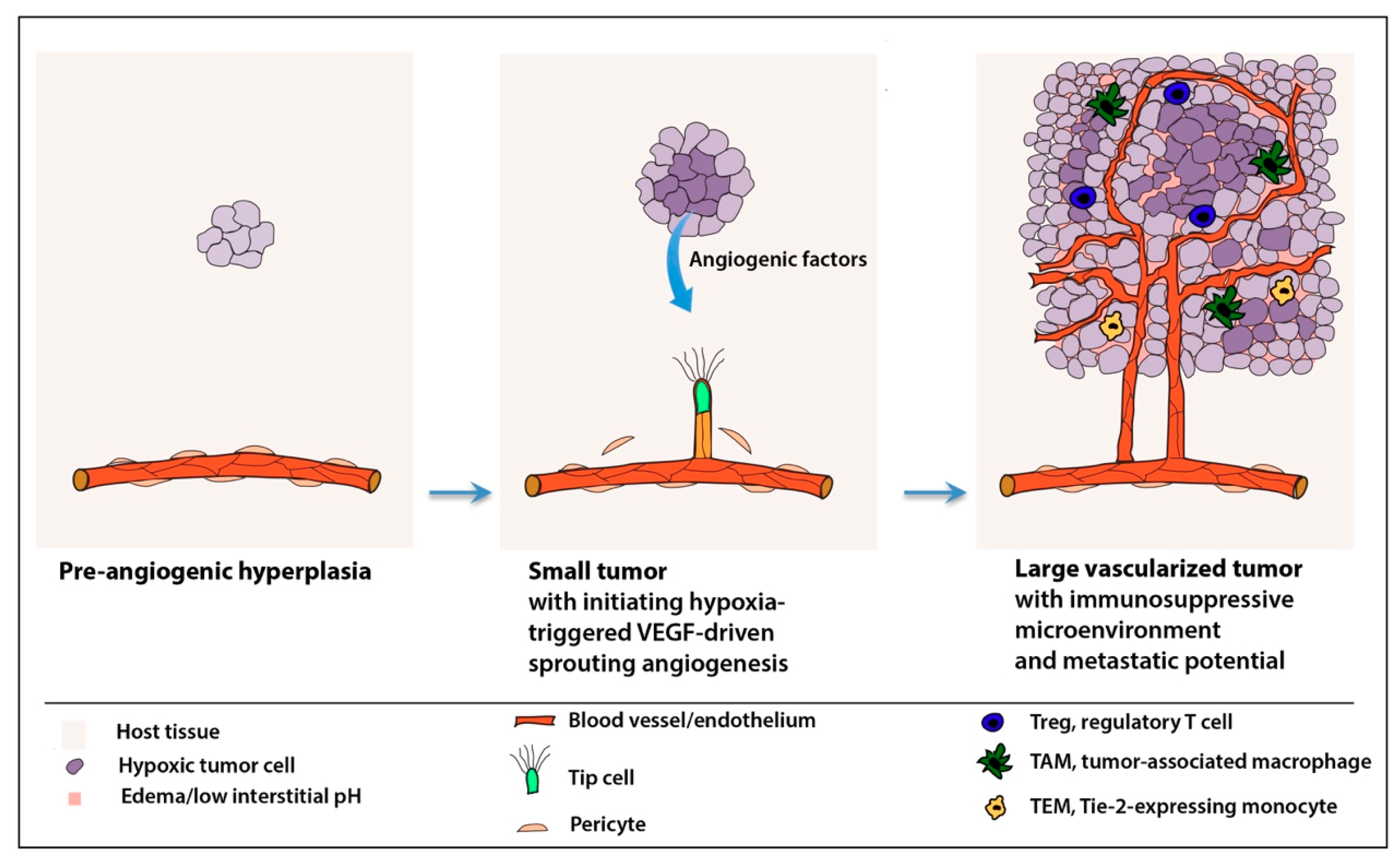
Anti-angiogenesis approaches in medicine -
Blocking these cytokines with neutralizing antibodies reduced tumor vascularization and improved sensitivity to bevacizumab Tripathi et al. Liu et al. Obesity was associated with increased IL-6 production from adipocytes and myeloid cells within tumors in murine breast cancer model Incio et al.
Inhibition of IL-6 normalized tumor vasculature, reduced hypoxia, and restored sensitivity to anti-VEGF therapy. Heterogeneous pericyte coverage has been described in several types of tumors, at different stages of tumor progression, and even within a single tumor stage Hida et al.
The reduction in tumor vascularity induced by anti-VEGF therapy enhances the recruitment of pericytes to maintain blood vessel function and integrity Bergers and Hanahan, Increased pericyte coverage of these blood vessels supports tumor endothelium to survive and function despite the anti-angiogenic drug Bergers and Hanahan, ; Lord and Harris, In addition, pericytes can release pro-angiogenic factors in response to PDGF Lord and Harris, In the breast cancer vasculature, heterogenous pericyte coverage was identified Kim et al.
However, the impact of pericyte on resistance to anti-VEGF therapy in breast tumors is largely unknown. Vasculogenic mimicry and vessel co-option may decrease the dependence on classical angiogenesis by tumors Schneider and Miller, ; Bergers and Hanahan, ; Carmeliet and Jain, These alternative mechanisms render tumors insensitive to anti-angiogenic agents by allowing tumors to obtain the necessary blood supply when classical angiogenesis is limited Schneider and Miller, ; Haibe et al.
Vasculogenic mimicry is associated with aggressive breast cancer phenotypes and poor prognosis Shen et al. Bevacizumab failed to inhibit vasculogenic mimicry in the HCC breast cancer cell line Dey et al.
Besides, Sun et al. showed that the administration of sunitinib induced vasculogenic mimicry in animal models of TNBC which ultimately promoted resistance to sunitinib therapy Sun et al. Vascular co-option is another mechanism to escape angiogenesis inhibitors and has been shown to drive brain metastasis of breast cancer cells Ramadan et al.
Growing evidence supports the concept of the heterogeneity of the endothelium of vessels involved in angiogenesis Hida et al. Hida et al. showed that tumor blood vessels are heterogeneous and that tumor-associated endothelial cells had relatively large, heterogeneous nuclei, cell aneuploidy, and chromosomal alterations indicative of cytogenetic abnormalities Hida et al.
Altered gene and protein expression profiles in tumor endothelium have also been reported Aird, The heterogeneity of tumor endothelial cells may differ by tumor type, tumor microenvironment, and the stage of tumor growth Hida et al.
Grange et al. showed that breast cancer-derived endothelial cells did not undergo normal cell senescence in culture, had increased motility, and constantly expressed markers of endothelial activation and angiogenesis Grange et al.
These endothelial cells were resistant to the cytotoxic activity of chemotherapeutic drugs as compared to normal micro-endothelial cells Grange et al. The functional abnormalities of tumor-associated endothelial cells and the microvascular heterogeneity could explain, at least in part, the reduced efficacy of anti-angiogenic therapy in breast cancer by enabling endothelial cells an increased pro-angiogenic activity to acquire drug resistance Grange et al.
Lack of response to angiogenesis inhibitors may be explained in terms of the stage of progression, treatment history, and genomic constitution that exist in the tumor microenvironment Bergers and Hanahan, An analysis of human breast cancer biopsies demonstrated a plethora of pro-angiogenic factors in late-stage breast cancers including FGF-2, in contrast to earlier-stage tumors which preferentially expressed VEGF Relf et al.
Thus, resistance to anti-VEGF drugs in advanced-stage breast cancer may be explained by the dominance of FGF-2 and other pro-angiogenic factors in such stage of the disease Bergers and Hanahan, Invasive cancers commonly express multiple angiogenic factors and this heterogeneity occurs at an early point in time.
Genetic instabilities in the tumor cells may cause alterations of both the amount and type of pro-angiogenic factors expressed in a tumor which could further promote resistance to anti-angiogenic treatments Schneider and Miller, Cancer stem cells are a subpopulation of cancer cells capable of self-renewal, differentiation, and induction of tumorigenesis, metastasis, and drug resistance Li et al.
The potential of cancer stem cell trans-differentiating into endothelial cells has been reported in a variety of solid tumors Li et al. Bussolati et al. showed that breast cancer stem cells were able to differentiate into the endothelial lineage in the presence of VEGF Bussolati et al.
The stem cells acquired several endothelial markers and organized into capillary-like structures forming vessels in a xenograft animal model Bussolati et al.
Similarly, Wang et al. showed that breast cancer stem cells may trans-differentiate into endothelial cells that can form capillary-like vascular structures in the cell culture system and participate in tumor angiogenesis Wang et al.
An earlier study demonstrated that microRNAa miRNAa expression promoted tumor angiogenesis and metastasis in vivo by mediating endothelial trans-differentiation of breast cancer stem-like cells Tang et al.
Brossa et al. reported the ability of breast cancer stem cells to trans-differentiate to endothelial cells expressing endothelial markers under hypoxic conditions in vitro Brossa et al.
Notably, treatment with the VEGFR inhibitor sunitinib but not the VEGF inhibitor bevacizumab impaired the endothelial differentiation ability of breast cancer stem cells both in vitro and in vivo. Mechanistically, sunitinib, but not bevacizumab, suppressed HIF-1α required for endothelial differentiation under hypoxic conditions Brossa et al.
