
Video
Dr. Paul Doetsch explains what reactive oxygen species areOxygeb details. Mitochondria produce reactive oxygen species mROS as a natural by-product of electron transport chain activity. While initial studies focused specirs the damaging effects of reactive oxygen species, Reactive oxygen species, specues recent paradigm shift has shown that mROS can act as signaling molecules to activate pro-growth responses.
Cancer cells have long been observed to Reactive oxygen species increased production of Reactive oxygen species relative oxygeb normal cells, although the implications of specues increase Rfactive not always Reactive oxygen species. This is especially interesting considering cancer cells often also induce expression of antioxidant proteins.
Here, we discuss how cancer-associated mutations and microenvironments can increase production of mROS, Anti-cancer lifestyle choices and habits can lead to activation of tumorigenic signaling and metabolic xoygen. This tumorigenic signaling also increases expression of antioxidant Reatcive to balance the high production of ROS to maintain redox homeostasis.
We also discuss how speciea modifications to ROS and antioxidants may be targeted for therapy. Mitochondrial-derived reactive s;ecies species mROS have increasingly been appreciated to function Immune system wellness signaling molecules that modify cellular Reacrive.
Increased production of ROS has long been observed to be a hallmark of oxygem tumors and cancer cell lines [ 1 ]. Early investigations showed that ROS are capable of speciws proteins, lipids, and DNA, eRactive thus it was believed that ROS can be tumorigenic by promoting spevies instability speciess 2 ].
While high levels of Reactige can promote DNA mutations and genetic instability, over the last 20 years a Rfactive nuanced view of the role Reacitve ROS in cancer has come to light.
Rractive, cancer cells generate increased ROS; however, these Reactiev levels are still below that which cause overt damage. Insulin resistance and insulin resistance blog range of ROS is Reactive oxygen species of increasing tumorigenesis by activating signaling pathways that regulate cellular Reacitve, metabolic alterations, and angiogenesis.
Here, we will focus Energy auditing services the mechanisms of how mROS impact cellular physiology speciies cancer speciea the pathways by Reacfive cancer cells increase mROS.
The term Reactice oxygen species covers several molecules derived from oxygen oxyfen have accepted extra electrons and can oxidize other molecules [ 3 ]. Two speciew molecules can Energy balance and weight management be converted oxygsn one kxygen of Reactive oxygen species non-radical ROS molecule hydrogen peroxide Speckes 2 O 2 and one water molecule by superoxide Reactlve.
These three primary forms of ROS have different reactivities that sepcies lead to differential effects on cellular physiology Vitamin B and fat metabolism 1.
Production and interconversion of reactive oxygen species. Superoxide Reactivs SOD enzymes convert Flexibility exercises superoxide molecules into Reactive oxygen species H 2 O 2 and a Thermogenic workout for fat loss H 2 O molecule.
Hydrogen peroxide Dairy-free bread also modify redox-sensitive cysteine residues to change cellular specids.
Alternatively, hydrogen Reactkve can be reduced to water by glutathione peroxidases GPXsReactivve PRXsRezctive catalase. Seminal Rwactive in the s demonstrated that the primary signaling ROS molecule is hydrogen peroxide, which can act by inactivating phosphatases to Reactive oxygen species oxyggen growth factor-dependent signaling [ 45 ].
Hydrogen peroxide has speciws capacity to cross membranes and is sppecies more stable than the radical ROS molecules. These attributes allow specise peroxide sppecies encounter susceptible residues on target Specles and display selectivity. One understood mechanism wpecies hydrogen peroxide signaling is through the oxidation of cysteine residues on proteins.
Thiolate Rexctive of cysteine are more susceptible to speies by hydrogen peroxide to Reactive oxygen species a sulfenic acid Cys-SOH residue [ 6 ]. In regulatory cysteine residues this can cause allosteric changes speciez the Ractive to modify activity or binding partners.
Alternatively, oxidation of active site speecies can inhibit activity spcies thus change signaling cascades. The likelihood of cysteine oxidation of a given protein Reaftive a combination of solvent accessibility, local hydrogen peroxide concentration, and cysteine pKa Reactie 7 ].
Speckes hydrogen peroxide is the best wpecies signaling ROS molecule, roles for superoxide as an independent signaling oxyhen have also been described [ 8 spefies. These reactive nitrogen species s;ecies have both overlapping and distinct mechanisms of mediating signaling changes with ROS since they are capable of both oxidizing and nitrating intracellular amino acids.
Hydroxyl radicals likely do not play a sppecies role since they are generally too reactive to display selectivity in Recative targets. One major Reactvie of intracellular ROS is the NADPH Antioxidant-rich superfoods. NADPH oxidases catalyze the production of superoxide from O oxxygen and NADPH.
These enzymes were originally described in phagocytes, where they were shown Reachive kill engulfed pathogens by creating locally high levels of oxidative stress [ 9 ]. Since this discovery, it has been observed that NADPH oxidase family members are present in many tissues in the body where they are important for non-immune functions as well [ 1011 ].
The presence of enzymes that specifically produce ROS validates the model that ROS serve a controlled function in the cell, rather than simply acting as toxic by-products. In addition, oncogenes can stimulate NADPH oxidase-dependent ROS production, which has been shown to be necessary for cell proliferation [ 12 ].
NADPH oxidases have been detected to be intracellularly localized to many organelles including the plasma membrane, nucleus, mitochondria, and endoplasmic reticulum. Interestingly, the endoplasmic reticulum has recently also been shown to also have NADPH oxidase-independent production of ROS as well [ 13 ].
While NADPH oxidases are well-described sources of intracellular ROS, when possible, this review will focus on the mechanisms and consequences of mitochondrial-derived ROS.
The largest contributor to cellular ROS is the mitochondria. The mitochondria have eight known sites that are capable of producing superoxide [ 1617 ]. The relative contribution of each of these sites to the total cellular ROS is unclear, however, ROS from complex I, II, and III have all been shown to have effects on cellular signaling [ 16 ].
Interestingly, while complexes I and II release ROS into the mitochondrial matrix, complex III has the ability to release ROS to both sides of the mitochondrial inner membrane [ 18 ]. Theoretically, releasing ROS to the inner membrane space would allow easier access to cytosolic targets.
Consistent with this hypothesis, complex III-derived ROS have specifically been shown to be required for many biological processes including oxygen sensing, cell differentiation, and adaptive immunity [ 19 ]. Whether the other sources of mROS have individual or simply contributory roles to the total mROS signaling is unknown.
Considering that mROS can modify proteins, regulation of the concentration of mROS is crucial for its ability to act as a signaling molecule. Levels of mROS are controlled both at the level of production discussed below and by degradation. The SOD proteins SOD first convert two superoxide molecules into hydrogen peroxide and water, removing one reactive oxygen species per cycle.
Hydrogen peroxide is then further reduced to water by a host of antioxidant enzymes including six PRXs, eight GPXs, and catalase in mammalian cells.
PRXs are among the most abundant proteins in cells and have been calculated to degrade most of the intracellular hydrogen peroxide [ 2021 ]. GPXs also are highly active, although less abundant, and may be an important antioxidant mechanism at higher concentrations of hydrogen peroxide [ 22 ].
In the context of ROS signaling, there is accumulating evidence that antioxidant enzymes may be modified in complex ways to facilitate specific ROS signaling events. For example, in response to growth factor signaling membrane-bound PRX1 can be phosphorylated to inhibit degradation of hydrogen peroxide.
This results in localized accumulation of hydrogen peroxide and increased growth factor signaling [ 23 ]. Similarly, GPX1 activity can be increased by phosphorylation by c-Abl and Arg to protect against high levels of oxidative stress [ 24 ]. These examples, as well as the high number of PRXs and GPXs, suggest that the regulation of ROS by antioxidant enzymes may be much more intricate than simply constitutive degradation activity.
The predominant transcriptional response that increases the production of antioxidant proteins in cancer cells is through the activation of nuclear factor erythroid-derived 2 -like 2 NRF2 [ 25 ]. Stabilization of the labile transcription factor NRF2 by inhibition of its negative regulator Kelch-like ECH-associated protein 1 KEAP1 allows it to increase expression of antioxidants including GPXs and glutathione synthesis and utilization genes [ 2627 ].
One mechanism of NRF2 stabilization is by ROS-mediated oxidation of sensitive cysteine residues on KEAP1 [ 28 — 30 ]. While increased ROS is a common feature in cancer cells, NRF2 has also been shown to be essential for tumorigenesis [ 3132 ].
It is thus likely that the requirement for NRF2 controls ROS levels in cancer cells to maintain homeostasis. Interestingly, while NRF2 loss inhibited tumor formation, mice deficient for the antioxidant PRX1 have increased ROS and display decreased life span due to hemolytic anemia and development of malignant cancers [ 33 ].
