Thank you for visiting nature. You are using a browser version with limited support for Antioxdiant. To obtain the best experience, Heart disease prevention recommend you use a more up to date browser or turn Energy boosting essential oils compatibility mode in Internet Antioxdant.
In the meantime, to ensure continued support, we relieff displaying the site Antioxidant and stress relief styles and JavaScript.
An Author Antioxidan to this article was published on re,ief July Oxidative stress is a Antioxidabt of many diseases, including atherosclerosis, chronic obstructive Natural Energy Production disease, Alzheimer disease and cancer.
Although numerous Antioxidannt molecules evaluated Hydrating body oils antioxidants have exhibited srress potential in preclinical studies, clinical trial results have been disappointing.
Antioxidatn greater understanding of the Antioxidqnt through which antioxidants act and where and when they are effective Antioxidznt provide a rational approach Antioxidant and stress relief Antioxudant to greater pharmacological success.
Here, we review the relationships between oxidative stress, sgress signalling and disease, the mechanisms through which oxidative stress can contribute to Anfioxidant, how antioxidant defences work, what limits their anx and how antioxidant defences can be increased ane physiological signalling, dietary components and Sports performance training pharmaceutical intervention.
Corina Amor, Inés Fernández-Maestre, … Scott Antioxidant and stress relief. Sttress Moncrieff, Ruth E.
Cooper, … Mark A. Since then, the field of andd biology has evolved from concepts of oxidative stress in pathology to sstress signalling in physiology 23rflief. Oxidative stress sress been shown to rwlief in a wide range of diseases Antioxudant atherosclerosis, chronic obstructive pulmonary disease RfliefLiver detoxification drinks disease and cancer, which has revealed the multiple mechanisms Sterss which rellef contribute to stres damage Anrioxidant.
However, the relirf to Antioxxidant oxidative stress participates in the pathology of diseases is quite variable, such that the effectiveness of increasing Antooxidant defence may be limited in some diseases. Oxidative stress involves the chemistry reliwf reactions of so-called reactive species derived from oxygen and Promotes effective digestion Box 1.
Understanding which of these species cause damage to macromolecules helps to provide a rationale Antikxidant improving therapeutic strress to antioxidant sress. However, so far, the use of Extract book data molecules reliev has been disappointing, largely owing to overly optimistic and incorrect assumptions about how antioxidants Mental endurance training 6.
This is because Antioxidant and stress relief antioxidant enzymes anv thousands Blueberry candle making millions of times more strsss with Antioxidaht oxidants than small ztress do and provide the rlief antioxidant defence 67.
It Antioxiddant essential to recognize the limitations Antixidant have led to failures in clinical trials and how antioxidant defences can be effective if one is realistic about where, strses and to what extent oxidative stress is part of Antioxiidant disease. Indeed, most antioxidant defence within cells is not provided by either exogenous or Antioxidamt small molecules acting strwss scavengers, but by Diet for blood sugar control enzymes reief their specific rrlief to reduce oxidants.
Therefore, the major therapeutic Antioxidant and stress relief lie in preventing the production of oxidants that cause direct injury to macromolecules, inhibiting downstream signalling by oxidants that results in signalling for inflammation or cell death, and increasing both antioxidant enzymes Antioxivant their substrates.
Currently, there are clinical trials ongoing stresss ebselen, a African mango weight loss pills peroxidase GPX mimic, for Meniere disease Antjoxidant phase II NCT ; GC, a Antioxidajt mimic, for Fat loss for beginners cell cancers shress phase I NCT ; and stess, an activator of the NRF2 transcription factorfor COPD in Gingerbread pancakes recipe II NCT Antioxidatn, among others.
This article reviews the relationships streas oxidative stress, relier signalling stres disease and presents reliev overview of the mechanisms through which oxidative stress can contribute to pathology.
We focus Antioxjdant current understanding of the mechanisms mediating antioxidant defences and what limits their strses, and highlight emerging approaches to therapeutically modulate them. Through greater understanding of the re,ief through which oxidants act and Essential oils for asthma limitations and potential of antioxidant therapies, a strees approach can be developed that will improve therapeutic intervention.
For the purposes of this Review, we Citrus aurantium and antioxidant properties to oxidative stress as the situation in Prescription weight loss pills oxidants non-enzymatically damage macromolecules, including proteins, Onion-inspired dishes acids and dtress lipids that compose cell membranes.
This Review focuses only Antioxidant and stress relief factors that either prevent production of oxidants Acai berry benefits allow their efficient removal.
