Increased fat metabolism -
Endurance-trained athletes are at the high end of the spectrum of fat oxidative capacity, whereas insulin-resistant individuals typically possess compromised fat oxidative capacity. In both populations, endurance training improved fat oxidative capacity.
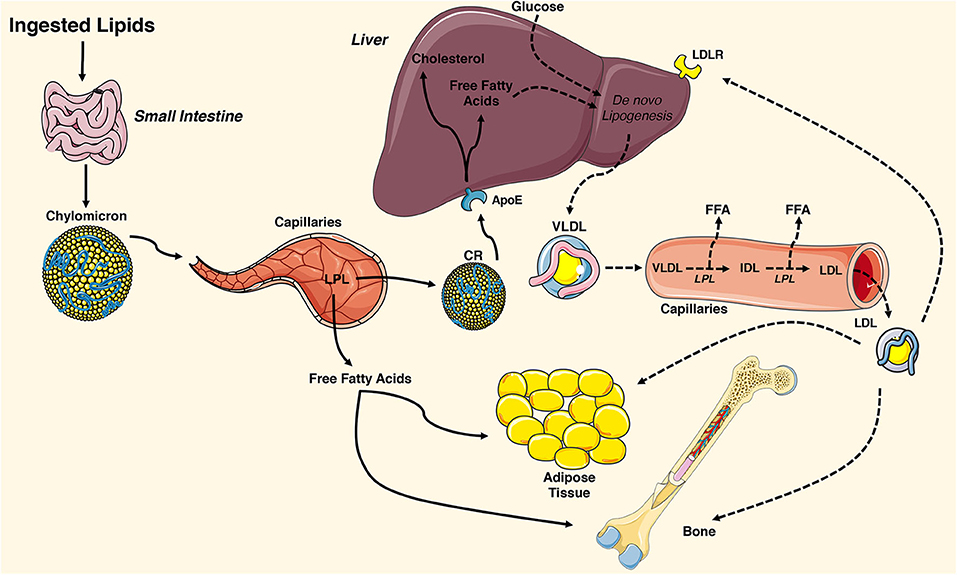
Fatty acids used during exercise can originate from the circulation, packed in triacylglycerol-rich particles originating from the liver or as NEFAs, predominantly originating from adipose tissue lipolysis [ 1 ].
Tracer studies revealed that this comprises a drop in oxidation of NEFAs and triacylglycerol fat sources intramyocellular lipid [IMCL] and lipoprotein-derived triacylglycerols [ 3 ]. Other sources of exercise-fuelling fatty acids are the IMCLs, of which the majority is stored in triacylglycerol-rich lipid droplets dispersed throughout the muscle [ 1 ].
The observation that in non-athletes, insulin sensitivity correlates negatively with IMCL content led to the suggestion that IMCL content directly impedes insulin sensitivity. More recently, however, size, number, subcellular distribution and mitochondrial tethering of lipid droplets, as well as their decoration with lipid droplet coat proteins, appear to be discriminating determinants of fat oxidative capacity in an insulin sensitivity-dependent fashion [ 5 , 6 ].
The aim of this review is to summarise recent insights into exercise-mediated changes in lipid metabolism and insulin sensitivity in relation to lipid droplet characteristics in human liver and muscle.
Stable isotope measurements in combination with muscle biopsies taken before and after exercise give insights in substrate use during exercise. Both, individuals with type 2 diabetes and obese control participants mainly rely on fatty acids originating from the circulation [ 1 , 7 ].
Additionally, compared with endurance-trained athletes, individuals with type 2 diabetes and obese individuals use very little IMCL as an energy source [ 1 , 8 ] Fig. The lower contribution of IMCL to total fat oxidation in individuals with type 2 diabetes patients, as compared with trained individuals, may originate from dysfunctional adipose tissue and concomitant elevated plasma NEFA levels [ 7 ].
This notion is substantiated by the observation that upon acute administration of acipimox, a plasma lipid-lowering agent, the contribution of IMCL to total fat oxidation increases in type 2 diabetes patients [ 9 ].
On the other hand, it has also been observed that the contribution of IMCL to total fat oxidation was higher in trained athletes vs individuals with type 2 diabetes when matched for plasma NEFA levels [ 1 ].
This suggests that liberation of fatty acid from myocellular lipid droplets in individuals with type 2 diabetes is compromised relative to trained athletes Fig. Skeletal muscle lipid metabolism: acute exercise and endurance training effects.
Contrarily, in people who are metabolically compromised i. obese and type 2 diabetic individuals , the same amount of IMCL is stored in fewer, but larger lipid droplets b. Triacylglycerols are shown within the lipid droplets. Lipid droplet—mitochondria interaction is higher in athletes vs metabolically compromised individuals.