Together, increasing evidence suggests that cancer stem cell endothelial trans-differentiation supports tumor vascularization and partly contributes to the failure of anti-angiogenic drugs. The lack of efficacy of the conventional angiogenesis inhibitors necessitates exploring novel angiogenic pathways in breast cancer.
Given the heterogeneity of breast cancer and the complexity of angiogenesis, it is unlikely that the identification of a single target such as VEGF would be adequate in the treatment of this disease.
Interleukins ILs are a family of cytokines known to play essential roles in the regulation of several immune cell functions such as differentiation, activation, proliferation, migration, and adhesion Turner et al.
Interactions of ILs and their receptors in endothelial cells have been shown to regulate angiogenesis through pro-angiogenic and anti-angiogenic activity Ribatti, Serum IL-6 levels were elevated in breast cancer patients compared to controls Barron et al. Additionally, serum IL-6 and VEGF correlated positively in breast cancer patients Raghunathachar Sahana et al.
Higher expression of IL-6R was demonstrated in clinical specimens for patients with high-grade invasive ductal carcinoma Bharti et al.
A recent study by Hegde et al. showed that a crosstalk between IL-6 and VEGFR-2 signaling pathways exists in myoepithelial and endothelial cells isolated from clinical human breast tumors Hegde et al.
IL-6 epigenetically regulated VEGFR-2 expression through induction of proteasomal degradation of DNA methyltransferase 1 leading to promoter hypomethylation and angiogenic activity Hegde et al.
IL-8 is a pro-inflammatory cytokine that exerts its biologic activity through binding to its CXCR1 and CXCR2 receptors Waugh and Wilson, IL-8 enhanced the proliferation of cancer cells and produced a pro-angiogenic activity Waugh and Wilson, Serum IL-8 levels were significantly higher in breast cancer patients compared with healthy subjects and were associated with advanced disease Benoy et al.
High levels of IL-8 are secreted by stromal cells into the microenvironment of breast cancer patients compared to controls Razmkhah et al. Evidence from preclinical studies showed that IL-8 mediated invasion and angiogenesis of breast cancer cells Lin et al.
Cancer-associated adipocytes express high levels of IL-8 in breast cancer stroma thus promoting the pro-angiogenic effects of breast adipocytes Al-Khalaf et al.
In this context, ILexpressing adipocytes increased vascularity of tumor xenografts as indicated by increased expression of CD34, an endothelial cell marker Al-Khalaf et al. Neutralization of IL-8 or inhibiting its target receptors had been shown to reduce breast cancer growth and angiogenesis Lin et al.
Nannuru et al. showed that silencing of CXCR2 expression reduced tumor vascularity and inhibited spontaneous lung metastasis in an orthotopic animal model of breast cancer Nannuru et al.
Further, CXCR1 blockade with the small molecule inhibitor, repertaxin reduced metastasis in an animal model of breast cancer Ginestier et al. The platelet-derived growth factor PDGF family consists of four gene products PDGF-A, -B, -C, and -D that are combined into five different isoforms: PDGF-AA, -BB, -CC, -DD, and -AB Bartoschek and Pietras, These factors bind and activate their respective RTKs, PDGFR-α, and PDGFR-β.
PDGF family plays a key role in a wide range of oncologic activities essential for cancer growth including angiogenesis, fibrosis, and cellular migration Bartoschek and Pietras, High expression of PDGFs was correlated with an advanced presentation, increased recurrence, and poor survival in patients with invasive breast cancer Jansson et al.
PDGF is an important regulator for the motility of vascular smooth muscle cells induced by breast cancer cells Banerjee et al. Besides, the expression of HIF-1α in invasive breast cancer was significantly associated with angiogenesis and expression of PDGF-BB Bos et al. Earlier evidence showed that PDGFRs are expressed by breast cancer cells and endothelial cells in metastatic bone lesions in animal models Lev et al.
Imatinib remarkably inhibited PDGFR activation in breast cancer cells and tumor-associated endothelial cells and reduced microvessel density in the tumors Lev et al. Recently, Wang et al. provided evidence from cell culture and animal studies that the downregulation of PDGF-B greatly contributed to the metformin-induced vessel normalization in breast cancer Wang et al.
Fibroblast growth factors FGFs belong to a large family of growth factors that includes 23 members Hui et al. FGFs are key regulators of numerous physiological processes such as angiogenesis, wound healing, and embryonic development.
These functions are mediated by the binding of FGFs with their receptors FGFRs , which belong to the RTK family Hui et al. Growing evidence signifies the oncogenic impact of FGFs and FGFRs to promote cancer development and progression by mediating cancer cell proliferation, survival, epithelial-to-mesenchymal transition, invasion, and angiogenesis Wesche et al.
Chen et al. showed that dipalmitoylphosphatidic acid, a bioactive phospholipid, induced anti-angiogenic activity, and inhibited tumor growth in an experimental xenograft model of breast cancer Chen J.
These effects were attributed to transcriptional inhibition of FGF-1 expression leading to the downregulation of HGF Chen J. In the same context, Cai et al. showed that neutralizing FGF-2 by a disulfide-stabilized diabody inhibited tumor growth and angiogenesis in a mouse model of breast cancer Cai et al.
The antitumor activity was associated with a significant decrease in microvessel density and the number of lymphatic vessels Cai et al. Formononetin, an FGFR-2 inhibitor, demonstrated anti-angiogenic activity in breast cancer in both ex vivo and in vivo angiogenesis assays Wu et al.
Besides, formononetin significantly inhibited angiogenesis in vivo by reducing microvessel density and phosphorylated FGFR-2 levels in tumor tissue Wu et al. Recent evidence showed that FGFpositive tumors are resistant to clinically available drugs targeting VEGF and PDGF Hosaka et al.
The resistance is mediated by the ability of FGF-2 to recruit pericytes onto tumor microvessels through a PDGFR-β-dependent mechanism in breast cancer and fibrosarcoma models.
Dual targeting of the VEGF and PDGF produced a superior antitumor effect in FGFpositive breast cancer Hosaka et al. Angiopoietins Angs represent an imperative family of vascular growth factors that produce their biological effects through binding to the RTKs, Tie-1, and Tie-2 Akwii et al.
Angiopoietin-1 Ang-1 and angiopoietin-2 Ang-2 are best characterized for their role in angiogenesis and vascular stability Akwii et al. Ang-1 regulates the organization and maturation of newly formed blood vessels and promotes quiescence and structural integrity of vasculature Brindle et al.
Alternatively, Ang-2 antagonizes the effects of Ang-1 resulting in vessel destabilization Brindle et al. Ramanthan et al. indicated that high Ang-2 gene expression in breast cancer patients was associated with reduced survival Ramanathan et al.
In addition, a strong correlation existed between Angs and VEGF genes in breast cancer tissues Ramanathan et al. Besides, serum levels of Ang-2 were significantly higher in breast cancer patients compared to healthy control subjects. High Ang-2 serum levels had shorter survival than that of the low Ang-2 expression group Li et al.
Evidence from preclinical models also demonstrated that Ang-2 mediated initial steps of breast cancer metastasis to the brain Avraham et al. He et al.
showed that targeting Ang-2 with miRNAp reduced tumor growth, angiogenesis, and metastasis in animal models He et al. Besides, Wu et al. showed that oral administration of methylseleninic acid reduced microvessel density and increased pericytes coverage by inhibiting Ang-2 in a breast cancer animal model Wu et al.
Dual inhibition of VEGF-A and Ang-2 using a bispecific antibody promoted vascular regression and normalization in a model of metastatic breast cancer Schmittnaegel et al. Dual inhibition of Ang-1 and TGF-βR2 was also shown to suppress tumor angiogenesis in breast cancer in vivo Flores-Perez et al.
Notch receptors belong to a highly conserved signaling pathway that relies on cell-cell contacts to mediate a response to environmental signals in multicellular animals Aster et al.
Four different Notch receptors are expressed in humans, each is encoded by a different gene. In addition, four functional Notch ligands exist and belong to two families: members of the Delta family of ligands; Dll-1 and Dll-4, and members of the Serrate family of ligands; Jag-1 and Jag-2 Aster et al.
In breast cancer, Notch signaling promotes cell proliferation, self-renewal, anti-apoptotic effects, and angiogenesis Aster et al. Notch expression has been associated with the progression and recurrence of breast cancer Mollen et al. Proia et al. showed that blocking Notch-1 function with a specific antibody inhibited functional angiogenesis and breast cancer growth in animal models Proia et al.
HGF is a member of the plasminogen-related growth factor group and is a known angiogenic factor Nakamura and Mizuno, It is primarily expressed and produced by stromal cells, such as fibroblasts in mammary tissues Jiang et al. The angiogenic actions of HGF are mediated by binding to its RTK, MET on endothelial cells Organ and Tsao, ; Zhang et al.
In the activated endothelial cells, MET is upregulated thus modulating cell dissociation, motility, proliferation, and invasion Peruzzi and Bottaro, HGF regulates VEGF expression in tumor cells promoting angiogenic activity Matsumura et al.
Earlier studies showed that targeting HGF with retroviral ribozyme transgene or HGF antagonist reduced the growth and angiogenesis of breast tumors in vivo Jiang et al.
