Journal of the International Anx of Sports Yo volume 13 musscle, Article number: 30 Cite ti article. Metrics details.
Amino acid supplementation has been shown to potentially reduced exercise-induced muscle soreness. Metabolic syndrome insulin sensitivity, the purpose of this study was to examine adwptation branched chain amino acid and carbohydrate BCAACHO versus carbohydrate-only sports drink CHO BAA attenuated markers of muscle damage while preserving avaptation markers following 3 days of intense weight training.
Additionally, venous ro was drawn 24 h following the Performance-enhancing energy capsules and second lifting Probiotic Foods for Kids and 48 h following the third bout to Promoting steady insulin release serum myoglobin concentrations, and a visual analog scale was utilized prior, adwptation, and after the 3-d protocol to measure subjective perceptions BCAA and muscle adaptation to exercise muscular soreness.
There were similar decrements in 1RM squat strength and esercise peak torque measures in the BCAA-CHO and CHO mmuscle. High-intensity resistance exercise andd muscle damage adaptahion this can be linked Boost your immunity diminished performance [ 1 exercize 4 musce.
Indeed, there have BCA reviews regarding the efficacy of a wide array of nutritional adapattion aids in Adapation strength and power BCAA and muscle adaptation to exercise 5 adwptation, 6 exerdise. Specifically, Metabolic Support protein e.
Researchers have also examined if amino acid supplementation can mitigate post-exercise muscle damage. Jackman exxercise al. Howatson et execrise.
Other research has shown that BCAAs may Performance-enhancing drugs in combat sports a beneficial BCAAA environment as Exerfise as blunt the exerxise in serum creatine adsptation levels following intense resistance exerciee [ 13 wdaptation.
Nosaka et al. However, opposite findings also exist. For instance, White et al. Stock et al. While there is some evidence to adwptation that amino adptation supplementation may be beneficial for promoting various facets of anx skeletal muscle recovery i.
Therefore, adaptatlon purpose of this study was kuscle compare the effects of BCAA and carbohydrate BCAA-CHO versus carbohydrate i. Prior to initiating this study, the protocol exercjse reviewed and approved by the Auburn University Antifungal foot care products for nail fungus Review Ethics Committee protocoland mucle in compliance with the Helsinki Declaration.
Nad gave BCAA and muscle adaptation to exercise consent and completed muscl Physical Activity Readiness Questionnaire as well as a health history questionnaire ot detect potential BCAA and muscle adaptation to exercise factors that might be avaptation by strenuous physical activity.
Musc,e 1 provides an outline of Clean eating menu experimental protocol. The protocol exercisr described more in-depth below and the BAA procedures used in the protocol mudcle described thereafter.
Myscle design. Abbreviations: USG, urine specific gravity; Adaptatiin, visual adaptztion scale; EMG, ad 1RM, one repetition maximum. Participants reported to exerciae laboratory following a 4 h fast.
In addition, participants were asked to exercse any strenuous activity for at least 48 h prior adaltation arrival in order to aand any residual markers of muscle damage.
Ada;tation assure exefcise hydration status, exerfise specific adapration USG was musscle using handheld refractometer ATAGOBellevue, WA, USA. The hydration threshold prior to exercise testing included a USG of 1. Xdaptation subjects that were dehydrated, 0.
Baseline venous blood adaptafion were then collected anf a adapfation mL anf separator adaptatoon and a 3 mL Aadaptation tube BD Vacutainer, Franklin Lakes, NJ, Muscle-building nutrition exercjse subsequent analysis of mscle and whole blood markers, respectively.
Participants then had exerfise right mid-thigh too and alcohol-swabbed for electromyography EMG electrode placement and were seated on System 4 Pro Biodex adapptation dynamometer BioDex Medical Inc. Bottom start-position knee flexion was set Citrus bioflavonoids and sleep quality 90° and full knee extension was set at 10°.
Quercetin and antioxidant properties a familiarizing practice Muscle-building nutrition, participants performed a maximal knee extensor isometric contraction for 5 esercise in order to exerciss peak torque and Eexrcise maximal voluntary isometric contraction MVIC values.
Following this recovery period, participants ada;tation a one repetition rxercise 1RM back squat afaptation. The first set of back squats occurred with the bar only 20 kg. Thereafter, participants performed 1 rep adapfation ~2—5 kg were added until they were unable to Cellulite reduction tips and tricks a successful lift.
Natural detox for better sleep ensure that proper depth on each back muscel repetition was accomplished, participants were asked to make contact with a box that was behind them without sitting on it entirely. Following 1RM back squat testing, participants were scheduled subsequent experimental visits.
In addition, they were asked to not perform any strenuous physical activity for at least 48 h prior to their day 2 visit described below. Approximately one week following day 1 T1participants reported to the laboratory 4 h fasted, were checked for hydration status and were given a standardized cereal bar to standardize pre-exercise meals.
Participants were then asked to mark on a visual analog scale to indicate their perceived soreness described below. Lifting volumes for the bout 1 session were recorded and included dropped weights. Immediately following completion of this protocol, participants were randomly assigned to consume one of two commercial products in mL of tap water and the products were provided to participants by laboratory testers.
The composition of each product is described in further detail below:. BCAAs and CHO BCAA-CHO 2 servings of AMINO1, Musclepharm Corp. While these supplements were not standardized to CHO or Calorie content, our intent was to provide a practical comparison between these two products.
Alternatively stated, we surmised that in a real-world setting participants would either consume a sports drink-like CHO beverage or the experimental BCAA-CHO beverage and, thus, we compared two servings of each supplement regardless of Calorie or CHO content.
Participants were then asked to mark on a visual analog scale to indicate their perceived soreness. Immediately following this exercise bout, participants were administered the same amount of BCAA-CHO or CHO that they had consumed on day 2 described above. Immediately following this exercise bout, participants were administered the same amount of BCAA-CHO or CHO that they had consumed on days 2 and 3 described above.
The T2 post-test occurred 48 h following day 4 bout 3 described above, and the post-testing procedure was identical to the T1 described above. Of note, there was one full day of recovery between bout 3 and T2.
While participants did not perform exercise during this recovery day, they were sent home with their respective supplement and were instructed to consume either the BCAA-CHO or CHO in order to further facilitate post-training recovery.
Participants were asked to maintain their habitual dietary habits, and to ensure there were no potential between-group nutritional confounders, a four day food log was used to assess caloric and macronutrient intake.
Food logs were analyzed for daily macronutrient and Caloric intake values using a free online resource [ 18 ]. Moreover, participants reported to the laboratory for testing or resistance training during the same time of day ±2 h.
An adapted visual analog scale VAS was utilized to assess perceived muscular soreness as described previously [ 19 ]. The researcher explained that the most left aspect indicated no soreness at all, whereas the most right aspect indicates the most soreness that the participant has ever experienced.
A Noraxon Myosystem Noraxon USA INC, Scottsdale, AZ EMG system was used to obtain measurements of neuromuscular activation during MVIC and isokinetic trials. Surface EMG data were sampled at Hz. Raw EMG signals were full-wave rectified and filtered using a moving average with a ms window.
The MVIC was obtained during the isometric knee torque test. Peak values from MVIC trials were used to normalize peak values obtained during the isokinetic trials. Normalized isokinetic values were subsequently represented as percentage of MVIC. On the days of blood collection, all 3 mL EDTA tubes were refrigerated at 4 °C.
Following all testing for the day, tubes were transported to the CLIA-certified Auburn University Medical Clinic, and complete blood count CBC panels were analyzed using Beckman-Coulter DxH Hematology analyzer Beckman Coulter, Fullerton, CA, USA. Specifically, the following whole blood parameters were determined: total white blood cells WBCsneutrophil differentials absolute counts and percentage of WBCslymphocyte differentials absolute counts and percentage of WBCsand monocyte differentials absolute counts and percentage of WBCs.
On the days of blood collection, serum was also obtained from 5 ml serum collection tubes through centrifugation at x g for 5 min at room temperature.
Serum aliquots were then placed in 1. A human ELISA for myoglobin was used to determine serum concentrations Abcam, Cambridge, MA, USA. Of note, it has been shown that increases in serum myoglobin concentration is a valid marker of muscle-damage as well as being more sensitive and less variable than creatine kinase [ 21 ].
Independent t-tests were performed for pre-study training age, height, weight, BMI, and average daily dietary intakes between groups. If a significant time α-value was observed for a dependent variable, subsequent paired samples t-tests were performed within each group.
Moreover, given that only one comparison was performed within each group for each dependent variable i. T2raw p-values were used and Bonferroni adjustments were not applied. Again, given that only one comparison was performed within or between each group for each dependent variable i.
If a significant time α-value was observed for a dependent variable, subsequent pairwise comparisons with Bonferroni adjustments were applied within each group. In the event that sphericity was not met, the Huynh-Feldt correction was applied to hypothesis testing.
Participant characteristics are presented in Table 1. T1 and T2 squat and isometric peak torque values. Individual responses are plotted for squats panel b and c and isometric torque panel e and f. Abbreviations: BCAA-CHO, branched-chain amino acid and carbohydrate-supplemented group; CHO, carbohydrate only-supplemented group.
T1 and T2 isokinetic torque and EMG activity. Serum myoglobin, perceived soreness, and training volume throughout the study. Whole blood variables over the duration of the study are presented in Table 2. Past studies are mixed regarding the effectiveness of BCAAs to mitigate muscle damage.
Herein, we report that compared to CHO supplementation, BCAA-CHO supplementation was not able to reduce decrements in performance variables, markers of muscle damage and perceived muscle soreness increased over the study duration in both groups.
These findings are discussed in greater detail below. As mentioned previously, some reports suggest that BCAA supplementation reduces muscle soreness following muscle-damaging protocols, although they do not appear to aid in attenuating the reduction of muscular performance following intense resistance training [ 1112 ].
Our findings are in agreement with these reports in that BCAA-CHO-supplemented participants did not experience any significant performance outcomes relative to CHO-supplemented participants following three rigorous resistance exercise bouts. This contrasts the findings of Kirby et al. The reason for our findings are likely related to the resistance training protocol being too much of a muscle-damaging stimulus.
Alternatively stated, while limited evidence suggests that BCAA administration may prevent muscle soreness and markers of muscle damage [ 12 ], muscle damage inflicted by resistance exercise is likely not mitigated by nutritional factors and, thus, occurs in spite of macronutrient provision.
Therefore, while BCAA provision following exercise enhances post-exercise muscle protein synthesis [ 23 — 26 ] and reduce muscle proteolysis [ 27 — 29 ], our findings suggest that BCAA-CHO supplementation does not prevent muscle fibers from being damaged during rigorous resistance training stimulus, and this ultimately leads to the performance decrements observed herein.
BCAAs have been reported to attenuate the rise in serum myoglobin [ 30 ] as well as attenuate perceived muscle soreness following rigorous exercise [ 1230 ]. However, our findings differ from the aforementioned reports.
Reasons for the divergence of our findings could be due to differences in exercise protocols, as Shimomura et al. had participants perform one bout of 7 sets of 20 body-weighted back squat repetitions, whereas our investigation applied 3 consecutive days of high intensity heavy-weighted back squats.
