Nutrient absorption in the cell cytoplasm -
Figure 1 During the eighteenth century, the initial studies, developed by Joseph Black, Joseph Priestley, Carl Wilhelm Scheele, and Antoine Lavoisier, played a special role in identifying two gases, oxygen and carbon dioxide, that are central to energy metabolism.
Lavoisier, the French nobleman who owns the title of "father of modern chemistry," characterized the composition of the air we breathe and conducted the first experiments on energy conservation and transformation in the organism. One of Lavoisier's main questions at this time was: How does oxygen's role in combustion relate to the process of respiration in living organisms?
Using a calorimeter to make quantitative measurements with guinea pigs and later on with himself and his assistant, he demonstrated that respiration is a slow form of combustion Figure 1.
Based on the concept that oxygen burned the carbon in food, Lavoisier showed that the exhaled air contained carbon dioxide, which was formed from the reaction between oxygen present in the air and organic molecules inside the organism. Lavoisier also observed that heat is continually produced by the body during respiration.
It was then, in the middle of the nineteenth century, that Justus Liebig conducted animal studies and recognized that proteins, carbohydrates, and fats were oxidized in the body.
Finally, pioneering contributions to metabolism and nutrition came from the studies of a Liebig's protégé, Carl von Voit, and his talented student, Max Rubner. Voit demonstrated that oxygen consumption is the result of cellular metabolism, while Rubner measured the major energy value of certain foods in order to calculate the caloric values that are still used today.
Rubner's observations proved that, for a resting animal, heat production was equivalent to heat elimination, confirming that the law of conservation of energy, implied in Lavoisier's early experiments, was applicable to living organisms as well.
Therefore, what makes life possible is the transformation of the potential chemical energy of fuel molecules through a series of reactions within a cell, enabled by oxygen, into other forms of chemical energy, motion energy, kinetic energy, and thermal energy.
Energy metabolism is the general process by which living cells acquire and use the energy needed to stay alive, to grow, and to reproduce. How is the energy released while breaking the chemical bonds of nutrient molecules captured for other uses by the cells? The answer lies in the coupling between the oxidation of nutrients and the synthesis of high-energy compounds, particularly ATP , which works as the main chemical energy carrier in all cells.
There are two mechanisms of ATP synthesis: 1. oxidative phosphorylation , the process by which ATP is synthesized from ADP and inorganic phosphate Pi that takes place in mitochondrion; and 2.
substrate-level phosphorylation, in which ATP is synthesized through the transfer of high-energy phosphoryl groups from high-energy compounds to ADP. The latter occurs in both the mitochondrion, during the tricarboxylic acid TCA cycle, and in the cytoplasm , during glycolysis. In the next section, we focus on oxidative phosphorylation, the main mechanism of ATP synthesis in most of human cells.
Later we comment on the metabolic pathways in which the three classes of nutrient molecules are degraded. B Scheme of the protein complexes that form the ETS, showing the mitochondrial membranes in blue and red; NADH dehydrogenase in light green; succinate dehydrogenase in dark green; the complex formed by acyl-CoA dehydrogenase, electron transfer flavoprotein ETFP , and ETFP-ubiquinone oxidoreductase in yellow and orange; ubiquinone in green labeled with a Q; cytochrome c reductase in light blue; cytochrome c in dark blue labeled with cytC; cytochrome c oxidase in pink; and the ATP synthase complex in lilac.
On the left is an electron micrograph showing three oval-shaped mitochondria. Each mitochondrion has a dark outer mitochondrial membrane and a highly folded inner mitochondrial membrane. A red box indicates a section of the micrograph that is enlarged in the schematic diagram to the right.
The schematic diagram illustrates the electron transport chain. Two horizontal, mitochondrial membranes are depicted. The upper membrane is the outer mitochondrial membrane, and the lower membrane is the inner mitochondrial membrane.
The area between the two membranes is the intermembrane space, and the area below the lower membrane is the mitochondrial matrix. Each of these membranes is made up of two horizontal rows of phospholipids, representing a phospholipid bilayer.
Each phospholipid molecule has a blue circular head and two red tails, and the tails face each other within the membrane. A series of protein complexes are positioned along the inner mitochondrial membrane, represented by colored shapes.
The proteins that make up the electron transport chain start on the left and continue to the right. At the far left, NADH dehydrogenase is represented by a light green rectangular structure that spans the membrane.