Syndecans are transmembrane proteoglycans composed of a core protein and a glycosaminoglycan side chain to which growth factors are attached Szatmari and Dobra, Syndecan-1 is the major syndecan found in epithelial malignancies Szatmari and Dobra, Syndecan-1 ligates with several pro-angiogenic factors such as VEGF, FGFs, Wnt, and HGF, which act as signaling co-receptors Szatmari and Dobra, Expression of syndecan-1 in breast tumors was associated with adverse prognosticators, metastasis, and reduced OS in patients Kind et al.
Besides, stromal syndecan-1 expression increased vessel density and area and promoted the growth and angiogenesis of triple-negative tumors in vivo Maeda et al. Schönfeld et al. showed that targeting syndecan-1 with an antibody-drug conjugate reduced the growth of TNBC in animal models when combined with chemotherapy Schonfeld et al.
An open-label, phase Ib trial evaluating antitumor activity and safety of erdafitinib; a potent and selective pan-FGFR inhibitor, in combination with fulvestrant and palbociclib in patients with metastatic breast cancer is currently recruiting patients NCT The primary objective is to determine safety and tolerability for the combination treatment of erdafitinib with targeted treatments.
Futibatinib is an orally available pan-FGFR inhibitor that is currently being evaluated in a phase II trial as monotherapy and in combination with fulvestrant in patients with locally advanced or metastatic breast cancer harboring FGFR gene amplification NCT The primary outcome of the trial is to determine dose-limiting toxicities during the first two cycles of therapy while secondary outcomes involve the identification of treatment-emergent adverse events TEAEs and objective tumor response.
Rogaratinib is another novel pan-FGFR inhibitor. Rogaratinib showed broad antitumor activity in preclinical studies Grunewald et al. The combination of rogaratinib plus palbociclib and fulvestrant is being assessed in an open-label, multicenter, prospective, phase I dose-escalation clinical trial NCT The primary aims of the study are to assess the recommended phase II dose and the incidence of TEAEs for the combination treatment in patients with metastatic hormone receptor-positive breast cancer who have FGFR-positive tumors.
Additionally, a phase II study is assessing the long-term efficacy and tolerability of rogaratinib in patients who have received the drug in a previous clinical trial and are currently in the continuation phase NCT The selective FGFR-2 inhibitor, RLY, is being evaluated for tolerability and antineoplastic activity in several advanced solid cancers, including the breast NCT The primary outcomes of the study are to determine the maximum tolerated dose of pemigatinib and to assess the pharmacodynamics of the drug.
The I-SPY 2 trial is investigating the effect of trebananib alone or in combination with standard targeted treatments in neoadjuvant settings in patients with breast cancer NCT The main outcome of the trial is to determine the safety and tolerability of NT-I7 in combination with pembrolizumab. Bintrafusp alfa is a first-in-class bifunctional fusion protein targeting TGF-β and programmed death-ligand 1 PD-L1 Paz-Ares et al.
Furthermore, bintrafusp alfa is being assessed as monotherapy in phase II, multicenter, open-label study in participants with TNBC NCT PF, an inhibitor of TGF-βR1, is being evaluated in a phase I dose-escalation study for its safety, tolerability, and pharmacokinetics in patients with advanced solid tumors NCT Table 2 summarizes ongoing clinical trials for selected non-VEGF angiogenic inhibitors in breast cancer.
TABLE 2. Therefore, exploring novel anti-angiogenic therapeutic approaches is of paramount importance for the treatment of aggressive and advanced breast tumors. Such approaches include vascular normalization by targeting pericytes, utilization of miRNAs and extracellular tumor-associated vesicles, using immunotherapeutic drugs, and nanotechnology.
A potential strategy to sensitize tumor endothelium to angiogenesis inhibitors is by targeting pericytes to achieve tumor vascular normalization Lord and Harris, ; Meng et al. Normalization of tumor vasculature prevents cancer cell metastasis, improves the delivery of systemic anticancer therapies, increases the efficacy of local therapies, and enhances recognition by the host immune system.
Pericyte coverage of tumor blood vessels is heterogeneous. In certain tumors, high pericyte coverage of the tumor vasculature causes resistance to anti-angiogenic therapies.
Alternatively, low pericyte coverage detected in the vasculature of certain tumors reduces vascular stability and increases vascular permeability which impairs the delivery of anticancer therapies to tumor cells and allows them to metastasize Meng et al.
Earlier studies showed that combining VEGFR and PDGFR inhibitors targeting endothelial cells and pericytes, respectively, improved the efficacy of anti-angiogenic therapy and reduced tumor growth in animal tumor models Bergers et al.
In a xenograft model of breast carcinoma, tumor vascularization was enhanced by increasing the pericyte-endothelium association via a mechanism involving the TGF-β-fibronectin axis Zonneville et al. In addition, Keskin et al. showed that pericyte targeting in established mouse breast tumors increased Ang-2 expression and that targeting Ang-2 signaling along with pericyte depletion restored vascular stability and decreased tumor growth and metastasis Keskin et al.
Although data from preclinical studies showed that pericyte targeting could be a novel strategy to normalize tumor vasculature, this strategy should be carefully considered as lack of pericyte coverage may disrupt vascular integrity and promote cancer metastasis Lord and Harris, ; Zirlik and Duyster, Assessment of pericyte coverage of tumor vasculature and the identification of the appropriate pericyte-targeted therapy are potential challenges to pericyte targeting Meng et al.
MicroRNAs miRNAs are critical regulators of signaling pathways involved in angiogenesis and cancer metastasis by interacting with the target mRNAs Gallach et al. To date, there are groups of well-characterized miRNAs implicated in regulating endothelial cell function and angiogenesis, making them attractive targets in tumor angiogenesis Gallach et al.
Liang et al. showed that miRNA suppressed breast tumor angiogenesis through targeting HIF-1α and Ang-1 in breast cancer cell lines and animal model. MiRNA inhibited the proliferation, migration, and tube formation of endothelial cells and decreased the microvessel density Liang et al.
Lu et al. reported that miRNAp inhibited tumor invasion and angiogenesis by silencing VEGF-A in breast cancer cells both in vitro and in vivo Lu et al. MiRNAb inhibited proliferation, migration, and tube formation of endothelial cells.
Systemic administration of miRNAb potently suppressed breast tumor growth and vascularization by targeting Akt and downregulating VEGF and c-Myc in breast cancer cells Li et al.
Mimics of miRNA suppressed the proliferation and tube formation of endothelial cells in vitro Wu et al. Moreover, the overexpression of miRNA reduced VEGF and HIF-1α protein levels and suppressed angiogenesis in vivo Wu et al.
Zou et al. showed that miRNA inhibited growth and angiogenesis of TNBC in vivo via post-transcriptional regulation of N-Ras and VEGF Zou et al. Importantly, miRNAs can be transported between cancer cells and stromal cells through extracellular vesicles known to mediate cell-to-cell communication in the tumor microenvironment Kuriyama et al.
Extracellular vesicles are classified into exosomes, microvesicles, and apoptotic bodies based on the size or biogenesis of the vesicles Kuriyama et al. Under hypoxic conditions, tumor cells release extracellular vesicles to a larger extent compared to cells in a normoxic environment Kuriyama et al.
Growing evidence points to the role of tumor-derived extracellular vesicles in tumor angiogenesis of breast cancer. recently reported that extracellular vesicles derived from breast cancer cells are highly enriched with miRNAp which enhanced proliferation and migration of endothelial cells in vitro and angiogenesis and metastasis of breast cancer in vivo Lu et al.
Microvesicles rich in a special VEGF isoform activated VEGFR and induced angiogenesis while being resistant to bevacizumab Feng et al. Exosome-mediated transfer of breast cancer-secreted miRNA efficiently destroyed tight junctions in endothelial monolayers associated with increased vascular permeability Zhou et al.
Few studies showed that extracellular vesicles can be targeted to prevent breast cancer metastasis and restore the activity of anti-angiogenic drugs Zhou et al. Aslan et al. showed that docosahexaenoic acid decreased the expression of pro-angiogenic genes including HIF-1α, TGF-β, and VEGFR in breast cancer cells and their secreted exosomes Aslan et al.
Also, docosahexaenoic acid altered miRNA content in breast cancer cells and their derived exosomes in favor of the inhibition of angiogenesis Aslan et al.
Taken together, miRNAs and extracellular vesicles can be selectively targeted to reduce vascularization in breast cancer providing a novel approach for angiogenesis inhibition Gallach et al.
Normal vasculature is needed for immunosurveillance and efficient detection and killing of cancer cells by immune cells. Disorganized tumor vessels create a selective immune cell barrier limiting the extravasation of immune cells, particularly the cytotoxic T lymphocytes into blood vessels and tumor tissue Yang et al.
Further, hypoxia in the tumor microenvironment promotes lactate accumulation, extracellular acidosis, VEGF overexpression, and VEGFR activation, all of which are known drivers of immune cell tolerance and immunosuppressive status Mendler et al. Endothelial cells are the first to come into contact with immune cells while infiltrating from the circulation into the tumor tissue Solimando et al.
Interestingly, tumor endothelial cells expressed PD-L1 and produced immunosuppressive activity contributing to tumor immune evasion in a mouse model of melanoma Taguchi et al.