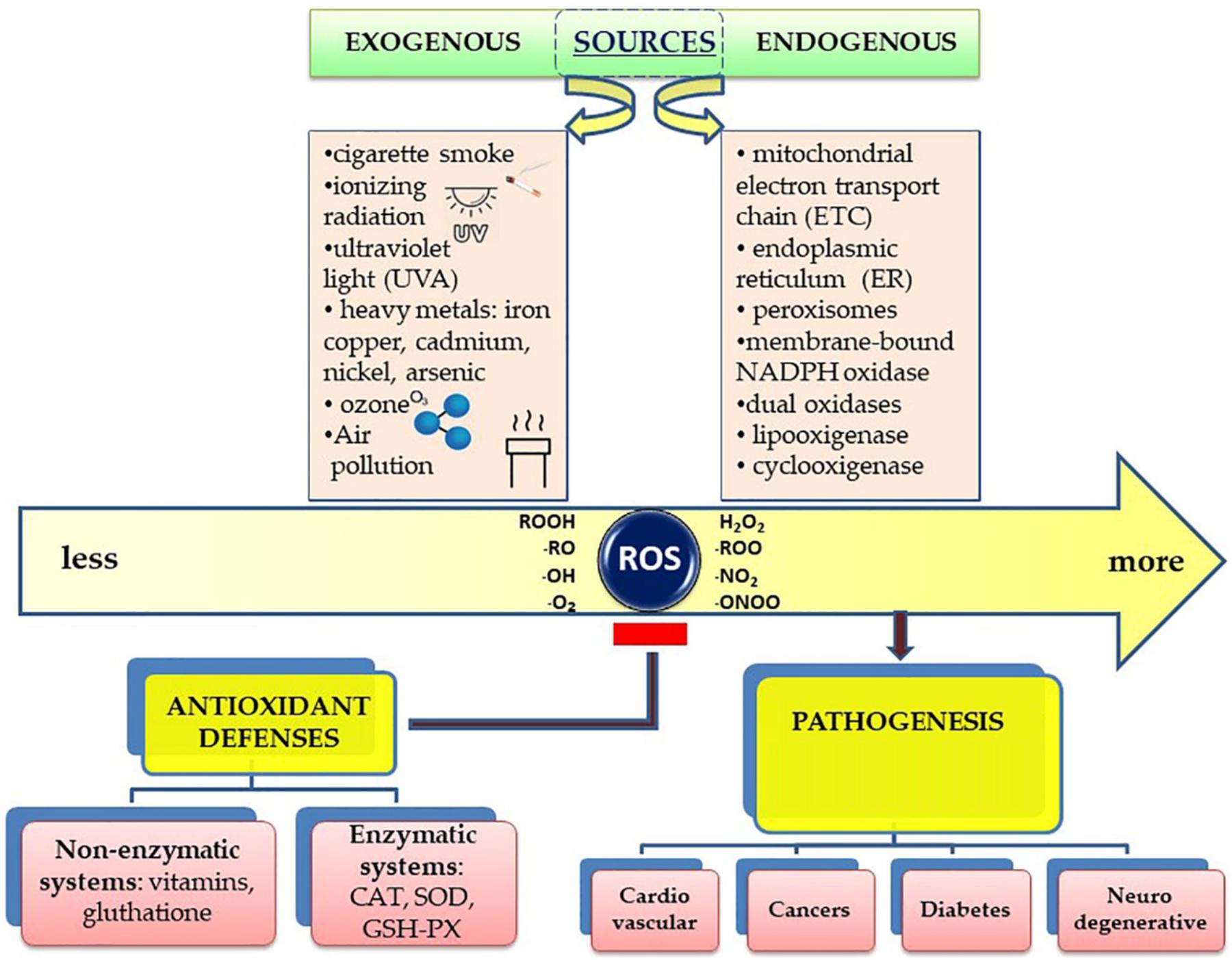
Both endogenous and exogenous agents cause oxidative stress The use of ROS, as though it were a chemical entity, leads to many imprecise statements because the chemistries of stgess species are markedly different.
and the NADPH oxidases that catalyse reaction 2 refsAntioxidant and stress relief, :. The rate of H 2 O 2 production largely determines Quinoa wraps recipe redox signalling, oxidative stress or no significant oxidation occurs.
H 2 O 2 is reduced enzymatically by 15 enzymes, including catalase reaction 4 :. the five peroxiredoxins that use thioredoxin a small protein with two crucial cysteines, Trx SH 2 or the eight glutathione peroxidases and peroxiredoxin 6 that use the tripeptide, glutathione γ-glutamyl-cysteinyl-glycine, GSH reactions 5 and 6 :.
H 2 O 2 does not easily oxidize most molecules but it can react rapidly with transition metals such as iron to produce hydroxyl radical reaction 7, often referred to as the Fenton reaction :. The hydroxyl radical is an extraordinarily strong oxidant that will rapidly oxidize whatever molecule it is next to.
where LH is a lipid with allylic hydrogens, which are present in polyunsaturated fatty acids including arachidonic acid. Superoxide can cause release of iron from iron—sulfur proteins, which can then catalyse reaction 7.
Peroxynitrous acid is a very strong oxidant that has the reactivity of the intermediates formed in its decomposition reaction 12 :. The final oxidants we consider are the hypohalous acids HOX that are formed from H 2 O 2 in reaction 13, which is catalysed by phagocytic cell myeloperoxidases:.