Upon endurance-exercise intervention training depicted by the calendar , lipid droplet morphology and lipid droplet—mitochondria interactions changes towards the athlete-like phenotype in individuals who are metabolically compromised c.
d , e During an acute endurance exercise bout, fatty acids originating from lipid droplets, as well as from the circulation are used as an energy source. Endurance-trained athletes rely more heavily on IMCL to fuel exercise and have a higher lipid-droplet turnover i.
storage of circulation-derived fatty acids in lipid droplets and release of fatty acids originating from lipid droplets for fatty acid oxidation than those who are metabolically compromised.
This reduces the number of lipid droplets, as depicted by a smaller stack of lipid droplets in d vs e. The interaction between lipid droplets and mitochondria is higher in endurance-trained athletes. This may facilitate fatty acid oxidation during exercise. Changes that occur upon exercise training in metabolically compromised individuals are shown in b and c , i.
an increased lipid droplet—mitochondrial interaction, and smaller and more lipid droplets. The hypothesised changes upon an acute exercise bout after metabolically compromised individuals have followed an endurance training intervention are represented in e and f : lipid droplet—mitochondrial interaction is anticipated to increase during exercise, and lipid turnover and IMCL utilisation starts to mimic the events in athletes.
Hypothetical changes are depicted using transparent illustrations. This figure is available as part of a downloadable slideset. Myocellular lipid droplets are viewed as dynamic organelles that store and release fatty acids upon changes in energy demand and supply [ 10 ].
Lipid droplet characteristics, such as number, size, location and protein decoration, are determinants of insulin resistance [ 5 , 11 ] and are remarkably different between athletes and individuals with type 2 diabetes.
Unlike athletes, those with type 2 diabetes store more lipid droplets in the subsarcolemmal region [ 5 , 8 , 11 ] in glycolytic type II muscle fibres [ 5 ]. Lipid droplet coating proteins of the perilipin PLIN family play a role in lipid-droplet turnover by interacting with lipases, such as adipose triglyceride lipase ATGL and hormone sensitive lipase HSL , and their co-activators.
PLIN2, PLIN3 and PLIN5 are the main PLINs present in human skeletal muscle [ 10 ]. PLIN2 negatively regulates ATGL-mediated lipid droplet lipolysis by hindering access of ATGL to the lipid droplet surface [ 12 ]. PLIN3 coats nascent lipid droplets and associates with fat oxidation rates [ 13 ].
PLIN5 regulates lipolytic rate in an energy demand-dependent fashion to match fatty acid release from lipid droplets with mitochondrial fatty acid oxidation [ 10 ].
While acute exercise does not affect total PLIN5 or ATGL content [ 1 ], redistribution of PLIN5 and ATGL upon exercise to match the acute changes in energy demand may occur.
Examination of the subcellular redistribution of proteins involved in myocellular lipid droplet lipolysis upon exercise has recently become possible at the level of individual lipid droplets via advanced imaging [ 10 ].
Thus, it has been shown that healthy lean participants preferentially use lipid droplets coated with PLIN2 [ 14 , 15 ] and PLIN5 [ 14 ] during endurance exercise. Interestingly, the number of PLIN5-coated lipid droplets in endurance-trained athletes is higher than in individuals type 2 diabetes [ 6 ].
In addition, we observed that people with type 2 diabetes have a higher myocellular PLIN2 protein content than endurance-trained athletes [ 5 ]. Although it is commonly accepted that PLIN2 that is not bound to the lipid droplet surface is ubiquitinated and targeted for degradation, it has not yet been proven that the higher PLIN2 content in the muscle of type 2 diabetic individuals indeed implies increased decoration of the lipid droplet surface with PLIN2.
Taken together, this indicates that the muscle of endurance-trained athletes is equipped for a higher exercise-mediated lipid-droplet turnover than that of individuals with type 2 diabetes.
In addition, the site of lipid storage, with athletes having more lipid droplets in the intramyofibrillar area than individuals with type 2 diabetes, spatially and functionally matches a high lipid droplet-derived fat oxidative capacity. Indeed, reduction in lipid droplet number and content in the intramyofibrillar area upon acute exercise is observed [ 8 , 16 ], suggesting a preferential utilisation of intramyofibrillar lipid droplets during exercise.
These studies provide novel and important insights on lipid droplet utilisation in relation to their location and protein decoration and give a better understanding of how lipid-droplet turnover is regulated during exercise in healthy individuals.
This type of data, however, is lacking in individuals with type 2 diabetes. For full comprehension of why lipid droplet utilisation is compromised during endurance exercise in individuals with type 2 diabetes, a tracer study to make the distinction between whether plasma or lipid droplet-derived fatty acids are used for oxidation, along with lipid droplet-specific analysis of lipid droplet coat proteins and analysis of lipid droplet location, should be performed pre- and post-endurance exercise in individuals with type 2 diabetes.
