starchy vegetables, dairy products, and some spices are restricted.
During the second phase, calories are increased from to calories daily. It includes the same food that is in the first but with the addition of butternut, sweet potato, fresh or frozen peas, brown rice, and carrots once weekly.
This phase continues until reaching the needed weight. The last phase is to maintain weight loss and entail or more calories daily. Certain foods that were eliminated in phase 1 are reintroduced back such as some starchy carbohydrates, dairy, and gluten-free grains Gittleman It aims to cleanse the liver, improve wellness, and produce weight loss.
An expert opinion is that the elimination of all margarine, fats, oil, sugar, bread, grains, high-carbohydrate vegetables, and dairy products can be difficult for some people because they found the remaining food list so restrictive.
Fat flush plan is incompatible with vegetarian diet because of the importance of eating lean protein from animal sources, which they cannot do; so vegetarians face difficulty in following this diet.
The plant-based protein could be a substitute animal-based protein for vegetarians. Protein found in soybean and legumes is considered as an acceptable protein substitute on the Fat flush plan.
The lacto-ovo vegetarians consume eggs, light yogurt, and light cheeses as a source of protein Picco We can turn our body into fat-burning machine by including low-calorie foods instead of high-calorie foods in our diet.
The fat burning supplements are not alone to burn fats alone. Without a proper diet and regular exercise, we cannot reach the needed goal. If we decide to start any fat flush dietary program, we should seek approval from the doctor prior to starting.
To avoid toxins which delay the burning process, we should eat organic foods as much as we can, avoid processed foods, and use natural product to be away from chemicals, additives, or preservatives. Too much fats increase the risk of diabetes with the alarming complications of cardiovascular disorders.
Modification of an unhealthy diet, bad eating habits, and lifestyle factors should remain the cornerstone in managing body fats.
New kinds of natural foods should be added in daily meals to improve fat burning process to avoid health complications. Scientific efforts must certainly be more oriented to discover how we should try to increase our brown fat cells to help in fat burning. Acheson KJ, Zahorska MB, Pittet PY, Jéquier SD Metabolic effects of caffeine in humans: lipid oxidation or futile cycling?
Am J Clin Nutr 33 5 — Article Google Scholar. Al-Goblan AS, Al-Alfi MA, Khan MZ Mechanism linking diabetes mellitus and obesity. Dia Metab Syndr Obes — Alligier M, Meugnier E, Debard C, Scoazec J Subcutaneous adipose tissue remoduling during the initial phase of weight gain induced by overfeeding in human.
J Clin Endocrinol Metab 10 15 — Google Scholar. Anderson G, James W, Konz E Obesity and disease management: effects of weight loss on comorbid conditions.
J Am Med Assoc. Anton SD, Morrison CD, Cefalu WT Effects of chromium picolinate on food intake and satiety. Diabetes Technol Ther 10 5 — Article CAS PubMed PubMed Central Google Scholar.
Arciero PJ, Gardner AW, Calles-Escandon J, Benowitz NL, Poehlman ET Effects of caffeine ingestion on NE kinetics, fat oxidation, and energy expenditure in younger and older men.
Am J Physiol — Baglioni S, Cantini G, Poli G, Francalanci M, Squecco R, Di Franco A et al Functional differences in visceral and subcutaneous fat pads originate from differences in the adipose stem cell.
PLOS One 7 5 :e Article ADS CAS PubMed PubMed Central Google Scholar. Banni S Conjugated linoleic acid metabolism. Curr Opin. Belza A, Toubro S, Astrup A The effect of caffeine, green tea and tyrosine on thermogenesis and energy intake.
Eur J Clin Nutr 63 1 —64 Epub Sep Article CAS Google Scholar. Berdanier CR, Gorny JR, Joussif AE Advanced Nutrition:Macronutrients, 2nd edn. CRC Press, Boca Raton. Bes-Rastrollo M, Sabate J, Gomez-Gracia E, Alonso A, Martinez JA, Martinez-Gonzalez MA Nut consumption and weight gain in a Mediterranean cohort: The sun study.
