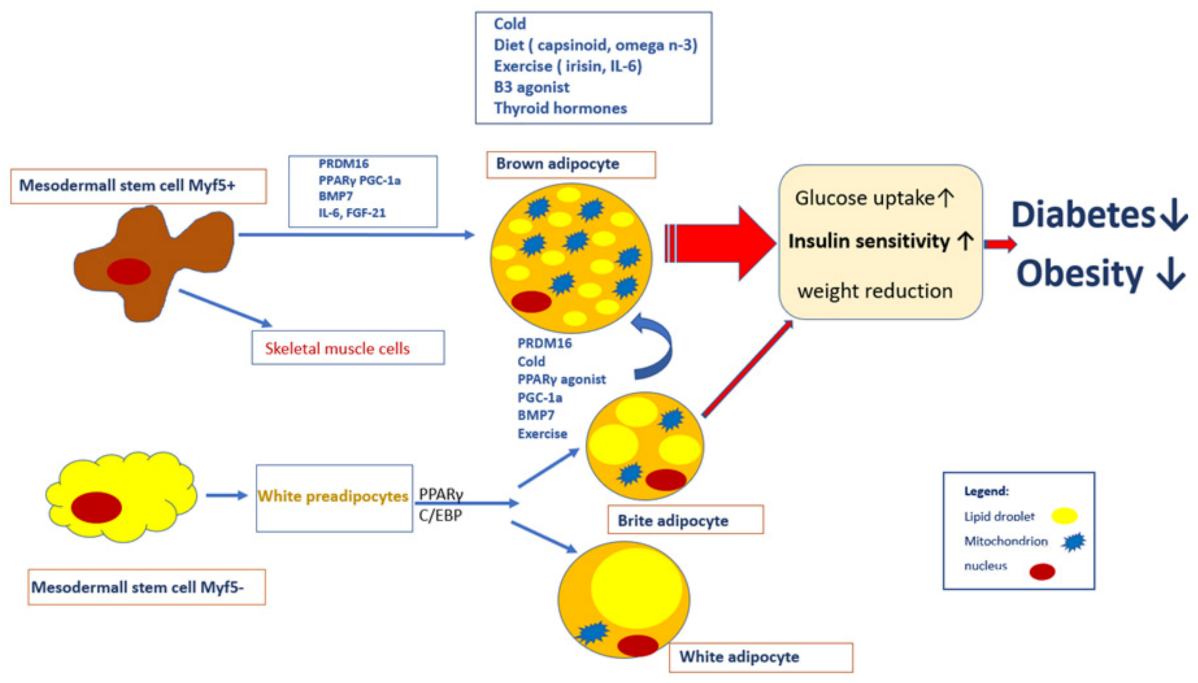
Thermogenesis and insulin sensitivity -
Find articles by Townsend, K. Find articles by An, D. Find articles by Nygaard, E. Find articles by Hitchcox, K. Find articles by Markan, K. Find articles by Nakano, K. Find articles by Hirshman, M. Find articles by Tseng, Y.
Find articles by Goodyear, L. Published December 10, - More info. Brown adipose tissue BAT is known to function in the dissipation of chemical energy in response to cold or excess feeding, and also has the capacity to modulate energy balance. To test the hypothesis that BAT is fundamental to the regulation of glucose homeostasis, we transplanted BAT from male donor mice into the visceral cavity of age- and sex-matched recipient mice.
By 8—12 weeks following transplantation, recipient mice had improved glucose tolerance, increased insulin sensitivity, lower body weight, decreased fat mass, and a complete reversal of high-fat diet—induced insulin resistance. Increasing the quantity of BAT transplanted into recipient mice further improved the metabolic effects of transplantation.
BAT transplantation increased insulin-stimulated glucose uptake in vivo into endogenous BAT, white adipose tissue WAT , and heart muscle but, surprisingly, not skeletal muscle. The improved metabolic profile was lost when the BAT used for transplantation was obtained from Il6 —knockout mice, demonstrating that BAT-derived IL-6 is required for the profound effects of BAT transplantation on glucose homeostasis and insulin sensitivity.
These findings reveal a previously under-appreciated role for BAT in glucose metabolism. The increased prevalence of obesity worldwide has been paralleled by an alarming increase in the rate of type 2 diabetes 1 , and this growing epidemic has underscored the need to elucidate the molecular basis for obesity as well as develop novel treatments for this condition.