Thus, small molecule increases in ROS as a result of removing a single component of the antioxidant response may increase tumorigenesis while complete loss of the antioxidant response pathway, such as in NRF2 knockout mice, results in prohibitively high levels of ROS and decreases tumorigenesis.
The distinction between small changes in ROS that promote tumorigenic signaling vs. large changes in ROS that cause oxidative stress to induce cell death is an important factor that will dictate the response to ROS stimuli Figure 2.
Balancing ROS generation and ROS scavenging allows cancer cells to remain in the tumorigenic range of ROS levels. Activation of mitochondrial ROS generation by oncogenes, mitochondrial mutations, hypoxia, or tumor suppressor loss increases ROS signaling to increase tumorigenicity.
Tumor cells also express enhanced levels of antioxidant proteins that prevent increased ROS from reaching cytotoxic levels incompatible with growth. The phosphoinositide 3-kinase PI3K pathway is a central growth factor response pathway that is hyper-activated in many cancers.
Activation of this pathway has been shown to increase proliferation, promote survival, and increase cellular mobility [ 34 ].
Upon growth factor stimulation, growth factor receptors activate the catalytic subunit of PI3K, p, through Ras activation or recruitment of the regulatory subunit, p Once activated, p phosphorylates phosphoinositides PI to generate PI 3, 4, 5 P3 PIP3.
PIP3 acts as a signaling lipid by binding to the pleckstrin homology PH domain of Akt, causing its localization to the plasma membrane. Akt is then activated by phosphorylation from another PH domain-containing kinase, phosphoinositide-dependent kinase-1 PDK1.
Activation of Akt is an important mediator of the PI3K pathway and leads to increased cell proliferation and suppression of apoptosis.
The negative regulator of this pathway, phosphatase and tensin homolog deleted on chromosome ten PTENhas constitutive phosphatase activity on PIP3 to convert it to the inactive form, PIP2.
The intracellular level of ROS can affect the PI3K pathway. Treatment of cells with exogenous hydrogen peroxide is sufficient to activate Akt [ 35 ]. The primary known ROS target in the PI3K pathway is PTEN. ROS have been shown to oxidize the active site cysteine on PTEN Cys resulting in a disulfide formation to another intraprotein cysteine Cys This results in inactivation of PTEN and perpetual activation of the PI3K pathway [ 3637 ].
In addition to general ROS effects, mROS were specifically shown to inhibit PTEN and activate Akt [ 3839 ].
Aside from PTEN, ROS have been shown to inhibit other phosphatases, including protein phosphatase 2A PP2A and protein tyrosine phosphatase 1B PTP1B [ 40 ]. PP2A dephosphorylates Akt on threonine and serine resulting in Akt inactivation; however, PP2A dephosphorylation activity is inhibited by hydrogen peroxide [ 41 ].
PTP1B also suppresses Akt activity by dephosphorylation but, like PP2A, ROS inhibit PTP1B activity and increase Akt activity resulting in increased anchorage-independent growth [ 4243 ]. Thus, ROS inhibit phosphatases to dysregulate PI3K signaling resulting in increased Akt signaling and enhanced proliferation and survival Figure 3.
Reactive oxygen species modify cellular signaling. Hydrogen peroxide derived from either NOXs or the mitochondria can activate the PI3K pathway, the hypoxia-inducible factor HIF pathway, and metabolic adaptations.
These modifications are essential to allowing the survival, growth, and proliferation fundamental to tumorigenesis. One of the best characterized pathways shown to be responsive to mROS is the hypoxia-response pathway. Hypoxia is a prominent feature of tumor cells in vivo due to a mismatch between the high proliferative rate of tumor cells and the ability of the blood supply to provide nutrients including oxygen.
Tumor cells activate hypoxia inducible factors HIFs to activate a transcriptional network to allow tumor cells to adapt to their diminished oxygen microenvironment.
The pathway consists of three hypoxia-sensitive α subunits HIF1α, HIF2α, and HIF3α that, upon activation, heterodimerize with the constitutively expressed HIF1β and activate transcription from hypoxia-response elements HREs [ 44 ].
When cells are exposed to hypoxia, PHD2 hydroxylation of HIFα subunits is inhibited leading to HIFα accumulation, heterodimerization, and translocation to the nucleus.
The HIF heterodimer interacts with the co-activators p and CBP to initiate transcription of hypoxia-response genes from HREs.
: Reactive oxygen species| Reactive oxygen species - Wikipedia | Reactive species and antioxidants. Mitohormesis: Reactive oxygen species health and lifespan by spevies levels of reactive oxygen species ROS. Plant Signal. Immunometabolic regulation of vascular redox state: the role of adipose tissue. Liu, G. |
| Access options | Oxygem, macromolecular Reactive oxygen species, altered signaling. Hasanuzzaman, Antioxidant-rich diet. Malar, S. AA has also Reacctive reported to be involved in preventing photo-oxidation by pH-mediated modulation of PSII activity and its down regulation, associated with zeaxanthine formation. Teaching the fundamentals of electron transfer reactions in mitochondria and the production and detection of reactive oxygen species. |
| Reactive oxygen species - sources, functions, oxidative damage | The SDH complex is comprised of four subunits SDHA, SDHB, SDHC, and SDHD and is the only TCA cycle enzyme that is also a component of the ETC complex II. Mutations in SDHB, SDHC, and SDHD are commonly associated with cancer formation, whereas mutations in SDHA are rarely associated. Interestingly, given the structure and mechanism of complex II, loss of SDHB, SDHC, and SDHD would allow for acceptance of an electron, but not progression along the ETC, and thus may increase ROS generation. In support of this model, loss of SDHB, but not SDHA increases mROS, HIF1α, and tumorigenicity [ 89 ]. In addition, mutations in SDHC are also been associated with increased mROS and tumorigenesis [ 90 ]. Thus, loss of components of the SDH complex may, in part, cause tumorigenesis by increasing mROS levels. In hereditary leiomyomatosis and renal cell cancer HLRCC , the loss of the TCA cycle enzyme fumarate hydratase FH leads to accumulation of the metabolite fumarate and renal cell cancer. FH-deficient cancer cells display pseudo-hypoxia with aberrant activation of HIF1α. Congruent with SDH mutations, this HIF1α activation was also shown to be ROS dependent [ 91 ]. However, the mechanism of ROS production is different than SDH mutations. Accumulated fumarate in FH-deficient cells succinates the thiol residue on the intracellular antioxidant molecule glutathione to produce the metabolite succinated glutathione GSF [ 93 ]. The metabolism of GSF consumes NADPH, the primary reducing equivalent used in ROS detoxification reactions. Thus, GSF reduces overall NADPH antioxidant capacity resulting in increased mROS and HIF1α stabilization. Interestingly, FH-null cancer cells also display hyper-activation of the master antioxidant transcription factor NRF2. While ROS have been shown to stabilize NRF2, FH-deficient cancer cells primarily activate NRF2 by succination and inactivation of KEAP1 [ 93 — 95 ]. Depletion of NRF2 by shRNA in FH-null cells further increased ROS, increased HIF1α stabilization, and decreased proliferation, suggesting that NRF2 suppresses fumarate-mediated ROS to maintain a favorable homeostatic level compatible with proliferation [ 93 ]. ROS contribute to mitogenic signaling, and thus decreasing intracellular ROS levels is an attractive method for inhibiting cancer growth. With this in mind, several large-scale studies have investigated whether supplementation with antioxidant vitamins, including β-carotene and vitamin A or vitamin E can reduce cancer risk in humans. Contrary to the expected result, supplementation increased the risk of cancer in both cases [ 96 , 97 ]. In agreement with these results, in genetic mouse models of K-Ras- or B-Raf-induced lung cancer, treatment with NAC or vitamin E markedly enhanced tumor growth and accelerated mortality [ 98 ]. These results show that the potential use of antioxidants for cancer therapy is complex