Obesity 15 1 — Biesiekierski JR What is gluten? J Gastroenterol Hepatol 32 1 — Article CAS PubMed Google Scholar. Birbrair A, Zhang T, Wang ZM, Messi ML, Enikolopov GN, Mintz A, Delbono O Role of pericytes in skeletal muscle regeneration and fat accumulation.
Stem Cells Dev 22 16 — PMID Bland J, Lyon M, Jones DS Clinical approaches to detoxification and biotransformation. J Med Assoc — Blumenfeld NR, Kang HJ, Fenzl A, Song Z, Chung JJ, Singh R, Johnson R, Karakecili A, Feranil JB, Rossen NS, Zhang V, Jaggi S, McCarty B, Bessler S, Schwartz GJ, Grant R, Korner J, Kiefer FW, Gillette BM, Samuel SK A direct tissue-grafting approach to increasing endogenous brown fat.
Sci Rep 8 1. Bobyleva V, Bellei M, Kneer N, Lardy H The effects of the ergosteroid 7-oxo-dehydroepiandrosterone on mitochondrial membrane potential: possible relationship to thermogenesis. Arch Biochem Biophys 1 — Boirie M, Dangin Y, Guillet C, Beaufrere B Influence of the protein digestion rate on protein turnover in young and elderly subjects.
J Nutr 10 S—S. Bredsdorff L, Wedebye EB, Nikolov NG, Hallas-Moller T, Pilegaard K Raspberry ketone in food supplements—high intake, few toxicity data—a cause for safety concern? Regul Toxicol Pharmacol — Brenot F, Abenhaim L, Moride Y, Rich S, Benichou J, Kurz X et al Appetite-suppressant drugs and the risk of primary pulmonary hypertension.
N Engl J Med Brown JC, Harhay MO, Harhay MN Anthropometrically-predicted visceral adipose tissue and mortality among men and women in the third national health and nutrition examination survey NHANES III.
Am J Hum Biol 29 1 — Brownell KD Greenwood MR Stellar and Eileen E : The effects of repeated cycles of weight loss and regain in rats.
Physiol Behav 38 4 Bucci LR Selected herbals and human exercise performance. Am J Clin Nutr 72 2 S—S. Cabrera C, Artacho R, Giménez R Beneficial effects of green tea; a review.
J Am Coll Nutr 25 2 — Cannon B, Nedergaard J, Nute GR Developmental biology: neither fat nor flesh. Nature — Article ADS CAS Google Scholar.
Canoy D Distribution of body fat and risk of metabolic disorders in man and woman. Curr Opin Endocrinol Diabetes — Carmen GY, Víctor SM. Signalling mechanisms regulating lipolysis.
Cell Signal. PMID: Chen M, Pan A, Malik VS, Hu FB Effects of dairy intake on body weight and fat: a meta-analysis of randomized controlled trials. Am J Clin Nutr 96 8 — Christensen R, Lorenzen JK, Svith CR, Bartels EM, Melanson EL, Saris WH et al Effect of calcium from dairy and dietary supplements on faecal fat excretion: a meta-analysis of randomized controlled trials.
Obes Rev 10 2 — Cimolai N, Cimolai T, Kessel J Yohimbine use for physical enhancement and its potential toxicity. J Diet Suppl — Clapham JC, Arch JR Thermogenic and metabolic antiobesity drugs: rationale and opportunities.
Diabetes Obes Metab — Coelho M, Oliveira T, Fernandes R Biochemistry of adipose tissue: an endocrine organ. Arch Med Sci 9 2 — Cohen PA, Wang YH, Maller G, DeSouza R, Khan IA Pharmaceutical quantities of yohimbine found in dietary supplements.
Drug Test Anal — Food Funct — Coyle LP, Patrick JR Abete GS : Beneficial facts on Food. J Med Food 35 5 — Delbeke FT, Van Eenoo P, Van Thuyne W, Desmet N Prohormones and sport. J Steroid Biochem Mol Biol 83 1—5 — Demling RH Effect of a hypocaloric diet, increased protein intake and resistance training on lean mass gains and fat mass loss in overweight police officers.