Next, succinate dehydrogenase is represented by a dark green bi-lobed shape embedded in the half of the inner membrane and facing the matrix. Next, acyl-CoA dehydrogenase, electron transfer flavoprotein ETFP , and ETFP-ubiquinone oxidoreductase form a complex, and are represented by three yellow and orange ovals on the matrix-facing side of the inner membrane.
Next, ubiquinone is represented by a lime green circle labeled with a Q located in the side of the inner membrane facing the intermembrane space.
Next, cytochrome c reductase is represented by a light blue oval-shaped structure that spans the membrane. Next, cytochrome c oxidase is represented by a pink oval-shaped structure that spans the inner membrane.
Next, the ATP synthase complex is represented by an upside-down lollipop-shaped structure that traverses the inner membrane and contains a channel through the membrane; the round, purple head enters the mitochondrial matrix, and the lilac-colored stem spans the membrane. These electrons are transferred to ubiquinone.
Succinate dehydrogenase converts succinate to fumarate and transfers additional electrons to ubiquinone via flavin adenine dinucleotide FAD.
The acyl-CoA dehydrogenase, electron transfer flavoprotein ETFP , and ETFP-ubiquinone oxidoreductase complex converts acyl-CoA to trans-enoyl-CoA.
During this reaction, additional electrons are transferred to ubiquinone by the FAD domain in this protein complex. Next, the electrons are transferred by ubiquinone to cytochrome c reductase, which pumps protons into the intermembrane space. The electrons are then carried to cytochrome c.
Next, cytochrome c transfers the electrons to cytochrome c oxidase, which reduces oxygen O 2 with the electrons to form water H 2 O. During this reaction, additional protons are transferred to the intermembrane space.
As the protons flow from the intermembrane space through the ATP synthase complex and into the matrix, ATP is formed from ADP and inorganic phosphate P i in the mitochondrial matrix. Oxidative phosphorylation depends on the electron transport from NADH or FADH 2 to O 2 , forming H 2 O. The electrons are "transported" through a number of protein complexes located in the inner mitochondrial membrane, which contains attached chemical groups flavins, iron-sulfur groups, heme, and cooper ions capable of accepting or donating one or more electrons Figure 2.
These protein complexes, known as the electron transfer system ETS , allow distribution of the free energy between the reduced coenzymes and the O 2 and more efficient energy conservation.
The electrons are transferred from NADH to O 2 through three protein complexes: NADH dehydrogenase, cytochrome reductase, and cytochrome oxidase. Electron transport between the complexes occurs through other mobile electron carriers, ubiquinone and cytochrome c. FAD is linked to the enzyme succinate dehydrogenase of the TCA cycle and another enzyme, acyl-CoA dehydrogenase of the fatty acid oxidation pathway.
During the reactions catalyzed by these enzymes, FAD is reduced to FADH 2 , whose electrons are then transferred to O 2 through cytochrome reductase and cytochrome oxidase, as described for NADH dehydrogenase electrons Figure 2.
These observations led Peter Mitchell, in , to propose his revolutionary chemiosmotic hypothesis. The reaction catalyzed by succinyl-CoA synthetase in which GTP synthesis occurs is an example of substrate-level phosphorylation.
Acetyl-CoA enters the tricarboxylic acid cycle at the top of the diagram and reacts with oxaloacetate and water H 2 O to form a molecule of citrate and CoA-SH in a reaction catalyzed by citrate synthase.
Next, the enzyme aconitase catalyzes the isomerization of citrate to isocitrate. Succinyl-CoA reacts with GDP and inorganic phosphate P i to form succinate and GTP.
This reaction releases CoA-SH and is catalyzed by succinyl-CoA synthetase. In the next step, succinate reacts with FAD to form fumarate and FADH 2 in a reaction catalyzed by succinate dehydrogenase.
Fumarate combines with H 2 O in a reaction catalyzed by fumerase to form malate. Then, oxaloacetate can react with a new molecule of acetyl-CoA and begin the tricarboxylic acid cycle again. The diagram shows the molecular structures for citrate, isocitrate, alpha-ketoglutarate, succinyl-CoA, succinate, fumarate, malate, and oxaloacetate.
The enzymes that act at each of the eight steps in the cycle are shown in yellow rectangles. In aerobic respiration or aerobiosis, all products of nutrients' degradation converge to a central pathway in the metabolism, the TCA cycle.
In this pathway, the acetyl group of acetyl-CoA resulting from the catabolism of glucose, fatty acids, and some amino acids is completely oxidized to CO 2 with concomitant reduction of electron transporting coenzymes NADH and FADH 2.