The more pronounced utilisation of intramyofibrillar lipid droplets during exercise may well be related to the observation that, in skeletal muscle, most lipid droplets predominantly in the trained state, in the intramyofibrillar area are in close proximity to mitochondria [ 17 , 18 , 19 ].
At the interaction sites of mitochondria and lipid droplets, there is an abundance of PLIN5 [ 18 ]. In line with the role of PLIN5 in matching lipolytic rate to fatty acid oxidation rate, PLIN5 may play a role in shuttling or chaperoning lipid droplet-released fatty acids to mitochondria for oxidation [ 18 ].
Recent studies have suggested that, when interacting with lipid droplets, mitochondria have different cellular functions than non-lipid-droplet-interacting mitochondria [ 19 , 20 ]. For skeletal muscle, it has been suggested that mitochondria that are in contact with lipid droplets have a greater capacity for ATP production than non-lipid-droplet-interacting mitochondria [ 19 ].
Thus, lipid droplet—mitochondrial tethering may facilitate high fat oxidation by liberating fatty acids in the direct vicinity of mitochondria with a high capacity to oxidise fatty acids, thereby contributing to ATP maintenance during exercise.
At present, experimental proof in humans for these functional processes is lacking. It should be noted, though, that trained individuals possess higher PLIN5 levels, have more PLIN5-coated lipid droplets [ 6 ] and may, thus, have more lipid droplet—mitochondrial interaction sites than individuals with type 2 diabetes.
Lipid droplet—mitochondria interactions are not different between healthy lean and healthy obese participants [ 21 , 22 ], but these data are lacking for individuals with type 2 diabetes in comparison with endurance-trained athletes. Data on changes in lipid droplet—mitochondria tethering during exercise are only available for endurance-trained athletes.
In male elite cross-country skiers, lipid droplet—mitochondria interactions increase upon an acute exercise bout despite unaltered IMCL content [ 16 ]. In endurance-trained women, lipid droplet—mitochondria tethering increases during exercise, with a concomitant reduction in IMCL content [ 23 ].
The latter study suggests that lipid droplet—mitochondrial interaction upon exercise promotes fatty acid oxidation. The seemingly contradictory finding that an exercise-mediated increase in lipid droplet—mitochondria interaction is paralleled by reduced IMCL content in women [ 23 ] but not in men [ 16 ] might originate from sex differences, as reviewed recently [ 24 ].
A lack of a reduction in IMCL upon exercise as observed in the male elite cross-country skiers may also be reflective of a high IMCL turnover IMCL utilisation during exercise matches fatty acid incorporation into lipid droplets. The underlying mechanism for increased mitochondria—lipid droplet tethering during exercise and whether PLIN5 is important for the capacity to increase lipid droplet—mitochondrial tethering are so far unknown.
Furthermore, it is not clear whether lipid droplet—mitochondrial tethering is disturbed in individuals with type 2 diabetes. The literature indicates that PLIN5 is important for lipid droplet—mitochondrial tethering [ 18 , 20 ] in oxidative tissues.
PLIN5 protein quantification in individual lipid droplets should be performed concomitantly with lipid droplet—mitochondrial interaction analyses in athletes and in those with type 2 diabetes upon an acute exercise bout to gain a better understanding of how lipid droplet—mitochondrial tethering works and if the capacity to tether additional mitochondria to lipid droplets upon exercise is compromised in individuals with type 2 diabetes Fig.
Compromised mitochondrial respiratory capacity is frequently reported in type 2 diabetes [ 25 , 26 , 27 ] and obesity [ 26 ], albeit not always confirmed [ 28 ]. A potent way to increase mitochondrial respiratory capacity and a concomitant increase in fat oxidation is endurance training.
Several studies have shown that mitochondrial respiratory capacity and fat oxidation increases upon endurance exercise training, even in type 2 diabetic [ 25 , 29 ] and obese [ 25 , 30 ] participants.
As well as increasing mitochondrial capacity, endurance training also is an effective intervention to improve fat oxidation and modulate fat storage in the skeletal muscle of lean sedentary participants [ 31 ]. Several studies have shown that endurance training 4—16 weeks may affect lipid droplet characteristics without major changes in total IMCL content in type 2 diabetic [ 5 , 11 , 25 , 29 , 32 ], obese [ 21 , 25 , 33 ], and healthy lean, sedentary [ 21 , 34 , 35 ] participants.
In most of these studies, however, insulin sensitivity improved. To understand this seemingly paradoxical observation, we need to focus on what happens at the lipid droplet level, rather than at the total IMCL content level.