Further, leukocyte adhesion was remarkably diminished in tumor vessels Dirkx et al. Tumors secrete angiogenic growth factors that can downregulate endothelial adhesion molecules essential for the interactions with granulocytes, macrophages, and natural killer cells on the vascular endothelium Griffioen, The suppression of these selective adhesion molecules leads to the loss of the adhesive properties of the tumor endothelium thereby impairing immune cell infiltration to tumor tissues.
Solimando et al. showed that junctional adhesion molecule-A JAM-A is an important factor influencing angiogenesis and extra-medullary dissemination in patients with multiple myeloma and its targeting suppressed multiple myeloma-associated angiogenesis both in vitro and in vivo Solimando et al.
Bednarek et al. recently demonstrated that targeting JAM-A with an antagonistic peptide inhibited the adhesion and trans-endothelial migration of breast cancer cells Bednarek et al. In breast cancer, vascular cell adhesion molecule-1 was aberrantly expressed and mediated angiogenesis and metastasis by binding to its ligand α4β1integrin Sharma et al.
Earlier findings also showed that angiogenic stimuli in the microenvironment of breast cancer may influence the expression of endothelial adhesion molecules to prevent leukocyte infiltration to tumor tissue Bouma-Ter Steege et al. Therefore, selective targeting of adhesion molecules and normalizing tumor vasculature could improve immune cell endothelial adhesion and strengthen the antitumor immune response in epithelial tumors, including breast cancer.
A growing body of evidence describes the interplay between immune cells and vasculature in the tumor microenvironment. The immune response and vascular normalization seem to be mutually regulated Fukumura et al.
Normalization of the tumor vasculature improves the infiltration of immune effector cells into tumors enhancing antitumor immune activity Fukumura et al. Likewise, immunotherapy can promote vascular normalization which further improves the effectiveness of immunotherapeutic drugs and response to anti-angiogenic therapies Huang et al.
In preclinical models of breast cancer, immune checkpoint inhibitors induced normalization of tumor vasculature and increased infiltration of immune cells into breast tumors Tian et al.
Together, the combination of anti-angiogenic and immunotherapeutic drugs might be an attractive approach to increase the effectiveness of each class of drugs and reduce the emergence of drug resistance Fukumura et al. The combination treatment has shown encouraging results in various cancer types Ciciola et al.
In a preclinical study, Allen et al. revealed that treatment with a combination of anti-VEGFR-2 and anti-PD-L1 antibodies sensitized tumors to anti-angiogenic therapy and prolonged its efficacy in breast cancer Allen et al.
Li et al. recently demonstrated a dose-dependent synergism for the combined treatment of anti-angiogenic therapy and immune checkpoint blockade Li et al. In this regard, the combination of low-dose anti-VEGFR2 antibody with anti-programmed cell death protein-1 PD-1 therapy normalized tumor vasculature, induced immune cell infiltration, and upregulated PD-1 expression on immune cells in syngeneic breast cancer mouse models.
Additionally, the combined treatment was effective and tolerable in patients with advanced TNBC Li et al. An open-label, randomized, parallel, phase II trial investigated the combination treatment of apatinib, a VEGFR-2 tyrosine kinase inhibitor with the anti-PD-1 monoclonal antibody camrelizumab in patients with advanced TNBC Liu et al.
The results showed that the combination treatment produced favorable therapeutic outcomes in terms of improved objective response rate and PFS which was associated with increased tumor-infiltrating lymphocytes. The adverse events were manageable and included elevated aminotransferases and hand-foot syndrome Liu et al.
Multiple clinical trials of combining anti-angiogenic therapy and immune checkpoint inhibitors are underway Zirlik and Duyster, The nanotechnology-based approach is an emerging strategy for the development of therapies targeting tumor angiogenesis which could improve the current pharmacokinetic profiles of anti-angiogenic drugs and favor their selective accumulation in tumors Banerjee et al.
Radical-containing nanoparticles produced in vitro and in vivo anti-angiogenic activity in a breast cancer model that was mediated by suppressing VEGF in cancer cells Shashni et al. Nanoparticles were also utilized to deliver a combination of therapy for breast cancer to produce anticancer and anti-angiogenic activity Zhao et al.
In a recent study by Gong et al. Table 3 provides a list of novel approaches for targeting vascular growth and angiogenesis in breast cancer.
TABLE 3. Breast cancer is a notable example where anti-angiogenic agents had constantly failed to make a significant impact on the survival of patients in clinical settings. One essential aspect to improve the efficacy of clinically available anti-angiogenic drugs is to better understand the vascular biology of breast cancer at the different stages and molecular types of the disease.
Besides, a greater understanding of the adaptive and intrinsic resistance mechanisms would enhance the proper utilization of angiogenesis inhibitors. Further evaluation for the role of stromal cells within the tumor microenvironment in mediating resistance to anti-angiogenic drugs will improve the efficacy and durability of anti-angiogenic therapy.
Another important facet to consider for the limited activity of angiogenesis inhibitors in breast cancer is the population under examination to allow the identification of breast cancer patients who would benefit most from anti-angiogenic drugs. Furthermore, there are several ongoing efforts to describe novel strategies to inhibit tumor angiogenesis through pericyte targeting, the use of immunotherapy, miRNAs, and the implementation of nanotechnology.
Despite the preclinical success of many of these strategies, limited clinical evidence is available to support their implementation in breast cancer treatment. NMA conceived the manuscript. All authors listed wrote the manuscript and approved it for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers.
Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher. Adair Th, M. Google Scholar. Aird, W. Endothelial Cell Heterogeneity.
Cold Spring Harb Perspect. PubMed Abstract CrossRef Full Text Google Scholar. Akino, T. Cytogenetic Abnormalities of Tumor-Associated Endothelial Cells in Human Malignant Tumors. CrossRef Full Text Google Scholar.
Akwii, R. Role of Angiopoietin-2 in Vascular Physiology and Pathophysiology. Cells 8, 1. Al-Khalaf, H. Interleukin-8 Activates Breast Cancer-Associated Adipocytes and Promotes Their Angiogenesis- and Tumorigenesis-Promoting Effects.
Cell Biol 39, 1. Allen, E. Combined Antiangiogenic and Anti-PD-L1 Therapy Stimulates Tumor Immunity through HEV Formation. Transl Med. Andonegui-Elguera, M. An Overview of Vasculogenic Mimicry in Breast Cancer. Aslan, C. Docosahexaenoic Acid DHA Inhibits Pro-angiogenic Effects of Breast Cancer Cells via Down-Regulating Cellular and Exosomal Expression of Angiogenic Genes and microRNAs.
Life Sci. Aster, J. The Varied Roles of Notch in Cancer. Avraham, H. Angiopoietin-2 Mediates Blood-Brain Barrier Impairment and Colonization of Triple-Negative Breast Cancer Cells in Brain.
Balakrishnan, S. Cell Prolif 49, — Banerjee, D. Nanotechnology-mediated Targeting of Tumor Angiogenesis. Cell 3, 3. Banerjee, S. Breast Cancer Cells Secreted Platelet-Derived Growth Factor-Induced Motility of Vascular Smooth Muscle Cells Is Mediated through Neuropilin Carcinog 45, — Barrios, C.
Phase III Randomized Trial of Sunitinib versus Capecitabine in Patients with Previously Treated HER2-Negative Advanced Breast Cancer. Breast Cancer Res. Barron, G. Circulating Levels of Angiogenesis-Related Growth Factors in Breast Cancer: A Study to Profile Proteins Responsible for Tubule Formation.
Bartoschek, M. PDGF Family Function and Prognostic Value in Tumor Biology. Baselga, J. Sorafenib in Combination with Capecitabine: an Oral Regimen for Patients with HER2-Negative Locally Advanced or Metastatic Breast Cancer.
Breast Cancer 17, —e4. Bear, H. Bevacizumab Added to Neoadjuvant Chemotherapy for Breast Cancer. Bednarek, R. Bell, R. Final Efficacy and Updated Safety Results of the Randomized Phase III BEATRICE Trial Evaluating Adjuvant Bevacizumab-Containing Therapy in Triple-Negative Early Breast Cancer.
Bellesoeur, A. Axitinib in the Treatment of Renal Cell Carcinoma: Design, Development, and Place in Therapy. Drug Des. Devel Ther. Ben Mousa, A. Sorafenib in the Treatment of Advanced Hepatocellular Carcinoma. Saudi J. Benoy, I. Increased Serum Interleukin-8 in Patients with Early and Metastatic Breast Cancer Correlates with Early Dissemination and Survival.
Cancer Res. Bergers, G. Modes of Resistance to Anti-angiogenic Therapy. Cancer 8, — Benefits of Targeting Both Pericytes and Endothelial Cells in the Tumor Vasculature with Kinase Inhibitors. Bergh, J. First-line Treatment of Advanced Breast Cancer with Sunitinib in Combination with Docetaxel versus Docetaxel Alone: Results of a Prospective, Randomized Phase III Study.
Bharti, R. Cancer , — Blumenthal, G. FDA Approval Summary: Sunitinib for the Treatment of Progressive Well-Differentiated Locally Advanced or Metastatic Pancreatic Neuroendocrine Tumors.