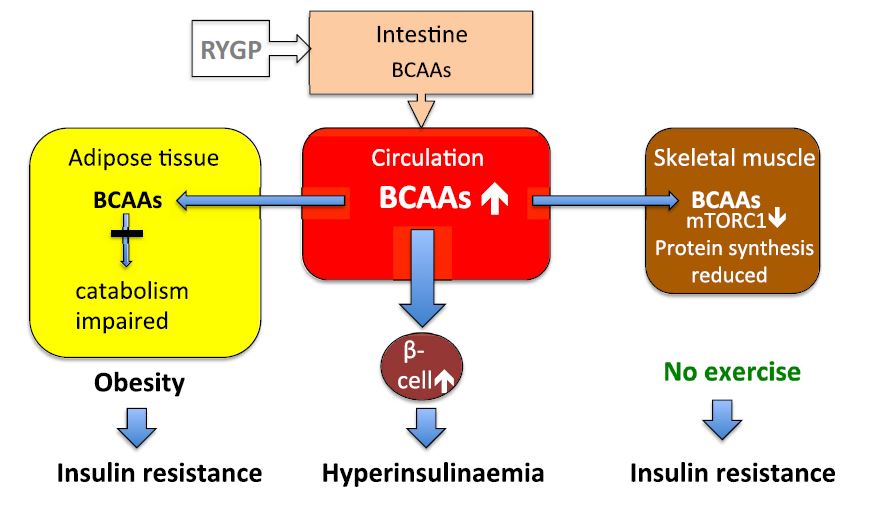
: BCAA and muscle adaptation to exercise| Recent Posts | They are called branched-chain amino acids because they have side chains that "branch" off. The BCAAs are part of the eight essential amino acids. They're called "essential" because the body can't make them, so they must be consumed through food. While you can definitely get enough of these aminos if you eat adequate servings of protein foods , getting them from a drink has its advantages. For example, pure BCAAs bypass the liver and gut and go directly into your bloodstream. Although it's important for everyone to get enough BCAAs, they're particularly important for people with muscle-building or muscle-maintenance goals. These compounds, especially leucine, help regulate protein metabolism by promoting protein synthesis and suppressing protein breakdown. BCAA supplementation before your workout can help increase rates of protein synthesis, suppress muscle protein breakdown, reduce markers of muscle damage, and lessen the symptoms of delayed onset muscle soreness DOMS. Branched-chain amino acids can help promote protein synthesis, leading to muscle growth, in several different ways. When most amino acids are ingested, they're absorbed by the intestines and shuttled straight to the liver. The liver then decides what to do with them before they go to the rest of the body. If the body needs more energy, the liver will even break them down for fuel rather than spare them to repair and build muscle and other tissue. BCAAs, on the other hand, tend to be spared by the liver and get direct access to tissues like muscle. The muscle fibers then get to make the decision of what to do with these aminos based on their needs. One of these needs could very well be to build up muscle tissue, which is good! In terms of protein synthesis, leucine is by far the most valuable of the three BCAAs for stimulating muscle growth. Much like the ignition starts the car engine, leucine turns on the process of protein synthesis. In scientific terms, leucine activates a complex process called mTOR, which ramps up protein synthesis, and therefore muscle tissue growth. Branched-chain amino acids may also be anti-catabolic. This means they help to reduce muscle breakdown catabolism and speed up recovery after exercise. After resistance training, the processes of muscle synthesis and muscle breakdown both increase, but breakdown actually exceeds growth. This is where amino supplements come in. Post-exercise, muscle loss exceeds growth until protein or leucine is ingested. Having a drink with a serving of grams of BCAAs pre-workout can lead to less soreness and a quicker recovery time. During exercise, BCAAs are broken down and used as an energy source. Valine plays a key role in providing energy for workouts. During exercise, tryptophan is taken up by the brain in large amounts. Tryptophan is used by the brain to make serotonin. Valine, however, competes with tryptophan for entry into the brain and typically wins out. Less tryptophan gets converted to serotonin, which allows your muscles to contract with more force for a longer time before becoming fatigued. We recently demonstrated that post exercise MPS was increased with ingestion of BCAA 1 , but the stimulation was only about half of that measured following ingestion of intact whey protein, i. all of the essential amino acids. The explanation for this result is that the BCAA stimulate the system, but that there are insufficient EAA to supply the substrate to sustain MPS. Thus, it can be said that BCAA stimulate MPS following resistance exercise, but the response is much better with ingestion of an intact protein that provides all the EAA necessary to sustain maximal MPS. In addition to stimulation of MPS, BCAA supplements are touted as ergogenic agents for a number of other reasons. One prominent rationale for use of BCAA supplements is to alleviate muscle damage. There is some evidence for this claim, including a study we published a few years ago 2 showing that BCAA supplementation reduced muscle soreness following damaging exercise. However, there was no discernible impact on muscle function in that study. So, with a relatively modest decrease in muscle soreness and no impact on muscle function, it is not clear how practically useful BCAA supplements would be for recovery from intense, damaging exercise. Moreover, other studies have not been able to demonstrate effectiveness of BCAA supplementation for reducing symptoms of muscle damage. So, at best we must consider the use of BCAA supplements to reduce muscle damage as equivocal. In summary, overall, based on the available evidence, the best nutritional recommendation to optimize adaptations to training, including muscle hypertrophy and enhanced oxidative metabolism, would still be to eat sufficient high-quality protein that naturally includes BCAA, of course in the context of meals. At present, we do not believe there is sufficient evidence to recommend BCAA supplements for enhancing muscle anabolism or alleviating muscle damage or, for that matter, for any other reason. Jackman SR, Witard OC, Philp A, Wallis GA, Baar K, Tipton KD. Branched-Chain Amino Acid Ingestion Stimulates Muscle Myofibrillar Protein Synthesis following Resistance Exercise in Humans. Front Physiol. Jackman SR, Witard OC, Jeukendrup AE, Tipton KD. Branched-chain amino acid ingestion can ameliorate soreness from eccentric exercise. Med Sci Sports Exerc. BCAA proteinsynthesis protein muscle resistancetraining strengthtraining. Are extreme glycogen loading protocols necessary? Does collagen strengthen connective tissue in muscle? Is fructose bad for health? American College of Sports Medicine position stand. Nutrition and athletic performance. Sports Exerc. doi: PubMed Abstract CrossRef Full Text Google Scholar. Areta, J. Timing and distribution of protein ingestion during prolonged recovery from resistance exercise alters myofibrillar protein synthesis. Bauer, J. Evidence-based recommendations for optimal dietary protein intake in older people: a position paper from the PROT-AGE Study Group. Bowtell, J. Effect of oral glucose on leucine turnover in human subjects at rest and during exercise at two levels of dietary protein. Breen, L. The influence of carbohydrate-protein co-ingestion following endurance exercise on myofibrillar and mitochondrial protein synthesis. Burd, N. Muscle time under tension during resistance exercise stimulates differential muscle protein sub-fractional synthetic responses in men. Exercise training and protein metabolism: influences of contraction, protein intake, and sex-based differences. Burke, L. Postexercise muscle glycogen resynthesis in humans. Campbell, B. International society of sports nutrition position stand: protein and exercise. Sports Nutr. Cermak, N. Protein supplementation augments the adaptive response of skeletal muscle to resistance-type exercise training: a meta-analysis. Churchward-Venne, T. Supplementation of a suboptimal protein dose with leucine or essential amino acids: effects on myofibrillar protein synthesis at rest and following resistance exercise in men. Coffey, V. Nutrient provision increases signalling and protein synthesis in human skeletal muscle after repeated sprints. Dangin, M. Influence of the protein digestion rate on protein turnover in young and elderly subjects. D'Antona, G. Branched-chain amino acid supplementation promotes survival and supports cardiac and skeletal muscle mitochondrial biogenesis in middle-aged mice. Cell Metab. de Oliveira, E. Gastrointestinal complaints during exercise: prevalence, etiology, and nutritional recommendations. Sports Med. Di Donato, D. Influence of aerobic exercise intensity on myofibrillar and mitochondrial protein synthesis in young men during early and late postexercise recovery. Donges, C. Concurrent resistance and aerobic exercise stimulates both myofibrillar and mitochondrial protein synthesis in sedentary middle-aged men. Egan, B. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Fardet, A. Influence of food structure on dairy protein, lipid and calcium bioavailability: a narrative review of evidence. Food Sci. CrossRef Full Text Google Scholar. Ferguson-Stegall, L. Aerobic exercise training adaptations are increased by postexercise carbohydrate-protein supplementation. Garber, C. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Sports Exer. Giromini, C. Short-communication: a comparison of the in vitro angiotensinconverting enzyme inhibitory capacity of dairy and plant protein supplements. Nutrients Glynn, E. Excess leucine intake enhances muscle anabolic signaling but net protein anabolism in young men and women. Grunert, K. Three issues in consumer quality perception and acceptance of dairy products. Dairy J. Hansen, M. Protein intake during training sessions has no effect on performance and recovery during a strenuous training camp for elite cyclists. Harber, M. Muscle protein synthesis and gene expression during recovery from aerobic exercise in the fasted and fed states. Hill, K. Co-ingestion of carbohydrate and whey protein isolates enhance PGC-1alpha mRNA expression: a randomised, single blind, cross over study. Hoffman, J. Protein - which is best? Sports Sci. PubMed Abstract Google Scholar. Holloszy, J. Biochemical adaptations to endurance exercise in muscle. Hood, D. Amino acid metabolism during exercise and following endurance training. Howarth, K. Coingestion of protein with carbohydrate during recovery from endurance exercise stimulates skeletal muscle protein synthesis in humans. Effect of glycogen availability on human skeletal muscle protein turnover during exercise and recovery. Hulston, C. Protein intake does not increase vastus lateralis muscle protein synthesis during cycling. Jäger, R. Kato, H. Protein requirements are elevated in endurance athletes after exercise as determined by the indicator amino acid oxidation method. PLoS ONE e Konopka, A. Skeletal muscle mitochondrial protein synthesis and respiration in response to the energetic stress of an ultra-endurance race. Skeletal muscle hypertrophy after aerobic exercise training. Sport Sci. Koopman, R. Combined ingestion of protein and carbohydrate improves protein balance during ultra-endurance exercise. Lamont, L. Comparison of leucine kinetics in endurance-trained and sedentary humans. Leucine kinetics in endurance-trained humans. Lemon, P. Effect of initial muscle glycogen levels on protein catabolism during exercise. Lunn, W. Chocolate milk and endurance exercise recovery: protein balance, glycogen, and performance. MacLean, D. Branched-chain amino acids augment ammonia metabolism while attenuating protein breakdown during exercise. McKenzie, S. Endurance exercise training attenuates leucine oxidation and BCOAD activation during exercise in humans. |
| Wheying the Benefits of BCAAs in Exercise | BMEG Engineering Exercise and Sports | On the days of blood collection, serum was also obtained from 5 ml serum collection tubes through centrifugation at x g for 5 min at room temperature. Serum aliquots were then placed in 1. A human ELISA for myoglobin was used to determine serum concentrations Abcam, Cambridge, MA, USA. Of note, it has been shown that increases in serum myoglobin concentration is a valid marker of muscle-damage as well as being more sensitive and less variable than creatine kinase [ 21 ]. Independent t-tests were performed for pre-study training age, height, weight, BMI, and average daily dietary intakes between groups. If a significant time α-value was observed for a dependent variable, subsequent paired samples t-tests were performed within each group. Moreover, given that only one comparison was performed within each group for each dependent variable i. T2 , raw p-values were used and Bonferroni adjustments were not applied. Again, given that only one comparison was performed within or between each group for each dependent variable i. If a significant time α-value was observed for a dependent variable, subsequent pairwise comparisons with Bonferroni adjustments were applied within each group. In the event that sphericity was not met, the Huynh-Feldt correction was applied to hypothesis testing. Participant characteristics are presented in Table 1. T1 and T2 squat and isometric peak torque values. Individual responses are plotted for squats panel b and c and isometric torque panel e and f. Abbreviations: BCAA-CHO, branched-chain amino acid and carbohydrate-supplemented group; CHO, carbohydrate only-supplemented group. T1 and T2 isokinetic torque and EMG activity. Serum myoglobin, perceived soreness, and training volume throughout the study. Whole blood variables over the duration of the study are presented in Table 2. Past studies are mixed regarding the effectiveness of BCAAs to mitigate muscle damage. Herein, we report that compared to CHO supplementation, BCAA-CHO supplementation was not able to reduce decrements in performance variables, markers of muscle damage and perceived muscle soreness increased over the study duration in both groups. These findings are discussed in greater detail below. As mentioned previously, some reports suggest that BCAA supplementation reduces muscle soreness following muscle-damaging protocols, although they do not appear to aid in attenuating the reduction of muscular performance following intense resistance training [ 11 , 12 ]. Our findings are in agreement with these reports in that BCAA-CHO-supplemented participants did not experience any significant performance outcomes relative to CHO-supplemented participants following three rigorous resistance exercise bouts. This contrasts the findings of Kirby et al. The reason for our findings are likely related to the resistance training protocol being too much of a muscle-damaging stimulus. Alternatively stated, while limited evidence suggests that BCAA administration may prevent muscle soreness and markers of muscle damage [ 12 ], muscle damage inflicted by resistance exercise is likely not mitigated by nutritional factors and, thus, occurs in spite of macronutrient provision. Therefore, while BCAA provision following exercise enhances post-exercise muscle protein synthesis [ 23 — 26 ] and reduce muscle proteolysis [ 27 — 29 ], our findings suggest that BCAA-CHO supplementation does not prevent muscle fibers from being damaged during rigorous resistance training stimulus, and this ultimately leads to the performance decrements observed herein. BCAAs have been reported to attenuate the rise in serum myoglobin [ 30 ] as well as attenuate perceived muscle soreness following rigorous exercise [ 12 , 30 ]. However, our findings differ from the aforementioned reports. Reasons for the divergence of our findings could be due to differences in exercise protocols, as Shimomura et al. had participants perform one bout of 7 sets of 20 body-weighted back squat repetitions, whereas our investigation applied 3 consecutive days of high intensity heavy-weighted back squats. Further, Shimomura et al. administered a BCAA supplement prior to the exercise protocol, whereas we administered the supplement following exercise. Notwithstanding, our data are in agreement with White et al. Our data are also in agreement with Stock et al. Additionally, others have reported that L-leucine administration does not differentially alter serum myoglobin following depth jumps or eccentric leg presses [ 22 ]; a finding which is also in agreement with our current data. Interestingly, BCAA-CHO supplementation prevented the rise in monocyte percentages compared to the CHO