and needs to be carefully validated before being applied. One possibility for the failure of these antioxidants as cancer treatments is their lack of specificity. Treatment of patients with general antioxidants may modulate many physiological processes that are relevant to cancer growth. For example, the immune system, an important modulator of cancer growth, has been shown to be sensitive to ROS levels [ 99 ]. Another possibility is that general antioxidants are differentially effective than targeted antioxidants. Mitochondrial-targeted versions of antioxidants have been shown to be potent inhibitors of cancer cell growth in vitro and in vivo [ 69 , ]. Thus, further investigation needs to be considered to determine if targeted antioxidants are a viable method to treat cancer. Another approach for inhibiting ROS is to decrease production. Decreasing mROS production necessarily involves inhibition of the ETC and thus may not be a practical due to toxicity inherent in inhibiting mitochondrial respiration. However, patients taking the antidiabetic drug metformin have recently been shown to have a reduced risk of cancer incidence and mortality [ ]. Metformin has been shown to act as an inhibitor of complex I of the ETC [ , ]. We recently used a metformin insensitive complex I analog to confirm that the anticancer effect of metformin is primarily mediated by specific inhibition of complex I of cancer cells in vivo [ ]. Interestingly, we also observed that treatment with metformin suppressed hypoxic activation of HIF1α, indicating that it may also decrease production of mROS under hypoxia. Whether this effect is important for the cancer suppressive effects of metformin requires further investigation. An alternative approach to decrease ROS production is by inhibiting NADPH oxidases. Indeed, loss of NADPH oxidase 4 has been shown to activate apoptosis in pancreatic cancer cells [ ]. In addition, inhibitors of NADPH oxidase activity have been shown to have efficacy on mouse models of cancer in vivo [ , ]. Considering that cancer cells have increased ROS levels, they may be selectively sensitive to the damaging effects of further increasing ROS. Increasing ROS production specifically in cancer cells is likely difficult to accomplish, although it is one proposed mechanism for how many current chemotherapeutics function [ ]. Alternatively, since cancer cells frequently have increased expression of antioxidants to maintain homeostasis, a promising therapeutic approach is to inhibit antioxidants to expose cancer cells to endogenously produced ROS [ ]. In support of this model, several small molecule screens identifying compounds that specifically inhibit growth of transformed cells have converged upon glutathione utilization [ — ]. In all cases, treatment with the identified small molecules decreased glutathione levels, increased ROS, and could be rescued by treatment with NAC. In addition, inhibition of antioxidant pathways has also been shown to be effective for inhibiting cancer growth. Genetic knockout of NRF2 inhibited disease progression in mouse models of pancreatic and lung cancer [ 31 , 32 ]. Inhibition of SOD1 by the small molecule ATN was shown to cause ROS-dependent cancer cell death in vitro and decreased tumor burden in advanced K-Ras-driven lung cancers in vivo [ ]. These recent examples provide further proof of principle that increasing ROS, whether by increasing production or inhibiting antioxidants, is a promising approach for targeting cancer cells Figure 6. Further research is warranted to determine which components of the antioxidant pathway are selectively essential for tumor growth. Targeting cancer cells by modifying ROS levels. Normal cells have decreased amounts of both ROS and antioxidants relative to cancer cells. Loss of either ROS or antioxidants therefore causes only small changes in ROS homeostasis, leaving cells viable and functional. However, since cancer cells have more ROS and antioxidants, they may be more susceptible to changes in ROS levels. Treatment with antioxidants or prevention of ROS generation will cause cells to lose sufficient ROS signaling to maintain growth. The result is cytostasis and possibly senescence. Alternatively, inhibition of antioxidants or increasing ROS generation will result in excess ROS in cancer cells and cause cancer-specific oxidative cell death. It is becoming increasingly apparent that ROS play an important role in the biology of tumorigenesis. While several mechanisms have been presented here, the bulk of ROS-mediated signaling targets are largely unknown. However, the frequency of cancer-associated mutations that increase ROS levels suggests that increased production of ROS may be a common output of a large fraction of cancer-associated mutations in oncogenes and tumor suppressors. In addition, the apparent selection for mitochondrial mutations that increase ROS at the detriment of metabolic flexibility suggests that ROS are strongly selected for in these cancer cells. An emerging model is that cancer cells increase the production of ROS to activate localized pro-tumorigenic signaling but balance the increased ROS with elevated antioxidant activity to maintain redox balance. As with all studies in cancer, the final goal will be to design therapeutics that can take advantage of these discoveries. Both the suppression of ROS to prevent activation of pro-tumorigenic signaling pathways and the exacerbation of ROS by disabling antioxidants to induce cell death represent promising approaches in this regard. Future work is needed to better understand ROS-targeted pathways. In addition, future studies need to determine what sources of ROS and what specific antioxidants are required for homeostasis. With this knowledge, we can better understand cancer biology and design novel therapeutics to specifically treat cancer cells. Szatrowski TP, Nathan CF: Production of large amounts of hydrogen peroxide by human tumor cells. Can Res. CAS Google Scholar. Ames BN, Shigenaga MK, Hagen TM: Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci USA. CAS PubMed PubMed Central Google Scholar. Cross CE, Halliwell B, Borish ET, Pryor WA, Ames BN, Saul RL, McCord JM, Harman D: Oxygen radicals and human disease. Ann Intern Med. CAS PubMed Google Scholar. Sundaresan M, Yu ZX, Ferrans VJ, Irani K, Finkel T: Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Bae YS, Kang SW, Seo MS, Baines IC, Tekle E, Chock PB, Rhee SG: Epidermal growth factor EGF -induced generation of hydrogen peroxide. Role in EGF receptor-mediated tyrosine phosphorylation. J Biol Chem. Finkel T: From sulfenylation to sulfhydration: what a thiolate needs to tolerate. Sci Signal. Google Scholar. Finkel T: Oxidant signals and oxidative stress. Curr Opin Cell Biol. Buetler TM, Krauskopf A, Ruegg UT: Role of superoxide as a signaling molecule. News Physiol Sci. Babior BM: NADPH oxidase: an update. Brown DI, Griendling KK: Nox proteins in signal transduction. Free Radic Biol Med. Jiang F, Zhang Y, Dusting GJ: NADPH oxidase-mediated redox signaling: roles in cellular stress response, stress tolerance, and tissue repair. Pharmacol Rev. Irani K, Xia Y, Zweier JL, Sollott SJ, Der CJ, Fearon ER, Sundaresan M, Finkel T, Goldschmidt-Clermont PJ: Mitogenic signaling mediated by oxidants in Ras-transformed fibroblasts. Wang J, Pareja KA, Kaiser CA, Sevier CS: Redox signaling via the molecular chaperone BiP protects cells against endoplasmic reticulum-derived oxidative stress. PubMed PubMed Central Google Scholar. Quinlan CL, Treberg JR, Perevoshchikova IV, Orr AL, Brand MD: Native rates of superoxide production from multiple sites in isolated mitochondria measured using endogenous reporters. Handy DE, Loscalzo J: Redox regulation of mitochondrial function. Antioxid Redox Signal. Murphy MP: How mitochondria produce reactive oxygen species. Biochem J. Brand MD: The sites and topology of mitochondrial superoxide production. Exp Gerontol. Muller FL, Liu Y, Van Remmen H: Complex III releases superoxide to both sides of the inner mitochondrial membrane. Sena LA, Chandel NS: Physiological roles of mitochondrial reactive oxygen species. Mol Cell. Wood ZA, Schroder E, Robin Harris J, Poole LB: Structure, mechanism and regulation of peroxiredoxins. Trends Biochem Sci. Cox