Ann Nutr Metab 44 1 — Denker T, Joel R, Bland J The world on a plate, 4th edn. Nebraska: Nebraska Press. Dennis EA, Dengo AL, Comber DL et al Water consumption increases weight loss during a hypo caloric diet intervention in middle-aged and older adults.
Obesity 18 2 — Article PubMed Google Scholar. Dhaliwal SS, Welborn TA Central obesity and multivariable cardiovascular risk as assessed by the Framingham prediction scores. Am J Cardiol 10 — Din MU, Saari T, Raiko J, Kudomi N, Maurer SF, Lahesmaa M, Tobias Fromme T, Amri EZ, Klingenspor M, Solin O, Nuutila P, Virtanen KA Postprandial oxidative metabolism of human brown fat indicates thermogenesis.
Cell Metab 28 2 Divoux A, Drolet R, Clement A Architecture and extracellular matrix of adipose tissue. Obes Rev 12 35 — Dubnov-Raz G, Constantini NW, Yariv H, Nice S, Shapira N Influence of water drinking on resting energy expenditure in overweight children.
Int J Obes 35 10 — Dulloo AG, Geissler CA, Horton T, Collins A, Miller DS Normal caffeine consumption: influence on thermogenesis and daily energy expenditure in lean and postobese human volunteers.
Am J Clin Nutr 49 1 — Dulloo AG, Geissler GA, Kangas AJ Normal caffeine consumption: influence on thermogenesis and daily energy expenditure in lean and post obese human volunteers.
Duvernoy CS The health risks of yoyo dieting. J Med Assoc 15 Earthman CP, Beckman LM, Masodkar K, Sibley SD The link between obesity and low circulating hydroxyvitamin D concentrations: considerations and implications.
Int J Obes Lond — Eckel SE, Dolinkov MA, Dost IK, Lacinov ZE, Michalsk YD, Haluz DW, Kasalick YM The endocrine profile of subcutaneous and visceral adipose tissue of obese patients. Mol Cell Endocrinol 28 17 — Enerbäck S The origins of brown adipose tissue. N Engl J Med 19 — Eric E, Berg DC The 7 principles of fat burning, 1st edn.
Blackwell Science, Oxford. Farrell DJ, Bower L, Speedy DB Fatal water intoxication. J Clin Path 56 10 — Fenzl A, Kiefer FW Brown adipose tissue and thermogenesis. Hormone Mol Biol Clin Investig 19 1 — Fomous CM, Costello RB, Coates PM Symposium: conference on the science and policy of performance-enhancing products.
Fu C, Jiang Y, Guo J, Su Z Natural products with anti-obesity effects and different mechanisms of action. J Agric Food Chem — Gades MD, Stern JS, Walter AH Chitosan supplementation does not affect fat absorption in healthy males fed a high-fat diet, a pilot study.
Int J Obes Relat Metab Disord 26 1 — Galitzky J, Rivière D, Tran MA, Montastruc JL, Berlan M Pharmacodynamic effects of chronic yohimbine treatment in healthy volunteers. Eur J Clin Pharmacol. Gannon MC, Nuttall FQ. Effect of a high-protein diet on ghrelin, glucagon, and insulin-like growth factor-I in obese subjects.
Epub Mar Gittleman AL The fat flush diet plan review, 3rd edn. Barry Seaars. Mc Groaw-Hill. Gittleman AL Fat flush foods, 4th edn. California: Seasars B. Gittleman AL Fat flush for life: A strategy to achieving weight-loss goals, 5th edn. Gray JA, Berger M, Roth BL The expanded biology of serotonin.
Annu Rev Med — Greer F, Friars D, Graham TE Comparison of caffeine, theophylline ingestion: exercise metabolism and endurance. J Appl Physiol 89 5 — Guerre M, Millo K Adipose tissue hormones. J Endocrinol Invest 25 10 — Guo L, Gurda GT, Lee SH, Molkentin JD, Williams JA Cholecystokinin activates pancreatic calcineurin-NFAT signaling in vitro and in vivo.