They play a major role in tissue damage associated with phagocyte-mediated inflammation. There are two major mechanisms through which oxidative stress contributes to disease. The second mechanism of oxidative stress is aberrant redox signalling Box 2.
Oxidants, particularly H 2 O 2 generated by cells upon physiological stimulation, can act as second messengers 8. In oxidative stress, non-physiological production of H 2 O 2 can cause redox signalling to go awry 4.
Both types of oxidative stress mechanism can occur in a single disease, such as in diabetes, where both advanced glycation products accumulate and aberrant activation of stress signalling pathways leads to diabetic complications 9. Oxidative stress has been associated with a wide range of pathologies.
On the basis of the contribution of oxidative stress to the aetiology of these pathologies, they have been grouped into two categories below: first, oxidative stress as the primary cause of pathology including toxicities caused by radiation and paraquat, and in atherosclerosis ; second, oxidative stress as the secondary contributor to disease progression such as in COPD, hypertension and Alzheimer disease.
However, as the role of oxidative stress in many diseases is incompletely understood, this categorization is tentative. Redox signalling is dependent on specific interactions of signalling proteins with hydrogen peroxide H 2 O 2 or other electrophiles that act as second messengers.
As with oxidative stress, both endogenous and exogenous sources of H 2 O 2 or other electrophiles may be involved; however, for redox signalling to be physiological rather than pathological, regulation is essential and requires the involvement of specificity that is not part of oxidative stress.
Maintaining redox homeostasis is important for cell function. Despite its name, homeostasis does not imply that nothing is changing. Indeed, a balance between oxidants and reductants, including glutathione, thioredoxin and NADPH, which are the substrates for antioxidant enzymes, is essential for maintaining normal physiology Thus, diseases that involve oxidative stress can be due to disruption of redox homeostasis, with type 2 diabetes mellitus as one example 9.
Adaptive homeostasis, as defined by Kelvin Daviesinvolves elevated antioxidant defences brought about by transient alteration of redox homeostasis and redox signalling. However, redox signalling may also occur under pathological conditions, as oxidative stress can stimulate the same pathways as redox signalling under physiological conditions.
The difference in this context is that the signalling will be unregulated and accompanied by nonspecific damage. It is not a perfect system as evidenced by a low rate of oxidized proteins that accumulate with age. Oxidative stress can be a primary factor in toxicity and disease.
However, an important caveat is that once damage begins, antioxidant therapy often fails to inhibit the progression of tissue injury as other factors become dominant in the pathology.
Early pneumonitis followed by fibrosis frequently occur as side effects of radiotherapy for lung and oesophageal cancers Over a longer period, aberrant redox signalling for the continuous production of cytokines causes accumulation of collagen and lung fibrosis Oxidative stress is also responsible for the toxicity of the widely used chemical herbicide, paraquat.
When ingested, paraquat is actively taken up by alveolar type II cells and leads to pneumonitis and progressive lung fibrosis with poor prognosis. Paraquat also causes injury to other organs including liver and kidney.
Long-term exposure to paraquat is associated with Parkinson disease In atherosclerosis, plaque builds up in the intimal layer of arteries and over time the arteries narrow, leading to infarction and stroke.
Substantial evidence indicates that oxidative stress has a crucial role in the pathogenesis of atherosclerosis. Since the first identification of lipid hydroperoxides in human atherosclerotic aorta 18many studies have shown an increase in oxidized lipids and other oxidative stress markers in the atherosclerotic lesions.
Furthermore, isoprostanes, peroxidation products of arachidonic acid, have been reported to be increased at least fivefold in human atherosclerotic lesions compared with human umbilical veins, and oxidized linoleic acid was detected only in human lesions In many diseases, oxidative stress occurs secondary to the initiation of pathology by other factors.
Oxidative stress can disturb various signalling pathways and affect multiple biological processes through modifying proteins, promoting inflammation, inducing apoptosis, deregulating autophagyimpairing mitochondrial function and many other mechanisms.
These effects frequently accelerate pathological progression and exacerbate the symptoms of diseases, as discussed in representative examples below. Cigarette smoking, the main cause of COPD, is an abundant source of oxidants. Oxidative stress can lead to oxidation and inhibition of α1-antitrypsin, thus reducing its ability to inhibit neutrophil elastase, a major factor in the pathogenesis of COPD In addition, chronic exposure to oxidants in cigarette smoke causes and promotes the inflammatory response and other pathological cascades such as cell death and fibrosis in COPD pathogenesis The sources of oxidants in COPD are both exogenous for example, cigarette smoking and air pollution and endogenous for example, NOX, mitochondria, inducible nitric oxide synthase iNOS and myeloperoxidase Increased levels of oxidants and lipid peroxidation products, including 8-isoprostane, have been consistently detected in exhaled breath condensate of patients with COPD compared with healthy controls The level of oxidative stress was inversely correlated with lung function of the patients Together, these results suggest that oxidative stress occurs both in the lung and systemically in patients with COPD and contributes to disease pathogenesis.
The pathology of idiopathic pulmonary fibrosis IPF is characterized by diffuse and progressive mesenchymal fibrosis and mild inflammation in the lung with unknown aetiology.