group, although monocyte counts were not altered between or within groups. While aerobic and resistance exercise have been reported to transiently increase leukocyte number and white blood cell differentials [ 31 , 32 ], levels typically return to baseline within hours of endurance [ 33 ] and resistance exercise [ 32 ]. Vigorous physical activity has also been shown to induce neutrophil proliferation [ 34 , 35 ], and intense cycling bouts have been shown to increase neutrophilia and alter neutrophil function [ 36 ]. Notwithstanding, exercise-induced increases in monocyte percentages can lead to their differentiating into macrophages which, like neutrophils, can infiltrate damaged skeletal muscle following eccentric contractions and potentiate the inflammatory response. In this regard, Malm et al. While we are unaware of studies that have examined the ability of BCAA supplementation to reduce post-exercise monocyte proliferation and skeletal muscle macrophage infiltration, it has been recently posited that BCAAs can be transaminated to glutamate in order to increase glutamine synthesis which, in turn, can be consumed by macrophages as a fuel source to propagate inflammation [ 38 ]. Notwithstanding, our findings that BCAAs are able to prevent the elevation in monocyte percentages over three days of rigorous weightlifting may suggest that BCAAs may possess an anti-inflammatory properties and should be further researched. Our study is not without limitations. First, and as described earlier, while these supplements were not standardized to CHO or Calorie content, our intent was to compare these two supplements in a practical or real-world setting. In this regard, our results may have differed had we had participants consume isocaloric amounts of each supplement. Notwithstanding, we posit that our administration of supplements ascribed to real-world application whereby participants in free-living environment would have consumed either the BCAA-CHO or CHO supplement. Second, the training protocol was very brief and does lack a certain degree of external validity i. However, we contend that this study is similar to other laboratory studies which have used comparable weightlifting protocols to elicit muscle damage over short periods of time. Thus, we contend that future research needs to employ such mechanistic protocols over longer and more practical training interventions i. Finally, it should be noted that there was not a non-supplemented control condition. Alternatively stated, it is possible that supplement conditions may have reduced the decrements in strength and markers similarly compared to a non-supplement group, but the current study design precludes us from reporting such data. As with previous studies reporting similar findings [ 15 , 16 , 22 ], we contend this is due to exercise-induced muscle damage occurring independent of BCAA or CHO provision. Clarkson PM, Hubal MJ. Exercise-induced muscle damage in humans. Am J Phys Med Rehabil. Article PubMed Google Scholar. Clarkson PM, Nosaka K, Braun B. Muscle function after exercise-induced muscle damage and rapid adaptation. Med Sci Sports Exerc. CAS PubMed Google Scholar. Kraemer WJ, Spiering BA, Volek JS, Martin GJ, Howard RL, Ratamess NA, et al. Recovery from a national collegiate athletic association division I football game: muscle damage and hormonal status. J Strength Cond Res. Twist C, Eston R. The effects of exercise-induced muscle damage on maximal intensity intermittent exercise performance. Eur J Appl Physiol. Juhn MS. Popular sports supplements and ergogenic aids. Sports Med. Maughan R. Nutritional ergogenic aids and exercise performance. Nutr Res Rev. Article CAS PubMed Google Scholar. Hulmi JJ, Lockwood CM, Stout JR. Nutr Metab. Article Google Scholar. Ha E, Zemel MB. Functional properties of whey, whey components, and essential amino acids: mechanisms underlying health benefits for active people review. J Nutr Biochem. Tang JE, Moore DR, Kujbida GW, Tarnopolsky MA, Phillips SM. Ingestion of whey hydrolysate, casein, or soy protein isolate: effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. J Appl Physiol. Nicastro H, da Luz CR, Chaves DFS, Bechara LRG, Voltarelli VA, Rogero MM et al. Does branched-chain amino acids supplementation modulate skeletal muscle remodeling through inflammation modulation? Possible mechanisms of action. J Nutr Metab. Jackman SR, Witard OC, Jeukendrup AE, Tipton KD. Branched-chain amino acid ingestion can ameliorate soreness from eccentric exercise. Howatson G, Hoad M, Goodall S, Tallent J, Bell PG, French DN. Exercise-induced muscle damage is reduced in resistance-trained males by branched chain amino acids: a randomized, double-blind, placebo controlled study. J Int Soc Sports Nutr. Article CAS PubMed PubMed Central Google Scholar. Sharp CP, Pearson DR. Amino acid supplements and recovery from high-intensity resistance training. Nosaka K, Sacco P, Mawatari K. Effects of amino acid supplementation on muscle soreness and damage. Int J Sport Nutr Exerc Metab. White JP, Wilson JM, Austin KG, Greer BK, St John N, Panton LB. Effect of carbohydrate-protein supplement timing on acute exercise-induced muscle damage. Stock MS, Young JC, Golding LA, Kruskall LJ, Tandy RD, Conway-Klaassen JM, et al. The effects of adding leucine to pre and postexercise carbohydrate beverages on acute muscle recovery from resistance training. Tipton KD, Ferrando AA, Phillips SM, Doyle Jr D, Wolfe RR. Postexercise net protein synthesis in human muscle from orally administered amino acids. Am J Phys. CAS Google Scholar. Data N. BOBBERT MF, HOLLANDER AP, Huijing P. Factors in delayed onset muscular soreness. Basmajian JV, De Luca C. Muscles alive. Proc R Soc Med. Google Scholar. Sorichter S, Puschendorf B, Mair J. Skeletal muscle injury induced by eccentric muscle action: muscle proteins as markers of muscle fiber injury. Exerc Immunol Rev. Kirby TJ, Triplett NT, Haines TL, Skinner JW, Fairbrother KR, McBride JM. Effect of leucine supplementation on indices of muscle damage following drop jumps and resistance exercise. Amino Acids. Blomstrand E, Eliasson J, Karlsson HK, Köhnke R. Branched-chain amino acids activate key enzymes in protein synthesis after physical exercise. J Nutr. Norton LE, Layman DK. Leucine regulates translation initiation of protein synthesis in skeletal muscle after exercise. Anthony JC, Anthony TG, Kimball SR, Jefferson LS. Signaling pathways involved in translational control of protein synthesis in skeletal muscle by leucine. Tipton K, Gurkin B, Matin S, Wolfe R. Nonessential amino acids are not necessary to stimulate net muscle protein synthesis in healthy volunteers. Matsumoto K, Mizuno M, Mizuno T, Dilling-Hansen B, Lahoz A, Bertelsen V, et al. Branched-chain amino acids and arginine supplementation attenuates skeletal muscle proteolysis induced by moderate exercise in young individuals. Int J Sports Med. Louard RJ, Barrett EJ, Gelfand RA. Overnight branched-chain amino acid infusion causes sustained suppression of muscle proteolysis. J Cell Physiol. Shimomura Y, Inaguma A, Watanabe S, Yamamoto Y, Muramatsu Y, Bajotto G, et al. Branched-chain amino acid supplementation before squat exercise and delayed-onset muscle soreness. Int J Sport Nutr. Field CJ, Gougeon R, Marliss EB. Circulating mononuclear cell numbers and function during intense exercise and recovery. Kraemer WJ, Clemson A, Triplett NT, Bush JA, Newton RU, Lynch JM. The effects of plasma cortisol elevation on total and differential leukocyte counts in response to heavy-resistance exercise. Eur J Appl Physiol Occup Physiol. Nieman DC, Nehlsen-Cannarella SL, Fagoaga OR, Henson DA, Utter A, Davis JM, et al. Effects of mode and carbohydrate on the granulocyte and monocyte response to intensive, prolonged exercise. Peake JM. Exercise-induced alterations in neutrophil degranulation and respiratory burst activity: possible mechanisms of action. PubMed Google Scholar. Suzuki K, Sato H, Kikuchi T, Abe T, Nakaji S, Sugawara K, et al. Capacity of circulating neutrophils to produce reactive oxygen species after exhaustive exercise. Suzuki K, Totsuka M, Nakaji S, Yamada M, Kudoh S, Liu Q, et al. Endurance exercise causes interaction among stress hormones, cytokines, neutrophil dynamics, and muscle damage. Malm C, Nyberg P, Engström M, Sjödin B, Lenkei R, Ekblom B, et al. Immunological changes in human skeletal muscle and blood after eccentric exercise and multiple biopsies. J Physiol. Chieng W-S, Lee S-C. Discrepancy between initial high expression of interest in clinical cancer genetic testing and actual low uptake in an Asian population. Genet Test Mol Biomarkers. Download references. Reagent costs and participant compensation costs were paid through a contract awarded to MDR through MusclePharm Corp. Denver, CO. WCK and PWM coordinated the study, recruited participants, and analyzed the data. WCK, PWM, AEM, AMH, JJS and CBM performed data collection. AEJ, WHW and GDO performed all EMG procedures and analyses. KCY and JJM assisted study design, data analyses and the drafting of the manuscript. MDR was the principal investigator of the study responsible for procuring funds, drafting the study design, and WCK and MDR drafted the first version of the manuscript. All authors read and approved the final manuscript. Besides JRM, none of the authors have conflicts of interest. JRM is a Ph. scientist that was employed by the MusclePharm Research Institute at the time of this study, but he substantially contributed to the study design and data write-up. Therefore, all co-authors agreed that his work into this project warranted co-authorship. Hence, consent for publication is not applicable. All participants gave their informed consent in writing prior to inclusion in the study. Identifying details names, dates of birth, identity numbers and other information of the participants are not published in the current work. The Auburn University Institutional Review Board approved the study on October 14, and the protocol number associated with this project was MR School of Kinesiology, Molecular and Applied Sciences Laboratory, Auburn University, Wire Road, Office , Auburn, AL, , USA. Wesley C. Kephart, Petey W. Significant difference between control and BDK-mKO mice. All of the control and BDK-mKO mice were trained for 2 weeks, as described in the Materials and Methods. One-half of the control and BDK-mKO mice were run for 32 min before being sacrificed, while the other half were sacrificed under the sedentary condition. No differences were observed in body and tissue weights on the final experimental day among the 4 groups of mice S1 Table. In BDK-mKO mice, BDK was not detected in heart and skeletal muscle, but was detected in other tissues examined S1 Fig , as reported previously [ 17 ]. The amounts of the subunits of muscle BCKDC were not affected by exercise training in either control and BDK-mKO mice S2 Fig. The concentrations of the three plasma BCAAs were significantly lower in the sedentary BDK-mKO mice than in the sedentary control mice Fig 2. These concentrations tended to be decreased after the exercise bout in control mice, but the opposite was observed in BDK-mKO mice Fig 2. The increases in the plasma BCAA concentrations in BDK-mKO mice might be related to the increases in the amino acid concentrations in tissues other than muscles such as liver [ 28 ]. The concentrations of other plasma amino acids S3 Fig were not different between the sedentary control and BDK-mKO mice, except that the aspartate concentration tended to be higher in BDK-mKO than in control. As observed for the concentrations of plasma BCAAs, the concentrations of many other plasma amino acids tended to be decreased by the exercise bout in control mice, but were not changed or rather increased by the exercise bout in BDK-mKO mice S3 Fig ; thus, the concentrations of alanine, arginine, aspartate, glutamate, glycine, histidine, lysine, and threonine tended to be higher in BDK-mKO mice than in control mice. Since muscle glycogen content has been reported to be a factor affecting exercise performance [ 29 ], we determined the contents in the hind-limb muscle. Glycogen content was significantly lower in sedentary BDK-mKO mice than in sedentary control mice Fig 3. The exercise bout significantly decreased the glycogen content in both groups of mice, resulting in lower glycogen content in BDK-mKO than in control mice after the exercise bout Fig 3. The levels of the three BCAAs were significantly less in BDK-mKO mice than in control mice with and without the exercise bout; specifically, leucine and isoleucine levels were less than half in the former compared to the latter Fig 4A—4C. These concentrations in both groups of mice were not affected by the exercise bout. The levels of alanine, aspartate, glutamate, histidine, lysine, and threonine tended to be higher in BDK-mKO mice than in control mice, but were not affected by the exercise bout S4 Fig. Only the tryptophan level was increased by the exercise bout in both groups of mice. Changes in the metabolite levels in the skeletal muscle of control and BDK-mKO mice with and without the exercise bout are shown. PMP, pyridoxamine 5'-phosphate; KIC, α-ketoisocaproate; KMV, α-keto-ß-methylvalerate; KIV, α-ketoisovalerate; ßMB-CAR, ß-methylbutyryl-carnitine; αMB-CAR, α-methylbutyryl-carnitine; IB-CAR, isobutyryl-carnitine; and αKG, α-ketoglutarate. The level of pyridoxamine 5-phosphate PMP , a derivative of vitamin B6, tended to be lower in BDK-mKO mice than in control mice with and without the exercise bout Fig 4D. α-Ketoisocaproic acid KIC and α-keto-ß-methylvaleric acid KMV were found at substantial levels in control mice, but were barely detected in BDK-mKO mice Fig 4E. On the other hand, the levels of ß-methylbutyryl-carnitine ßMB-CAR , α-methylbutyryl-carnitine αMB-CAR Fig 4F and isobutyryl-carnitine IB-CAR Fig 4G were significantly higher in BDK-mKO mice than in control mice. Only the IB-CAR level in control mice was affected by the exercise bout: the level was higher in control mice with exercise bout than in the same group of mice without exercise. There was no difference in glucose 6-phosphate G6P level in each group Fig 5A. The levels of fructose 1,6-bisphosphate F1,6BP decreased in BDK-mKO mice with and without the exercise bout compared with control mice Fig 5B. The levels of dihydroxyacetone phosphate DHAP , 2-phosphoglycerate 2PG , and phosphoenolpyruvate PEP tended to be less in BDK-mKO mice than in control mice without the exercise bout Fig 5C—5E , and these levels were largely not affected by the exercise bout. Muscle lactate also showed no difference in each group Fig 5F. Changes in metabolite levels in skeletal muscle of BDK-mKO mice and control mice with and without the exercise bout are shown. G6P, glucose 6-phosphate; F1,6BP, fructose 1,6-bisphosphate; DHAP, dihydroxyacetone phosphate; 2PG, 2-phosphoglycerate; and PEP, phosphoenolpyruvate. The levels of acetyl-CoA, citrate, and isocitrate were lower in BDK-mKO mice than in control mice without the exercise bout, and only the acetyl-CoA level in control mice was significantly decreased by the exercise bout Fig 6A—6C. On the other hand, other metabolites succinate, fumarate, or malate in the cycle were not affected by BDK-mKO or the exercise bout Fig 6D—6F. αKG, α-ketoglutarate. The exercise bout tended to decrease the NADH level only in control mice. The levels of ATP, GTP, and phosphocreatine were the same between the control and BDK-mKO mice with and without the exercise bout Fig 7C—7E. We measured the activities of citrate synthase and cytochrome