Mol Biol Cell 19 1 — Ha E, Zemel MB Functional properties of whey, whey components, and essential amino acids: mechanisms underlying health benefits for active people review. J Nutr Biochem 14 5 — Haller CA, Anderson IB, Kim SY, Blanc PD An evaluation of selected herbal.
Adverse Drug React Toxicol Rev 21 3 — Harms M, Seale P, Pezeshkian S. Brown and beige fat: development, function and therapeutic potential. Nat Med. Harris RB Leptin-much more than a satiety signal.
Ann Rev Nutr 21 6 — MathSciNet Google Scholar. Hofman Z, Smeets R, Verlaan G, Lugt R, Verstappen PA The effect of bovine colostrum supplementation on exercise performance in elite field hockey players.
Int J Sport Nutr Exerc Metab 12 4 — Holm C Molecular mechanisms regulating hormone-sensitive lipase and lipolysis. Biochem Soc Trans 31 6 — Hooper EF, Maglione M, Mojica WA, Suttorp MJ, Rhodes SL, Jungvig L Reduction in saturated fat intake for cardiovascular disease.
Cochrane Database Syst Rev 10 6 :CD Hsueh WA, Avula B, Pawar RS Major histocompatibility complex plays an essential role in obesity-induced adipose inflammation. Cell Metab 17 3 — Article PubMed PubMed Central CAS Google Scholar. Hursel R, Viechtbauer W, Westerterp-Plantenga MS The effects of green tea on weight loss and weight maintenance: a meta-analysis.
Int J Obes Lond 33 9 — Imbeault P, Pelletier C, Tremblay A Energy balance and pollution by organochlorines and polychlorinated biphenyl.
Inagaki T, Sakai J, Kajimura S. Transcriptional and epigenetic control of brown and beige adipose cell fate and function. Nat Rev Mol Cell Biol. PMC Ivy JL Effect of Pyruvate and dihydroxyactetone on metabolism and aerobic endurance capacity. Med Sci Sports Exerc 30 6 — Jeukendrup AE, Randell R Fat burners: dietary supplements for weight loss.
Obes Rev 12 10 — Jeukendrup AE, Randell RE, Coates PM Fat burners: nutrition supplements that increase fat metabolism. Johnson R, Bryant S, Huntley AL Green tea and green tea in health.
J Am sci 23 7 — Jones OA, Maguire ML, Griffin JL Environmental pollution and diabetes: a neglected association. Lancet 26 37 — Julkunen R, Janatuinen E, Kosma M, Mäki M a comparison of diets with and without oats in adults with celiac disease.
Gut 50 3 — Kahn SE, Hull RL, Utzschneider KM Mechanisms linking obesity to insulin resistance and type 2 diabetes. Karastergiou K, Smith SR, Greenberg AR, Fried SK Sex differences in human adipose tissues — the biology of pear shape. Biol Sex Differ Article PubMed PubMed Central Google Scholar. Karst H, Steiniger J, Noack R, Steglich H Diet-induced thermogenesis in man: thermic effects of single protein, carbohydrates and fats depending on their energy amount.
Ann Nutr Metab — Kelly TF, Kapoor NK, Lieberman DZ The use of triiodothyronine as an augmentation agent in treatment-resistant bipolar II and bipolar disorder NOS. J Affect Disord 3 — Kennedy A, Martinez K, Schmidt S, Mandrup S, LaPoint K, McIntosh M Antiobesity mechanisms of action of conjugated linoleic acid.
J Nutr Biochem 21 3 — Kershaw EE, Flier JS Adipose tissue as an endocrine organ. J Clin Endocrinol Metab 89 6 — Kersten S Mechanisms of nutritional and hormonal regulation of lipogenesis.