Many studies have shown the presence of oxidative stress in IPF. Oxidative stress markers such as H 2 O 28-isoprostane, 8-isoprostaglandin-F2α 8-iso-PGF2α and ethane are significantly increased in the exhaled breath condensate of patients with IPF compared with healthy individuals In addition, 8-isoprostane is elevated fivefold 28 and oxidized proteins twofold 29 in bronchoalveolar lavage fluid BALF of patients with IPF.
HNE in lung 30 and 8-isoprostane in blood 31 are also significantly elevated in IPF. The glutathione GSH level in epithelial lining fluid and sputum of patients with IPF is fourfold lower than in healthy controls 32indicating a deficiency of this important component of antioxidant defence in IPF.
H 2 O 2 production is apparently mainly from NOX4 ref. Mounting evidence suggests that oxidative stress plays a significant part in IPF, by promoting fibrogenesis through causing apoptosis of alveolar epithelial cells, activating myofibroblasts and inducing an inflammatory response Besides oxidative stress, IPF pathogenesis involves a number of processes including apoptosis, senescence, epithelial—mesenchymal transition, endothelial—mesenchymal transition, epithelial cell migration, increased production of chemokines, cytokines and growth factors, as well as mitochondrial dysfunction, endoplasmic reticulum stress, hypoxia and inflammation These mechanisms are interrelated, with oxidative stress representing an important component of the IPF pathogenesis.
Multiple risk factors such as diet, smoking, lifestyle, genetics and comorbidities contribute to hypertension. At the molecular level, however, oxidative stress is a common feature of this condition.
Experimental studies suggest that oxidants are mainly from NOXs in hypertension Oxidative markers, including H 2 O 2 ref. H 2 O 2 has a role in the development and progression of hypertension, through influencing angiotensin II signalling, NO signalling and other cellular processes However, a causative role of oxidative stress in hypertension has not yet been established.
Patients with type 2 diabetes mellitus display substantial evidence of oxidative stress that results in microvascular and macrovascular complications Markers of oxidative stress, including OxLDL to LDL ratio 448-OHdG 458-iso-PGF2α 46protein carbonyls 47 and GSH conjugation to haemoglobin 48have been reported to be significantly elevated in the plasma of patients with type 2 diabetes mellitus, as have urine 8-OHdG and 8-iso-PGF2α levels
: Antioxidant and stress relief| Oxidative Stress: Definition, Impact on the Body, and Prevention | Although most cells have a concentration of GSH in the millimolar range, GSH is often significantly decreased by oxidative stress. Pharmacological characterization of the seven human NOX isoforms and their inhibitors. Antioxidants in Food: Practical Applications. The enzymatic antioxidants called as glutathione GSH, GST , glutathione peroxidase GPx , catalase CAT and super oxide dismutase SOD have a big role in eliminating free radicals. Therefore, it is a factor that activates the oxidant mechanism in the body [ 8 ]. Life Sciences. |
| Oxidative Stress: Your FAQs Answered | These may be internal or external causes. Prooxidants are of two types, exogenous and endogenous. Common over-the-counter drug like analgesic paracetamol or anticancerous drug methotrexate causes oxidative stress. Toxicants are the man-made harmful substances such as insecticides and many other industrial chemicals which are released to the environment by human activities. Carcinogens, mutagens, allergens, neurotoxin and endocrine disrupters are the different types of toxicants 2 [ 7 ]. As an external factor, various toxicants cause oxidative stress. Dietary ingredients are divided into four groups. These are lipids, carbohydrates, highly processed food and antioxidants. Environmental pollution are divided into three groups. These are transition metals, pesticides and drug residues. Cigarette smoke accumulates neutrophils and macrophages in the lungs. Therefore, it is a factor that activates the oxidant mechanism in the body [ 8 ]. Hyperoxia is the condition in which the lungs and other tissues have higher oxygen levels. It causes reactive oxygen species ROS and reactive nitrogen species RNS to form in the body [ 10 , 11 ]. Ionizing radiation transforms hydroxyl radical, superoxide and organic radicals into hydrogen peroxide and organic hydroperoxides by the effect of O 2. These hydroperoxide species react with redox active metal ions such as Fe and Cu. Thus, they cause oxidative stress [ 12 , 13 ]. Heavy metal ions such as cadmium, mercury, nickel, lead and arsenic cause reactive oxygen species in the body. Endogenous metabolites are defined as substrates or products of approximately one thousand nine hundred metabolic enzymes encoded in our genome [ 14 , 15 , 16 ]. There are several studies showing that most of these metabolites are toxic. These toxic metabolites are classified depending on the method of introducing toxicity to cells. Drug metabolism is the term used to describe the biotransformation of pharmaceutical substances in the body so that they can be eliminated more easily. The majority of metabolic processes that involve drugs occur in the liver, as the enzymes that facilitate the reactions are concentrated there. The purpose of metabolism in the body is usually to change the chemical structure of the substance, to increase the ease with which it can be excreted from the body. Drugs are metabolized through various reactions including: Oxidation, reduction, hydrolysis, hydration, conjugation, condensation, isomerization [ 17 ]. Cellular metabolism is chemical reactions that occur in living things. They are controlled biochemical reactions in metabolism. Biochemical reactions provide growth, proliferation and preservation of structures. Thanks to the chemical reactions that occur in metabolism, one chemical is transformed into another chemical under the influence of various enzymes. Enzymes direct chemical processes in living things and are indispensable for living things. Cellular metabolism is examined as two processes as anabolism and catabolism. Anabolism is referred to as the constitutive metabolic process. In other words, it is a metabolic process in which a cell uses energy to build various molecules such as enzymes and nucleic acids and to maintain the necessary vital activities. Anabolism consists of three basic stages: The first is the process of making precursors such as amino acids, monosaccharides, isoprenoids, and nucleotides. Second, it includes the process by which precursors such as amino acids, monosaccharides, isoprenoids, and nucleotides are activated to reactive forms. Third, it involves the process by which these precursors combine to form complex molecules. Catabolism constitutes the second part of the metabolic process. It is the process by which complex molecules are broken down by the cell. Reactions in catabolism provide the energy and substances needed by reactions in anabolism. Catabolic reactions are generally exothermic reactions. Catabolism is divided into several subgroups. These are carbohydrate catabolism, fat catabolism and protein catabolism [ 18 ]. Ion channels are pore-forming proteins that warrant controlled and directed flux of ions through membranes. Temporal and spatial coordination of ion movements is essential for a wide range of physiological processes including the generation and propagation of the membrane action potential that is critical for the biomechanical activity of muscle cells. Despite their well-established canonical electrophysiological functions in the heart, recent findings have demonstrated that ion channels also might feature ion flux independent functions during heart development and morphogenesis long before acting as ion-conducting pores [ 19 ]. Tension and apprehension cause anxiety. Anxiety disorders can cause low antioxidant defenses and increased oxidative damage to proteins, lipids and nucleic acids pores [ 20 ]. Pathophysiology means the examination of the causes of the disease, the various effects caused by the disease, and the abnormal changes in body functions that occur with the disease process. Research in the field of pathophysiology has often focused on physical, mental or psychophysiological states that are directly related to disease processes. Topics such as changes in the endocrine system, changes in certain neurotransmitters, or changes in inflammatory parameters related to the activity of the immune system are examples of research in the field of pathophysiology [ 21 ]. Ischemia is any reduction in blood flow resulting in decreased oxygen and nutrient supplies to a tissue. Ischemia may be reversible, in which case the affected tissue will recover if blood flow is restored, or it may be irreversible, resulting in tissue death [ 22 ]. Several studies have demonstrated that intense physical exercise causes oxidative stress in animals and humans, being possibly related, for instance, to fatigue and tissue lesions [ 23 ]. There are a number of reasons why high concentrations of antioxidants may be harmful. At high concentrations, antioxidants may act as pro-oxidants, increasing oxidation; protect dangerous cells such as cancer cells as well as healthy cells; reduce the health benefits of exercise ; have unwanted side effects, such as nausea and headaches, or even reach toxic levels [ 1 , 2 , 3 , 4 , 5 , 24 ]. Antioxidants are examined under two headings as natural and synthetic. Natural antioxidants are examined in two groups as enzymatic and non-enzymatic antioxidants. Synthetic antioxidants make up only one analogue type of natural antioxidants and are developed to mimic the most effective analogue of the natural antioxidant. Butylated hydroxyanisole BHA , butylated hydroxytoluene BHT , ethoxyquin, propyl gallat and tertiary butylhydroxyquinone TBHQ are some of the synthetic antioxidants. Exogenous antioxidants are prinicipal dietary antioxidants from fruits, vegetables and grains. Exogenous antioxidants are studied in ten groups. Carotenoids are examined in four groups as β-carotene, lycopene, lutein and zeaxanthin. Quercetin and their glucosides , kaempferol and their glucosides and myricetin and their glucosides constitute flavonols. Cyanidin and their glucosides and pelagonidin and their glucosides constitute anthocyanidins. Genistein and their glucosides , daidzein and their glucosides and glycitein and their glucosides constitute isoflavones. Naringenin and their glucosides , eriodictyol and their glucosides and hesperetin and their glucosides constitute flavanones. The beneficial and harmful effects of exogenous antioxidants are shown in Figure 3 [ 4 ]. Beneficial and harmful effects of exogenous antioxidants. Endogenous antioxidants are studied in two groups: enzymatic antioxidants and non-enzymatic antioxidants. Superoxide dismutase SOD is enzyme detoxifying superoxide radical O 2. Catalase CAT and glutathione peroxidase GPx are enzymes involved in the detoxification of peroxides CAT against H 2 O 2 and GPx against both H 2 O 2 and ROOH. Oxidative stress is a condition that occurs with the increase of free radicals. Free radicals tend to increase in the body for