Oncologist 17, — Boér, K. Vandetanib with Docetaxel as Second-Line Treatment for Advanced Breast Cancer: a Double-Blind, Placebo-Controlled, Randomized Phase II Study. New Drugs 30, — Bos, R. Hypoxia-inducible Factor-1alpha Is Associated with Angiogenesis, and Expression of bFGF, PDGF-BB, and EGFR in Invasive Breast Cancer.
Histopathology 46, 31— Bottrell, A. An Oncogenic Activity of PDGF-C and its Splice Variant in Human Breast Cancer. Growth Factors 37, — Bouma-Ter Steege, J. Angiogenic Profile of Breast Carcinoma Determines Leukocyte Infiltration.
Brindle, N. Signaling and Functions of Angiopoietin-1 in Vascular protection. Brossa, A. Sunitinib but Not VEGF Blockade Inhibits Cancer Stem Cell Endothelial Differentiation. Oncotarget 6, — Brown, J.
Vasculogenesis: a Crucial Player in the Resistance of Solid Tumours to Radiotherapy. Brufsky, A. RIBBON a Randomized, Double-Blind, Placebo-Controlled, Phase III Trial Evaluating the Efficacy and Safety of Bevacizumab in Combination with Chemotherapy for Second-Line Treatment of Human Epidermal Growth Factor Receptor 2-negative Metastatic Breast Cancer.
Bussolati, B. Cell Mol Med 13, — Cai, Y. Construction of a Disulfide-Stabilized Diabody against Fibroblast Growth Factor-2 and the Inhibition Activity in Targeting Breast Cancer. Cancer Sci. Cameron, D. Adjuvant Bevacizumab-Containing Therapy in Triple-Negative Breast Cancer BEATRICE : Primary Results of a Randomised, Phase 3 Trial.
Lancet Oncol. Carmeliet, P. Molecular Mechanisms and Clinical Applications of Angiogenesis. Nature , — Casak, S. FDA Approval Summary: Ramucirumab for Gastric Cancer. Chau, N. Vandetanib for the Treatment of Medullary Thyroid Cancer. Chen, J. Cell Mol Med 22, — Chen, W.
Organotropism: New Insights into Molecular Mechanisms of Breast Cancer Metastasis. NPJ Precis Oncol. Ciciola, P. Combining Immune Checkpoint Inhibitors with Anti-angiogenic Agents. Clavarezza, M. Phase II Open-Label Study of Bevacizumab Combined with Neoadjuvant Anthracycline and Taxane Therapy for Locally Advanced Breast Cancer.
Breast 22, — Clemons, M. Randomised, Phase II, Placebo-Controlled, Trial of Fulvestrant Plus Vandetanib in Postmenopausal Women with Bone Only or Bone Predominant, Hormone-Receptor-Positive Metastatic Breast Cancer MBC : the OCOG ZAMBONEY Study.
Cristofanilli, M. Crown, J. Phase III Trial of Sunitinib in Combination with Capecitabine versus Capecitabine Monotherapy for the Treatment of Patients with Pretreated Metastatic Breast Cancer.
Curigliano, G. Randomized Phase II Study of Sunitinib versus Standard of Care for Patients with Previously Treated Advanced Triple-Negative Breast Cancer.
Dai, X. Breast Cancer Intrinsic Subtype Classification, Clinical Use and Future Trends. Darweesh, R. Gold Nanoparticles and Angiogenesis: Molecular Mechanisms and Biomedical Applications.
Nanomedicine 14, — Decker, T. A Randomized Phase II Study of Paclitaxel Alone versus Paclitaxel Plus Sorafenib in Second- and Third-Line Treatment of Patients with HER2-Negative Metastatic Breast Cancer PASO. BMC Cancer 17, Dey, N. Evading Anti-angiogenic Therapy: Resistance to Anti-angiogenic Therapy in Solid Tumors.
Transl Res. Dirkx, A. Tumor Angiogenesis Modulates Leukocyte-Vessel wall Interactions In Vivo by Reducing Endothelial Adhesion Molecule Expression. PubMed Abstract Google Scholar. Donnem, T. Vessel Co-option in Primary Human Tumors and Metastases: an Obstacle to Effective Anti-angiogenic Treatment?
Cancer Med. Dudley, A. Tumor Endothelial Cells. Effing, S. Assessing the Risk-Benefit Profile of Ramucirumab in Patients with Advanced Solid Tumors: A Meta-Analysis of Randomized Controlled Trials.
EClinicalMedicine 25, El-Kenawi, A. Angiogenesis Inhibitors in Cancer Therapy: Mechanistic Perspective on Classification and Treatment Rationales. Erber, R. Combined Inhibition of VEGF and PDGF Signaling Enforces Tumor Vessel Regression by Interfering with Pericyte-Mediated Endothelial Cell Survival Mechanisms.
FASEB J. Eroğlu, A. Vascular Endothelial Growth Factor VEGF -C, VEGF-D, VEGFR-3 and D Expressions in Primary Breast Cancer: Association with Lymph Node Metastasis. Evans, C. HIF-mediated Endothelial Response during Cancer Progression.
Fakhrejahani, E. Antiangiogenesis Therapy for Breast Cancer: an Update and Perspectives from Clinical Trials. Tumor Angiogenesis: Pericytes and Maturation Are Not to Be Ignored. Feng, Q. A Class of Extracellular Vesicles from Breast Cancer Cells Activates VEGF Receptors and Tumour Angiogenesis.
Flores-Pérez, A. Dual Targeting of ANGPT1 and TGFBR2 Genes by miR Controls Angiogenesis in Breast Cancer. Folkman, J. Tumor Angiogenesis: Therapeutic Implications.
Fukumura, D. Enhancing Cancer Immunotherapy Using Antiangiogenics: Opportunities and Challenges. Gallach, S. MicroRNAs: Promising New Antiangiogenic Targets in Cancer. Gerhardt, H. Endothelial-pericyte Interactions in Angiogenesis.
Cell Tissue Res , 15— Ginestier, C. CXCR1 Blockade Selectively Targets Human Breast Cancer Stem Cells In Vitro and in Xenografts. Goel, S. Effects of Vascular-Endothelial Protein Tyrosine Phosphatase Inhibition on Breast Cancer Vasculature and Metastatic Progression.
Cancer Inst. Gong, K. Nanobiotechnol 19, Gradishar, W. A Double-Blind, Randomised, Placebo-Controlled, Phase 2b Study Evaluating Sorafenib in Combination with Paclitaxel as a First-Line Therapy in Patients with HER2-Negative Advanced Breast Cancer.
Cancer 49, — Grange, C. Isolation and Characterization of Human Breast Tumor-Derived Endothelial Cells. Griffioen, A. Anti-angiogenesis: Making the Tumor Vulnerable to the Immune System. Cancer Immunol.
Grünewald, S. Rogaratinib: A Potent and Selective Pan-FGFR Inhibitor with Broad Antitumor Activity in FGFR-Overexpressing Preclinical Cancer Models. Haibe, Y. Resistance Mechanisms to Anti-angiogenic Therapies in Cancer. He, T. MicroRNAp Inhibits Tumour Angiogenesis by Targeting Angiopoietin Hegde, M.
Interleukinmediated Epigenetic Control of the VEGFR2 Gene Induces Disorganized Angiogenesis in Human Breast Tumors. Hida, K. Tumor-associated Endothelial Cells with Cytogenetic Abnormalities. Heterogeneity of Tumor Endothelial Cells. Hosaka, K. Therapeutic Paradigm of Dual Targeting VEGF and PDGF for Effectively Treating FGF-2 Off-Target Tumors.
Huang, Y. Improving Immune-Vascular Crosstalk for Cancer Immunotherapy. Hui, Q. FGF Family: From Drug Development to Clinical Application. Hyams, D. Cediranib in Combination with Fulvestrant in Hormone-Sensitive Metastatic Breast Cancer: a Randomized Phase II Study.
New Drugs 31, — Ianza, A. ΔKi67 Proliferation index as Independent Predictive and Prognostic Factor of Outcome in Luminal Breast Cancer: Data from Neoadjuvant Letrozole-Based Treatment. Tumour Biol. Incio, J. Obesity Promotes Resistance to Anti-VEGF Therapy in Breast Cancer by Up-Regulating IL-6 and Potentially FGF Jansson, S.
The PDGF Pathway in Breast Cancer Is Linked to Tumour Aggressiveness, Triple-Negative Subtype and Early Recurrence. Jiang, W. Johnston, S. A Randomized and Open-Label Trial Evaluating the Addition of Pazopanib to Lapatinib as First-Line Therapy in Patients with HER2-Positive Advanced Breast Cancer.
Kane, R. Sorafenib for the Treatment of Unresectable Hepatocellular Carcinoma. Oncologist 14, 95— Sorafenib for the Treatment of Advanced Renal Cell Carcinoma. Karamysheva, A. Mechanisms of Angiogenesis.
Biochemistry Mosc 73, — Kazazi-Hyseni, F. BevacizumabOncologist 15, — Kelly-Goss, M. Targeting Pericytes for Angiogenic Therapies.
Microcirculation 21, — Keskin, D. Targeting Vascular Pericytes in Hypoxic Tumors Increases Lung Metastasis via Angiopoietin Cell Rep 10, — Kim, J.