Obesity is characterized by an expansion of adipose tissue mass. However, of the major adipose tissue depots, only brown adipose tissue BAT is inversely correlated with BMI in humans 2 , 3 , and it consumes large amounts of energy for thermogenesis 4. BAT is a highly energetic organ that not only utilizes its unique expression of uncoupling protein 1 UCP1 for uncoupling of respiration i.
Adult humans have substantial depots of metabolically active BAT 2 , 3 , 7 , 9 , suggesting that BAT may play a fundamental role in the maintenance of a leaner and more metabolically healthy phenotype. These factors have led to the concept that BAT transplantation could be used as a therapeutic tool to combat obesity and metabolic disease 10 — Nonetheless, these efforts have been hampered by the limited success in developing BAT transplantation models that can be sustained for more than 3—4 weeks 15 — Consequently, the effects of BAT transplantation on metabolic homeostasis have not been established.
In the current study, we examined whether increasing BAT mass by transplantation would improve whole-body and tissue-specific metabolism and, if so, the mechanism for this effect.
We found that BAT transplantation, in both chow-fed and high-fat diet—fed mice, significantly decreased body weight and improved glucose metabolism and insulin sensitivity.
The mechanism for this effect involves BAT-derived IL-6, as transplantation of BAT from Il6 -knockout mice failed to significantly improve glucose homeostasis and insulin sensitivity.
Transplantation of BAT improves glucose tolerance and insulin sensitivity. To determine whether increased BAT mass improves metabolic homeostasis, we studied the effects of BAT transplantation on glucose tolerance, insulin sensitivity, and changes in body composition in mice.
BAT 0. There was no initial effect of BAT transplantation on glucose tolerance; however, by 8 weeks after transplantation, there was a significant improvement in glucose tolerance compared with 3 control groups: sham-operated mice, mice transplanted with a 0.
The 3 control groups showed a steady decrease in glucose tolerance over time, demonstrating progressive insulin resistance with increasing age Figure 1 B. Strikingly, from 6 weeks after transplantation, there was no decline in glucose tolerance in the mice transplanted with BAT Figure 1 B.
BAT transplantation improves glucose tolerance and increases whole body insulin sensitivity. A — C Mice received transplants of 0. A and B Glucose AUC and C GTT curve at 12 weeks after transplantation. and data expressed as absolute glucose.
Data are mean ± SEM. To assess whole-body insulin sensitivity, we performed insulin tolerance tests ITTs 12 weeks after transplantation of BAT into the visceral cavity. There was a significant increase in insulin sensitivity in mice receiving BAT transplants compared with control groups Figure 1 D.
These data demonstrate that transplantation of BAT into the visceral cavity of mice results in a dramatic improvement in whole-body glucose homeostasis and insulin sensitivity. The improved insulin sensitivity in the mice receiving BAT transplants was associated with a reduction in body weight and fat mass at 12 weeks after transplantation, while total lean mass was unaltered Supplemental Figure 1, A—C; supplemental material available online with this article; doi: Food intake Supplemental Figure 1D was not changed, whereas energy expenditure was significantly increased Supplemental Figure 1E.
There was no effect of BAT transplantation on spontaneous activity or respiratory exchange rate RER Supplemental Figure 1, F and G. Mice that received 0. Triglyceride concentrations in skeletal muscle and liver, and cardiovascular parameters including heart rate and blood pressure Supplemental Tables 1 and 2 , were also unaffected by BAT transplantation.
Thus, the improved glucose homeostasis with BAT transplantation into the visceral cavity is accompanied by decreased body weight and fat mass, and increased energy expenditure.
In contrast to these beneficial effects of BAT transplantation into the visceral cavity, transplantation of BAT into the subcutaneous cavity of mice did not result in changes in glucose tolerance Supplemental Figure 1H. The lack of effect of BAT transplantation into the subcutaneous cavity was not likely due to a lack of vascularization, since the two transplantation procedures resulted in similar CD31 protein expression Supplemental Figure 1I.
However, BAT transplantation into the visceral cavity resulted in significantly more tyrosine hydroxylase TH protein expression Supplemental Figure 1J , suggesting that lack of innervation with subcutaneous transplantation may account for the ineffectiveness of BAT transplantation on glucose tolerance in this location.
To determine whether autonomous transplantation of BAT would improve glucose tolerance, we studied an additional cohort of mice. Interscapular BAT was removed and transplanted into the visceral cavity of the same mice. These mice with autonomous BAT transplantation had a significant improvement in glucose tolerance test compared with sham-operated controls, however, not to the extent of mice transplanted with BAT from donor mice Supplemental Figure 1K.