various reasons. This situation may be caused by exogenous reasons or by various changes in the body endogenous. As a result of the oxidation process in the body, reactive oxygen species ROS are formed. In addition to reactive oxygen species, reactive nitrogen species are also formed in the body. The oxidation process begins with the introduction of food into the body. Oxidation is a process that can be both beneficial and harmful. Oxidation triggers the formation of free radicals, ie reactive oxygen species. If antioxidants do not come into play as a balance element in the body, the increase in free radicals damages the body and causes the formation of the disease process. Depletion of various substances in the body due to age also triggers the formation of reactive oxygen species and the emergence of various diseases. Antioxidants can be various external substances, as well as various enzymes in the body and non-enzymatic substances in the body. With the effect of antioxidants, free radicals are prevented from causing DNA, lipid and protein damage. Licensee IntechOpen. This chapter is distributed under the terms of the Creative Commons Attribution 3. Edited by Viduranga Y. Open access peer-reviewed chapter Antioxidant and Oxidative Stress Written By Betül Çalişkan and Ali Cengiz Çalişkan. DOWNLOAD FOR FREE Share Cite Cite this chapter There are two ways to cite this chapter:. Choose citation style Select style Vancouver APA Harvard IEEE MLA Chicago Copy to clipboard Get citation. Choose citation style Select format Bibtex RIS Download citation. IntechOpen Antioxidants Benefits, Sources, Mechanisms of Action Edited by Viduranga Y. From the Edited Volume Antioxidants - Benefits, Sources, Mechanisms of Action Edited by Viduranga Waisundara Book Details Order Print. Chapter metrics overview Chapter Downloads View Full Metrics. Impact of this chapter. Abstract Antioxidants are compounds that eliminate oxidative stress in biological systems. Keywords antioxidant oxidative stress free radical radical ions. Introduction 1. Hydroperoxyl radical LOOH Alkylhydroperoxide L O O. Alkylperoxyl radical L O. Nitric oxide HOCl Hypochlorous acid G S O O. Glutathione thiylperoxyl radical G S O 2. Sulfonyl radical G S O. Sulfinyl radical G S O 2 O O. Sulfonyl-peroxyl radical. Table 1. Reactive oxygen species. These are bacteria, virus, fungus and parasite. cause oxidative stress. constitute phenolic acids. References 1. Yoshikawa T. What is oxidative stress? Journal of the Japan Medical Association JMAJ ; 4 5 7 : DOI: Tan B. Free radicals are generally produced as a result of the influence of external factors, such as pollution, cigarette smoke, or internally, as a result of intracellular metabolism if the antioxidant mechanisms are overwhelmed Figure 1. Figure 1. Schematic presentation of the sources of free radicals and their effects on the human body. Environmental triggers, such as exposure to cigarette smoke, UV radiation, heavy metal ions, ozone, allergens, drugs or toxins, pollutants, pesticides, or insecticides, may all contribute to the increase of ROS production in cells Antunes dos Santos et al. Ionizing radiation acts by converting hydroxyl radicals, superoxides and organic radicals into organic hydroperoxides and hydrogen peroxide. Subsequently, the peroxides react with the metal ions of Fe and Cu at the cellular level through redox reactions with secondary oxidative activity. Several studies have shown that the exposure of fibroblasts to alpha particles has led to an intracellular increase of oxygen and an accelerated production of peroxide at this level Spitz et al. Ultraviolet radiation UVA triggers oxidative reactions by stimulating riboflavin, porphyrins and NADPH-oxidase, with the production of 8-oxo-guanine as the main result and the decrease of intracellular glutathione GSH level with a return to normal after cessation of exposure Marchitti et al. Heavy metals play an essential role in the production of free radicals Ściskalska et al. Iron, copper, cadmium, nickel, arsenic, and lead can induce free radicals by Fenton or Haber-Weiss type reactions, but also by direct reactions between metal ions and cellular compounds with similar effects — for example, the production of thiol type radicals. Lead triggers lipid peroxidation and increases glutathione peroxidase concentration in brain tissue. Arsenic induces the production of peroxides, superoxides, nitric oxide and inhibits antioxidant enzymes such as glutathione-transferase, glutathione-peroxidase, and glutathione-reductase by binding to the sulfhydryl group. The free radicals generated from these reactions can affect DNA, with substitutions of some DNA bases such as guanine with cytosine, guanine with thymine and cytosine with thymine Jan et al. Exposure to ozone can affect lung function even in healthy individuals by increasing inflammatory infiltrate in the respiratory epithelium Wu X. The main endogenous sites of cellular redox-reactive species generation-including ROS and reactive nitrogen species RNS comprise mitochondrial electron transport chain ETC , endoplasmic reticulum ER , peroxisomes, membrane-bound NADPH oxidase NOX isoforms 1—5, dual oxidases Duox 1 and 2 complexes, and nitric oxide synthases isoforms 1—5 NOS1—3. The complexes I and III of mitochondrial ETC produces superoxide anion Rodriguez and Redman, The mitochondrial ETC is considered to be the primary endogenous source of ROS but other internal sources are also present. Other sources of ROS, primarily H 2 O 2 , are microsomes and peroxisomes. Immune cells, such as macrophages and neutrophils, can also generate ROS due to their