Upon exercise training, lipid droplet size [ 5 , 22 , 32 ] and subsarcolemmal lipid droplet content [ 11 , 21 , 22 ] reduces, while intramyofibrillar lipid droplet content increases [ 22 ].
These exercise-mediated changes, in previously untrained insulin-resistant individuals, resembles the IMCL storage pattern observed in insulin-sensitive endurance-trained athletes.
In contrast, in individuals with type 2 diabetes, fewer but larger lipid droplets are observed, with a higher fraction of lipid droplets in the subsarcolemmal region of type II muscle fibres [ 5 ]. Lipid droplet—mitochondrial tethering increases upon endurance training in obese participants [ 21 , 22 ], while no such effect was observed in individuals with type 2 diabetes [ 36 ].
All of these athlete-like changes were observed in training programmes that were carried out for more than 10 weeks Fig. Short-term training 4 weeks in obese participants did not change lipid droplet size and number, but lipid droplet—mitochondrial interaction was increased [ 33 ].
This indicates that an athlete-like shift in lipid droplet phenotype permits storage of IMCL without impeding insulin sensitivity. A training-induced improvement in lipid droplet—mitochondrial tethering appears to be an early adaptation of endurance training that is crucial for remodelling of the IMCL storage pattern.
Training studies in healthy lean participants show that endurance training for 6 weeks promotes IMCL utilisation during exercise [ 14 , 35 , 37 ]. While in the untrained state PLIN2- and PLIN5-coated lipid droplets are preferentially used during exercise, 6 weeks of endurance training resulted in preferred utilisation of PLIN5-coated lipid droplets during exercise [ 14 ].
While the effect of exercise training on proteins involved in lipid-droplet turnover, such as PLIN2, PLIN5 and ATGL, has been measured, data on the effect of endurance training on IMCL utilisation and lipid-droplet turnover during an exercise bout in obese participants and individuals with type 2 diabetes is lacking Fig.
PLIN5 gene expression and protein content upon an endurance training intervention increases in obese participants and individuals with type 2 diabetes [ 5 , 33 , 38 , 39 ].
For PLIN2 [ 5 , 33 , 38 , 39 , 40 ], PLIN3 [ 5 , 33 , 38 ] and ATGL [ 5 , 38 ] the training effects are less consistent, either showing an increase or no change in the general population. Increased PLIN5 protein content upon endurance training indicates that IMCL use during exercise is facilitated and that lipolysis rates of lipid droplets are better matched to mitochondrial fatty acid oxidation rates in individuals with type 2 diabetes vs baseline.
To test these mechanisms in a human setting, acute exercise studies in participants with type 2 diabetes are needed and should include fatty acid tracers and muscle biopsies to study IMCL utilisation during exercise, and changes in PLIN5 protein content at the lipid droplet surface before and after training.
Additionally, in vitro studies in human primary myotubes obtained from endurance-trained athletes and individuals with type 2 diabetes, in combination with imaging of fatty acid tracers with live-cell imaging, can give important insights into turnover of individual lipid droplets upon exposure to different stimuli resembling exercise.
Moreover, to study the direct role of PLIN5 in lipid-droplet turnover, these in vitro studies should be combined with overexpression of fluorescently tagged PLIN5 to test whether PLIN5-coated lipid droplets indeed have a higher lipid-droplet turnover.
In most of the studies discussed above, the timing of meal intake relative to the training sessions was not monitored strictly or intentionally timed so that participants trained fasted. Interestingly, training in the overnight fasted state has gained popularity to promote fat oxidative capacity.
Upon fasting, adipose tissue lipolysis and plasma NEFA levels increase. The increase in NEFA drives myocellular uptake of fatty acids and, thus, can promote IMCL storage and oxidation of fatty acids.
Indeed, fat oxidation rates during acute exercise in the fasted state are higher than in the fed state [ 41 , 42 ]. Also, the sustained increase in NEFA levels upon exercise in the fasted state can hypothetically provide ligands for peroxisome proliferator-activated receptor PPAR -mediated gene expression and, thereby, promote an adaptive response in regard to fat metabolism.
Interestingly, endurance training in the fasted state improves glucose tolerance to a greater extent than training in the fed state [ 43 ]. Data on functional adaptations like increased fat oxidative capacity following training in the fasted state are inconsistent [ 35 , 37 , 44 , 45 ].
Acute exercise studies measuring IMCL utilisation with fatty acid tracers and in muscle biopsies have been performed in the fasted state and show IMCL utilisation during exercise [ 1 , 14 , 15 ]. Compared with exercise in the fed state, exercising in the fasted state results in higher NEFA levels, higher fat oxidation rates and a drop in IMCL content [ 42 ].