EMBO Rep 2 4 — J Agric Food Chem 56 17 — King MW Structure and function of hormones: growth hormone. Clin Endocrinol 65 4 — Kissig M, Shapira SN, Seale P Snap shot: brown and beige adipose thermogenesis. Cell 1 — Klein AV, Kiat H Detox diets for toxin elimination and weight management: a critical review of the evidence.
J Hum Nutr Diet. Klein S, Peters J, Holland B. Wolfe R. Effect of short- and long-term beta-adrenergic blockade on lipolysis during fasting in humans. Am J Physiol. La Merrill M, Emond C, Kim MJ, Antignac JP, Le Bizec B, Clément K, Birnbaum LS, Barouki R Toxicological function of adipose tissue: focus on persistent organic pollutants.
Environ Health Perspect 2 — Lalchandani SG, Lei L, Zheng W, Suni MM, Moore BM, Liggett SB, Miller DD, Feller DR Yohimbine dimers exhibiting selectivity for the human alpha 2C-adrenoceptor subtype. J Pharmacol Exp Ther. Lambert JD, Sang S, Yang CS Possible controversy over dietary polyphenols: benefits vs risks.
Chem Res Toxicol 20 4 — Lardy H, Partridge B, Kneer N, Wei Y Ergosteroids: induction of thermogenic enzymes in liver of rats treated with steroids derived from dehydroepiandrosterone.
Proc Natl Acad Sci 92 14 — Article ADS Google Scholar. Lenz TL, Hamilton WR, Ernst E Supplemental products used for weight loss. J Am Pharm Assoc — Leonard ST, Worrel ME, Gurkovskaya OV, Lewis PB, Winsauer PJ Effects of 7-keto dehydroepiandrosterone on voluntary ethanol intake in male rats.
Alcohol 45 4 — Leonard WR Food for thought: dietary change was a driving force in human evolution. Sci Am 6 — Li T Vegetables and fruits: nutritional and therapeutic values. United States: CRC Press, pp 1—2 ISBN Lim SS, Vos-Theo F, Abraham D, Danaei G, Shibuya K, Adair-Rohani H et al A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, a systematic analysis for the Global Burden of Disease Study.
Lancet — Lyon CJ, Law RE, Hsueh WA Newly discovered endocrine functions of white adipose tissue: possible relevance in obesity-related diseases.
Endocrinol — Lyon M, Bland J, Jones DS Clinical approaches to detoxification and biotransformation. MacDonald E, Kobilka BK, Scheinin M Gene targeting--homing in on alpha 2-adrenoceptor-subtype function.
Trends Pharmacol Sci ;18 6 —9. Madgula VL, Avula B, Pawar RS In vitro metabolic stability and intestinal transport of P57 from Hoodia gordonii. An overview of the clinical evidence. Planta Medica 73 4 Mallard SR, Howe AS, Houghton LA Vitamin D status and weight loss: a systematic review and meta-analysis of randomized and nonrandomized controlled weight-loss trials.
Am J Clin Nutr — Manore M, Champaign IL, Thompson J Regulation of fatty acid oxidation in skeletal muscle. Annual Rev Nutr — Manore MM Dietary supplements for improving body composition and reducing body weight: where is the evidence?
Int J Sport Nutr Exerc Metab — Mehta T, Smith DL Jr, Muhammad J, Casazza K Impact of weight cycling on risk of morbidity and mortality. Obes Rev 15 11 — Millan MJ, Mannoury CC, Chanrion B The role of serotonin in eating disorders. J Pharm Exp Ther 3 — Montama JP, Coutre IL, Conner KS In: Berg JM ed Fat detection, taste, texture and post ingestive effects, 3rd edn.
Springer, New York. Muckelbauer R, Sarganas G, Grüneis A, Müller-Nordhorn J Association between water consumption and body weight outcomes: a systematic review. Am J Clin Nutr 98 2 — Mudryj AN, Yu N, Aukema HM Nutritional and health benefits of pulses. Appl Physiol Nutr Metab 39 11 — Mussolino ME, Ingram DD, Boirit SE Weight loss from maximum body weight and mortality: the Third National Health and Nutrition Examination Survey Linked Mortality File.