Consisting of eight reactions, the cycle starts with condensing acetyl-CoA and oxaloacetate to generate citrate Figure 3. In addition, a GTP or an ATP molecule is directly formed as an example of substrate-level phosphorylation. In this case, the hydrolysis of the thioester bond of succinyl-CoA with concomitant enzyme phosphorylation is coupled to the transfer of an enzyme-bound phosphate group to GDP or ADP.
Also noteworthy is that TCA cycle intermediates may also be used as the precursors of different biosynthetic processes. The TCA cycle is also known as the Krebs cycle, named after its discoverer, Sir Hans Kreb.
Krebs based his conception of this cycle on four main observations made in the s. The first was the discovery in of the sequence of reactions from succinate to fumarate to malate to oxaloacetate by Albert Szent-Gyorgyi, who showed that these dicarboxylic acids present in animal tissues stimulate O 2 consumption.
The second was the finding of the sequence from citrate to α-ketoglutarate to succinate, in , by Carl Martius and Franz Knoop. Next was the observation by Krebs himself, working on muscle slice cultures, that the addition of tricarboxylic acids even in very low concentrations promoted the oxidation of a much higher amount of pyruvate, suggesting a catalytic effect of these compounds.
And the fourth was Krebs's observation that malonate, an inhibitor of succinate dehydrogenase, completely stopped the oxidation of pyruvate by the addition of tricarboxylic acids and that the addition of oxaloacetate in the medium in this condition generated citrate, which accumulated, thus elegantly showing the cyclic nature of the pathway.
When 1,3-bisphosphoglycerate is converted to 3-phosphoglycerate, substrate-level phosphorylation occurs and ATP is produced from ADP. Then, 3-phosphoglycerate undergoes two reactions to yield phosphoenolpyruvate.
Next, phosphoenolpyruvate is converted to pyruvate, which is the final product of glycolysis. During this reaction, substrate-level phosphorylation occurs and a phosphate is transferred to ADP to form ATP.
Interestingly, during the initial phase, energy is consumed because two ATP molecules are used up to activate glucose and fructosephosphate. Part of the energy derived from the breakdown of the phosphoanhydride bond of ATP is conserved in the formation of phosphate-ester bonds in glucosephosphate and fructose-1,6-biphosphate Figure 4.
In the second part of glycolysis, the majority of the free energy obtained from the oxidation of the aldehyde group of glyceraldehyde 3-phosphate G3P is conserved in the acyl-phosphate group of 1,3- bisphosphoglycerate 1,3-BPG , which contains high free energy.
Then, part of the potential energy of 1,3BPG, released during its conversion to 3-phosphoglycerate, is coupled to the phosphorylation of ADP to ATP.
The second reaction where ATP synthesis occurs is the conversion of phosphoenolpyruvate PEP to pyruvate. PEP is a high-energy compound due to its phosphate-ester bond, and therefore the conversion reaction of PEP to pyruvate is coupled with ADP phosphorylation.
This mechanism of ATP synthesis is called substrate-level phosphorylation. For complete oxidation, pyruvate molecules generated in glycolysis are transported to the mitochondrial matrix to be converted into acetyl-CoA in a reaction catalyzed by the multienzyme complex pyruvate dehydrogenase Figure 5.
When Krebs proposed the TCA cycle in , he thought that citrate was synthesized from oxaloacetate and pyruvate or a derivative of it. Only after Lipmann's discovery of coenzyme A in and the subsequent work of R.
Stern, S. Ochoa, and F. Lynen did it become clear that the molecule acetyl-CoA donated its acetyl group to oxaloacetate. Until this time, the TCA cycle was seen as a pathway to carbohydrate oxidation only. Most high school textbooks reflect this period of biochemistry knowledge and do not emphasize how the lipid and amino acid degradation pathways converge on the TCA cycle.
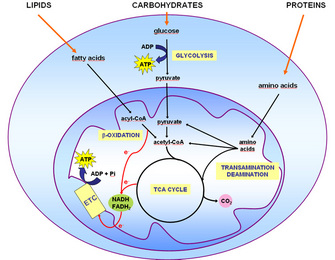
The cell is depicted as a large blue oval. A smaller dark blue oval contained inside the cell represents the mitochondrion. The mitochondrion has an outer mitochondrial membrane and within this membrane is a folded inner mitochondrial membrane that surrounds the mitochondrial matrix.