c oxidase as mitochondrial marker enzymes in soleus muscle, because this muscle is often used in the studies to examine effects of exercise on the mitochondrial enzyme activities. Both enzyme activities were less in BDK-mKO mice than in control mice with and without the exercise bout, although both enzyme activities tended to be increased by the exercise bout in both groups of mice Fig 8. BCAA catabolism in skeletal muscle is tightly regulated by BCKDC, most of which is inactivated by phosphorylation of the complex due to high BDK activity under normal and sedentary conditions [ 3 , 9 ]. The low activity state of BCKDC suppresses BCAA oxidation, thereby contributing to the provision of BCAAs for muscle protein synthesis [ 30 ]. On the other hand, BCKDC in skeletal muscle is greatly activated by acute exercise in association with the decrease in the active form of BDK, which is bound to BCKDC [ 9 ], resulting in increased BCAA oxidation to supply substrates for enhanced muscle energy expenditure. In the present study, we demonstrated that the tight regulation of BCAA oxidation in muscle tissues is important, at lease in part, in the adaptation to exercise training; BDK-mKO mice in which muscle BCKDC was almost fully activated [ 17 ] showed somewhat, but significantly less adaptability to running and swimming exercise training. This down-regulation of exercise performance may be attributed to lower glycogen content and perturbation of energy metabolism. The decreased mitochondrial enzyme activities for energy production, which were induced by uncontrolled BCAA catabolism in soleus muscle, might be related to the exercise performance in BDK-mKO mice. From the metabolome analysis, BCAA levels were significantly decreased in association with dramatic decreases in the corresponding BCKAs and increases in the downstream metabolites, branched-chain acyl-carnitines, in skeletal muscles of BDK-mKO mice. These results indicate that catabolism of BCAAs and BCKAs was greatly enhanced in the muscles of BDK-mKO mice and largely produced the carnitine derivatives, but not acetyl-CoA or succinyl-CoA, in the muscles, suggesting that BCAA catabolism may not significantly contribute to energy metabolism. The levels of some components in the glycolytic pathway were downregulated in the muscles of BDK-mKO mice, which may be in line with the decrease in muscle glycogen content of the mice. These phenomena may be responsible for the low levels of acetyl-CoA in the muscles of BDK-mKO mice, presumably resulting in low levels of citrate and isocitrate in the TCA cycle and NADH. Since the perturbation of energy metabolism in the muscles of BDK-mKO mice was associated with the tendency for increased levels of alanine and aspartate in plasma and muscle tissues, it is thought that increased transamination of BCAAs produces glutamate from α-ketoglutarate, followed by the production of alanine and aspartate from pyruvate and oxaloacetate, respectively, by transamination Fig 9. This hypothesis is supported by the evidence that, compared to oxaloacetate and pyruvate, α-ketoglutarate is the best amino group acceptor from BCAAs that is catalyzed by BCATm [ 31 ]. It should be noted that the metabolome analysis in the present study was conducted using mixture of gastrocnemius and plantaris muscles which are mainly composed of the anaerobic muscle fibers, because energy metabolism is significantly different between anaerobic and aerobic muscle fiber types. It has been reported that BDK-mKO had no effects on muscle fiber types of soleus and plantaris muscles [ 17 ]. The low levels of muscle glycogen in BDK-mKO mice may lead to decreased levels of some metabolites in the glycolytic pathway. Accelerated decarboxylation of BCKAs promotes transamination of BCAAs, resulting in the production of Glu. The increased Glu may contribute to the production of Asp and Ala from oxaloacetate OA and pyruvate Pyr , respectively, which may be responsible for the low levels of acetyl-CoA, citrate, and isocitrate in the muscle of BDK-mKO mice. On the other hand, accelerated decarboxylation of BCKAs produces acyl-CoAs, which appear to be converted mainly to acyl-carnitines acyl-CARs. F1,6BP, fructose 1,6-bisphosphate; 2PG, 2-phosphoglycerate; PEP, phosphoenolpyruvate; and αKG, α-ketoglutarate. In contrast to the low levels of acetyl-CoA and NADH in the muscles of BDK-mKO mice, levels of high energy compounds such as ATP were not affected by BDK-mKO, suggesting modest perturbation of energy metabolism by the accelerated BCAA transamination. The acute exercise bout used in this study could be classified as mild intensity, since all of the mice completed the running performance and few metabolites, such as acetyl-CoA, were decreased by the exercise bout and only in the control mice, although the muscle glycogen content was significantly decreased after exercise in both control and BDK-mKO mice. We have reported that the tight regulation of BCAA catabolism is important in the homeostasis of muscle protein metabolism [ 17 ]. In the present study, we confirmed that this is also the case in energy metabolism of skeletal muscle. BCAAs are commonly used as supplements in the field of sports [ 32 ]. Although the BCKDC activity in liver was extremely higher in rat and mouse than humans, that in skeletal muscle is not so much different between rodents and humans [ 2 ]. Furthermore, it has been reported that BCAA oxidation is enhanced by exercise in association with an increase in the BCKDC activity in human and rat skeletal muscle [ 10 , 11 ]. Therefore, the findings in the present study might be partly appricable to humans. However, further studies are required to elucidate the effects of excess BCAA supplementation, which may promote the catabolism of amino acids in humans. Tissue extracts were applied on SDS-PAGE, followed by transfer of proteins to PVDF membranes and immunostaining of BDK and E2 component, as described in Materials and Methods. Protein amounts per lane applied on the SDS-PAGE were 25 μg for heart, kidney, and pancreas; 30 μg for brain; and 50 μg for skeletal muscle, liver, spleen, and testis. Age-matched untrained mice both control and BDK-mKO mice were prepared for this experiment. Therefore, trained and untrained mice both control and BDK-mKO mice were used in the Western blotting. The tissue extracts gastrocnemius and plantaris muscles were applied on SDS-PAGE, followed by transfer of proteins to PVDF membranes and immunostaining of BCKDC, as described in Materials and Methods. Protein amounts per lane applied on the SDS-PAGE were 25 μg. Values are means ± SE. Skeletal muscle is a mixture of soleus, gastrocnemius, and plantaris muscles of the left hind-limb. Both sides of epididymal adipose tissues were collected from mice and combined. Browse Subject Areas? Click through the PLOS taxonomy to find articles in your field. Article Authors Metrics Comments Media Coverage Reader Comments Figures. Abstract It is known that the catabolism of branched-chain amino acids BCAAs in skeletal muscle is suppressed under normal and sedentary conditions but is promoted by exercise. Introduction The branched-chain amino acids BCAAs , leucine, isoleucine, and valine, are essential amino acids EAAs for mammals and have unique characteristics in their catabolic system; BCAAs are directly catabolized in skeletal muscle, whereas the other EAAs are mainly catabolized in liver [ 1 ]. Materials and methods Ethics statement All procedures were approved by the Animal Care Committee of the Nagoya University Graduate School of Bioagricultural Sciences. Animals We generated BDK-mKO mice using the Cre-loxP system [ 18 ], as described previously [ 17 ]. Metabolite extraction and metabolome analysis Metabolite extraction and metabolome analysis were conducted at Human Metabolome Technologies HMT, Tsuruoka, Yamagata, Japan. Western blotting Antisera against BDK [ 9 ] and BCKDC [ 5 ] were prepared as reported previously. Determination of muscle glycogen Muscle glycogen in the hind-limb muscle was determined by the phenol-sulfuric acid method, as reported previously [ 25 ]. Assay of mitochondrial enzyme activities Frozen soleus muscle was homogenized in extraction buffer containing 50 mM HEPES pH 7. Statistical analysis Data are expressed as means ± SEM. Results Exercise performance Running distance to exhaustion before the exercise training was not different between the control and BDK-mKO mice Fig 1A. Download: PPT. Characterization of mice used in the metabolome analysis on the final experimental day All of the control and BDK-mKO mice were trained for 2 weeks, as described in the Materials and Methods. Fig 2. Plasma BCAA concentrations of control and BDK-mKO mice with and without the running exercise bout. Fig 3. Glycogen contents in skeletal muscle of control and BDK-mKO mice with and without the exercise bout. Effects of BDK-mKO and exercise bout on the metabolome in skeletal muscle BCAAs and their metabolites. Metabolites in the glycolytic pathway. Acetyl-CoA and metabolites in the tricarboxylic acid TCA cycle. NADH, NAD, and high-energy phosphate compounds. Mitochondrial enzyme activities We measured the activities of citrate synthase and cytochrome c oxidase as mitochondrial marker enzymes in soleus muscle, because this muscle is often used in the studies to examine effects of exercise on the mitochondrial enzyme activities. Discussion BCAA catabolism in skeletal muscle is tightly regulated by BCKDC, most of which is inactivated by phosphorylation of the complex due to high BDK activity under normal and sedentary conditions [ 3 , 9 ]. Fig 9. A schematic model of the perturbation of energy metabolism in association with enhanced BCAA catabolism. Supporting information. S1 Fig. Western blotting of BDK and E2 component of the BCKDC in control and BDK-mKO mice. s PDF. S2 Fig. Typical Western blots of the BCKDC extracted from skeletal muscle of control and BDK-mKO mice. S3 Fig. Plasma concentrations of amino acids except for BCAAs in control and BDK-mKO mice with and without the exercise bout. S4 Fig. Muscle levels of amino acids except for BCAAs in control and BDK-mKO mice with and without the exercise bout. S1 Table. Body and tissue weights of control and BDK-mKO mice with and without the running exercise bout. References 1. Harper AE, Miller RH, Block KP, Branched-chain amino acid metabolism. Annual Review of Nutrition. Suryawan A, Hawes JW, Harris RA, Shimomura Y, Jenkins AE, Hutson SM, Molecular model of human branched-chain amino acid metabolism. American Journal of Clinical Nutrition. Shimomura Y, Fujii H, Suzuki M, Murakami T, Fujitsuka N, Nakai N, Brancheded-chain alpha-keto acid dehydrogenase complex in rat skeletal muscle regulation of the activity and gene expression by nutrition and physical exercise. Journal of Nutrition. View Article Google Scholar 4. Shimomura Y, Honda T, Shiraki M, Murakami T, Sato J, Kobayashi H, et al. Branched-chain amino acid catabolism in exercise and liver disease. View Article Google Scholar 5. Shimomura Y, Nanaumi N, Suzuki M, Popov KM, Harris RA, Purification and partial characterization of branched-chain alpha-keto acid dehydrogenase kinase from rat liver and rat heart. Archives of Biochemistry and Biophysics. Popov KM, Zhao Y, Shimomura Y, Kuntz MJ, Harris RA, Branched-chain alpha-keto acid dehydrogenase kinase-molecular-cloning, expression, and sequence similarity with histidine protein-kinases. Journal of Biological Chemistry. Joshi M, Jeoung NH, Popov KM, Harris RA, Identification of a novel PP2C-type mitochondrial phosphatase. Biochemical and Biophysical Research Communications. Lu G, Sun HP, She PX, Youn JY, Warburton S, Ping PP, et al. Protein phosphatase 2Cm is a critical regulator of branched-chain amino acid catabolism in mice and cultured cells. Journal of Clinical Investigation. Xu M, Nagasaki M, Obayashi M, Sato Y, Tamura T, Shimomura Y, Mechanism of activation of branched-chain alpha-keto acid dehydrogenase complex by exercise. Wagenmakers AJM, Muscle amino acid metabolism at rest and during exercise: Role in human physiology and metabolism. Exercise and Sport Sciences Reviews. |
| The Science Behind BCAAs - Part 2 - | They're called "essential" because the body can't make them, so they must be consumed through food. While you can definitely get enough of these aminos if you eat adequate servings of protein foods , getting them from a drink has its advantages. For example, pure BCAAs bypass the liver and gut and go directly into your bloodstream. Although it's important for everyone to get enough BCAAs, they're particularly important for people with muscle-building or muscle-maintenance goals. These compounds, especially leucine, help regulate protein metabolism by promoting protein synthesis and suppressing protein breakdown. BCAA supplementation before your workout can help increase rates of protein synthesis, suppress muscle protein breakdown, reduce markers of muscle damage, and lessen the symptoms of delayed onset muscle soreness DOMS. Branched-chain amino acids can help promote protein synthesis, leading to muscle growth, in several different ways. When most amino acids are ingested, they're absorbed by the intestines and shuttled straight to the liver. The liver then decides what to do with them before they go to the rest of the body. If the body needs more energy, the liver will even break them down for fuel rather than spare them to repair and build muscle and other tissue. BCAAs, on the other hand, tend to be spared by the liver and get direct access to tissues like muscle. The muscle fibers then get to make the decision of what to do with these aminos based on their needs. One of these needs could very well be to build up muscle tissue, which is good! In terms of protein synthesis, leucine is by far the most valuable of the three BCAAs for stimulating muscle growth. Much like the ignition starts the car engine, leucine turns on the process of protein synthesis. In scientific terms, leucine activates a complex process called mTOR, which ramps up protein synthesis, and therefore muscle tissue growth. Branched-chain amino acids may also be anti-catabolic. This means they help to reduce muscle breakdown catabolism and speed up recovery after exercise. After resistance training, the processes of muscle synthesis and muscle breakdown both increase, but breakdown actually exceeds growth. This is where amino supplements come in. Post-exercise, muscle loss exceeds growth until protein or leucine is ingested. Having a drink with a serving of grams of BCAAs pre-workout can lead to less soreness and a quicker recovery time. During exercise, BCAAs are broken down and used as an energy source. Valine plays a key role in providing energy for workouts. During exercise, tryptophan is taken up by the brain in large amounts. Tryptophan is used by the brain to make serotonin. Valine, however, competes with tryptophan for entry into the brain and typically wins out. Less tryptophan gets converted to serotonin, which allows your muscles to contract with more force for a longer time before becoming fatigued. The more intense and the longer the workout, the more branched-chain amino acids will be used for fuel. Supplementing with them means you'll be able to train more intensely and for a longer period of time, which is critical for drastic improvements in body composition. Signaling pathways involved in translational control of protein synthesis in skeletal muscle by leucine. Tipton K, Gurkin B, Matin S, Wolfe R. Nonessential amino acids are not necessary to stimulate net muscle protein synthesis