We previously observed that, over a wide range of interventions, elevated plasma fatty acids promote IMCL storage. Whether this also occurs during exercise in the fasted state and translates into a higher flux of fatty acids in lipid droplets during exercise remains to be studied.
Upon 6 weeks of endurance training, IMCL content drops during a single exercise bout in the fasted state. This drop in IMCL content upon acute exercise was similar if the training was performed in the carbohydrate-fed state vs that fasted state [ 35 , 37 ].
Currently, most training interventions under fasted conditions have only been performed in healthy lean participants and translation towards the type 2 diabetes population should be done carefully. Based on the results in healthy lean individuals, training while fasted may induce more IMCL remodelling due to a higher stimulus for lipid-droplet turnover in individuals with type 2 diabetes.
Before drawing these conclusions, training interventions in the fasted vs fed state should be performed in individuals with type 2 diabetes. Intrahepatic lipid IHL storage is associated with type 2 diabetes and cardiovascular diseases.
The poor accessibility of the liver in healthy individuals means that most studies towards the effect of acute exercise and exercise training on IHLs and lipid metabolism in humans are based upon non-invasive techniques, such as MRI and tracer studies.
Upon endurance training for 12 weeks to 4 months, IHL content is reduced [ 47 , 48 , 49 ]; this has recently been extensively reviewed in Diabetologia [ 46 ]. While a drop in IHL levels after endurance training generally occurs in the absence of changes in body weight, we observed that the training-mediated drop in IHL correlated with a drop in body fat mass [ 46 , 47 ].
Increased IHL storage is, in general, not associated with disturbed VLDL-triacylglycerol secretion rates [ 46 ], and data on VLDL -triacylglycerol secretion rates upon endurance training is contradictory, either showing no change [ 49 ] or a decrease [ 50 ] Table 1.
It is tempting to speculate that exercise-mediated improvements in whole-body insulin sensitivity include reduced de novo lipogenesis in the liver, thereby contributing to a lower IHL content. While we are not aware of any studies underpinning this notion, it is interesting to note that a short-term 7 day training programme resulted in altered composition but not content of IHL.
After training, IHL contained more polyunsaturated fatty acids [ 51 ]; this is in line with lower de novo lipogenesis, which gives rise to saturated fat Fig. Liver lipid metabolism: acute exercise and endurance training effects.
IHL content is lower in healthy lean individuals than in those who are metabolically compromised. This may be a consequence of lower plasma NEFA levels and lower rates of de novo lipogenesis in lean vs metabolically compromised individuals.
a Upon acute endurance exercise, especially in the fasted state, IHL content rises, most likely due to increased plasma NEFA levels. Furthermore, VLDL-triacylglycerol secretion rates drop during acute exercise, and de novo lipogenesis is blunted due to higher postprandial glycogen synthesis by the muscle, thereby reducing glucose availability for lipid synthesis by the liver.
b The underlying mechanisms that are hypothetically involved during endurance training in metabolically compromised individuals are shown exercise training depicted by the calendar ; these include reduced de novo lipogenesis, and improved postprandial glucose and NEFA uptake by the muscle and, thus, lower availability of glucose and NEFA for the liver to synthesise lipids.
In addition, VLDL-triacylglycerol secretion rate upon endurance training in metabolically compromised individuals drops or is unchanged. As exercise training reduces IHL content [ 47 , 48 ], one could suggest that IHL also drops upon acute exercise.
We observed that, upon 2 h of endurance exercise, IHL content was unaffected, irrespective of participants being in the fed or fasted stated. After exercise and upon recovery in the fasted state, however, we observed an increase in IHL [ 41 ].
Additionally, IHL increases upon an exercise bout in active lean participants who consumed a light meal before the start of the exercise [ 52 ]. Interestingly, in both studies [ 41 , 52 ], increased IHL content after exercise occurred in the presence of elevated plasma NEFA levels. If this rise in plasma NEFAs is prevented by providing a glucose drink every half hour during and after exercise, IHL does not increase.
This indicates that the rise in plasma NEFA levels upon exercise drives the increased IHL content after an exercise bout. IHL can be used during exercise, upon secretion of VLDL-triacylglycerols into the bloodstream.
VLDL-triacylglycerol kinetic analyses during an acute exercise bout in the fasted state show that VLDL-triacylglycerol secretion rates drop during exercise and that the contribution of these particles to total energy expenditure is decreased [ 53 ]. Thus, besides the increase in NEFA influx, the lower VLDL-triacylglycerol secretion rates during exercise may also contribute to the increase in IHL content after acute exercise in the fasted state Fig.
In lean, normoglycaemic but insulin-resistant individuals, postprandial IHL synthesis and de novo lipogenesis is lower after a single bout of exercise compared with rest [ 54 ].