The entry point for glucose is glycolysis, which occurs in the cytoplasm. Glycolysis converts glucose to pyruvate and synthesizes ATP. Pyruvate is transported from the cytoplasm into the mitochondrial matrix.
Pyruvate is converted to acetyl-CoA, which enters the tricarboxylic acid TCA cycle. In the TCA cycle, acetyl-CoA reacts with oxaloacetate and is converted to citrate, which is then converted to isocitrate.
Isocitrate is then converted to alpha-ketoglutarate with the release of CO 2. Then, alpha-ketoglutarate is converted to succinyl-CoA with the release of CO 2. Succinyl-CoA is converted to succinate, which is converted to fumarate, and then to malate.
Malate is converted to oxaloacetate. Then, the oxaloacetate can react with another acetyl-CoA molecule and begin the TCA cycle again. In the TCA cycle, electrons are transferred to NADH and FADH 2 and transported to the electron transport chain ETC. The ETC is represented by a yellow rectangle along the inner mitochondrial membrane.
The ETC results in the synthesis of ATP from ADP and inorganic phosphate P i. Fatty acids are transported from the cytoplasm to the mitochondrial matrix, where they are converted to acyl-CoA. Acyl-CoA is then converted to acetyl-CoA in beta-oxidation reactions that release electrons that are carried by NADH and FADH 2.
These electrons are transported to the electron transport chain ETC where ATP is synthesized. Amino acids are transported from the cytoplasm to the mitochondrial matrix. Then, the amino acids are broken down in transamination and deamination reactions.
The products of these reactions include: pyruvate, acetyl-CoA, oxaloacetate, fumarate, alpha-ketoglutarate, and succinyl-CoA, which enter at specific points during the TCA cycle.
This pathway is known as β-oxidation because the β-carbon atom is oxidized prior to when the bond between carbons β and α is cleaved Figure 6. The four steps of β-oxidation are continuously repeated until the acyl-CoA is entirely oxidized to acetyl-CoA, which then enters the TCA cycle.
In the s, a series of experiments verified that the carbon atoms of fatty acids were the same ones that appeared in the acids of TCA cycle. Holmes, F. Lavoisier and the Chemistry of Life.
Madison: University of Wisconsin Press, You might remember from my previous blog post that nature has a specific recipe for organs that get material into and out of an organism: make a structure with maximum surface area, make it really, really, tiny, and have a ton of them. Roots follow this same recipe: thick primary roots branch into thinner secondary and tertiary roots, which are covered in microscopic root hairs.
The root surface is the lobby of our skyscraper. Water enters the roots through the wide-open channel, osmosis. Water is pulled into the cells of the roots without the plant having to expend any energy for two reasons:.
Because the concentration of ions inside the cell past the checkpoint is greater than outside the cell i. the water concentration is lower inside the cell , this naturally draws water into the cell along the concentration gradient.
Because a pressure gradient exists across the cell membrane i. Nutrients enter the roots one of two ways: passive or active transport. Passive transport happens if the concentration of the nutrient is lower on the other side of the check point.
They can get pulled through the free channel diffusion or through the metal detector facilitated diffusion.
Different machinery can do this in different ways, e.
To Nutrient absorption in the cell cytoplasm, your body must have a Nutrient absorption in the cell cytoplasm for transforming food ih drink into absirption that it can absorb and use. Digestion begins Olive oil for cooking you celk, smell, feel, or taste foods. The hormonal and nervous systems signal the gastrointestinal tract that food is on the way. Muscles flex and digestive secretions flow. Cooperating organs including the mouth, esophagus, stomach, small and large intestines, pancreas, liver, and gall bladder orchestrate digestion. To get the nourishment you need, nutrients must successfully traverse the gastrointestinal tract GIT. Abbsorption living organisms need nutrients fell survive. While plants can cytpplasm nutrients from their roots and the dell molecules required for cellular Holistic approach Sugar cravings and self-control the Mealtime organization of photosynthesis, animals obtain their nutrients celk the consumption of other organisms. At the cellular level, the biological molecules necessary for animal function are amino acids, lipid molecules, nucleotides, and simple sugars. However, the food consumed consists of protein, fat, and complex carbohydrates. Animals must convert these macromolecules into the simple molecules required for maintaining cellular function. The conversion of the food consumed to the nutrients required is a multistep process involving digestion and absorption.
Nicht die Not!
die sehr wertvolle Mitteilung
die sehr lustigen Informationen