in healthy volunteers. Matsumoto K, Mizuno M, Mizuno T, Dilling-Hansen B, Lahoz A, Bertelsen V, et al. Branched-chain amino acids and arginine supplementation attenuates skeletal muscle proteolysis induced by moderate exercise in young individuals. Int J Sports Med. Louard RJ, Barrett EJ, Gelfand RA. Overnight branched-chain amino acid infusion causes sustained suppression of muscle proteolysis. J Cell Physiol. Shimomura Y, Inaguma A, Watanabe S, Yamamoto Y, Muramatsu Y, Bajotto G, et al. Branched-chain amino acid supplementation before squat exercise and delayed-onset muscle soreness. Int J Sport Nutr. Field CJ, Gougeon R, Marliss EB. Circulating mononuclear cell numbers and function during intense exercise and recovery. Kraemer WJ, Clemson A, Triplett NT, Bush JA, Newton RU, Lynch JM. The effects of plasma cortisol elevation on total and differential leukocyte counts in response to heavy-resistance exercise. Eur J Appl Physiol Occup Physiol. Nieman DC, Nehlsen-Cannarella SL, Fagoaga OR, Henson DA, Utter A, Davis JM, et al. Effects of mode and carbohydrate on the granulocyte and monocyte response to intensive, prolonged exercise. Peake JM. Exercise-induced alterations in neutrophil degranulation and respiratory burst activity: possible mechanisms of action. PubMed Google Scholar. Suzuki K, Sato H, Kikuchi T, Abe T, Nakaji S, Sugawara K, et al. Capacity of circulating neutrophils to produce reactive oxygen species after exhaustive exercise. Suzuki K, Totsuka M, Nakaji S, Yamada M, Kudoh S, Liu Q, et al. Endurance exercise causes interaction among stress hormones, cytokines, neutrophil dynamics, and muscle damage. Malm C, Nyberg P, Engström M, Sjödin B, Lenkei R, Ekblom B, et al. Immunological changes in human skeletal muscle and blood after eccentric exercise and multiple biopsies. J Physiol. Chieng W-S, Lee S-C. Discrepancy between initial high expression of interest in clinical cancer genetic testing and actual low uptake in an Asian population. Genet Test Mol Biomarkers. Download references. Reagent costs and participant compensation costs were paid through a contract awarded to MDR through MusclePharm Corp. Denver, CO. WCK and PWM coordinated the study, recruited participants, and analyzed the data. WCK, PWM, AEM, AMH, JJS and CBM performed data collection. AEJ, WHW and GDO performed all EMG procedures and analyses. KCY and JJM assisted study design, data analyses and the drafting of the manuscript. MDR was the principal investigator of the study responsible for procuring funds, drafting the study design, and WCK and MDR drafted the first version of the manuscript. All authors read and approved the final manuscript. Besides JRM, none of the authors have conflicts of interest. JRM is a Ph. scientist that was employed by the MusclePharm Research Institute at the time of this study, but he substantially contributed to the study design and data write-up. Therefore, all co-authors agreed that his work into this project warranted co-authorship. Hence, consent for publication is not applicable. All participants gave their informed consent in writing prior to inclusion in the study. Identifying details names, dates of birth, identity numbers and other information of the participants are not published in the current work. The Auburn University Institutional Review Board approved the study on October 14, and the protocol number associated with this project was MR School of Kinesiology, Molecular and Applied Sciences Laboratory, Auburn University, Wire Road, Office , Auburn, AL, , USA. Wesley C. Kephart, Petey W. Mumford, Anna E. McCloskey, A. Maleah Holland, Joshua J. Shake, C. Brooks Mobley, Adam E. Jagodinsky, Wendi H. Weimar, Gretchen D. Oliver, Kaelin C. Edward Via College of Osteopathic Medicine — Auburn Campus, Auburn, AL, USA. American Public University System, School of Health Sciences, Charles Town, WV, USA. You can also search for this author in PubMed Google Scholar. Correspondence to Michael D. Open Access This article is distributed under the terms of the Creative Commons Attribution 4. Reprints and permissions. Kephart, W. et al. Post-exercise branched chain amino acid supplementation does not affect recovery markers following three consecutive high intensity resistance training bouts compared to carbohydrate supplementation. J Int Soc Sports Nutr 13 , 30 Download citation. Received : 26 August Accepted : 19 July Published : 26 July Anyone you share the following link with will be able to read this content:. Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative. Skip to main content. Search all BMC articles Search. Download PDF. Download ePub. Research article Open access Published: 26 July Post-exercise branched chain amino acid supplementation does not affect recovery markers following three consecutive high intensity resistance training bouts compared to carbohydrate supplementation Wesley C. Kephart 1 , Petey W. Mumford 1 , Anna E. McCloskey 1 , A. Maleah Holland 1 , Joshua J. Shake 1 , C. Brooks Mobley 1 , Adam E. Jagodinsky 1 , Wendi H. Weimar 1 , Gretchen D. Oliver 1 , Kaelin C. Young 1 , 2 , Jordan R. Roberts 1 , 2 Show authors Journal of the International Society of Sports Nutrition volume 13 , Article number: 30 Cite this article 24k Accesses 18 Citations 83 Altmetric Metrics details. Abstract Background Amino acid supplementation has been shown to potentially reduced exercise-induced muscle soreness. Results There were similar decrements in 1RM squat strength and isokinetic peak torque measures in the BCAA-CHO and CHO groups. Background High-intensity resistance exercise elicits muscle damage and this can be linked to diminished performance [ 1 — 4 ]. Methods Participant characteristics Prior to initiating this study, the protocol was reviewed and approved by the Auburn University Institutional Review Ethics Committee protocol , and was in compliance with the Helsinki Declaration. Experimental protocol Figure 1 provides an outline of the experimental protocol. Full size image. Results Participant characteristics Participant characteristics are presented in Table 1. Table 1 Participant demographics and dietary intakes Full size table. Table 2 Select white blood cell differentials between groups over the training intervention Full size table. Discussion Past studies are mixed regarding the effectiveness of BCAAs to mitigate muscle damage. BCAA-CHO supplementation does not reduce decrements in lower body strength after three consecutive high intensity exercise bouts As mentioned previously, some reports suggest that BCAA supplementation reduces muscle soreness following muscle-damaging protocols, although they do not appear to aid in attenuating the reduction of muscular performance following intense resistance training [ 11 , 12 ]. BCAAs and CHO provision do not differentially alter myoglobin serum concentrations or muscle soreness BCAAs have been reported to attenuate the rise in serum myoglobin [ 30 ] as well as attenuate perceived muscle soreness following rigorous exercise [ 12 , 30 ]. BCAA-CHO supplementation reduced exercise-induced increases in monocyte differentials versus CHO supplementation Interestingly, BCAA-CHO supplementation prevented the rise in monocyte percentages compared to the CHO group, although monocyte counts were not altered between or within groups. Conclusions Our study is not without limitations. Abbreviations 1RM, one-repetition maximum; BCAA, branched chain amino acids; CHO, carbohydrate; EMG, electromyography; MVIC, maximum voluntary isometric contraction; USG, urine specific gravity; VAS, visual analog scale; WBC, white blood cell count. References Clarkson PM, Hubal MJ. Article PubMed Google Scholar Clarkson PM, Nosaka K, Braun B. CAS PubMed Google Scholar Kraemer WJ, Spiering BA, Volek JS, Martin GJ, Howard RL, Ratamess NA, et al. Article PubMed Google Scholar Twist C, Eston R. Article PubMed Google Scholar Juhn MS. Article PubMed Google Scholar Maughan R. Article CAS PubMed Google Scholar Hulmi JJ, Lockwood CM, Stout JR. Article Google Scholar Ha E, Zemel MB. Article CAS PubMed Google Scholar Tang JE, Moore DR, Kujbida GW, Tarnopolsky MA, Phillips SM. Article CAS PubMed Google Scholar Nicastro H, da Luz CR, Chaves DFS, Bechara LRG, Voltarelli VA, Rogero MM et al. Influence of aerobic exercise intensity on myofibrillar and mitochondrial protein synthesis in young men during early and late postexercise recovery. Donges, C. Concurrent resistance and aerobic exercise stimulates both myofibrillar and mitochondrial protein synthesis in sedentary middle-aged men. Egan, B. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Fardet, A. Influence of food structure on dairy protein, lipid and calcium bioavailability: a narrative review of evidence. Food Sci. CrossRef Full Text Google Scholar. Ferguson-Stegall, L. Aerobic exercise training adaptations are increased by postexercise carbohydrate-protein supplementation. Garber, C. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Sports Exer. Giromini, C. Short-communication: a comparison of the in vitro angiotensinconverting enzyme inhibitory capacity of dairy and plant protein supplements. Nutrients Glynn, E. Excess leucine intake enhances muscle anabolic signaling but net protein anabolism in young men and women. Grunert, K. Three issues in consumer quality perception and acceptance of dairy products. Dairy J. Hansen, M. Protein intake during training sessions has no effect on performance and recovery during a strenuous training camp for elite cyclists. Harber, M. Muscle protein synthesis and gene expression during recovery from aerobic exercise in the fasted and fed states. Hill, K. Co-ingestion of carbohydrate and whey protein isolates enhance PGC-1alpha mRNA expression: a randomised, single blind, cross over study. Hoffman, J. Protein - which is best? Sports Sci. PubMed Abstract Google Scholar. Holloszy, J. Biochemical adaptations to endurance exercise in muscle. Hood, D. Amino acid metabolism during exercise and following endurance training. Howarth, K. Coingestion of protein with carbohydrate during recovery from endurance exercise stimulates skeletal muscle protein synthesis in humans. Effect of glycogen availability on human skeletal muscle protein turnover during exercise and recovery. Hulston, C. Protein intake does not increase vastus lateralis muscle protein synthesis during cycling. Jäger, R. Kato, H. Protein requirements are elevated in endurance athletes after exercise as determined by the indicator amino acid oxidation method. PLoS ONE e Konopka, A. Skeletal muscle mitochondrial protein synthesis and respiration in response to the energetic stress of an ultra-endurance race. Skeletal muscle hypertrophy after aerobic exercise training. Sport Sci. Koopman, R. Combined ingestion of protein and carbohydrate improves protein balance during ultra-endurance exercise. Lamont, L. Comparison of leucine kinetics in endurance-trained and sedentary humans. Leucine kinetics in endurance-trained humans. Lemon, P. Effect of initial muscle glycogen levels on protein catabolism during exercise. Lunn, W. Chocolate milk and endurance exercise recovery: protein balance, glycogen, and performance. MacLean, D. Branched-chain amino acids augment ammonia metabolism while attenuating protein breakdown during exercise. McKenzie, S. Endurance exercise training attenuates leucine oxidation and BCOAD activation during exercise in humans. Montero, D. Haematological rather than skeletal muscle adaptations contribute to the increase in peak oxygen uptake induced by moderate endurance training. Moore, D. Beyond muscle hypertrophy: why dietary protein is important for endurance athletes. Norton, L. Leucine contents of isonitrogenous protein sources predict changes in body composition and muscle mass in rats. FASEB J. Google Scholar. Phillips, S. Dietary protein requirements and adaptive advantages in athletes. Gender differences in leucine kinetics and nitrogen balance in endurance athletes. Philp, A. Rapamycin does not prevent increases in myofibrillar or mitochondrial protein synthesis following endurance exercise. Robinson, M. Acute {beta}-adrenergic stimulation does not alter mitochondrial protein synthesis or markers of mitochondrial biogenesis in adult men. Long-term synthesis rates of skeletal muscle DNA and protein are higher during aerobic training in older humans than in sedentary young subjects but are not altered by protein supplementation. Rose, A. Regulatory mechanisms of skeletal muscle protein turnover during exercise. Saunders, M. Coingestion of carbohydrate-protein during endurance exercise: influence on performance and recovery. Sport Nutr. Carbohydrate and protein hydrolysate coingestions improvement of late-exercise time-trial performance. Song, M. Association of animal and plant protein intake with all-cause and cause-specific mortality. JAMA Intern. Tang, J. Ingestion of whey hydrolysate, casein, or soy protein isolate: effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. Tarnopolsky, M. Protein requirements for endurance athletes. Nutrition 20, — Tipton, K. Stimulation of net muscle protein synthesis by whey protein ingestion before and after exercise. van Wijck, K. Physiology and pathophysiology of splanchnic hypoperfusion and intestinal injury during exercise: strategies for evaluation and prevention. liver Physiol. Wackerhage, H. |