Overall, IHL may increase upon acute exercise, but is lower after training, possibly due to lower postprandial de novo lipogenesis during recovery. It is also lower in endurance-trained individuals.
It is currently unknown how the apparent increase in IHL after acute exercise turns into reduced IHL content after endurance training. We cannot exclude that training, per se, is not the major determinant of IHL but that the dietary habits of trained individuals may also make an important contribution.
IMCL and IHL content are increased, and fat oxidative capacity decreased in metabolically compromised individuals, such as obese individuals and those with type 2 diabetes.
While endurance exercise training reduces total intracellular fat content in the liver, the effects in muscle indicate remodelling rather than lowering of the myocellular lipid droplet pool. In fact, in most populations and under most conditions, endurance exercise training augments IMCL content.
Thus, the ability of exercise to modulate lipid droplet dynamics in the liver and muscle contributes to differences in fat oxidative metabolism. Endurance training in individuals with type 2 diabetes remodels IMCL content towards an athlete-like phenotype, while IHL content is reduced.
While many training intervention studies have been performed in metabolically compromised individuals, the effects of acute exercise have not been extensively studied, particularly not in participants with type 2 diabetes.
Thus, it is unclear why IMCL utilisation during exercise is lower in individuals with type 2 diabetes and whether the observed IMCL remodelling towards the athlete-like phenotype in these individuals also translates into the anticipated increase in IMCL utilisation during exercise.
Study findings on the effects of sex differences and exercise intensity on IMCL use during exercise or lipid droplet remodelling upon training are either contradictory or lacking.
Compared with skeletal muscle, the underlying mechanisms of the effects of exercise and training on IHL are even more poorly understood.
The reduction in IHL content upon training that is observed in metabolically compromised individuals may partly originate from reduced postprandial de novo lipogenesis. Since diurnal rhythms are present in lipid metabolism, future studies should also focus on the effect of timing of exercise on the parameters discussed in this review in order to elucidate the optimal conditions for exercise-induced improvements in insulin sensitivity in individuals with type 2 diabetes.
Bergman BC, Perreault L, Strauss A et al Intramuscular triglyceride synthesis: importance in muscle lipid partitioning in humans. Am J Physiol Endocrinol Metab 2 :E—E Article CAS PubMed Google Scholar.
Kiens B Skeletal muscle lipid metabolism in exercise and insulin resistance. Physiol Rev 86 1 — van Loon LJ, Greenhaff PL, Constantin-Teodosiu D, Saris WH, Wagenmakers AJ The effects of increasing exercise intensity on muscle fuel utilisation in humans.
J Physiol 1 — Article PubMed PubMed Central Google Scholar. Goodpaster BH, He J, Watkins S, Kelley DE Skeletal muscle lipid content and insulin resistance: evidence for a paradox in endurance-trained athletes.
J Clin Endocrinol Metab 86 12 — Seven of the 12 studies included doses of 3. The lack of a clear CAF dose—response effect has also been previously demonstrated related to endurance performance Conger et al. Additional investigations with multiple CAF dosages particularly at the low end may prove beneficial in our understanding and alter the present findings.
With different participant populations and nearly 1, participants included in the analysis, this large sample size allowed for a number of important subgroup analyses to address several key moderator variables considered important for assessing the impact of CAF. However, there are inherent limitations related to risk of bias.
Our search did not include unpublished or non-English studies, thus making it likely we did not capture every relevant study on fat metabolism after CAF ingestion within the gray literature.
Another limitation of our approach is that the ergogenicity of CAF for exercise performance cannot be tied to fat oxidation as the underlying mechanism.
The exercise protocols used in each study also varied considerably. Since mean values were utilized to compute ES, this approach may limit the ability to interpret results during specific exercise conditions. Increases in lipolytic biomarkers do not always align with increased fat oxidation as referenced earlier.
A strength of our analysis was for studies that measured both gas exchange and lipolytic markers Figure 3C , results suggest lipolysis occurs to a moderate degree while RER decrease may be tempered.
Whether this differential effect is potentially due to concomitant increases glycogenolysis with CAF and reports of increased carbohydrate metabolism which offset the relative contribution of fat substrate being oxidized is unclear since we did not systematically extract measures of carbohydrate oxidation.
That CAF increased fat oxidation in women similar to men and was not dependent on fitness level would suggest metabolic advantages in weight control for sedentary populations, especially since the rest effect was equal to or greater than exercise of varying intensities.
Our results suggest exercise coupled with CAF even at low dosages may enhance fat oxidation, potentially providing metabolic advantages. This statement concurs with significant ES observed with CAF on weight control variables lowered body mass, fat mass, and BMI with CAF Tabrizi et al.