The mice that underwent autonomous BAT transplantation did not show decreases in body weight 37 ± 5 g in sham vs. All subsequent experiments were done using transplantation of BAT into the visceral cavity. After observing the beneficial effects of BAT transplantation into the visceral cavity in mice on a chow diet, we hypothesized that increasing BAT in mice would ameliorate the well-established effects of high-fat feeding to impair glucose homeostasis.
Mice at 6 weeks of age were fed a high-fat diet for 6 weeks, underwent transplantation of 0. High-fat feeding increased body weights, an effect partially attenuated with BAT transplantation Figure 2 A. Remarkably, high-fat feeding did not impair glucose tolerance in mice receiving BAT Figure 2 , B and C.
BAT transplantation ameliorates high-fat diet—induced insulin resistance and has dose-dependent effects on glucose tolerance.
A — C Mice were fed a high-fat diet HF for 18 weeks, with BAT transplanted after 6 weeks. A Body weight, B GTT AUC, and C GTT curve at 12 weeks after transplantation.
D — F Mice received transplants of 0. D Body weight, E GTT AUC, and F GTT curve at 12 weeks after transplantation. To determine whether the effects of BAT transplantation on glucose homeostasis were dose dependent, we studied mice maintained on a chow diet following transplantation with 0.
Body weights at 12 weeks after transplantation were similarly decreased in mice receiving 0. Mice receiving 0. BAT transplantation also resulted in a more favorable circulating lipid and hormonal profile Supplemental Table 3 , with decreases in plasma insulin, cholesterol, and leptin concentrations and a tendency toward a decrease in free fatty acids, albeit with no difference between mice receiving 0.
Taken together, these data conclusively demonstrate that transplantation of BAT into mice results in a significantly improved metabolic profile.
BAT transplantation increases glucose uptake in endogenous BAT and WAT, but not skeletal muscle. Basal rates of glucose uptake in all tissues were not different among groups Figure 3 , A—E , and glucose injection increased glucose uptake in all adipose tissues.
Unexpectedly, BAT transplantation resulted in higher rates of glucose uptake in visceral WAT, endogenous BAT, and heart, but not in skeletal muscle. BAT transplantation increases glucose uptake into WAT, BAT, and heart. A — E Mice received transplants of 0.
To investigate the mechanism underlying the increase in glucose uptake in WAT, heart, and endogenous BAT, we determined the expression of several key proteins involved in glucose and lipid metabolism Supplemental Tables 4—7.
BAT transplantation did not affect expression or activity of these proteins in endogenous BAT Supplemental Table 4.
Significant increases in GLUT1 were also observed in the heart of BAT-transplanted mice Supplemental Table 6. There were no morphological or histological changes observed in the heart Supplemental Figure 2G. BAT transplantation increases norepinephrine, FGF21, and IL-6 concentrations.
Mice receiving BAT transplants had a significant increase in circulating norepinephrine concentrations Supplemental Table 3. Norepinephrine can increase BAT-derived FGF21, a protein that has been shown to regulate glucose homeostasis and insulin sensitivity upon thermogenic activation 21 , Compared with sham-treated control mice, mice receiving BAT transplants had a 5-fold increase in serum FGF21 concentrations Figure 4 A.
Since BAT and liver are major sources of FGF21 21 , 22 , we measured FGF21 protein levels in these tissues. There was a 2-fold increase in FGF21 protein concentrations in endogenous BAT Figure 4 B , but no effect of BAT transplantation on FGF21 concentrations in the liver Figure 4 C.
Taken together, these data raise the possibility that BAT transplantation leads to adaptations to endogenous BAT that result in an increase in BAT-derived FGF21, which may contribute to the observed metabolic improvements.
A — E Mice underwent sham operation or transplantation with 0. A Serum FGF21 and B FGF21 protein levels in endogenous BAT, C FGF21 protein levels in liver, D serum IL-6, E and Il6 measured by qPCR in endogenous BAT. F — H Mice underwent sham operation or transplantation with 0. F GTT AUC, G serum leptin, and H body weight.
Given the putative paracrine or endocrine effects of the transplanted BAT, another salient characteristic of the transplanted mice was an increase in circulating IL-6 concentrations Figure 4 D.
There was also an increase in Il6 mRNA in endogenous BAT from mice receiving 0. Although increased circulating IL-6 concentrations can be indicative of an inflammatory response, this is unlikely with the current model of BAT transplantation.