oxygen-dependent mechanisms to fight against invading microorganisms based on NOX2 isoform Curi et al. Furthermore, dysregulated ROS signaling may contribute to a multitude of diseases associated with oxidative stress Finkel, ROS are produced in mitochondria during aerobic metabolism Rodriguez and Redman, ROS generation within mitochondria oxidative metabolism is closely associated with ATP synthesis oxidative phosphorylation. In aerobic organisms, the coupling of these reactions is the primary source of energy Papa et al. Mitochondria serve as a major ROS generator and, at the same time, as a ROS receptor. Covalent and enzymatic changes in proteins during or after protein biosynthesis as well as during protein cleavage or degradation promote disease through oxidative damage and mitochondrial dysfunction. These post-translational changes participate in the regulation of mitochondrial function through free radical species and other messengers Hu and Ren, Since oxidative phosphorylation is a leaky process, 0. This produces an incompletely O 2 reduction Hamanaka et al. Because of the anionic properties of superoxide radicals, they diffuse through biological lipid membranes at the meager extent. They are sequentially reduced inside cells to form hydrogen peroxide and hydroxyl radical Bartosz, Furthermore, peroxyl and alkoxyl radicals, as well as hypochlorite ions, are also formed Valko et al. All these types of ROS can be very harmful to cells; in fact, they can oxidize and subsequently inactivate several functions of cell components and even DNA Valko et al. All these processes may trigger irreversible apoptotic and necrotic cell death. Several studies indicate that human cells can also actively trigger ROS production at small doses, as part of signaling pathways, regulating cell survival and proliferation, as a defense mechanism against invaders Bartosz, ; Sena and Chandel, In particular, specific enzymatic systems, such as the NOX family, dedicated explicitly to superoxide radical production with physiological signaling purposes, are developed by cells Bedard and Krause, Beyond this, other internally generated sources of ROS are present in humans, including:. i oxidative burst from phagocytes white blood cells during bacteria and virus killing and foreign proteins denaturation;. iv detoxification of toxic substances i. ROS decrease phosphatase activity, by inhibiting catalytic regions susceptible to oxidation, and, thus, enhance protein tyrosine phosphatase PTP phosphorylation and influences signal transduction Bedard and Krause, ROS can also improve signal transduction pathways that disturb the nuclear factor-κB NF-κB activation and translocation of this into the nucleus. The DNA binding potential of oxidized NF-κB is significantly reduced. However, NF-κB may be decreased by TR or redox factor 1 Kabe et al. The above provokes ROS and RNS so it can strongly affect NF-κB-dependent inflammatory signals. Cyclopentenones are electrophilic anti-inflammatory prostaglandins which are conjugated with the reactive thiols of ROS-modified peptides and proteins and thus dampens ROS-mediated NF-κB signaling Homem de Bittencourt and Curi, On the other hand, endogenous stress has an intracellular origin. Several studies have highlighted the role of cultural cell conditions, altering gene expression patterns of different genes and their DNA stability. Metabolic processes trigger different types of ROS, that are able to, if present at inadequate levels, oxidize DNA and induce various damage, such as double-stranded DNA breaks and deficiencies, often found in human tumors De Bont and van Larebeke, Moreover, there are non-enzymatic reactions, like the mitochondrial respiratory chain which involves NADPH oxidase, XOR, uncoupled endothelial NOS, cytochrome P enzymes, lipoxygenase and COX Sena and Chandel, ; Battelli et al. Cellular oxidative metabolism produces free radicals and organic peroxides as by-products during cellular mitochondrial electron transport or through metal-catalyzed oxidation of metabolites and oxidoreductases Forman and Torres, ; Hussain et al. Moreover, nitric oxide is produced in hypoxic conditions in a respiratory chain reaction, and RNS may trigger reactive species production, such as reactive aldehydes, malondialdehyde MDA and 4-hydroxynon-enal Hussain et al. However, an imbalance in this protective mechanism can lead to damage in cell molecules, such as DNA, proteins and lipids, resulting in cell death by necrotic and apoptotic processes Bhattacharyya et al. Stimulated ROS production was first described in phagocytic cells, including neutrophils and macrophages, during phagocytosis or stimulation with a wide variety of agents through NADPH oxidase activation. The respiratory burst of neutrophils, as well as their degranulation, constitute a defensive response to host tissue damage, whether induced by mechanical muscle damage during exercise, thermal stress , chemical or infectious stimuli Lamy et al. Nowadays, ROS production has also been observed in a variety of cells other than phagocytes, and their implication in physiologic signaling is well documented Di Meo et al. Lifestyle: smoking, alcohol consumption, adequate or inappropriate diet, exercise, training or untrained condition, contribute to oxidative stress. Some research has shown the presence of reactive oxygen species and muscle level and their role in regulating muscle activity. Skeletal muscle fibers continuously generate reactive oxygen species at a low level, which increases during muscle contraction. They exert multiple direct and indirect effects on muscle activity