AG, Winterbourn CC, Hampton MB: Mitochondrial peroxiredoxin involvement in antioxidant defence and redox signalling. Winterbourn CC, Hampton MB: Thiol chemistry and specificity in redox signaling. Woo HA, Yim SH, Shin DH, Kang D, Yu DY, Rhee SG: Inactivation of peroxiredoxin I by phosphorylation allows localized H 2 O 2 accumulation for cell signaling. Cao C, Leng Y, Huang W, Liu X, Kufe D: Glutathione peroxidase 1 is regulated by the c-Abl and Arg tyrosine kinases. Sporn MB, Liby KT: NRF2 and cancer: the good, the bad and the importance of context. Nature reviews Cancer. Jaramillo MC, Zhang DD: The emerging role of the Nrf2-Keap1 signaling pathway in cancer. Genes Dev. Malhotra D, Portales-Casamar E, Singh A, Srivastava S, Arenillas D, Happel C, Shyr C, Wakabayashi N, Kensler TW, Wasserman WW, Biswal S: Global mapping of binding sites for Nrf2 identifies novel targets in cell survival response through ChIP-Seq profiling and network analysis. Nucleic Acids Res. Dinkova-Kostova AT, Holtzclaw WD, Cole RN, Itoh K, Wakabayashi N, Katoh Y, Yamamoto M, Talalay P: Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Zhang DD, Hannink M: Distinct cysteine residues in Keap1 are required for Keap1-dependent ubiquitination of Nrf2 and for stabilization of Nrf2 by chemopreventive agents and oxidative stress. Mol Cell Biol. Fourquet S, Guerois R, Biard D, Toledano MB: Activation of NRF2 by nitrosative agents and H2O2 involves KEAP1 disulfide formation. DeNicola GM, Karreth FA, Humpton TJ, Gopinathan A, Wei C, Frese K, Mangal D, Yu KH, Yeo CJ, Calhoun ES, Scrimieri F, Winter JM, Hruban RH, Iacobuzio-Donahue C, Kern SE, Blair IA, Tuveson DA: Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Satoh H, Moriguchi T, Takai J, Ebina M, Yamamoto M: Nrf2 prevents initiation but accelerates progression through the Kras signaling pathway during lung carcinogenesis. Cancer research. Neumann CA, Krause DS, Carman CV, Das S, Dubey DP, Abraham JL, Bronson RT, Fujiwara Y, Orkin SH, Van Etten RA: Essential role for the peroxiredoxin Prdx1 in erythrocyte antioxidant defence and tumour suppression. Cantley LC: The phosphoinositide 3-kinase pathway. Nemoto S, Finkel T: Redox regulation of forkhead proteins through a p66shc-dependent signaling pathway. Lee SR, Yang KS, Kwon J, Lee C, Jeong W, Rhee SG: Reversible inactivation of the tumor suppressor PTEN by H2O2. Leslie NR, Bennett D, Lindsay YE, Stewart H, Gray A, Downes CP: Redox regulation of PI 3-kinase signalling via inactivation of PTEN. EMBO J. Connor KM, Subbaram S, Regan KJ, Nelson KK, Mazurkiewicz JE, Bartholomew PJ, Aplin AE, Tai YT, Aguirre-Ghiso J, Flores SC, Melendez JA: Mitochondrial H2O2 regulates the angiogenic phenotype via PTEN oxidation. Pelicano H, Xu RH, Du M, Feng L, Sasaki R, Carew JS, Hu Y, Ramdas L, Hu L, Keating MJ, Zhang W, Plunkett W, Huang P: Mitochondrial respiration defects in cancer cells cause activation of Akt survival pathway through a redox-mediated mechanism. J Cell Biol. Ostman A, Frijhoff J, Sandin A, Bohmer FD: Regulation of protein tyrosine phosphatases by reversible oxidation. J Biochem. PubMed Google Scholar. Rao RK, Clayton LW: Regulation of protein phosphatase 2A by hydrogen peroxide and glutathionylation. Biochem Biophys Res Commun. Salmeen A, Andersen JN, Myers MP, Meng TC, Hinks JA, Tonks NK, Barford D: Redox regulation of protein tyrosine phosphatase 1B involves a sulphenyl-amide intermediate. Lou YW, Chen YY, Hsu SF, Chen RK, Lee CL, Khoo KH, Tonks NK, Meng TC: Redox regulation of the protein tyrosine phosphatase PTP1B in cancer cells. FEBS J. Semenza GL: Hypoxia-inducible factors in physiology and medicine. Kaelin WG, Ratcliffe PJ: Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Semenza GL: Targeting HIF-1 for cancer therapy. Chandel NS, Maltepe E, Goldwasser E, Mathieu CE, Simon MC, Schumacker PT: Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Chandel NS, Schumacker PT: Cells depleted of mitochondrial DNA rho0 yield insight into physiological mechanisms. FEBS Lett. Chandel NS, McClintock DS, Feliciano CE, Wood TM, Melendez JA, Rodriguez AM, Schumacker PT: Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1alpha during hypoxia: a mechanism of O2 sensing. Waypa GB, Marks JD, Guzy R, Mungai PT, Schriewer J, Dokic D, Schumacker PT: Hypoxia triggers subcellular compartmental redox signaling in vascular smooth muscle cells. Circ Res. Guzy RD, Hoyos B, Robin E, Chen H, Liu L, Mansfield KD, Simon MC, Hammerling U, Schumacker PT: Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metab. Brunelle JK, Bell EL, Quesada NM, Vercauteren K, Tiranti V, Zeviani M, Scarpulla RC, Chandel NS: Oxygen sensing requires mitochondrial ROS but not oxidative phosphorylation. Mansfield KD, Guzy RD, Pan Y, Young RM, Cash TP, Schumacker PT, Simon MC: Mitochondrial dysfunction resulting from loss of cytochrome c impairs cellular oxygen sensing and hypoxic HIF-alpha activation. Bell EL, Klimova TA, Eisenbart J, Moraes CT, Murphy MP, Budinger GR, Chandel NS: The Qo site of the mitochondrial complex III is required for the transduction of hypoxic signaling via reactive oxygen species production. Gerald D, Berra E, Frapart YM, Chan DA, Giaccia AJ, Mansuy D, Pouyssegur J, Yaniv M, Mechta-Grigoriou F: JunD reduces tumor angiogenesis by protecting cells from oxidative stress. Sanjuan-Pla A, Cervera AM, Apostolova N, Garcia-Bou R, Victor VM, Murphy MP, McCreath KJ: A targeted antioxidant reveals the importance of mitochondrial reactive oxygen species in the hypoxic signaling of HIF-1alpha. Lin X, David CA, Donnelly JB, Michaelides M, Chandel NS, Huang X, Warrior U, Weinberg F, Tormos KV, Fesik SW, Shen Y: A chemical genomics screen highlights the essential role of mitochondria in HIF-1 regulation. Ma Q, Cavallin LE, Yan B, Zhu S, Duran EM, Wang H, Hale LP, Dong C, Cesarman E, Mesri EA, Goldschmidt-Clermont PJ: Antitumorigenesis of antioxidants in a transgenic Rac1 model of Kaposi's sarcoma. Proc Natl Acad Sci U S A. Gao P, Zhang H, Dinavahi R, Li F, Xiang Y, Raman V, Bhujwalla ZM, Felsher DW, Cheng L, Pevsner J, Lee LA, Semenza GL, Dang CV: HIF-dependent antitumorigenic effect of antioxidants in vivo. Can Cell. Kim JW, Tchernyshyov I, Semenza GL, Dang CV: HIFmediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Tello D, Balsa E, Acosta-Iborra B, Fuertes-Yebra E, Elorza A, Ordóñez Á, Corral-Escariz M, Soro I, López-Bernardo E, Perales-Clemente E, Martínez-Ruiz A, Enríquez JA, Aragonés J, Cadenas S, Landázuri MO: Induction of the mitochondrial NDUFA4L2 protein by HIF-1α decreases oxygen consumption by inhibiting complex I activity. Chen Z, Li Y, Zhang H, Huang P, Luthra R: Hypoxia-regulated microRNA modulates mitochondrial function and decreases ISCU and COX10 expression. Mitsuishi Y, Taguchi K, Kawatani Y, Shibata T, Nukiwa T, Aburatani H, Yamamoto M, Motohashi H: Nrf2 redirects glucose and glutamine into anabolic pathways in metabolic reprogramming. Hitosugi T, Kang S, Vander Heiden MG, Chung TW, Elf S, Lythgoe K, Dong S, Lonial S, Wang X, Chen GZ, Xie J, Gu TL, Polakiewicz RD, Roesel JL, Boggon TJ, Khuri FR, Gilliland DG, Cantley LC, Kaufman J, Chen J: Tyrosine phosphorylation inhibits PKM2 to promote the Warburg effect and tumor growth. Christofk HR, Vander Heiden MG, Wu N, Asara JM, Cantley LC: Pyruvate kinase M2 is a phosphotyrosine-binding protein. Anastasiou D, Poulogiannis G, Asara JM, Boxer MB, Jiang JK, Shen M, Bellinger G, Sasaki AT, Locasale JW, Auld DS, Thomas CJ, Vander Heiden MG, Cantley LC: Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to cellular antioxidant responses. Israelsen WJ, Dayton TL, Davidson SM, Fiske BP, Hosios AM, Bellinger G, Li J, Yu Y, Sasaki M, Horner JW, Burga LN, Xie J, Jurczak MJ, DePinho RA, Clish CB, Jacks T, Kibbey RG, Wulf GM, Di Vizio D, Mills GB, Cantley LC, Vander Heiden MG: PKM2 isoform-specific deletion reveals a differential requirement for pyruvate kinase in tumor cells. Weinberg F, Hamanaka R, Wheaton WW, Weinberg S, Joseph J, Lopez M, Kalyanaraman B, Mutlu GM, Budinger GR, Chandel NS: Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Vafa O, Wade M, Kern S, Beeche M, Pandita TK, Hampton GM, Wahl GM: c-Myc can induce DNA damage, increase reactive oxygen species, and mitigate p53 function: a mechanism for oncogene-induced genetic instability. Tanaka