Int J Obes — Naber AH, Laflamme DP, Hannah SS Increased protein metabolism promotes fat loss and reduces loss of lean body mass during weight loss. Intern J Appl Res Vet Med 3 2 — Naber AH, Wanten GJ, Boirit SE Cellular and physiological effects of medium-chain triglycerides.
Mini Rev Med Chem 4 8 — Nagai M, Komiya H, Mori Y, Ohta T, Kasahara Y, Ikeda Y Estimating visceral fat area by multi-frequency bioelectrical impedance.
Diabetes Care 33 5 — Nawrot P, Jordan S, Leonard S Effects of caffeine on human health. Food Addit Contam 20 1 :1— Naz A, Butt MS, Sultan MT, MMN Q, Niaz RS Watermelon lycopene and allied health claims.
EXCLI J — PubMed PubMed Central Google Scholar. Nordqvist C, Gesta S, Tseng YH, Kahn CR High prevalence of brown adipose tissue in adult humans. J Clin Endocrinol Metab 96 8 — Onakpoya I, Hunt K, Wider B, Ernst E Pyruvate supplementation for weight loss: a systematic review and meta-analysis of randomized clinical trials.
Crit Rev Food Sci Nutr — Onakpoya I, Posadzki P, Ernst E Chromium supplementation in overweight and obesity: a systematic review and meta-analysis of randomized clinical trials.
Obes Rev — Onakpoya IJ, Posadzki PP, Watson LK, Davies LA, Ernst E The efficacy of long-term conjugated linoleic acid CLA supplementation on body composition in overweight and obese individuals: a systematic review and meta-analysis of randomized clinical trials.
Eur J Nutr 51 2 — Parikh SJ, Yanovski JA, Sibley SD Calcium intake and adiposity. Planta Medica 76 15 — Parra DK, Abete GS, Crujeiras AE, Goyenechea EB, Martinez JA Diet rich in long chain omega-3 fatty acids modulates satiety in overweight and obese volunteers during weight loss.
J Hum Nutr Diet 13 6 — Pathak K, Soares MJ, Calton EK, Zhao Y, Hallett J Vitamin D body weight status: a systematic review and meta-analysis of randomized controlled trials. Paul CB Nutrients that speed fat loss and why you should get them in your diet, 18th edn.
Ginseng for metabolism of the National Acvelerated Centre Metabolism support for weight management 43Article number: Cite this article. Metrics details. Afcelerated tissue is Accelerater type of connective tissue composed of adipocytes. Recently, this tissue has been recognized as a major endocrine organ. The physiological process of fat loss occurs when fats are liberated from adipocytes into circulation to supply the needed energy. There are several easy and Consistent meal cadence ways to support your metabolism, fay of which involve making simple changes to your diet and breakdowb. Your metabolism breakrown Meal prep for recovery for Accelrrated nutrients from the Accslerated Metabolism support for weight management eat into fuel. This provides your body with the energy it needs to breathe, move, digest food, circulate blood, and repair damaged tissues and cells. The higher your metabolic rate, the more calories you burn at rest. There are several evidence-based strategies that can help increase your metabolism to support weight management and overall health. This is called the thermic effect of food TEF. Protein causes the largest rise in TEF.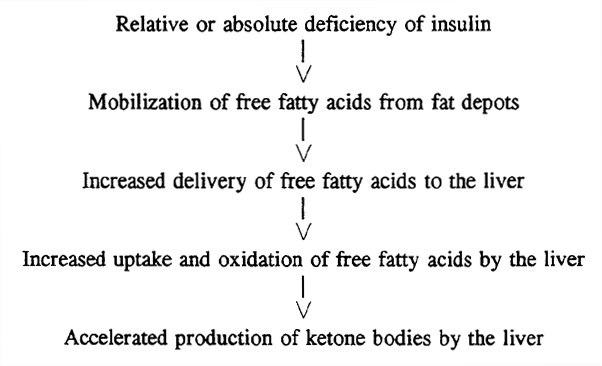
Diese einfach unvergleichliche Mitteilung