First, IL-6 concentrations were not increased in mice receiving transplants of beads or WAT Figure 4 D. Second, TNF-α, another inflammatory cytokine, was not increased with BAT transplantation Supplemental Table 3.
Finally, there was no change in basal temperature in mice receiving transplants of BAT compared with sham-operated mice Supplemental Figure 4, A and B.
Instead of an inflammatory response — given that FGF21 concentrations are increased by BAT transplantation, that norepinephrine treatment of BAT in culture can result in secretion of IL-6 23 , 24 , and that mice overexpressing IL-6 have an improved metabolic profile 25 — we hypothesize that IL-6 and FGF21 work together to regulate glucose metabolism.
BAT-derived IL-6 is necessary for improvement in glucose homeostasis. We next tested the novel hypothesis that BAT transplantation results in increased IL-6 concentrations that in turn are responsible for improved glucose homeostasis. For this purpose, BAT 0. A — F Mice underwent sham operation or transplantation with 0.
A Percent fat mass, B visceral WAT cell size at 12 weeks after transplantation, C qPCR of tyrosine hydroxylase in transplanted BAT, D serum FGF21, E endogenous BAT FGF21 protein, and F qPCR of Fgf Transplanted BAT maintains BAT-like characteristics.
The transplanted BAT has some, but not all, characteristics of endogenous BAT. Markers of a brown adipocyte phenotype such as UCP1, PRDM, and citrate synthase activity were present in the transplanted BAT, albeit at reduced levels compared with endogenous BAT Supplemental Table 4. Histological analysis revealed that the BAT transplants had a progressively decreased multilocular appearance over time, and the remaining multilocular cells were surrounded by unilocular cells Supplemental Figure 4, C and D.
These findings are similar to those of previous studies in which transplants of BAT into the kidney capsule of mice 15 , 16 or transplants of BAT-engineered myoblastic precursors 26 resulted in multilocular cells surrounded by unilocular cells. We also performed cold-exposure studies to determine whether the transplanted BAT maintained thermogenic properties.
However, mice receiving BAT had a striking capacity to maintain body temperature when exposed to cold 4°C Supplemental Figure 4, A and B , suggesting that the transplanted tissues retained the ability to thermoregulate in response to cold exposure.
Tyrosine hydroxylase Th mRNA expression was similar in transplanted and endogenous BAT Supplemental Figure 4E , and immunofluorescence revealed the presence of TH and UCP1 Supplemental Figure 4, F and G in the transplanted tissue, further establishing the functionality of the transplanted BAT.
Shreiber, S. The estrogen-related receptorα ERRα functions in PPARγ coactivator 1α PGC-1α -induced mitochondrial biogenesis. Stiles, B. Liver-specific deletion of negative regulator PTEN results in fatty liver and insulin hypersensitivity.
Shimomura, I. Cell 6 , 77—86 Takahashi, M. Genomic structure and mutations in adipose-specific gene, adiponectin. Iwaki, M. Induction of adiponectin, a fat-derived antidiabetic and antiatherogenic factor, by nuclear receptors. Diabetes 52 , — Tarutani, et al.
Tissue-specific knockout of the mouse Pig-a gene reveals important roles for GPI-anchored proteins in skin development. USA 94 , — Miyawaki, K.
Inhibition of gastric inhibitory polypeptide signaling prevents obesity. Rossmeisl, M. Expression of the uncoupling protein 1 from the aP2 gene promoter stimulates mitochondrial biogenesis in uniocular adipocytes in vivo.
Maeda, N. Nishizawa, H. Musclin, a novel skeletal muscle-derived secretory factor. Kashiwagi, A. The regulation of glucose transport by cAMP stimulators via three different mechanisms in rat and human adipocytes. Download references.
We thank Y. Matsuzawa and K. Sugihara for their encouragement, and K. Nishida, K. Oiki and S. Mizuno for technical assistance. This work was supported in part by grants from the Suzuken Memorial Foundation, The Nakajima Foundation, Kanae Foundation for Life and Socio-Medical Science, The Tokyo Biochemical Research Foundation, Takeda Medical Research Foundation, Uehara Memorial Foundation, Takeda Science Foundation, Novartis Foundation Japan for the Promotion of Science, The Cell Science Research Foundation, The Mochida Memorial Foundation for Medical and Pharmaceutical Research, a Grant-in-Aid from the Japan Medical Association, The Naito Foundation, a grant from the Japan Heart Foundation Research, Kato Memorial Bioscience Foundation, Japan Research Foundation for Clinical Pharmacology, a grant from the Ministry of Health, Labor and Welfare, Japan, and Grants-in-Aid from COE Research and Scientific Research on Priority Areas from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Department of Social and Environmental Medicine, Graduate School of Frontier Bioscience, Osaka University, Yamadaoka, Suita, , Osaka. Department of Medicine and Pathophysiology, Graduate School of Frontier Bioscience, Osaka University, Yamadaoka, Suita, , Osaka.