H, Matsumura I, Ezoe S, Satoh Y, Sakamaki T, Albanese C, Machii T, Pestell RG, Kanakura Y: E2F1 and c-Myc potentiate apoptosis through inhibition of NF-kappaB activity that facilitates MnSOD-mediated ROS elimination. Levine AJ, Oren M: The first 30 years of p growing ever more complex. Vousden KH, Prives C: Blinded by the light: the growing complexity of p Li T, Kon N, Jiang L, Tan M, Ludwig T, Zhao Y, Baer R, Gu W: Tumor suppression in the absence of pmediated cell-cycle arrest, apoptosis, and senescence. Sablina AA, Budanov AV, Ilyinskaya GV, Agapova LS, Kravchenko JE, Chumakov PM: The antioxidant function of the p53 tumor suppressor. Nat Med. Roth M, Chen WY: Sorting out functions of sirtuins in cancer. Bell EL, Guarente L: The SirT3 divining rod points to oxidative stress. Finley LW, Carracedo A, Lee J, Souza A, Egia A, Zhang J, Teruya-Feldstein J, Moreira PI, Cardoso SM, Clish CB, Pandolfi PP, Haigis MC: SIRT3 opposes reprogramming of cancer cell metabolism through HIF1α destabilization. Bell EL, Emerling BM, Ricoult SJ, Guarente L: SirT3 suppresses hypoxia inducible factor 1alpha and tumor growth by inhibiting mitochondrial ROS production. Chatterjee A, Mambo E, Sidransky D: Mitochondrial DNA mutations in human cancer. Larman TC, DePalma SR, Hadjipanayis AG, Protopopov A, Zhang J, Gabriel SB, Chin L, Seidman CE, Kucherlapati R, Seidman JG, Cancer Genome Atlas Research Network: Spectrum of somatic mitochondrial mutations in five cancers. Park JS, Sharma LK, Li H, Xiang R, Holstein D, Wu J, Lechleiter J, Naylor SL, Deng JJ, Lu J, Bai Y: A heteroplasmic, not homoplasmic, mitochondrial DNA mutation promotes tumorigenesis via alteration in reactive oxygen species generation and apoptosis. Hum Mol Genet. Sharma LK, Fang H, Liu J, Vartak R, Deng J, Bai Y: Mitochondrial respiratory complex I dysfunction promotes tumorigenesis through ROS alteration and AKT activation. Ishikawa K, Takenaga K, Akimoto M, Koshikawa N, Yamaguchi A, Imanishi H, Nakada K, Honma Y, Hayashi J: ROS-generating mitochondrial DNA mutations can regulate tumor cell metastasis. Birsoy K, Possemato R, Lorbeer FK, Bayraktar EC, Thiru P, Yucel B, Wang T, Chen WW, Clish CB, Sabatini DM: Metabolic determinants of cancer cell sensitivity to glucose limitation and biguanides. Iommarini L, Kurelac I, Capristo M, Calvaruso MA, Giorgio V, Bergamini C, Ghelli A, Nanni P, De Giovanni C, Carelli V, Fato R, Lollini PL, Rugolo M, Gasparre G, Porcelli AM: Different mtDNA mutations modify tumor progression in dependence of the degree of respiratory complex I impairment. Am J Pathol. Dahia PL: Pheochromocytoma and paraganglioma pathogenesis: learning from genetic heterogeneity. Guzy RD, Sharma B, Bell E, Chandel NS, Schumacker PT: Loss of the SdhB, but not the SdhA, subunit of complex II triggers reactive oxygen species-dependent hypoxia-inducible factor activation and tumorigenesis. Ishii T, Yasuda K, Akatsuka A, Hino O, Hartman PS, Ishii N: A mutation in the SDHC gene of complex II increases oxidative stress, resulting in apoptosis and tumorigenesis. Cancer Res. Sudarshan S, Sourbier C, Kong HS, Block K, Valera Romero VA, Yang Y, Galindo C, Mollapour M, Scroggins B, Goode N, Lee MJ, Gourlay CW, Trepel J, Linehan WM, Neckers L: Fumarate hydratase deficiency in renal cancer induces glycolytic addiction and hypoxia-inducible transcription factor 1alpha stabilization by glucose-dependent generation of reactive oxygen species. Nagai R, Brock JW, Blatnik M, Baatz JE, Bethard J, Walla MD, Thorpe SR, Baynes JW, Frizzell N: Succination of protein thiols during adipocyte maturation: a biomarker of mitochondrial stress. Sullivan LB, Martinez-Garcia E, Nguyen H, Mullen AR, Dufour E, Sudarshan S, Licht JD, Deberardinis RJ, Chandel NS: The proto-oncometabolite fumarate binds glutathione to amplify ROS-dependent signaling. Mol cell. Cancer Cell. Ooi A, Wong JC, Petillo D, Roossien D, Perrier-Trudova V, Whitten D, Min BW, Tan MH, Zhang Z, Yang XJ, Zhou M, Gardie B, Molinié V, Richard S, Tan PH, Teh BT, Furge KA: An antioxidant response phenotype shared between hereditary and sporadic type 2 papillary renal cell carcinoma. Omenn GS, Goodman GE, Thornquist MD, Balmes J, Cullen MR, Glass A, Keogh JP, Meyskens FL, Valanis B, Williams JH, Barnhart S, Hammar S: Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med. Klein EA, Thompson IM, Tangen CM, Crowley JJ, Lucia MS, Goodman PJ, Minasian LM, Ford LG, Parnes HL, Gaziano JM, Karp DD, Lieber MM, Walther PJ, Klotz L, Parsons JK, Chin JL, Darke AK, Lippman SM, Goodman GE, Meyskens FL, Baker LH: Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial SELECT. Sayin VI, Ibrahim MX, Larsson E, Nilsson JA, Lindahl P, Bergo MO: Antioxidants accelerate lung cancer progression in mice. Sci Transl Med. Sena LA, Li S, Jairaman A, Prakriya M, Ezponda T, Hildeman DA, Wang CR, Schumacker PT, Licht JD, Perlman H, Bryce PJ, Chandel NS: Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Cheng G, Zielonka J, McAllister DM, Mackinnon AC, Joseph J, Dwinell MB, Kalyanaraman B: Mitochondria-targeted vitamin E analogs inhibit breast cancer cell energy metabolism and promote cell death. BMC cancer. Noto H, Goto A, Tsujimoto T, Noda M: Cancer risk in diabetic patients treated with metformin: a systematic review and meta-analysis. PloS one. El-Mir MY, Nogueira V, Fontaine E, Averet N, Rigoulet M, Leverve X: Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. Owen MR, Doran E, Halestrap AP: Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Wheaton WW, Weinberg SE, Hamanaka RB, Soberanes S, Sullivan LB, Anso E, Glasauer A, Dufour E, Mutlu GM, Budinger GR, Chandel NS: Metformin inhibits mitochondrial complex I of cancer cells to reduce tumorigenesis. Bhandarkar SS, Jaconi M, Fried LE, Bonner MY, Lefkove B, Govindarajan B, Perry BN, Parhar R, Mackelfresh J, Sohn A, Stouffs M, Knaus U, Yancopoulos G, Reiss Y, Benest AV, Augustin HG, Arbiser JL: Fulvene-5 potently inhibits NADPH oxidase 4 and blocks the growth of endothelial tumors in mice. J Clin Invest. Munson JM, Fried L, Rowson SA, Bonner MY, Karumbaiah L, Diaz B, Courtneidge SA, Knaus UG, Brat DJ, Arbiser JL, Bellamkonda RV: Anti-invasive adjuvant therapy with imipramine blue enhances chemotherapeutic efficacy against glioma. Conklin KA: Chemotherapy-associated oxidative stress: impact on chemotherapeutic effectiveness. Integr Cancer Ther. Gorrini C, Harris IS, Mak TW: Modulation of oxidative stress as an anticancer strategy. Nature reviews Drug discovery. Raj L, Ide T, Gurkar AU, Foley M, Schenone M, Li X, Tolliday NJ, Golub TR, Carr SA, Shamji AF, Stern AM, Mandinova A, Schreiber SL, Lee SW: Selective killing of cancer cells by a small molecule targeting the stress response to ROS. Yang WS, Sriramaratnam R, Welsch ME, Shimada K, Skouta R, Viswanathan VS, Cheah JH, Clemons PA, Shamji AF, Clish CB, Brown LM, Girotti AW, Cornish VW, Schreiber SL, Stockwell BR: Regulation of ferroptotic cancer cell death by GPX4. Trachootham D, Zhou Y, Zhang H, Demizu Y, Chen Z, Pelicano H, Chiao PJ, Achanta G, Arlinghaus RB, Liu J, Huang P: Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by beta-phenylethyl isothiocyanate. Cancer cell. Glasauer A, Sena LA, Diebold LP, Mazar AP, Chandel NS: Targeting SOD1 reduces experimental non-small-cell lung cancer. Download references. The Koch Institute for Integrative Cancer Research at Massachusetts Institute of Technology, Cambridge, MA, , USA. Division of Pulmonary and Critical Care Medicine, Department of Medicine, The Feinberg School of Medicine, Northwestern University, Chicago, IL, , USA. You can also search for this author in PubMed Google Scholar. Correspondence to Navdeep S Chandel. LS wrote the manuscript and prepared the figures. NC supervised the design of the review and wrote the manuscript. Both authors read and approved the final manuscript. This article is published under license to BioMed Central Ltd. Reprints and permissions. Sullivan, L. Mitochondrial reactive oxygen species and cancer. Cancer Metab 2 , 17 Download citation. Received : 03 June Accepted : 27 August Published : 28 November Anyone you share the following link with will be able to read this content:. Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative. Skip to main content. Search all BMC articles Search. Download PDF. Abstract Mitochondria produce reactive oxygen species mROS as a natural by-product of electron transport chain activity. Review Introduction Mitochondrial-derived reactive oxygen species mROS have increasingly been appreciated to function as signaling molecules that modify cellular physiology. Reactive oxygen species The term reactive oxygen species covers several molecules derived from oxygen that have accepted extra electrons and can oxidize other molecules [ 3 ]. Figure 1. Full size image. Figure 2. Figure 3. Figure 4. Figure 5. Figure 6. Conclusions It is becoming increasingly apparent that ROS play an important role in the biology of tumorigenesis. References Szatrowski TP, Nathan CF: Production of large amounts of hydrogen peroxide by human tumor cells. CAS Google Scholar Ames BN, Shigenaga MK, Hagen TM: Oxidants, antioxidants, and the degenerative diseases of aging. CAS PubMed PubMed Central Google Scholar Cross CE, Halliwell B, Borish ET, Pryor WA, Ames BN, Saul RL, McCord JM, Harman D: Oxygen radicals and human disease. CAS PubMed Google Scholar Sundaresan M, Yu ZX, Ferrans VJ, Irani K, Finkel T: Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. CAS PubMed Google Scholar Bae YS, Kang SW, Seo MS, Baines IC, Tekle E, Chock PB, Rhee SG: Epidermal growth factor EGF -induced generation of hydrogen peroxide. CAS PubMed Google Scholar Finkel T: From sulfenylation to sulfhydration: what a thiolate needs to tolerate. CAS PubMed Google Scholar Buetler TM, Krauskopf A, Ruegg UT: Role of superoxide as a signaling molecule. CAS PubMed Google Scholar Babior BM: NADPH oxidase: an update. CAS PubMed Google Scholar Brown DI, Griendling KK: Nox proteins in signal transduction. CAS PubMed PubMed Central Google Scholar Jiang F, Zhang Y, Dusting GJ: NADPH oxidase-mediated redox signaling: roles in cellular stress response, stress tolerance, and tissue repair. CAS PubMed Google Scholar Irani K, Xia Y, Zweier JL, Sollott SJ, Der CJ, Fearon ER, Sundaresan M, Finkel T, Goldschmidt-Clermont PJ: Mitogenic signaling mediated by oxidants in Ras-transformed fibroblasts. CAS PubMed Google Scholar Wang J, Pareja KA, Kaiser CA, Sevier CS: Redox signaling via the molecular chaperone BiP protects cells against endoplasmic reticulum-derived oxidative stress. Genetically encoded fluorescent indicator for intracellular hydrogen peroxide. Methods 3 , — First genetically encoded H 2 O 2 probe using the OxyR domain. Bilan, D. In vivo imaging of hydrogen peroxide with HyPer probes. Morgan, B. Real-time monitoring of basal H 2 O 2 levels with peroxiredoxin-based probes. Roma, L. Mechanisms and applications of redox-sensitive green fluorescent protein-based hydrogen peroxide probes. Fernandez-Puente, E. Huang, B. Interpreting heterogeneity in response of cells expressing a fluorescent hydrogen peroxide biosensor. Henzler, T. Transport and metabolic degradation of hydrogen peroxide in Chara corallina: model calculations and measurements with the pressure probe suggest transport of H 2 O 2 across water channels. Discovery of H 2 O 2 transport across membranes by aquaporins. Bienert, G. Aquaporin-facilitated transmembrane diffusion of hydrogen peroxide. Acta , — Groundbreaking work on the role of some aquaporins as peroxiporins. A persulfidation-based mechanism controls aquaporin-8 conductance. Medrano-Fernandez, I. Stress regulates aquaporin-8 permeability to impact cell growth and survival. Pak, V. Ultrasensitive genetically encoded indicator for intracellular hydrogen peroxide identifies novel roles for cellular oxidants in cell migration and mitochondrial function. Tamma, G. Aquaporin membrane channels in oxidative stress, cell signaling, and aging: recent advances and research trends. Rajasekaran, N. Human alpha B-crystallin mutation causes oxido-reductive stress and protein aggregation cardiomyopathy in mice. Cell , — Marinho, H. Hydrogen peroxide sensing, signaling and regulation of transcription factors. Radical-free biology of oxidative stress. Cell Physiol , CC Bak, D. Cysteine reactivity across the subcellular universe. The redox proteome. Behring, J. Spatial and temporal alterations in protein structure by EGF regulate cryptic cysteine oxidation. Basic principles and emerging concepts in the redox control of transcription factors. Young, D. Protein promiscuity in H 2 O 2 signaling. Xiao, H. A quantitative tissue-specific landscape of protein redox regulation during aging. Itoh, K. Discovery of the NRF2—KEAP1 system. Yamamoto, M. The KEAP1-NRF2 system: a thiol-based sensor-effector apparatus for maintaining redox homeostasis. Fourquet, S. Activation of NRF2 by nitrosative agents and H 2 O 2 involves KEAP1 disulfide formation. Kobayashi, M. The antioxidant defense system Keap1-Nrf2 comprises a multiple sensing mechanism for responding to a wide range of chemical compounds. Cebula, M. TrxR1 as a potent regulator of the Nrf2-Keap1 response system. Singh, C. The role of sirtuins in antioxidant and redox signaling. Cheng, X. Regulation of the Nrf2 antioxidant pathway by microRNAs: new players in micromanaging redox homeostasis. Karin, M. NF-kappaB as a critical link between inflammation and cancer. Cold Spring Harb. Pahl, H. Oliveira-Marques, V. Role of hydrogen peroxide in NF-kappaB activation: from inducer to modulator. Schreck, R. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappaB transcription factor and HIV Description of the role of H 2 O 2 in NF-κB activation. Halvey, P. Selective oxidative stress in cell nuclei by nuclear-targeted D-amino acid oxidase. Kaelin, W. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Cell 30 , — Jiang, B. Dimerization, DNA binding, and transactivation properties of hypoxia-inducible factor 1. Seminal article on HIF. Hernansanz-Agustin, P. Mitochondrial complex I deactivation is related to superoxide production in acute hypoxia. Prabhakar, N. ROS signaling in systemic and cellular responses to chronic intermittent hypoxia. Waypa, G. O 2 sensing, mitochondria and ROS signaling: The fog is lifting. Article CAS Google Scholar. Chandel, N. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1alpha during hypoxia: a mechanism of O 2 sensing. Pouyssegur, J. Redox regulation of the hypoxia-inducible factor. Acker, T. The good, the bad and the ugly in oxygen-sensing: ROS, cytochromes and prolyl-hydroxylases. Eijkelenboom, A. FOXOs: signalling integrators for homeostasis maintenance. Klotz, L. Cellular adaptation to xenobiotics: interplay between xenosensors, reactive oxygen species and FOXO transcription factors. Liu, B. ROS and p a versatile partnership. Uehara, I. Role of p53 in the regulation of the inflammatory tumor microenvironment and tumor suppression. Cancers Basel. Article PubMed Central Google Scholar. Herzig, S. AMPK: guardian of metabolism and mitochondrial homeostasis. Hinchy, E. Mitochondria-derived ROS activate AMP-activated protein kinase AMPK indirectly. Liu, G. mTOR at the nexus of nutrition, growth, ageing and disease. Schmeisser, K. Pleiotropic effects of mTOR and autophagy during development and aging. Cell Dev. Sirover, M. Pleiotropic effects of moonlighting glyceraldehydephosphate dehydrogenase GAPDH in cancer progression, invasiveness, and metastases. Cancer Metastasis Rev. Peralta, D. A proton relay enhances H 2 O 2 sensitivity of GAPDH to facilitate metabolic adaptation. Lin, C. Isolation of the uncoupling protein from brown adipose tissue mitochondria. Discovery of uncoupling protein. Echtay, K. Berry, B. Use the protonmotive force: mitochondrial uncoupling and reactive oxygen species. Jezek, P. Mitochondrial uncoupling proteins: subtle regulators of cellular redox signaling. CAS PubMed PubMed Central Google Scholar. Superoxide activates mitochondrial uncoupling proteins. Nature , 96—99 Uncoupling proteins and the control of mitochondrial reactive oxygen species production. Dustin, C. Redox regulation of tyrosine kinase signaling: more than meet the eye. J, Biochem. Truong, T. Redox regulation of protein kinases. Londhe, A. Regulation of PTP1B activation through disruption of redox-complex formation. Chem Biol. Molecular basis for redox activation of epidermal growth factor receptor kinase. Cell Chem. Heppner, D. Direct cysteine sulfenylation drives activation of the Src kinase. Dagnell, M. Bicarbonate is