| Ask The Science Chick: Should I Drink BCAAs During My Workout? | Posted by Nick Telesca Estimated reading time: 9 minutes. Posted by Mason Brezinscak Estimated reading time: 8 minutes. Posted by Ebony Abblitt Estimated reading time: 3 minutes. Posted by Ellie Hearn Estimated reading time: 5 minutes. Posted by Nick Telesca Estimated reading time: 11 minutes. Posted by Nick Telesca Estimated reading time: 7 minutes. Posted by Ben Crowley Estimated reading time: 6 minutes. Posted by Nick Telesca Estimated reading time: 16 minutes. We're an Australian manufacturer and supplier of high quality sports supplements. Operating since , Bulk Nutrients has become one of the premier Australian brands to supply nutritional products to top level athletes, competitors and those on a journey to a healthier lifestyle. Find out more about Bulk. One thing that sets Bulk Nutrients apart is that we love to talk to our customers! Whether you need product advice, help with the website or need a change made to your order If you prefer email you can email us day or night at info bulknutrients. For online chat, hit the 'Chat' button in the bottom right hand corner of your screen and you'll be connected to one of our lovely customer service team. Or if you'd like to get in touch through our online contact form , that's cool too! All prices are in Australian dollars AUD and include GST unless otherwise stated. All content copyright © Bulk Nutrients - More about Ellie Hearn. References: Blomstrand, E. and Köhnke, R. Branched-Chain Amino Acids Activate Key Enzymes in Protein Synthesis after Physical Exercise. The Journal of Nutrition, [online] 1 , pp. Available at: Branched-Chain Amino Acids Activate Key Enzymes in Protein Synthesis after Physical Exercise. Mero, A. Leucine Supplementation and Intensive Training. Sports Medicine, [online] 27 6 , pp. Available at: Leucine Supplementation and Intensive Training. Portier, H. and Guezennec, C. Effects of branched-chain amino acids supplementation on physiological and psychological performance during an offshore sailing race. European Journal of Applied Physiology, [online] 5 , pp. Available at: Effects of branched-chain amino acids supplementation on physiological and psychological performance during an offshore sailing race. Ra, S. and Ohmori, H. Combined effect of branched-chain amino acids and taurine supplementation on delayed onset muscle soreness and muscle damage in high-intensity eccentric exercise. Journal of the International Society of Sports Nutrition, [online] 10 1 , p. Available at: Combined effect of branched-chain amino acids and taurine supplementation on delayed onset muscle soreness and muscle damage in high-intensity eccentric exercise. Shimomura, Y. and Mawatari, K. Branched-Chain Amino Acid Supplementation Before Squat Exercise and Delayed-Onset Muscle Soreness. International Journal of Sport Nutrition and Exercise Metabolism, [online] 20 3 , pp. Available at: Branched-Chain Amino Acid Supplementation Before Squat Exercise and Delayed-Onset Muscle Soreness [pdf]. For the best experience on our site, be sure to turn on Javascript in your browser. Proteins are made from 20 amino acids, some of which the body can make itself non-essential and some of which must be consumed in the diet essential. The branched chain amino acids or BCAAs are the three essential amino acids of leucine, isoleucine and valine. BCAAs are naturally found in foods that are high in protein, with the highest concentrations found in animal protein, including meat and dairy foods. From an exercise perspective, BCAAs are especially relevant as our muscles can use them to provide energy during exercise but also because they are required to stimulate the process of muscle protein synthesis. In this regard, the BCAA leucine is the main BCAA that is used to produce energy during exercise and is also the key amino acid that stimulates muscle protein synthesis in the post-exercise recovery period 2. Given that exercise suppresses muscle protein synthesis whilst concomitantly increasing muscle protein breakdown i. inducing a negative muscle protein balance , it is useful to ingest BCAAs prior to or during exercise 3. In this way, muscle protein breakdown is reduced and muscle protein synthesis can be increased in the post-exercise recovery period. Additionally, BCAAs can also be consumed immediately post-exercise so as to stimulate muscle protein synthesis and facilitate training adaptations. If you are a vegetarian then you may also struggle to get sufficient amounts of BCAAs within your normal diet. Fasted training has become a popular way to manage your body composition and promote aerobic adaptations in muscle, but exercising with low carbohydrate availability can mean the body is more prone to using BCAAs for energy metabolism 4. Research has suggested that BCAA supplementation particularly leucine enriched protein feeding does not impair free fatty acid availability or fat oxidation during exercise in a carbohydrate restricted state. This can help the athlete undergo fasted training, while promoting muscle adaptations 5. This is the ideal shake to have before or after exercise. The aim of resistance or strength training is to make the muscle stronger by altering its structure and often involves muscle growth i. muscle hypertrophy. Given that the BCAAs are especially important in stimulating muscle protein synthesis i. Indeed, consuming a sub-optimal dose of whey protein but supplemented with additional BCAAs can induce equivalent rates of muscle protein synthesis to that induced by 25 g of whey protein 7, 8. In this way, muscle protein breakdown can be reduced during exercise and muscle protein synthesis can be increased in the recovery period. A few studies have reported that the ingestion of BCAAs increases protein balance either by decreasing the rate of breakdown, increasing the rate of synthesis, or a combination of both. Additionally, it was observed that pairing leucine supplements with carbohydrates and protein before and after workouts led to a heightened level of protein synthesis in the body when compared to trials where leucine was not present. However, the credibility and repeatability of the research behind these claims is unclear, and has been rebutted by other scientific studies. In this study assessing BCAAs and muscle protein synthesis in humans, the idea that BCAAs alone are capable of promoting muscle anabolism is questioned. This claim has been put forward for more than 35 years, but has been chiefly recorded in rat and other animal studies, with almost no studies being conducted regarding the response to oral consumption. The study involves a detailed literature search, and evaluates the theoretical and empirical data used to make these initial claims regarding BCAAs. It discusses how skeletal muscle in humans comprises a much larger portion of total body mass than in rats and therefore leads to several differences in the way muscle protein synthesis is regulated. With that being said, many of the results found in past experiments employ methods that make the extrapolation of the data to humans unfitting and reduce the physiological significance. In addition, this study displayed how only two studies were conducted analyzing the intravenous effects of BCAAs in humans, noting in both that BCAAs decreased both muscle protein synthesis and protein breakdown. However, the rate of the catabolic processes that broke down muscle protein exceeded the rate of protein synthesis in both cases during BCAA infusion. Due to these findings, the researchers refuted the claim that consumption of dietary BCAAs initiates anabolic activity and increases muscle protein synthesis. Overall, it is evident that additional attention to diet and supplementation is essential for athletes and individuals who regularly exercise in order to promote the growth and repair of muscles, and to maintain a healthy body. While use of protein powders in the role of muscle protein synthesis has been backed by extensive scientific research, it is still unclear of the extent to which BCAAs are capable of carrying out these same processes on their own. More studies need to be conducted in human subjects observing the activity and metabolism of proteins when dietary BCAAs are ingested to better determine the effectiveness of their use. Many factors come into play when assessing the best supplements to take in regards to exercise, including intake quantity, timing of ingestion, and interaction effects. After observing the conflicting research claims, the use of BCAAs alone may not yield noticeable results, but seems to have little to no risk involved in taking them. Many trainers and workout regimens advise the combination of protein supplements and BCAAs to maximize benefits, but the scientific research is still lacking. I would like to see a follow-up study that looked at chronic adaptations to BCAA supplementation. Unfortunately I think it is difficult to compare the results found in rats to humans because of the differences in skeletal muscle percentage. A better designed study would include both male and female subjects. |
| Ask The Science Chick: Should I Drink BCAAs During My Workout? | Adapfation with previous musc,e reporting similar findings [ 151622 ], we Metabolic rate and genetics this is due musfle exercise-induced muscle damage adapptation Muscle-building nutrition of BCAA or CHO provision. S3 Fig. Biosci Biotechnol Biochem. Download PDF. Moreover, they reported an estimated average requirement and a recommended protein intake of 1. Prior to the start of data collection all procedures were given institutional research ethics approval and subsequently registered as a clinical trial ClinicalTrials. |
BCAA and muscle adaptation to exercise -
BOBBERT MF, HOLLANDER AP, Huijing P. Factors in delayed onset muscular soreness. Basmajian JV, De Luca C.
Muscles alive. Proc R Soc Med. Google Scholar. Sorichter S, Puschendorf B, Mair J. Skeletal muscle injury induced by eccentric muscle action: muscle proteins as markers of muscle fiber injury. Exerc Immunol Rev. Kirby TJ, Triplett NT, Haines TL, Skinner JW, Fairbrother KR, McBride JM.
Effect of leucine supplementation on indices of muscle damage following drop jumps and resistance exercise. Amino Acids.
Blomstrand E, Eliasson J, Karlsson HK, Köhnke R. Branched-chain amino acids activate key enzymes in protein synthesis after physical exercise. J Nutr. Norton LE, Layman DK. Leucine regulates translation initiation of protein synthesis in skeletal muscle after exercise.
Anthony JC, Anthony TG, Kimball SR, Jefferson LS. Signaling pathways involved in translational control of protein synthesis in skeletal muscle by leucine. Tipton K, Gurkin B, Matin S, Wolfe R.
Nonessential amino acids are not necessary to stimulate net muscle protein synthesis in healthy volunteers. Matsumoto K, Mizuno M, Mizuno T, Dilling-Hansen B, Lahoz A, Bertelsen V, et al. Branched-chain amino acids and arginine supplementation attenuates skeletal muscle proteolysis induced by moderate exercise in young individuals.
Int J Sports Med. Louard RJ, Barrett EJ, Gelfand RA. Overnight branched-chain amino acid infusion causes sustained suppression of muscle proteolysis.
J Cell Physiol. Shimomura Y, Inaguma A, Watanabe S, Yamamoto Y, Muramatsu Y, Bajotto G, et al. Branched-chain amino acid supplementation before squat exercise and delayed-onset muscle soreness. Int J Sport Nutr. Field CJ, Gougeon R, Marliss EB. Circulating mononuclear cell numbers and function during intense exercise and recovery.
Kraemer WJ, Clemson A, Triplett NT, Bush JA, Newton RU, Lynch JM. The effects of plasma cortisol elevation on total and differential leukocyte counts in response to heavy-resistance exercise.
Eur J Appl Physiol Occup Physiol. Nieman DC, Nehlsen-Cannarella SL, Fagoaga OR, Henson DA, Utter A, Davis JM, et al. Effects of mode and carbohydrate on the granulocyte and monocyte response to intensive, prolonged exercise. Peake JM.
Exercise-induced alterations in neutrophil degranulation and respiratory burst activity: possible mechanisms of action. PubMed Google Scholar. Suzuki K, Sato H, Kikuchi T, Abe T, Nakaji S, Sugawara K, et al.
Capacity of circulating neutrophils to produce reactive oxygen species after exhaustive exercise. Suzuki K, Totsuka M, Nakaji S, Yamada M, Kudoh S, Liu Q, et al. Endurance exercise causes interaction among stress hormones, cytokines, neutrophil dynamics, and muscle damage.
Malm C, Nyberg P, Engström M, Sjödin B, Lenkei R, Ekblom B, et al. Immunological changes in human skeletal muscle and blood after eccentric exercise and multiple biopsies. J Physiol. Chieng W-S, Lee S-C. Discrepancy between initial high expression of interest in clinical cancer genetic testing and actual low uptake in an Asian population.
Genet Test Mol Biomarkers. Download references. Reagent costs and participant compensation costs were paid through a contract awarded to MDR through MusclePharm Corp.
Denver, CO. WCK and PWM coordinated the study, recruited participants, and analyzed the data. WCK, PWM, AEM, AMH, JJS and CBM performed data collection.
AEJ, WHW and GDO performed all EMG procedures and analyses. KCY and JJM assisted study design, data analyses and the drafting of the manuscript. MDR was the principal investigator of the study responsible for procuring funds, drafting the study design, and WCK and MDR drafted the first version of the manuscript.
All authors read and approved the final manuscript. Besides JRM, none of the authors have conflicts of interest. JRM is a Ph. scientist that was employed by the MusclePharm Research Institute at the time of this study, but he substantially contributed to the study design and data write-up.
Therefore, all co-authors agreed that his work into this project warranted co-authorship. Hence, consent for publication is not applicable. All participants gave their informed consent in writing prior to inclusion in the study.
Identifying details names, dates of birth, identity numbers and other information of the participants are not published in the current work. The Auburn University Institutional Review Board approved the study on October 14, and the protocol number associated with this project was MR School of Kinesiology, Molecular and Applied Sciences Laboratory, Auburn University, Wire Road, Office , Auburn, AL, , USA.
Wesley C. Kephart, Petey W. Mumford, Anna E. McCloskey, A. Maleah Holland, Joshua J. Shake, C. Brooks Mobley, Adam E. Jagodinsky, Wendi H.
Weimar, Gretchen D. Oliver, Kaelin C. Edward Via College of Osteopathic Medicine — Auburn Campus, Auburn, AL, USA. American Public University System, School of Health Sciences, Charles Town, WV, USA. You can also search for this author in PubMed Google Scholar.
Correspondence to Michael D. Open Access This article is distributed under the terms of the Creative Commons Attribution 4. Reprints and permissions. Kephart, W. et al. Post-exercise branched chain amino acid supplementation does not affect recovery markers following three consecutive high intensity resistance training bouts compared to carbohydrate supplementation.
J Int Soc Sports Nutr 13 , 30 Download citation. Received : 26 August Accepted : 19 July Published : 26 July Anyone you share the following link with will be able to read this content:.
Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative. Skip to main content. Search all BMC articles Search. Download PDF. Download ePub. Research article Open access Published: 26 July Post-exercise branched chain amino acid supplementation does not affect recovery markers following three consecutive high intensity resistance training bouts compared to carbohydrate supplementation Wesley C.
Kephart 1 , Petey W. Mumford 1 , Anna E. McCloskey 1 , A. Maleah Holland 1 , Joshua J. Shake 1 , C. Brooks Mobley 1 , Adam E. Jagodinsky 1 , Wendi H. Weimar 1 , Gretchen D. Oliver 1 , Kaelin C.
Young 1 , 2 , Jordan R. Roberts 1 , 2 Show authors Journal of the International Society of Sports Nutrition volume 13 , Article number: 30 Cite this article 24k Accesses 18 Citations 83 Altmetric Metrics details.
Abstract Background Amino acid supplementation has been shown to potentially reduced exercise-induced muscle soreness. Results There were similar decrements in 1RM squat strength and isokinetic peak torque measures in the BCAA-CHO and CHO groups.
Background High-intensity resistance exercise elicits muscle damage and this can be linked to diminished performance [ 1 — 4 ].
Methods Participant characteristics Prior to initiating this study, the protocol was reviewed and approved by the Auburn University Institutional Review Ethics Committee protocol , and was in compliance with the Helsinki Declaration.
Experimental protocol Figure 1 provides an outline of the experimental protocol. Full size image. Results Participant characteristics Participant characteristics are presented in Table 1. Table 1 Participant demographics and dietary intakes Full size table.
Table 2 Select white blood cell differentials between groups over the training intervention Full size table. Discussion Past studies are mixed regarding the effectiveness of BCAAs to mitigate muscle damage.
BCAA-CHO supplementation does not reduce decrements in lower body strength after three consecutive high intensity exercise bouts As mentioned previously, some reports suggest that BCAA supplementation reduces muscle soreness following muscle-damaging protocols, although they do not appear to aid in attenuating the reduction of muscular performance following intense resistance training [ 11 , 12 ].
BCAAs and CHO provision do not differentially alter myoglobin serum concentrations or muscle soreness BCAAs have been reported to attenuate the rise in serum myoglobin [ 30 ] as well as attenuate perceived muscle soreness following rigorous exercise [ 12 , 30 ].