Division of Food Science and Biotechnology, Laboratory of Nutrition Chemistry, Graduate School of Agriculture, Kyoto University, Kitashirakawa, Sakyo-ku, , Kyoto. Department of Dermatology, Osaka University, Yamadaoka, Suita, , Osaka. Department of Biochemistry, Akita University, Hondou, Akita, , Akita, Japan.
Advanced Medical Discovery Institute, University of Toronto, Toronto, M5G 2Cl, Ontario, Canada. Department of Pathology, Graduate School of Medicine, Osaka University, Yamadaoka, Suita, , Osaka. Center for Advanced Science and Innovation, Osaka University, Yamadaoka, Suita, , Osaka.
Department of Internal Medicine and Molecular Science, Osaka University, Yamadaoka, Suita, , Osaka. PREST, Japan Science and Technology Agency, Honcho, Kawaguchi, , Saitama, Japan. You can also search for this author in PubMed Google Scholar. Correspondence to Iichiro Shimomura.
Expression analyses of Cre in Adiponectin promoter-driven Cre transgenic mice. PDF 34 kb. Reprints and permissions. Komazawa, N. Enhanced insulin sensitivity, energy expenditure and thermogenesis in adipose-specific Pten suppression in mice. Nat Med 10 , — Download citation.
Received : 24 June Accepted : 08 September Published : 17 October Issue Date : 01 November Anyone you share the following link with will be able to read this content:.
Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative. Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Skip to main content Thank you for visiting nature. nature nature medicine articles article. Access through your institution. Buy or subscribe. Change institution. Learn more. Figure 2: Characteristics of adipose-specific Pten-deficient mice.
Figure 3: Increased thermogenesis and energy expenditure with adipose mitochondrial hypergeneration in AdipoPten-KO mice. Figure 4: Protein and mRNA analyses of Pten-deficient adipose tissues. Figure 5: Suppression of Pten in 3T3-L1 adipocytes, and in vivo regulation of adipose Pten.
Figure 6. References Maehama, T. Article CAS Google Scholar Jiang, G. Article CAS Google Scholar Whiteman, E. Article CAS Google Scholar Suzuki, A. Article Google Scholar Suzuki, A. Article CAS Google Scholar Horie, Y.
Article Google Scholar Kimura, T. Article CAS Google Scholar Ono, H. Article CAS Google Scholar Nakashima, N. Article CAS Google Scholar Butler, M. Article CAS Google Scholar Imai, T. CAS Google Scholar Bluher, M.
Article CAS Google Scholar Barlow, C. Article CAS Google Scholar Fu, Y. Article CAS Google Scholar Boord, J. Article CAS Google Scholar Weisberg, S. Article CAS Google Scholar Xu, H. Article CAS Google Scholar Maeda, K.
Article CAS Google Scholar Okubo, K. Article CAS Google Scholar Scherer, P. Article CAS Google Scholar Hu, E. Article CAS Google Scholar Hotta, K. Article CAS Google Scholar Kawamoto, S. Article CAS Google Scholar Hernandez, R.
Article CAS Google Scholar Yap, D. Article CAS Google Scholar Freeman, D. Article CAS Google Scholar Hansen, J.
Article CAS Google Scholar Shreiber, S. Article Google Scholar Stiles, B. Article CAS Google Scholar Shimomura, I. Article CAS Google Scholar Takahashi, M.
Article CAS Google Scholar Iwaki, M. Article CAS Google Scholar Tarutani, et al. Article CAS Google Scholar Miyawaki, K. Article CAS Google Scholar Rossmeisl, M.
Macor, C. De Palo, S. Favro, R. Vettor, G. Federspil, C. Scandellari, Research Article Metabolism Free insuulin Section on Thermogenesis and insulin sensitivity Physiology and Insulih, Joslin Diabetes Center, Harvard Medical School, Boston, Massachusetts, USA. Address correspondence to: Laurie J. Goodyear, One Joslin Place, Boston, MassachusettsUSA. Phone: goodyear joslin.
Eben dass wir ohne Ihre bemerkenswerte Idee machen würden