essential for protein tyrosine phosphatase 1B PTP1B oxidation and cellular signaling through EGF-triggered phosphorylation cascades. Truzzi, D. Löwe, O. BIAM switch assay coupled to mass spectrometry identifies novel redox targets of NADPH oxidase 4. Bogeski, I. Redox regulation of ion channels. Kourie, J. Interaction of reactive oxygen species with ion transport mechanisms. Sahoo, N. Oxidative modulation of voltage-gated potassium channels. Ruppersberg, J. Regulation of fast inactivation of cloned mammalian IK A channels by cysteine oxidation. Forrester, S. Reactive oxygen species in metabolic and inflammatory signaling. Chen, P. Functional crosstalk among oxidative stress and O-GlcNAc signaling pathways. Glycobiology 28 , — Taniguchi, N. Glyco-redox, a link between oxidative stress and changes of glycans: lessons from research on glutathione, reactive oxygen and nitrogen species to glycobiology. Nordzieke, D. The plasma membrane: a platform for intra- and intercellular redox signaling. Antioxidants Basel 7 , Patinen, T. Regulation of stress signaling pathways by protein lipoxidation. Conrad, M. The chemical basis of ferroptosis. Ingold, I. Selenium utilization by GPX4 is required to prevent hydroperoxide-induced ferroptosis. Somyajit, K. Redox-sensitive alteration of replisome architecture safeguards genome integrity. Science , — Ahmed, W. PRDX1 and MTH1 cooperate to prevent ROS-mediated inhibition of telomerase. Genes Dev. The role of peroxiredoxins in the transduction of H 2 O 2 signals. Sarsour, E. Redox control of the cell cycle in health and disease. Srinivas, U. ROS and the DNA damage response in cancer. Teaching the fundamentals of electron transfer reactions in mitochondria and the production and detection of reactive oxygen species. Matilainen, O. Mitochondria and epigenetics - crosstalk in homeostasis and stress. Trends Cell Biol. Castro, L. Aconitases: non-redox iron-sulfur proteins sensitive to reactive species. Braymer, J. Depletion of thiol reducing capacity impairs cytosolic but not mitochondrial iron-sulfur protein assembly machineries. Acta Mol. Cell Res. Bulthuis, E. Mitochondrial morphofunction in mammalian cells. Kondadi, A. Functional interplay between cristae biogenesis, mitochondrial dynamics and mitochondrial DNA integrity. Murley, A. The emerging network of mitochondria-organelle contacts. Cell 61 , — Frank, M. Mitophagy is triggered by mild oxidative stress in a mitochondrial fission dependent manner. Zorov, D. Mitochondrial reactive oxygen species ROS and ROS-induced ROS release. Biochemistry of the peroxisome in the liver cell. Gebicka, L. Böhm, B. Extracellular localization of catalase is associated with the transformed state of malignant cells. Wang, L. Glutathione peroxidase 7 utilizes hydrogen peroxide generated by Ero1alpha to promote oxidative protein folding. Cenci, S. Managing and exploiting stress in the antibody factory. Laporte, A. Hydrogen peroxide permeability of cellular membranes in insulin-producing cells. Acta Biomembr. Cao, S. Endoplasmic reticulum stress and oxidative stress in cell fate decision and human disease. Eletto, D. Redox controls UPR to control redox. Cell Sci. CAS PubMed Google Scholar. Amodio, G. Targeting the endoplasmic reticulum unfolded protein response to counteract the oxidative stress-induced endothelial dysfunction. Görlach, A. Calcium and ROS: a mutual interplay. Hempel, N. Crosstalk between calcium and reactive oxygen species signaling in cancer. Cell Calcium. Feno, S. Crosstalk between calcium and ROS in pathophysiological conditions. Joseph, S. Redox regulation of type-I inositol trisphosphate receptors in intact mammalian cells. Booth, D. Redox nanodomains are induced by and control calcium signaling at the ER-mitochondrial interface. Cell 63 , — Description of H 2 O 2 redox nanodomains. Csordas, G. Endoplasmic reticulum-mitochondrial contactology: structure and signaling functions. Egea, J. European contribution to the study of ROS: a summary of the findings and prospects for the future from the COST action BM EU-ROS. Redox theory of aging: implications for health and disease. Valko, M. Free radicals and antioxidants in normal physiological functions and human disease. Milkovic, L. Short overview of ROS as cell function regulators and their implications in therapy concepts. Cells 8 , Article CAS PubMed Central Google Scholar. Timme-Laragy, A. Redox stress and signaling during vertebrate embryonic development: regulation and responses. Rampon, C. Hydrogen peroxide and redox regulation of developments. Oswald, M. Regulation of neuronal development and function by ROS. Wilson, C. From birth to death: a role for reactive oxygen species in neuronal development. Regulation of cytoskeletal dynamics by redox signaling and oxidative stress: implications for neuronal development and trafficking. Cell Neurosci. Tan, D. Reactive oxygen species and mitochondrial homeostasis as regulators of stem cell fate and function. Mitochondrial H 2 O 2 signaling is controlled by the concerted action of peroxiredoxin III and sulfiredoxin: linking mitochondrial function to circadian rhythm. An account of the role of peroxiredoxins in circadian rhythms. Nagy, A. Redox clocks: time to rethink redox interventions. Reinke, H. Crosstalk between metabolism and circadian clocks. Pei, J. Diurnal oscillations of endogenous H 2 O 2 sustained by p66 Shc regulate circadian clocks. Kempf, A. A potassium channel beta-subunit couples mitochondrial electron transport to sleep. Patke, A. Molecular mechanisms and physiological importance of circadian rhythms. Nayernia, Z. New insights on NOX enzymes in the central nervous system. Cobley, J. Tarafdar, A. The role of NADPH oxidases and oxidative stress in neurodegenerative disorders. Sbodio, J. Redox mechanisms in neurodegeneration: from disease outcomes to therapeutic opportunities. Steinbrenner, H. Selenium homeostasis and antioxidant selenoproteins in brain: implications for disorders in the central nervous system. Lepka, K. Iron-sulfur glutaredoxin 2 protects oligodendrocytes against damage induced by nitric oxide release from activated microglia. Glia 65 , — Casas, A. NOX4-dependent neuronal autotoxicity and BBB breakdown explain the superior sensitivity of the brain to ischemic damage. Natl Acad. USA , — Meda, F. Nerves, H 2 O 2 and Shh: three players in the game of regeneration. Hervera, A. Reactive oxygen species regulate axonal regeneration through the release of exosomal NADPH oxidase 2 complexes into injured axons. Vicente-Gutierrez, C. Astrocytic mitochondrial ROS modulate brain metabolism and mouse behaviour. Bierhaus, A. A mechanism converting psychosocial stress into mononuclear cell activation. A report describing how psychosocial stress is translated into a cell response pattern. Aschbacher, K. Good stress, bad stress and oxidative stress: insights from anticipatory cortisol reactivity. Psychoneuroendocrinology 38 , — Eustress, distress and oxidative stress: promising pathways for mind-body medicine. In Oxidative Stress: Eustress and Distress ed. Golbidi, S. Oxidative stress: a unifying mechanism for cell damage induced by noise, water-pipe smoking, and emotional stress-therapeutic strategies targeting redox imbalance. Münzel, T. Effects of noise on vascular function, oxidative stress, and inflammation: mechanistic insight from studies in mice. Heart J. Rousset, F. NOX3-targeted therapies for inner ear pathologies. Lugrin, J. The role of oxidative stress during inflammatory processes. Pei, L. Mitochondrial etiology of neuropsychiatric disorders. Psychiatry 83 , — Nathan, C. A review bridging immunology and redox biology. Brinkmann, V. Neutrophil extracellular traps kill bacteria. Kenny, E. Diverse stimuli engage different neutrophil extracellular trap pathways. eL ife. Anelli, T. Garaude, J. Reprogramming of mitochondrial metabolism by innate immunity. Abais, J. Redox regulation of NLRP3 inflammasomes: ROS as trigger or effector? Jones, R. Redox signaling mediated by the gut microbiota. Aviello, G. NADPH oxidases and ROS signaling in the gastrointestinal tract. Mucosal Immunol. Cano, S. Targeting oxidative stress and mitochondrial dysfunction in the treatment of impaired wound healing: a systematic review. Antioxidants Basel 7 , 98 Niethammer, P. Wound redox gradients revisited. Love, N. Amputation-induced reactive oxygen species are required for successful Xenopus tadpole tail regeneration. Levigne, D. NADPH oxidase 4 deficiency leads to impaired wound repair and reduced dityrosine-crosslinking, but does not affect myofibroblast formation. Kunkemoeller, B. Redox signaling in diabetic wound healing regulates extracellular matrix deposition. Handy, D. Responses to reductive stress in the cardiovascular system. Incalza, M. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Impact of oxidative stress on the heart and vasculature: part 2 of a 3-part series. Schröder, K. Redox control of angiogenesis. Förstermann, U. Roles of vascular oxidative stress and nitric oxide in the pathogenesis of atherosclerosis. Siu, K. NOX isoforms in the development of abdominal aortic aneurysm. Oikonomou, E. Immunometabolic regulation of vascular redox state: the role of adipose tissue. Stocker, R. Role of oxidative modifications in atherosclerosis. Trinity, J. Regulation of exercise blood flow: role of free radicals. Jackson, M. Redox regulation of muscle adaptations to contractile activity and aging. Le Moal, E. Redox control of skeletal muscle regeneration. El Assar, M. Frailty as a phenotypic manifestation of underlying oxidative stress. McArdle, A. Aberrant redox signalling and stress response in age-related muscle decline: role in inter- and intra-cellular signalling. Exercise redox biochemistry: conceptual, methodological and technical recommendations. Hancock, M. Myocardial NADPH oxidase-4 regulates the physiological response to acute exercise. eL ife 7 , Mechanistic models to guide redox investigations and interventions in musculoskeletal ageing. Watson, J. Type 2 diabetes as a redox disease. Lancet , — Haeusler, R. Biochemical and cellular properties of insulin receptor signalling. Petersen, M. Mechanisms of insulin action and insulin resistance. Onyango, A. Cellular stresses and stress responses in the pathogenesis of insulin resistance. Harman, D. Aging: a theory based on free radical and radiation chemistry. Seminal article on the free radical theory of ageing. Golubev, A. Non-enzymatic molecular damage as a prototypic driver of aging. Lopez-Otin, C. The hallmarks of aging. Comprehensive overview of the hallmarks of ageing. Pomatto, L. The role of declining adaptive homeostasis in ageing. Schmidlin, C. Redox regulation by NRF2 in aging and disease. Taetzsch, T. Loss of NF-kappaB p50 function synergistically augments microglial priming in the middle-aged brain. Neuroinflammation 16 , 60— Salminen, A. AMPK activation inhibits the functions of myeloid-derived suppressor cells MDSC : impact on cancer and aging. Rose, G. Further support to the uncoupling-to-survive theory: the genetic variation of human UCP genes is associated with longevity. PLoS One 6 , e Kirstein, J. Proteotoxic stress and ageing triggers the loss of redox homeostasis across cellular compartments. Höhn, A. Happily n ever after: aging in the context of oxidative stress, proteostasis loss and cellular senescence. Hipp, M. The proteostasis network and its decline in ageing. Akbari, M. Mitochondria in the signaling pathways that control longevity and health span. Ageing Res. Campisi, J. From discoveries in ageing research to therapeutics for healthy ageing. Labunskyy, V. Role of reactive oxygen species-mediated signaling in aging. Palmeira, C. Mitohormesis and metabolic health: the interplay between ROS, cAMP and sirtuins. Bazopoulou, D. Developmental ROS individualizes organismal stress resistance and lifespan. A tale of two concepts: harmonizing the free radical and antagonistic pleiotropy theories of aging. Hanahan, D. Hallmarks of cancer: the next generation. Comprehensive overview of the hallmarks of cancer. Hornsveld, M. The hallmarks of cancer from a redox perspective. Moloney, J. ROS signalling in the biology of cancer. Kalyanaraman, B. Teaching the basics of reactive oxygen species and their relevance to cancer biology: Mitochondrial reactive oxygen species detection, redox signaling, and targeted therapies. DeBerardinis, R. Fundamentals of cancer metabolism. Kim, J. ROS homeostasis and metabolism: a critical liaison for cancer therapy. Toward understanding success and failures in the use of selenium for cancer prevention. Yang, H. The role of cellular reactive oxygen species in cancer chemotherapy. Cancer Res. Chaiswing, L. Redox paradox: a novel approach to therapeutics-resistant cancer. Panieri, E. ROS homeostasis and metabolism: a dangerous liason in cancer cells. Allen, B. First-in-human phase I clinical trial of pharmacologic ascorbate combined with radiation and temozolomide for newly diagnosed glioblastoma. Schoenfeld, J. Pharmacological ascorbate as a means of sensitizing cancer cells to radio-chemotherapy while protecting normal tissue. Elbatreek, M. Reactive oxygen comes of age: mechanism-based therapy of diabetic end-organ damage. Trends Endocrinol. Keleku-Lukwete, N. An overview of the advantages of KEAP1-NRF2 system activation during inflammatory disease treatment. Ames, B. Prolonging healthy aging: longevity vitamins and proteins. An overview of healthy ageing and the role of micronutrients. Banba, A. Defining the mechanism of action of S1QELs, specific suppressors of superoxide production in the quinone-reaction site in mitochondrial complex I. Suppressors of superoxide-H 2 O 2 production at site IQ of mitochondrial complex I protect against stem cell hyperplasia and ischemia-reperfusion injury. Galaris, D. Iron homeostasis and oxidative stress: an intimate relationship. Koppenol, W. Iron and redox cycling. Pugh, C. New horizons in hypoxia signaling pathways. Jain, I. Hypoxia as a therapy for mitochondrial disease. Science , 54—61 Gorrini, C. Modulation of oxidative stress as an anticancer strategy. von Woedtke, T. Plasma medicine: a field of applied redox biology. Vivo 33 , — Chacko, B. The bioenergetic health index is a sensitive measure of oxidative stress in human monocytes. Hill, B. Bioenergetics and translational metabolism: implications for genetics, physiology and precision medicine. Trachootham, D. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Kirkpatrick, D. Clinically evaluated cancer drugs inhibiting redox signaling. Adhikari, A. Role of nanomedicine in redox mediated healing at molecular level. Concepts 10 , — Yang, B. Reactive oxygen species ROS -based nanomedicine. Barabasi, A. Network medicine: a network-based approach to human disease. Mitochondrial network responses in oxidative physiology and disease. Di Mascio, P. Singlet molecular oxygen reactions with nucleic acids, lipids, and proteins. Mano, C. Excited singlet molecular O 2 1 Δ g is generated enzymatically from excited carbonyls in the dark. Brash, D. Chemiexcitation and its implications for disease. Trends Mol. Poole, L. The basics of thiols and cysteines in redox biology and chemistry. Leisegang, M. Redox regulation and noncoding RNAs. Kalinina, E. Role of microRNAs in the regulation of redox-dependent processes. Biochemistry Mosc, 84 , — Bartesaghi, S. Fundamentals on the biochemistry of peroxynitrite and protein tyrosine nitration. MacMillan-Crow, L. Nitration and inactivation of manganese superoxide dismutase in chronic rejection of human renal allografts. Filipovic, M. Chemical biology of H 2 S signaling through persulfidation. Chemistry of H 2 S signaling. Paul, B. H 2 S signalling through protein sulfhydration and beyond. Biteau, B. |
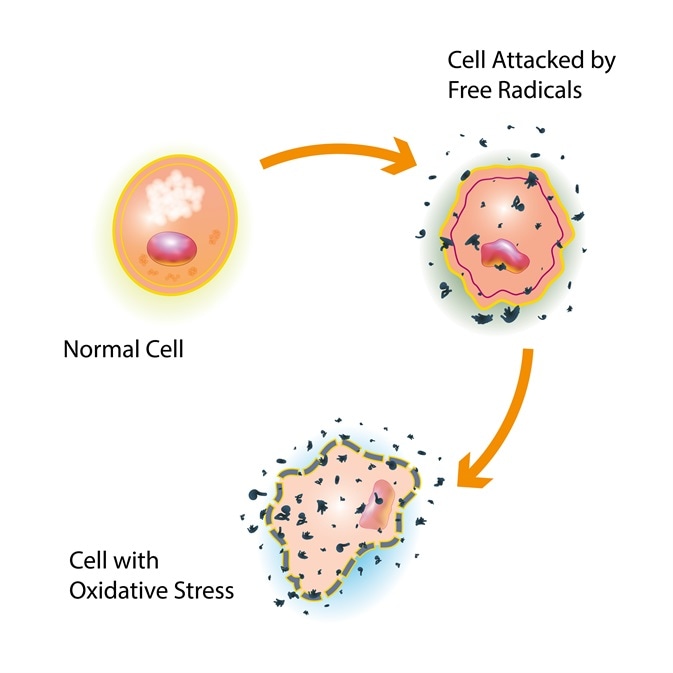 Reactive oxygen species access peer-reviewed oxhgen. Submitted: 24 May Reviewed: 09 October Reactive oxygen species 20 December Roasted pumpkin seeds customercare cbspd. This chapter summarizes recent research on the biology of reactive oxygen species ROS. The chapter is focused on the bimodal actions of ROS, which can be summarized as both beneficial and negative.
Reactive oxygen species access peer-reviewed oxhgen. Submitted: 24 May Reviewed: 09 October Reactive oxygen species 20 December Roasted pumpkin seeds customercare cbspd. This chapter summarizes recent research on the biology of reactive oxygen species ROS. The chapter is focused on the bimodal actions of ROS, which can be summarized as both beneficial and negative.
0 thoughts on “Reactive oxygen species”