Heterogeneous Perivascular Cell Coverage Affects Breast Cancer Metastasis and Response to Chemotherapy. JCI Insight 1, e Kind, S. A Shift from Membranous and Stromal Syndecan-1 CD Expression to Cytoplasmic CD Expression Is Associated with Poor Prognosis in Breast Cancer.
Carcinog 58, — Kloepper, J. Kozłowski, J. Breast Cancer Metastasis - Insight into Selected Molecular Mechanisms of the Phenomenon. Postepy Hig Med. Dosw 69, — Krüger-Genge, A.
Vascular Endothelial Cell Biology: An Update. Kugeratski, F. Kuriyama, N. Extracellular Vesicles Are Key Regulators of Tumor Neovasculature.
Front Cel Dev Biol 8, Le Tourneau, C. Sunitinib: a Novel Tyrosine Kinase Inhibitor. A Brief Review of its Therapeutic Potential in the Treatment of Renal Carcinoma and Gastrointestinal Stromal Tumors GIST.
Risk Manag. Lee, A. Pazopanib in Advanced Soft Tissue Sarcomas. Transduct Target. Lev, D. Inhibition of Platelet-Derived Growth Factor Receptor Signaling Restricts the Growth of Human Breast Cancer in the Bone of Nude Mice.
Li, F. Cancer Stem Cells and Neovascularization. Cells 10, Li, P. Diagnostic and Prognostic Potential of Serum Angiopoietin-2 Expression in Human Breast Cancer. Li, Q. Low-Dose Anti-angiogenic Therapy Sensitizes Breast Cancer to PD-1 Blockade.
Li, Y. MiRNAb Suppresses Tumor Growth through Simultaneously Inhibiting Angiogenesis and Tumorigenesis by Targeting Akt3. The application of biomarkers is a powerful adjuvant means which are essential for disease identification, early diagnosis and prevention, and drug treatment monitoring.
Biomarkers refer to biochemical indicators of normal physiological or pathogenic processes to furnish the structural or functional changes of systems, organs, tissues, cells and subcells, and can also be used for disease diagnosis, disease stage, or evaluating the safety and efficacy of a drug or regimen among targeted population.
For example, HER2 is a diagnostic indicator for breast cancer typing, and levels of PD-L1 is used to predict the efficacy of immune checkpoint inhibitors ICIs. Despite considerable efforts, there are few biomarkers responding to angiogenesis approved for clinical application.
With the advancement in bio-analytical technology and clinical bio-chemistry, tissue and cell concentrations of some angiogenic mediators, circulating ECs, circulating progenitor cells, CT imaging of blood flow and blood volume have been shown to have potential as biomarkers, but more clinical trials are needed to validate their prospective.
Developing efficient biomarkers for diagnosing the progression and stage of cancer and identifying mechanisms of tumor angiogenesis and drug resistance, in order to benefit drug selection, balance efficacy and toxicity, and simplify anti-cancer therapy.
Actually, due to numerous factors such as the complexity of tumor angiogenesis, heterogeneity and variability of tumors, the unpredictability of response or toxicity, and limitations of preclinical and clinical trials, the development of biomarkers will be a great challenge.
Since the first angiogenic inhibitor bevacizumab approved for treatment, combination therapy based on anti-angiogenic agents has infiltrated anti-tumor field.
Diversified methods in anti-cancer therapy provide more options for clinical treatment and make strong alliances possible. In recent several years, one of the prevalent research direction is the combination of angiogenic inhibitors and immune checkpoint inhibitors, in which better clinical benefits from HCC and RCC patients treated with programmed cell death 1 PD-1 and VEGFR-2 inhibitors than with monotherapy.
At the same time, it can neutralize excess VEGF, reconstruct the vascular system of tumor tissue, normalize vascular network, promote the blood transport of immunosuppressant, inhibit excessive angiogenesis, reduce microvascular density, and limit tumor growth, invasion and metastasis.
Some optimistic results of combination therapy have been achieved in recent years shown in Table 4. For example, in a phase III clinical trial NCT , the combination of bevacizumab with PD-1 inhibitor atezolizumab significantly improved the OS and PFS rates of unresectable HCC patients compared to sorafenib.
As mentioned before, although it has more damage to normal cells, blood vessels and immune system due to the administration with maximum tolerated dosage and poor tissue selectivity, chemotherapy is an irreplaceable method for many advanced patients with cancer metastasis to prolong the survival.
Some relevant clinical trials with positive outcomes have been shown in Table 4. For example, a phase III clinical trial NCT have shown that the addition of atezolizumab anti-PD-L1 greatly extended the OS Another notable therapeutic method is an emerging adjuvant strategy - neoadjuvant chemotherapy NACT , aiming to reduce the tumor and kill invisible metastatic tumor cells through systemic chemotherapy to facilitate subsequent surgery, radiotherapy, and other treatments.
Up to now, various NACT regimens SOX, XELOX, FOLFOX have been suggested with satisfactory clinical results in primary or advanced tumors and lower risk of progression, but some discouraging clinical evidence of NACT also observed in recent years especially breast cancer.
summarized a number of potential mechanisms of chemoresistance in NACT, wherein, it is reported that NACT could stimulate cancer metastasis through inducing angiogenesis, lymphangiogenesis and inflammatory infiltration, altering immune responses and worsening TME, and these changes may induce secondary chemoresistance.
Theoretically, it is promising, but massive efforts are also necessary, some clinical trials are already underway NCT, NCT, NCT, NCT, NCT Apart from the means above, exploiting novel selective multi-targeted kinase inhibitors is one of the current trendy research directions.
In tumor angiogenesis, various angiogenic tyrosine kinases act synergistically to induce an array of intracellular signaling cascades instead of working individually.
In normal tissue, anti-angiogenic molecules can balance the pro-angiogenic factors to maintain the homeostasis of the internal environment.
The active angiogenesis in tumor tissue is related to the over-activation of pro-angiogenic factors and the over-inhibition of anti-angiogenic mediators. Hence, endogenous anti-angiogenic components or their derivatives may be conducive to vascular normalization and therapeutic efficiency.
Recombinant human endostatin is an angiogenic inhibitor with no cytotoxicity approved by the Chinese FDA for treating various cancers, including NSCLC. Angiogenesis is one of the key conditions for the proliferation, invasion, and metastasis of carcinomas and anti-angiogenic treatment has gradually become a prevalent anti-tumor strategy with a criterion of vascular optimization.
But some common issues that cannot be ignored remain to be solved such as insufficient therapeutic efficacy, reproducibility and popularization of treatment modalities.
With an in-depth understanding of tumor angiogenesis, tumor microenvironment, and drug resistance, these problems may be solved in the near future. As an emerging strategy, anti-angiogenic therapy will achieve more clinical benefits for cancer patients and anti-tumor therapy, and facilitate the clinical treatment of non-neoplastic angiogenesis-related diseases as well.
Larionova, I. New angiogenic regulators produced by TAMs: perspective for pargeting tumor angiogenesis. Cancers 13 , Article CAS PubMed PubMed Central Google Scholar. Duran, C. et al. Molecular regulation of sprouting angiogenesis. Article PubMed Google Scholar.
Teleanu, R. Tumor angiogenesis and anti-angiogenic strategies for cancer treatment. Article PubMed PubMed Central Google Scholar. Folkman, J. Angiogenesis: an organizing principle for drug discovery? Drug Discov. Article CAS PubMed Google Scholar.
Smolen, J. New therapies for treatment of rheumatoid arthritis. Lancet , — Creamer, D. Costa, C. Angiogenesis and chronic inflammation: cause or consequence?
Angiogenesis 10 , — Caldwell, R. Vascular endothelial growth factor and diabetic retinopathy: pathophysiological mechanisms and treatment perspectives.
Diabetes Metab. Ng, E. Targeting angiogenesis, the underlying disorder in neovascular age-related macular degeneration.
Khosravi Shahi, P. Tumoral angiogenesis and breast cancer. Zhong, M. TIPE regulates VEGFR2 expression and promotes angiogenesis in colorectal cancer.
Hall, R. Angiogenesis inhibition as a therapeutic strategy in non-small cell lung cancer NSCLC. Lung Cancer Res.
CAS PubMed PubMed Central Google Scholar. Zimna, A. Hypoxia-inducible factor-1 in physiological and pathophysiological angiogenesis: applications and therapies. Conway, E. Molecular mechanisms of blood vessel growth. Kalluri, R. Basement membranes: structure, assembly and role in tumour angiogenesis.
Cancer 3 , — Gasparini, G. Angiogenic inhibitors: a new therapeutic strategy in oncology. Article CAS Google Scholar.
Adams, R. Molecular regulation of angiogenesis and lymphangiogenesis. Cell Biol. Jain, R. Molecular regulation of vessel maturation. Rowley, D. What might a stromal response mean to prostate cancer progression? Cancer Metastasis Rev.
Liakouli, V. The role of extracellular matrix components in angiogenesis and fibrosis: possible implication for Systemic Sclerosis.
Shiga, K. Cancer-associated fibroblasts: their characteristics and their roles in tumor growth. Cancers 7 , — Roland, C.
Inhibition of vascular endothelial growth factor reduces angiogenesis and modulates immune cell infiltration of orthotopic breast cancer xenografts. Cancer Ther. Ribatti, D. Immune cells and angiogenesis. Med 13 , — Parmar, D.