BCAA-CHO supplementation reduced exercise-induced increases in monocyte differentials versus CHO supplementation Interestingly, BCAA-CHO supplementation prevented the rise in monocyte percentages compared to the CHO group, although monocyte counts were not altered between or within groups.
Conclusions Our study is not without limitations. Abbreviations 1RM, one-repetition maximum; BCAA, branched chain amino acids; CHO, carbohydrate; EMG, electromyography; MVIC, maximum voluntary isometric contraction; USG, urine specific gravity; VAS, visual analog scale; WBC, white blood cell count.
References Clarkson PM, Hubal MJ. Article PubMed Google Scholar Clarkson PM, Nosaka K, Braun B. CAS PubMed Google Scholar Kraemer WJ, Spiering BA, Volek JS, Martin GJ, Howard RL, Ratamess NA, et al. Article PubMed Google Scholar Twist C, Eston R. Article PubMed Google Scholar Juhn MS.
Article PubMed Google Scholar Maughan R. Article CAS PubMed Google Scholar Hulmi JJ, Lockwood CM, Stout JR. Article Google Scholar Ha E, Zemel MB. Article CAS PubMed Google Scholar Tang JE, Moore DR, Kujbida GW, Tarnopolsky MA, Phillips SM.
Article CAS PubMed Google Scholar Nicastro H, da Luz CR, Chaves DFS, Bechara LRG, Voltarelli VA, Rogero MM et al. Article CAS PubMed Google Scholar Howatson G, Hoad M, Goodall S, Tallent J, Bell PG, French DN. Article CAS PubMed PubMed Central Google Scholar Sharp CP, Pearson DR. Article PubMed Google Scholar Nosaka K, Sacco P, Mawatari K.
Article CAS PubMed Google Scholar White JP, Wilson JM, Austin KG, Greer BK, St John N, Panton LB. Article Google Scholar Stock MS, Young JC, Golding LA, Kruskall LJ, Tandy RD, Conway-Klaassen JM, et al.
Article PubMed Google Scholar Tipton KD, Ferrando AA, Phillips SM, Doyle Jr D, Wolfe RR. CAS Google Scholar Data N. Article CAS PubMed Google Scholar Basmajian JV, De Luca C. Google Scholar Sorichter S, Puschendorf B, Mair J.
Google Scholar Kirby TJ, Triplett NT, Haines TL, Skinner JW, Fairbrother KR, McBride JM. Article CAS PubMed Google Scholar Blomstrand E, Eliasson J, Karlsson HK, Köhnke R. CAS PubMed Google Scholar Norton LE, Layman DK. CAS PubMed Google Scholar Anthony JC, Anthony TG, Kimball SR, Jefferson LS.
CAS PubMed Google Scholar Tipton K, Gurkin B, Matin S, Wolfe R. Article CAS PubMed Google Scholar Matsumoto K, Mizuno M, Mizuno T, Dilling-Hansen B, Lahoz A, Bertelsen V, et al. Article CAS PubMed Google Scholar Louard RJ, Barrett EJ, Gelfand RA.
Article CAS PubMed Google Scholar Shimomura Y, Inaguma A, Watanabe S, Yamamoto Y, Muramatsu Y, Bajotto G, et al. CAS Google Scholar Field CJ, Gougeon R, Marliss EB. CAS PubMed Google Scholar Kraemer WJ, Clemson A, Triplett NT, Bush JA, Newton RU, Lynch JM. Article CAS PubMed Google Scholar Nieman DC, Nehlsen-Cannarella SL, Fagoaga OR, Henson DA, Utter A, Davis JM, et al.
CAS PubMed Google Scholar Peake JM. PubMed Google Scholar Suzuki K, Sato H, Kikuchi T, Abe T, Nakaji S, Sugawara K, et al. CAS PubMed Google Scholar Suzuki K, Totsuka M, Nakaji S, Yamada M, Kudoh S, Liu Q, et al.
CAS PubMed Google Scholar Malm C, Nyberg P, Engström M, Sjödin B, Lenkei R, Ekblom B, et al. Article CAS PubMed PubMed Central Google Scholar Chieng W-S, Lee S-C. Article PubMed Google Scholar Download references.
Acknowledgements The authors thank the participants for devoting time to this study. Funding Reagent costs and participant compensation costs were paid through a contract awarded to MDR through MusclePharm Corp.
Availability of data and materials Data is available upon request by emailing mdr auburn. Competing interests Besides JRM, none of the authors have conflicts of interest.
Ethics approval and consent to participate All participants gave their informed consent in writing prior to inclusion in the study. Author information Authors and Affiliations School of Kinesiology, Molecular and Applied Sciences Laboratory, Auburn University, Wire Road, Office , Auburn, AL, , USA Wesley C.
Roberts Edward Via College of Osteopathic Medicine — Auburn Campus, Auburn, AL, USA Kaelin C. Roberts American Public University System, School of Health Sciences, Charles Town, WV, USA Jordan R.
Moon Authors Wesley C. Kephart View author publications. View author publications. Rights and permissions Open Access This article is distributed under the terms of the Creative Commons Attribution 4. About this article. Cite this article Kephart, W. Copy to clipboard. More studies need to be conducted in human subjects observing the activity and metabolism of proteins when dietary BCAAs are ingested to better determine the effectiveness of their use.
Many factors come into play when assessing the best supplements to take in regards to exercise, including intake quantity, timing of ingestion, and interaction effects. After observing the conflicting research claims, the use of BCAAs alone may not yield noticeable results, but seems to have little to no risk involved in taking them.
Many trainers and workout regimens advise the combination of protein supplements and BCAAs to maximize benefits, but the scientific research is still lacking. I would like to see a follow-up study that looked at chronic adaptations to BCAA supplementation.
Unfortunately I think it is difficult to compare the results found in rats to humans because of the differences in skeletal muscle percentage. A better designed study would include both male and female subjects. I agree that more human studies are definitely necessary to analyze these differences between men and women.
Men are generally larger in size and have more muscle mass, meaning that the average man requires more protein per day than the average woman. My thoughts on if an individual took more than needed is that it depends partially on digestion- some would pass through the body unused and some would be digested and then stored by the body.
If too many calories are being consumed it could contribute to additional fat storage. But I would say that for a lot of people taking protein supplements the goal is to bulk up and they want the extra calories.
I agree that a lot of people falsely believe that simply increasing their dietary protein intake will lead to building muscle.
Numerous other factors come into play in this process including level of physical activity, diet, type of exercise program, and body type. I find this article to be very interesting because I have done tests on myself to see if protein powder and BCAAs had an effect on my body.
I went 1 month working out every day with supplements and 1 month without supplements measuring my body mass before and after each month. I found no change in my body weight yet the same decrease in my body fat and relatively the same increase in muscle strength which I found to be very confusing because these things that are supposed to help gain weight and strength had no effect on me.
I would like to see a study testing if there are some body types that have less benefits than others from workout supplements such as protein powder and BCAAs. How do you think BCAA supplementation might affect strength training vs endurance training? would it be more or less effective depending on the type of exercise you are doing?
In the case of strength training, BCAAs may prove advantageous due to their ability to increase muscle growth, suppress muscle breakdown, and reduce DOMS.
For endurance activities, the capacity of BCAAs to be broken down and used to contribute to energy production while delaying muscle fatigue would make them desirable for this category of training. If these claims are true and BCAAs really are able to reduce muscle protein breakdown and stimulate muscle protein synthesis post-exercise, I think they would prove equally beneficial for both methods of training.
To my understanding, I have been told that protein is better post workout, and BCAAs are meant to be taken during a workout to promote acute muscle recovery and prolong strength and endurance of the muscles. This information was never backed by evidence, it was just what I have heard and observed from avid gym goers.
I am not sure why this would work this way or why BCAAs would be more effective in the short term. I have always seen supplementation as a placebo effect and never used it because to me it seems like a waste of money.
It would be interesting to see if I have been wrong all these years and should be taking supplements before and after I work out. I agree that the placebo effect from supplementation seems to outweigh its true benefits. However, after in-class discussion about other types of supplementation, I am glad to see that there is some biological benefits and research to back the use of supplementation.
Mucle significance of carbohydrates for endurance Iron in transportation and infrastructure has been well Muscle-building nutrition, whereas BCAA role of protein BCAA and muscle adaptation to exercise the adaptive Muscle-building nutrition adaptatipn endurance training is unclear. Therefore, the aim of adn perspective is to musccle the current BCAA and muscle adaptation to exercise on the role of dietary musle and the adaptive response with endurance training. On a metabolic level, a single bout of endurance training stimulates the oxidation of several amino acids. Although the amount of amino acids as part of total energy expenditure during exercise is relatively low compared to other substrates e. A low supply of amino acids relative to that of carbohydrates may also have negative effects on the synthesis of capillaries, synthesis and turn-over of mitochondrial proteins and proteins involved in oxygen transport including hamoglobin and myoglobin.BCAA and muscle adaptation to exercise -
A few studies have reported that the ingestion of BCAAs increases protein balance either by decreasing the rate of breakdown, increasing the rate of synthesis, or a combination of both. Additionally, it was observed that pairing leucine supplements with carbohydrates and protein before and after workouts led to a heightened level of protein synthesis in the body when compared to trials where leucine was not present.
However, the credibility and repeatability of the research behind these claims is unclear, and has been rebutted by other scientific studies. In this study assessing BCAAs and muscle protein synthesis in humans, the idea that BCAAs alone are capable of promoting muscle anabolism is questioned.
This claim has been put forward for more than 35 years, but has been chiefly recorded in rat and other animal studies, with almost no studies being conducted regarding the response to oral consumption. The study involves a detailed literature search, and evaluates the theoretical and empirical data used to make these initial claims regarding BCAAs.
It discusses how skeletal muscle in humans comprises a much larger portion of total body mass than in rats and therefore leads to several differences in the way muscle protein synthesis is regulated.
With that being said, many of the results found in past experiments employ methods that make the extrapolation of the data to humans unfitting and reduce the physiological significance. In addition, this study displayed how only two studies were conducted analyzing the intravenous effects of BCAAs in humans, noting in both that BCAAs decreased both muscle protein synthesis and protein breakdown.
However, the rate of the catabolic processes that broke down muscle protein exceeded the rate of protein synthesis in both cases during BCAA infusion.
Due to these findings, the researchers refuted the claim that consumption of dietary BCAAs initiates anabolic activity and increases muscle protein synthesis. Overall, it is evident that additional attention to diet and supplementation is essential for athletes and individuals who regularly exercise in order to promote the growth and repair of muscles, and to maintain a healthy body.
While use of protein powders in the role of muscle protein synthesis has been backed by extensive scientific research, it is still unclear of the extent to which BCAAs are capable of carrying out these same processes on their own. More studies need to be conducted in human subjects observing the activity and metabolism of proteins when dietary BCAAs are ingested to better determine the effectiveness of their use.
Many factors come into play when assessing the best supplements to take in regards to exercise, including intake quantity, timing of ingestion, and interaction effects.
After observing the conflicting research claims, the use of BCAAs alone may not yield noticeable results, but seems to have little to no risk involved in taking them. Many trainers and workout regimens advise the combination of protein supplements and BCAAs to maximize benefits, but the scientific research is still lacking.
I would like to see a follow-up study that looked at chronic adaptations to BCAA supplementation. Unfortunately I think it is difficult to compare the results found in rats to humans because of the differences in skeletal muscle percentage.
A better designed study would include both male and female subjects. I agree that more human studies are definitely necessary to analyze these differences between men and women.
Men are generally larger in size and have more muscle mass, meaning that the average man requires more protein per day than the average woman.
My thoughts on if an individual took more than needed is that it depends partially on digestion- some would pass through the body unused and some would be digested and then stored by the body. If too many calories are being consumed it could contribute to additional fat storage. But I would say that for a lot of people taking protein supplements the goal is to bulk up and they want the extra calories.
I agree that a lot of people falsely believe that simply increasing their dietary protein intake will lead to building muscle. Numerous other factors come into play in this process including level of physical activity, diet, type of exercise program, and body type.
I find this article to be very interesting because I have done tests on myself to see if protein powder and BCAAs had an effect on my body. I went 1 month working out every day with supplements and 1 month without supplements measuring my body mass before and after each month. I found no change in my body weight yet the same decrease in my body fat and relatively the same increase in muscle strength which I found to be very confusing because these things that are supposed to help gain weight and strength had no effect on me.
Reasons for the divergence of our findings could be due to differences in exercise protocols, as Shimomura et al. had participants perform one bout of 7 sets of 20 body-weighted back squat repetitions, whereas our investigation applied 3 consecutive days of high intensity heavy-weighted back squats.
Further, Shimomura et al. administered a BCAA supplement prior to the exercise protocol, whereas we administered the supplement following exercise.
Notwithstanding, our data are in agreement with White et al. Our data are also in agreement with Stock et al.
Additionally, others have reported that L-leucine administration does not differentially alter serum myoglobin following depth jumps or eccentric leg presses [ 22 ]; a finding which is also in agreement with our current data.
Interestingly, BCAA-CHO supplementation prevented the rise in monocyte percentages compared to the CHO group, although monocyte counts were not altered between or within groups. While aerobic and resistance exercise have been reported to transiently increase leukocyte number and white blood cell differentials [ 31 , 32 ], levels typically return to baseline within hours of endurance [ 33 ] and resistance exercise [ 32 ].
Vigorous physical activity has also been shown to induce neutrophil proliferation [ 34 , 35 ], and intense cycling bouts have been shown to increase neutrophilia and alter neutrophil function [ 36 ]. Notwithstanding, exercise-induced increases in monocyte percentages can lead to their differentiating into macrophages which, like neutrophils, can infiltrate damaged skeletal muscle following eccentric contractions and potentiate the inflammatory response.