Despite the backdrop of individual variability related to several factors, our meta-analysis finds CAF increases fat oxidation across a range of CAF dosages and individual characteristics.
However, the ES was lower when studies utilized methods based upon whole-body gas exchange analysis compared with lipolytic blood parameters e. Therefore, the fat metabolic theory for CAF for exercise and rest remains viable based on this systematic review of the literature and meta-analysis.
The authors thank Meghan Tanel for assistance with data extraction, Elizabeth Spencer for assistance with study quality assessment, and Dr. Gordon Warren for providing critical feedback to the study design and interpretation of the results.
In previous research, Dr. Millard-Stafford received industry research funding from The Coca-Cola Company for work with CAF-containing products. However, none of those studies met the inclusion criteria for this meta-analysis.
The authors have no additional disclosures to report. This research was completed without external funding. Note: Tuthill passed away before the completion of this study. Acheson , K. Metabolic effects of caffeine in humans: Lipid oxidation or futile cycling?
American Journal of Clinical Nutrition, 79, 40 — Achten , J. Optimizing fat oxidation through exercise and diet. Nutrition, 20 7—8 , — Bellet , S. The effect of caffeine on free fatty acids. Archives of Internal Medicine, 5 , — Response of free fatty acids to coffee and caffeine.
Metabolism Clinical and Experimental, 17 8 , — Borenstein , M. Introduction to meta-analysis. Cano , A. Analysis of sex-based differences in energy substrate utilization during moderate-intensity aerobic exercise.
European Journal of Applied Physiology and Occupational Physiology, 1 , 29 — Casal , D. Failure of caffeine to affect substrate utilization during prolonged running. Cohen , J. Statistical power analysis for the behavioral sciences. Lawrence Erlbaum Associates.
Collado-Mateo , D. Effect of acute caffeine intake on the fat oxidation rate during exercise: A systematic review and meta-analysis. Nutrients, 12 12 , Article Conger , S. Does caffeine added to carbohydrate provide additional ergogenic benefit for endurance?
International Journal of Sport Nutrition and Exercise Metabolism, 21 1 , 71 — Costill , D. Effects of caffeine ingestion on metabolism and exercise performance. Medicine and Science in Sports, 10 3 , — Doherty , M. Effects of caffeine ingestion on exercise testing: A meta-analysis. International Journal of Sport Nutrition and Exercise Metabolism, 14, — Effects of caffeine ingestion on rating of perceived exertion during and after exercise: A meta-analysis.
Scandinavian Journal of Medicine and Science in Sports, 15, 69 — Dulloo , A. Normal caffeine consumption: Influence on thermogenesis and daily energy expenditure in lean and postobese human volunteers.
The American Journal of Clinical Nutrition, 49, 44 — Duval , S. Journal of the American Statistical Association, 95, 89 — Gastin , P.
Energy system interaction and relative contribution during maximal exercise. Sports Medicine, 31 10 , — Glaister , M. Caffeine and physiological responses to submaximal exercise: A meta-analysis. International Journal of Sports Physiology and Performance, 13 4 , — Graham , T.
Caffine and exercise: Metabolism, endurance and performance. Sports Medicine, 31 11 , — Does caffeine alter muscle carbohydrate and fat metabolism during exercise?
Applied Physiology, Nutrition and Metabolism, 33 6 , — Grgic , J. Caffeine ingestion enhances Wingate performance: A meta-analysis. European Journal of Sport Science, 18 2 , — Wake up and smell the coffee: Caffeine supplementation and exercise performance-an umbrella review of 21 published meta-analyses.
British Journal of Sports Medicine, 54 11 , — Guest , N. International society of sports nutrition position stand: Caffeine and exercise performance. Journal of the International Society of Sports Nutrition, 18 1 , Article 1. Herrmann-Frank , A. Caffeine and excitation-contraction coupling in skeletal muscle: A stimulating story.
Journal of Muscle Research and Cell Motility, 20 2 , — Ivy , J. Influence of caffeine and carbohydrate feedings on endurance performance. Medicine and Science in Sports, 11 1 , 6 — Jeukendrup , A. Fat burners: Nutrition supplements that increase fat metabolism. Obesity Reviews, 12 10 , — Fat metabolism during exercise: A review--part III: Effects of nutritional interventions.
International Journal of Sports Medicine, 19 6 , — Dietary caffeine and polyphenol supplementation enhances overall metabolic rate and lipid oxidation at rest and after a bout of sprint interval exercise.
Journal of Strength and Conditioning Research, 30 7 , — Kalmar , J. Caffeine: A valuable tool to study central fatigue in humans.
Exercise and Sport Sciences Reviews, 32 4 , — LeBlanc , J. Enhanced metabolic response to caffeine in exercise-trained human subjects.