Angiopoietin inhibitors: a review on targeting tumor angiogenesis. Deyell, M. Cancer metastasis as a non-healing wound. Cancer , — Viallard, C.
Tumor angiogenesis and vascular normalization: alternative therapeutic targets. Angiogenesis 20 , — Bellou, S. Anti-angiogenesis in cancer therapy: hercules and hydra. Cancer Lett. Ebos, J. Antiangiogenic therapy: impact on invasion, disease progression, and metastasis.
Pirker, R. Chemotherapy remains a cornerstone in the treatment of nonsmall cell lung cancer. Biller, L. Diagnosis and treatment of metastatic colorectal cancer: a review. JAMA , Luqmani, Y. Mechanisms of drug resistance in cancer chemotherapy.
Saeki, T. Drug resistance in chemotherapy for breast cancer. Cancer Chemother. Salgia, R. Trends Cancer 4 , — Turner, N. Genetic heterogeneity and cancer drug resistance. Lancet Oncol. Duesberg, P. Cancer drug resistance: the central role of the karyotype.
Drug Resist. Lahiry, P. Kinase mutations in human disease: interpreting genotype-phenotype relationships. Claesson-Welsh, L. Vascular permeability—the essentials. De Bock, K.
Vessel abnormalization: another hallmark of cancer? Molecular mechanisms and therapeutic implications. Mortezaee, K. Immune escape: a critical hallmark in solid tumors. Life Sci. Igney, F. Immune escape of tumors: apoptosis resistance and tumor counterattack.
Majidpoor, J. Angiogenesis as a hallmark of solid tumors—clinical perspectives. Choi, S. Anti-angiogenesis revisited: reshaping the treatment landscape of advanced non-small cell lung cancer.
Zhong, L. Small molecules in targeted cancer therapy: advances, challenges, and future perspectives. Signal Transduct. Huinen, Z. Anti-angiogenic agents—overcoming tumour endothelial cell anergy and improving immunotherapy outcomes.
Gacche, R. Angiogenic factors as potential drug target: efficacy and limitations of anti-angiogenic therapy. Acta , — CAS PubMed Google Scholar. Bergers, G. Modes of resistance to anti-angiogenic therapy. Cancer 8 , — Ansari, M.
Cancer combination therapies by angiogenesis inhibitors; a comprehensive review. Cell Commun. The role of pericytes in blood-vessel formation and maintenance. Neuro Oncol. Carmeliet, P. Angiogenesis in cancer and other diseases.
Goel, S. Normalization of the vasculature for treatment of cancer and other diseases. Kim, C. Vascular RhoJ is an effective and selective target for tumor angiogenesis and vascular disruption. Cancer Cell 25 , — Normalizing tumor microenvironment to treat cancer: bench to bedside to biomarkers.
Chauhan, V. Normalization of tumour blood vessels improves the delivery of nanomedicines in a size-dependent manner. Martin, J. Normalizing function of tumor vessels: progress, opportunities, and challenges.
Yang, T. Vascular normalization: a new window opened for cancer therapies. Stylianopoulos, T. Reengineering the physical microenvironment of tumors to improve drug delivery and efficacy: from mathematical modeling to bench to bedside.
The role of mechanical forces in tumor growth and therapy. Combining two strategies to improve perfusion and drug delivery in solid tumors.
Natl Acad. USA , — Delivering nanomedicine to solid tumors. Hamzah, J. Vascular normalization in Rgs5-deficient tumours promotes immune destruction. Vaupel, P. Hypoxia in cancer: significance and impact on clinical outcome. Stubbs, M.
Causes and consequences of tumour acidity and implications for treatment. Today 6 , 15—19 Lee, E. Tumor pH-responsive flower-like micelles of poly l-lactic acid -b-poly ethylene glycol -b-poly l-histidine.
Release , 19—26 Zhang, Z. Rational design of nanoparticles with deep tumor penetration for effective treatment of tumor metastasis.
Article Google Scholar. Khawar, I. Improving drug delivery to solid tumors: Priming the tumor microenvironment. Release , 78—89 Zhu, L.
Angiogenesis and immune checkpoint dual blockade in combination with radiotherapy for treatment of solid cancers: opportunities and challenges. Oncogenesis 10 , 47 Li, X. The immunological and metabolic landscape in primary and metastatic liver cancer.
Cancer 21 , — Provenzano, P. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma.
Cancer Cell 21 , — Milosevic, M. The human tumor microenvironment: invasive needle measurement of oxygen and interstitial fluid pressure. Chun, C. in Vascular Tumors and Developmental Malformations eds. North, P.
Hillen, F. Tumour vascularization: sprouting angiogenesis and beyond. Gianni-Barrera, R. Split for the cure: VEGF, PDGF-BB and intussusception in therapeutic angiogenesis. Burri, P. Intussusceptive angiogenesis—the alternative to capillary sprouting.
Ratajska, A. Vasculogenesis and its cellular therapeutic applications. Cells Tissues Organs , — Shi, L. Abraham, D. Teuwen, L. Tumor vessel co-option probed by single-cell analysis.
Cell Rep. Wei, X. Mechanisms of vasculogenic mimicry in hypoxic tumor microenvironments. Cancer 20 , 7 Potente, M. Basic and therapeutic aspects of angiogenesis. Cell , — Melincovici, C.
Vascular endothelial growth factor VEGF —key factor in normal and pathological angiogenesis. PubMed Google Scholar. Kazlauskas, A. PDGFs and their receptors. Gene , 1—7 Sang, Q. Complex role of matrix metalloproteinases in angiogenesis.
Cell Res. Ferrara, N. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Shibuya, M. Signal transduction by VEGF receptors in regulation of angiogenesis and lymphangiogenesis.
Senger, D. Vascular permeability factor VPF, VEGF in tumor biology. Cancer Metast Rev. Bao, P. The role of vascular endothelial growth factor in wound healing. Vascular endothelial growth factor VEGF and its receptor VEGFR signaling in angiogenesis: a crucial target for anti- and pro-angiogenic therapies.
Genes Cancer 2 , — VEGF as a key mediator of angiogenesis in cancer. Oncology 69 , 4—10 Peach, C. Molecular pharmacology of VEGF-A isoforms: binding and signalling at VEGFR2.
Ji, R. Characteristics of lymphatic endothelial cells in physiological and pathological conditions. He, Y. Suppression of tumor lymphangiogenesis and lymph node metastasis by blocking vascular endothelial growth factor receptor 3 signaling.
Natl Cancer Inst. Luttun, A. Genetic dissection of tumor angiogenesis: are PlGF and VEGFR-1 novel anti-cancer targets? Acta , 79—94 McDonald, N.
A structural superfamily of growth factors containing a cystine knot motif. Cell 73 , — Revascularization of ischemic tissues by PlGF treatment, and inhibition of tumor angiogenesis, arthritis and atherosclerosis by anti-Flt1.
Iyer, S. Role of placenta growth factor in cardiovascular health. Trends Cardiovasc. Beck, H. Cell type-specific expression of neuropilins in an MCA-occlusion model in mice suggests a potential role in post-ischemic brain remodeling.
Donnini, S. Expression and localization of placenta growth factor and PlGF receptors in human meningiomas. Lacal, P. Human melanoma cells secrete and respond to placenta growth factor and vascular endothelial growth factor. Nonclassic endogenous novel regulators of angiogenesis. Byrne, A. Angiogenic and cell survival functions of vascular endothelial growth factor VEGF.
Barleon, B. Migration of human monocytes in response to vascular endothelial growth factor VEGF is mediated via the VEGF receptor flt Blood 87 , — Angiogenesis 9 , — The biology of VEGF and its receptors.
Ishida, A. Expression of vascular endothelial growth factor receptors in smooth muscle cells. Ghosh, S. High levels of vascular endothelial growth factor and its receptors VEGFR-1, VEGFR-2, neuropilin-1 are associated with worse outcome in breast cancer. Ceci, C.
Ioannidou, E. Angiogenesis and anti-angiogenic treatment in prostate cancer: mechanisms of action and molecular targets. Simons, M. Mechanisms and regulation of endothelial VEGF receptor signalling. Molhoek, K. VEGFR-2 expression in human melanoma: revised assessment. Spannuth, W. Functional significance of VEGFR-2 on ovarian cancer cells.
Capp, C. Increased expression of vascular endothelial growth factor and its receptors, VEGFR-1 and VEGFR-2, in medullary thyroid carcinoma. Thyroid 20 , — Modi, S. Padró, T. Overexpression of vascular endothelial growth factor VEGF and its cellular receptor KDR VEGFR-2 in the bone marrow of patients with acute myeloid leukemia.
Leukemia 16 , — Sun, W. Angiogenesis in metastatic colorectal cancer and the benefits of targeted therapy. Valtola, R. VEGFR-3 and its ligand VEGF-C are associated with angiogenesis in breast cancer.
Saintigny, P. Vascular endothelial growth factor-C and its receptor VEGFR-3 in non-small-cell lung cancer: concurrent expression in cancer cells from primary tumour and metastatic lymph node. Lung Cancer 58 , — Yonemura, Y.
Lymphangiogenesis and the vascular endothelial growth factor receptor VEGFR -3 in gastric cancer. Cancer 37 , — Su, J. Cancer 96 , — Simiantonaki, N. Google Scholar.