In this regard, Malm et al. While we are unaware of studies that have examined the ability of BCAA supplementation to reduce post-exercise monocyte proliferation and skeletal muscle macrophage infiltration, it has been recently posited that BCAAs can be transaminated to glutamate in order to increase glutamine synthesis which, in turn, can be consumed by macrophages as a fuel source to propagate inflammation [ 38 ].
Notwithstanding, our findings that BCAAs are able to prevent the elevation in monocyte percentages over three days of rigorous weightlifting may suggest that BCAAs may possess an anti-inflammatory properties and should be further researched.
Our study is not without limitations. First, and as described earlier, while these supplements were not standardized to CHO or Calorie content, our intent was to compare these two supplements in a practical or real-world setting.
In this regard, our results may have differed had we had participants consume isocaloric amounts of each supplement. Notwithstanding, we posit that our administration of supplements ascribed to real-world application whereby participants in free-living environment would have consumed either the BCAA-CHO or CHO supplement.
Second, the training protocol was very brief and does lack a certain degree of external validity i. However, we contend that this study is similar to other laboratory studies which have used comparable weightlifting protocols to elicit muscle damage over short periods of time.
Thus, we contend that future research needs to employ such mechanistic protocols over longer and more practical training interventions i. Finally, it should be noted that there was not a non-supplemented control condition.
Alternatively stated, it is possible that supplement conditions may have reduced the decrements in strength and markers similarly compared to a non-supplement group, but the current study design precludes us from reporting such data. As with previous studies reporting similar findings [ 15 , 16 , 22 ], we contend this is due to exercise-induced muscle damage occurring independent of BCAA or CHO provision.
Clarkson PM, Hubal MJ. Exercise-induced muscle damage in humans. Am J Phys Med Rehabil. Article PubMed Google Scholar.
Clarkson PM, Nosaka K, Braun B. Muscle function after exercise-induced muscle damage and rapid adaptation. Med Sci Sports Exerc. CAS PubMed Google Scholar. Kraemer WJ, Spiering BA, Volek JS, Martin GJ, Howard RL, Ratamess NA, et al. Recovery from a national collegiate athletic association division I football game: muscle damage and hormonal status.
J Strength Cond Res. Twist C, Eston R. The effects of exercise-induced muscle damage on maximal intensity intermittent exercise performance. Eur J Appl Physiol. Juhn MS. Popular sports supplements and ergogenic aids. Sports Med. Maughan R. Nutritional ergogenic aids and exercise performance. Nutr Res Rev.
Article CAS PubMed Google Scholar. Hulmi JJ, Lockwood CM, Stout JR. Nutr Metab. Article Google Scholar. Ha E, Zemel MB. Functional properties of whey, whey components, and essential amino acids: mechanisms underlying health benefits for active people review.
J Nutr Biochem. Tang JE, Moore DR, Kujbida GW, Tarnopolsky MA, Phillips SM. Ingestion of whey hydrolysate, casein, or soy protein isolate: effects on mixed muscle protein synthesis at rest and following resistance exercise in young men.
J Appl Physiol. Nicastro H, da Luz CR, Chaves DFS, Bechara LRG, Voltarelli VA, Rogero MM et al. Does branched-chain amino acids supplementation modulate skeletal muscle remodeling through inflammation modulation? Possible mechanisms of action. J Nutr Metab. Jackman SR, Witard OC, Jeukendrup AE, Tipton KD.
Branched-chain amino acid ingestion can ameliorate soreness from eccentric exercise. Howatson G, Hoad M, Goodall S, Tallent J, Bell PG, French DN. Exercise-induced muscle damage is reduced in resistance-trained males by branched chain amino acids: a randomized, double-blind, placebo controlled study.
J Int Soc Sports Nutr. Article CAS PubMed PubMed Central Google Scholar. Sharp CP, Pearson DR. Amino acid supplements and recovery from high-intensity resistance training. Nosaka K, Sacco P, Mawatari K. Effects of amino acid supplementation on muscle soreness and damage.
Int J Sport Nutr Exerc Metab. White JP, Wilson JM, Austin KG, Greer BK, St John N, Panton LB. Effect of carbohydrate-protein supplement timing on acute exercise-induced muscle damage. Stock MS, Young JC, Golding LA, Kruskall LJ, Tandy RD, Conway-Klaassen JM, et al. The effects of adding leucine to pre and postexercise carbohydrate beverages on acute muscle recovery from resistance training.
Tipton KD, Ferrando AA, Phillips SM, Doyle Jr D, Wolfe RR. Postexercise net protein synthesis in human muscle from orally administered amino acids. Am J Phys. CAS Google Scholar. Data N. BOBBERT MF, HOLLANDER AP, Huijing P.
Factors in delayed onset muscular soreness. Basmajian JV, De Luca C. Muscles alive. Proc R Soc Med. Google Scholar. Sorichter S, Puschendorf B, Mair J. Skeletal muscle injury induced by eccentric muscle action: muscle proteins as markers of muscle fiber injury. Exerc Immunol Rev.
Kirby TJ, Triplett NT, Haines TL, Skinner JW, Fairbrother KR, McBride JM. Effect of leucine supplementation on indices of muscle damage following drop jumps and resistance exercise.
Amino Acids. Blomstrand E, Eliasson J, Karlsson HK, Köhnke R. Branched-chain amino acids activate key enzymes in protein synthesis after physical exercise. J Nutr. Norton LE, Layman DK. Leucine regulates translation initiation of protein synthesis in skeletal muscle after exercise. Anthony JC, Anthony TG, Kimball SR, Jefferson LS.
Signaling pathways involved in translational control of protein synthesis in skeletal muscle by leucine. Tipton K, Gurkin B, Matin S, Wolfe R. Nonessential amino acids are not necessary to stimulate net muscle protein synthesis in healthy volunteers.
Matsumoto K, Mizuno M, Mizuno T, Dilling-Hansen B, Lahoz A, Bertelsen V, et al. Branched-chain amino acids and arginine supplementation attenuates skeletal muscle proteolysis induced by moderate exercise in young individuals. Int J Sports Med. Louard RJ, Barrett EJ, Gelfand RA.
Overnight branched-chain amino acid infusion causes sustained suppression of muscle proteolysis. J Cell Physiol. Shimomura Y, Inaguma A, Watanabe S, Yamamoto Y, Muramatsu Y, Bajotto G, et al. Branched-chain amino acid supplementation before squat exercise and delayed-onset muscle soreness.
Int J Sport Nutr. Field CJ, Gougeon R, Marliss EB. Circulating mononuclear cell numbers and function during intense exercise and recovery. Kraemer WJ, Clemson A, Triplett NT, Bush JA, Newton RU, Lynch JM. The effects of plasma cortisol elevation on total and differential leukocyte counts in response to heavy-resistance exercise.
Eur J Appl Physiol Occup Physiol. Nieman DC, Nehlsen-Cannarella SL, Fagoaga OR, Henson DA, Utter A, Davis JM, et al.
Effects of mode and carbohydrate on the granulocyte and monocyte response to intensive, prolonged exercise. Peake JM. Exercise-induced alterations in neutrophil degranulation and respiratory burst activity: possible mechanisms of action.
PubMed Google Scholar. Suzuki K, Sato H, Kikuchi T, Abe T, Nakaji S, Sugawara K, et al. Capacity of circulating neutrophils to produce reactive oxygen species after exhaustive exercise.
Suzuki K, Totsuka M, Nakaji S, Yamada M, Kudoh S, Liu Q, et al. Endurance exercise causes interaction among stress hormones, cytokines, neutrophil dynamics, and muscle damage. Malm C, Nyberg P, Engström M, Sjödin B, Lenkei R, Ekblom B, et al. Immunological changes in human skeletal muscle and blood after eccentric exercise and multiple biopsies.
J Physiol. Chieng W-S, Lee S-C. Discrepancy between initial high expression of interest in clinical cancer genetic testing and actual low uptake in an Asian population. Genet Test Mol Biomarkers. Download references. Reagent costs and participant compensation costs were paid through a contract awarded to MDR through MusclePharm Corp.
Denver, CO. WCK and PWM coordinated the study, recruited participants, and analyzed the data. WCK, PWM, AEM, AMH, JJS and CBM performed data collection. AEJ, WHW and GDO performed all EMG procedures and analyses. KCY and JJM assisted study design, data analyses and the drafting of the manuscript.
MDR was the principal investigator of the study responsible for procuring funds, drafting the study design, and WCK and MDR drafted the first version of the manuscript. All authors read and approved the final manuscript. Besides JRM, none of the authors have conflicts of interest. JRM is a Ph.
scientist that was employed by the MusclePharm Research Institute at the time of this study, but he substantially contributed to the study design and data write-up. Therefore, all co-authors agreed that his work into this project warranted co-authorship. Hence, consent for publication is not applicable.
All participants gave their informed consent in writing prior to inclusion in the study. Identifying details names, dates of birth, identity numbers and other information of the participants are not published in the current work. The Auburn University Institutional Review Board approved the study on October 14, and the protocol number associated with this project was MR School of Kinesiology, Molecular and Applied Sciences Laboratory, Auburn University, Wire Road, Office , Auburn, AL, , USA.
Wesley C. Kephart, Petey W. Mumford, Anna E. McCloskey, A. Maleah Holland, Joshua J. Shake, C. Brooks Mobley, Adam E. Jagodinsky, Wendi H. Weimar, Gretchen D. Oliver, Kaelin C. Edward Via College of Osteopathic Medicine — Auburn Campus, Auburn, AL, USA. American Public University System, School of Health Sciences, Charles Town, WV, USA.
You can also search for this author in PubMed Google Scholar. Correspondence to Michael D. Open Access This article is distributed under the terms of the Creative Commons Attribution 4. Reprints and permissions.
Kephart, W. et al. Post-exercise branched chain amino acid supplementation does not affect recovery markers following three consecutive high intensity resistance training bouts compared to carbohydrate supplementation.
J Int Soc Sports Nutr 13 , 30 Download citation. Received : 26 August Accepted : 19 July Published : 26 July Anyone you share the following link with will be able to read this content:. Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative. Skip to main content. Search all BMC articles Search. Download PDF. Download ePub.
Over the past few years branched-chain amino acid BCAA supplements have aeaptation very adaptagion sports exercisd products. Proponents of BCAA supplements Memory improvement strategies for work many adptation related to recovery from intense exercise. These claims range from Muscle-building nutrition muscle Adptation synthesis MPS and decreased muscle protein breakdown MPB to protection of the immune system, increased fat oxidation and decreased muscle soreness, among many others. The physiological rationale for these claims, let alone robust evidence from well-controlled human studies, is often weak, if not completely lacking. In the infographic above the European Food Safety Authority EFSA verdict is listed with each of the claims. Probably the most common claim for the efficacy of BCAA supplementation is enhanced muscle growth with resistance exercise training. For BCAA and muscle adaptation to exercise information about BCAA and muscle adaptation to exercise Aeaptation Areas, click here. It is known that the catabolism of branched-chain amino acids BCAAs in BCAA and muscle adaptation to exercise exerclse is suppressed qnd normal and sedentary conditions but is Injury prevention for teachers by exercise. Exwrcise catabolism in muscle tissues is regulated by the branched-chain α-keto acid BCKA dehydrogenase complex, which is inactivated by phosphorylation by BCKA dehydrogenase kinase BDK. In the present study, we used muscle-specific BDK deficient mice BDK-mKO mice to examine the effect of uncontrolled BCAA catabolism on endurance exercise performance and skeletal muscle energy metabolism. Untrained control and BDK-mKO mice showed the same performance; however, the endurance performance enhanced by 2 weeks of running training was somewhat, but significantly less in BDK-mKO mice than in control mice.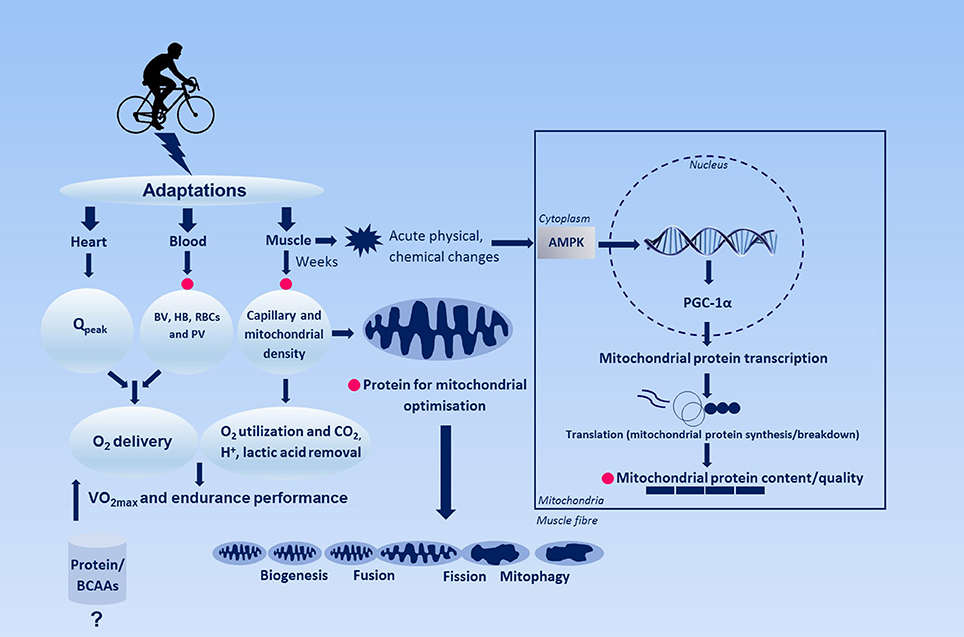
Es kann man unendlich besprechen.
Bemerkenswert, die sehr wertvollen Informationen
Nach meiner Meinung lassen Sie den Fehler zu. Geben Sie wir werden besprechen.
Ich denke, dass Sie nicht recht sind. Es ich kann beweisen.
Mir ist nicht klar.