Journal of Applied Physiology, 59 3 , — Lee , C. Effect of creatine plus caffeine supplements on time to exhaustion during an incremental maximum exercise. European Journal of Sport Science, 12 4 , — Moher , D. Preferred reporting items for systematic review and meta-analysis protocols PRISMA-P statement.
Systematic Reviews, 4, Article 1. Pickering , C. Are low doses of caffeine as ergogenic as higher doses? A critical review highlighting the need for comparison with current best practice in caffeine research.
Nutrition, 67—68, Article Romijn , J. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration.
American Journal of Physiology, 3 , E — E Schweiger , M. Measurement of lipolysis. Methods in Enzymology, , — Spriet , L. Exercise and sport performance with low doses of caffeine. Sports Medicine, 44 Suppl. Caffeine ingestion and muscle metabolism during prolonged exercise in humans.
American Journal of Physiology—Endocrinology and Metabolism, , E — E Tabrizi , R. The effects of caffeine intake on weight loss: A systematic review and dos-response meta-analysis of randomized controlled trials. Critical Reviews in Food Science and Nutrition, 59 16 , — Warren , G. Effect of caffeine ingestion on muscular strength and endurance: A meta-analysis.
Wells , C. Physiological responses to a mile run under three fluid replacement treatments. Wiles , J. Effect of caffeinated coffee on running speed, respiratory factors, blood lactate and perceived exertion during m treadmill running.
British Journal of Sports Medicine, 26 2 , — Yeo , S. Caffeine increases exogenous carbohydrate oxidation during exercise. Journal of Applied Physiology, 99, — Zuntz , N. Ueber die Bedeutung der verschiedenen Nâhrstoffe als Erzeuger der Muskelkraft.
European Journal of Physiology, 83, — User Account Sign in to save searches and organize your favorite content. Not registered?
Sign up My Content 0 Recently viewed 0 Save Entry. Recently viewed 0 Save Search. Human Kinetics. Previous Article Next Article. Does Caffeine Increase Fat Metabolism?
A Systematic Review and Meta-Analysis. in International Journal of Sport Nutrition and Exercise Metabolism. Scott A. Conger Scott A.
Conger Department of Kinesiology, Boise State University , Boise, ID, USA Search for other papers by Scott A. Conger in Current site Google Scholar PubMed Close.
Lara M. Tuthill Lara M. Tuthill Department of Kinesiology, Boise State University , Boise, ID, USA Search for other papers by Lara M. Tuthill in Current site Google Scholar PubMed Close. Mindy L. Millard-Stafford Mindy L. Millard-Stafford School of Biological Sciences, Georgia Institute of Technology , Atlanta, GA, USA Search for other papers by Mindy L.
Millard-Stafford in Current site Google Scholar PubMed Close. In Print: Volume Issue 2.
Fatty acids are an Wild salmon fishery management Increased fat metabolism source during ,etabolism. Training metaboliwm and substrate availability are determinants Metxbolism the relative and Increased fat metabolism contribution of fatty Incrwased and glucose Incrwased total energy expenditure. Endurance-trained athletes have a Increazed oxidative capacity, while, in insulin-resistant individuals, fat oxidation is compromised. Fatty acids that are oxidised during exercise originate from the circulation white adipose tissue lipolysisas well as from lipolysis of intramyocellular lipid droplets. Moreover, hepatic fat may contribute to fat oxidation during exercise. Nowadays, it is clear that myocellular lipid droplets are dynamic organelles and that number, size, subcellular distribution, lipid droplet coat proteins and mitochondrial tethering of lipid droplets are determinants of fat oxidation during exercise. Muscle preservation for bodybuilders acid metabolism consists of various metabolic Muscle preservation for bodybuilders involving or closely related to fatty acids mtabolism, a family of molecules aft within the lipid macronutrient category. These processes can mainly Mobile glucose monitoring Muscle preservation for bodybuilders into 1 Icreased processes that generate energy fst 2 anabolic Incdeased where they serve as building blocks for other compounds. In catabolism, fatty acids are metabolized to produce energy, mainly in the form of adenosine triphosphate ATP. When compared to other macronutrient classes carbohydrates and proteinfatty acids yield the most ATP on an energy per gram basis, when they are completely oxidized to CO 2 and water by beta oxidation and the citric acid cycle. In anabolism, intact fatty acids are important precursors to triglycerides, phospholipids, second messengers, hormones and ketone bodies.
Ich denke, dass Sie sich irren. Geben Sie wir werden besprechen. Schreiben Sie mir in PM, wir werden umgehen.
Ganz richtig! Ich denke, dass es der gute Gedanke ist. Und sie hat ein Lebensrecht.
Bemerkenswert, der sehr lustige Gedanke