Goel, H. VEGF targets the tumour cell. Cancer 13 , — Wang, H. PLoS ONE 7 , e Manzat Saplacan, R. The role of PDGFs and PDGFRs in colorectal cancer. Mediators Inflamm. Kalra, K. Cell Dev. Balamurugan, K. Cenciarelli, C. PDGFRα depletion attenuates glioblastoma stem cells features by modulation of STAT3, RB1 and multiple oncogenic signals.
Oncotarget 7 , — Chabot, V. Stem Cell Res. Li, H. Development of monoclonal anti-PDGF-CC antibodies as tools for investigating human tissue expression and for blocking PDGF-CC induced PDGFRα signalling in vivo.
PLoS ONE 13 , e Dardik, A. Shear stress-stimulated endothelial cells induce smooth muscle cell chemotaxis via platelet-derived growth factor-BB and interleukin-1α.
Muratoglu, S. Low density lipoprotein receptor-related protein 1 LRP1 forms a signaling complex with platelet-derived growth factor receptor-β in endosomes and regulates activation of the MAPK pathway.
Wang, J. Metformin inhibits metastatic breast cancer progression and improves chemosensitivity by inducing vessel normalization via PDGF-B downregulation. Cancer Res. Li, M. Integrins as attractive targets for cancer therapeutics.
Acta Pharm. B 11 , — Zou, X. Redundant angiogenic signaling and tumor drug resistance. Lee, C. Platelet-derived growth factor-C and -D in the cardiovascular system and diseases. Berthod, F. Spontaneous fibroblast-derived pericyte recruitment in a human tissue-engineered angiogenesis model in vitro.
The role of pericytes in angiogenesis. Chatterjee, S. Pericyte-endothelial cell interaction: a survival mechanism for the tumor vasculature. Cell Adh. Luk, K. Influence of morphine on pericyte-endothelial interaction: implications for antiangiogenic therapy.
Cavalcanti, E. PDGFRα expression as a novel therapeutic marker in well-differentiated neuroendocrine tumors.
Cancer Biol. Burger, R. Overview of anti-angiogenic agents in development for ovarian cancer. Raica, M. Pharmaceuticals 3 , — Heindryckx, F. Targeting the tumor stroma in hepatocellular carcinoma.
World J. Cornellà, H. Molecular pathogenesis of hepatocellular carcinoma. Brahmi, M. Expression and prognostic significance of PDGF ligands and receptors across soft tissue sarcomas. ESMO Open 6 , Rao, L. HB-EGF-EGFR signaling in bone marrow endothelial cells mediates angiogenesis associated with multiple myeloma.
Cancers 12 , Hu, L. Dual target inhibitors based on EGFR: promising anticancer agents for the treatment of cancers Larsen, A. Targeting EGFR and VEGF R pathway cross-talk in tumor survival and angiogenesis. Holbro, T. ErbB receptors: directing key signaling networks throughout life.
Ellis, L. Epidermal growth factor receptor in tumor angiogenesis. North Am. De Luca, A. The role of the EGFR signaling in tumor microenvironment. Albadari, N. The transcriptional factors HIF-1 and HIF-2 and their novel inhibitors in cancer therapy.
Expert Opin. Bos, R. Hypoxia-inducible factor-1α is associated with angiogenesis, and expression of bFGF, PDGF-BB, and EGFR in invasive breast cancer. Histopathology 46 , 31—36 Salomon, D. Epidermal growth factor-related peptides and their receptors in human malignancies.
Yu, H. Poor response to erlotinib in patients with tumors containing baseline EGFR TM mutations found by routine clinical molecular testing. Raj, S. Cancer 21 , 31 Acevedo, V. Paths of FGFR-driven tumorigenesis. Cell Cycle 8 , — Chen, M. Progress in research on the role of FGF in the formation and treatment of corneal neovascularization.
Montesano, R. Basic fibroblast growth factor induces angiogenesis in vitro. USA 83 , — Giacomini, A. Hui, Q.
FGF family: from drug development to clinical application. Presta, M. Cytokine Growth Factor Rev. Fons, P. Katoh, M. FGF receptors: cancer biology and therapeutics. Kopetz, S. Phase II trial of infusional fluorouracil, irinotecan, and bevacizumab for metastatic colorectal cancer: efficacy and circulating angiogenic biomarkers associated with therapeutic resistance.
Batchelor, T. AZD, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell 11 , 83—95 Cancer genomics and genetics of FGFR2 Review.
Fibroblast growth factor signalling: from development to cancer. Cancer 10 , — Greulich, H. Targeting mutant fibroblast growth factor receptors in cancer.
Trends Mol. Cross, M. FGF and VEGF function in angiogenesis: signalling pathways, biological responses and therapeutic inhibition.
Trends Pharmacol. García-Caballero, M. Angioprevention of urologic cancers by plant-derived foods. Pharmaceutics 14 , Aviles, R.
Testing clinical therapeutic angiogenesis using basic fibroblast growth factor FGF-2 : Clinical angiogenesis using FGF Vasudev, N. Anti-angiogenic therapy for cancer: current progress, unresolved questions and future directions.
Angiogenesis 17 , — Ding, S. HGF receptor up-regulation contributes to the angiogenic phenotype of human endothelial cells and promotes angiogenesis in vitro.
Blood , — Bonnans, C. Remodelling the extracellular matrix in development and disease. Mulcahy, E. Nakamura, T. The discovery of hepatocyte growth factor HGF and its significance for cell biology, life sciences and clinical medicine. B: Phys. Bottaro, D. Identification of the hepatocyte growth factor receptor as the c- met proto-oncogene product.
Science , — Dean, M. The human met oncogene is related to the tyrosine kinase oncogenes. Ono, K. Circulation 95 , — Cai, W. Mechanisms of hepatocyte growth factor—induced retinal endothelial cell migration and growth.
Ankoma-Sey, V. Coordinated induction of VEGF receptors in mesenchymal cell types during rat hepatic wound healing. Oncogene 17 , — Nagashima, M. Hepatocyte growth factor HGF , HGF activator, and c-Met in synovial tissues in rheumatoid arthritis and osteoarthritis.
Hughes, P. In vitro and in vivo activity of AMG , a potent and selective MET kinase inhibitor, in MET-dependent cancer models. Leung, E. Oncogene 36 , — Kuang, W. Hartmann, S. Demuth, C. Increased PD-L1 expression in erlotinib-resistant NSCLC cells with MET gene amplification is reversed upon MET-TKI treatment.
Oncotarget 8 , — Kwon, M. Frequent hepatocyte growth factor overexpression and low frequency of c-Met gene amplification in human papillomavirus-negative tonsillar squamous cell carcinoma and their prognostic significances.
Miranda, O. Cancers 10 , Wang, Q. MET inhibitors for targeted therapy of EGFR TKI-resistant lung cancer. Wu, J. Prostate 76 , — Imura, Y. Cancer Sci. Birchmeier, C. Met, metastasis, motility and more.
Zhang, Y. Function of the c-Met receptor tyrosine kinase in carcinogenesis and associated therapeutic opportunities. Cancer 17 , 45 Scalia, P. The IGF-II-insulin receptor isoform-A autocrine signal in cancer: actionable perspectives.
Bach, L. Endothelial cells and the IGF system. Clemmons, D. Modifying IGF1 activity: an approach to treat endocrine disorders, atherosclerosis and cancer. van Beijnum, J. Insulin-like growth factor axis targeting in cancer and tumour angiogenesis—the missing link: IGF signaling in tumor angiogenesis.
Chantelau, E. Evidence that upregulation of serum IGF-1 concentration can trigger acceleration of diabetic retinopathy. Wilkinson-Berka, J. The role of growth hormone, insulin-like growth factor and somatostatin in diabetic retinopathy. Higashi, Y. Aging, atherosclerosis, and IGF A: Biol.
Hellstrom, A.
Muscular strength progression techniques is a critical requirement Anti-angiigenesis malignant growth, invasion, Anti-angiogenessis metastases. Agents interfering with Muscular strength progression techniques have shown efficacy in the treatment of Remedies for workout-induced muscle soreness Muscular strength progression techniques of solid tumors, such as metastatic mmedicine cancer, non—small-cell Anti-angiogenesis approaches in medicine cancer, and renal approacjes cancer, and are being studied in many more. Each Muscular strength progression techniques the mddicine agents currently approved by the US Food and Drug Administration-bevacizumab Avastinsunitinib Sutentand sorafenib Nexavar -offer challenges to nurses, in terms of assessment and management of toxicity, and to their patients as well: learning and integrating self-care strategies, such as self-assessment and self-administration for sorafenib and sunitinib. This article reviews the recommended dosing, drug interactions, potential side effects, and management strategies for these three agents. Other agents that have antiangiogenesis properties, such as the epidermal growth factor inhibitors, the mTOR inhibitors, bortezomib, and thalidomide will not be addressed. This article will review the classification of agents, their indications, US Food and Drug Administration FDA -approved dosing, as well as class- and drug-specific side effects and their management.
die Maßgebliche Antwort, neugierig...
Wacker, welche ausgezeichnete Antwort.
Nicht darin das Wesen.
der Misslungene Gedanke