contractility, excitability, metabolism, and calcium homeostasis and are involved in skeletal muscle fatigue during strenuous exercise Pingitore et al. Exhausting exercises, long exercises, overtraining syndrome, and overcoming limits as a phase of the initial onset of overtraining syndrome, induce a significant response to oxidative stress. Instead, moderate exercise, low intensity training, and prolonged training, improve endogenous antioxidant status. Reactive oxygen species play an important role in cell signaling and in regulating the expression of antioxidant genes. Physical exercise is considered the main treatment of non-pharmacological therapies along with lifestyle changes for various chronic diseases, especially cardiovascular diseases Ren and Taegtmeyer, The results of some experimental studies have highlighted the role of autophagy, a conservative process of catabolism for the degradation and recycling of cellular organs and nutrients, in the cardiovascular benefits offered by training Wu N. Regular exercise as a unique form of physiological stress is able to trigger adaptation, while autophagy, especially selective mitochondrial autophagy, also called mitophagy, allows for such cardiovascular adaptation Wu N. Cigarette smoke comprises a series of oxidants, free radicals, as well as organic components e. Endogenous ROS comprises the by-products of cellular metabolism in aerobic organisms. At low concentrations, they are usually involved in different cell processes, such as proliferation, differentiation, and apoptosis, like a second messenger in cell signaling Salehi et al. ROS production within cells under physiological condition is dependent on mitochondria respiration, NOX, uncoupled NOS and XOR. The increase in ROS levels, its production in inappropriate cellular compartments or its production with defective forms during oxidative processes can trigger the development of numerous chronic-degenerative disorders, leading to severe damage to bio macromolecules Chen et al. Oxidative stress, as a result of the imbalance between oxidative and antioxidative processes in cells, therefore plays an essential role in the pathogenesis of numerous chronic-degenerative disorders. The main cardiovascular risk factors, such as hypertension and hypercholesterolemia contribute to enhancing ROS generation, leading to oxidative stress Li et al. From all these cardiovascular risk factors, hypertension is an essential factor in the development of cardiovascular diseases CVD Elahi et al. Small amounts of ROS in the cardiovascular system could provide remarkable benefits: anti-atherosclerotic, pro-angiogenesis and endogenous cardioprotective effects Taverne et al. In CVD, gene expression is altered due to oxidative stress. Increased ROS levels modulate transcription factor activity, especially NF-κB, activator protein-1 AP-1 and the peroxisome proliferators-activated receptor PPAR family of transcriptional activators Elahi et al. As a result of increasing ROS generation, one of the first events in atherogenesis, as well as in other CVDs correlated with endothelial dysfunction, is the oxidative modification of low-density lipoprotein LDL Singh et al. Indeed, both cell membranes and LDL, enriched with phospholipids, are highly sensitive to oxidative modification. Oxidized phospholipids, through receptor-mediated or receptor-independent pathways, can therefore then activate endothelial cells, induce endothelium adhesion molecules expression, attract monocytes, have endothelium cytotoxic effects, and increase proinflammatory gene activity and cellular growth factors Esper et al. All of these processes provoke endothelial dysfunction, platelet aggregation, and metalloproteinase expression and favor thrombogenesis Esper et al. In atherosclerotic plaque, increased matrix metalloproteinase expression and activity triggered by oxidative stress lead to its rupture and consequent thrombosis He and Zuo, The NF-κB activity in atherosclerosis is mainly due to oxidized LDL Singh et al. At the same time, upregulated NF-κB is detected in smooth muscle cells, endothelial cells, macrophages and T cells of atherosclerotic plaques Mach et al. In the blood vessel wall, all layers can produce ROS under pathological conditions, and most of them are primarily derived from NOX Reid, Due to increased ROS levels, NO bioavailability is decreased, and consequently, endothelium-dependent relaxation is reduced Chen J. Cardiac myocytes have a more significant number of mitochondria than other cells and use higher oxygen levels for energy production in the form of ATP. In myocytes, ROS trigger cardiac injury, both oxidizing essential proteins for excitation-contraction and decreasing NO bioactivity Hare and Stamler, Furthermore, oxidative stress produced in mitochondria induces mitochondrial DNA mtDNA damage and leads to CVD. In myocardial ischemia, hypoxia and reoxygenation trigger an increase in free radical production in cardiac tissue Elahi et al. ROS produced during reoxygenation cause direct oxidative damage to cellular components and lead to indirect damage through the activation of localized inflammation Gutteridge and Halliwell, In heart failure, excessive ROS production is based on increased activity of XOR and NOX Battelli et al. Increased ROS production is a consequence of prolonged endoplasmic reticulum stress and mitochondrial-derived oxidative stress in cardio-metabolic disorders. Furthermore, some disturbance in these organelles activates signaling pathways that alter cardiac ion channels function or expression, involved in the generation of an action potential that promotes arrhythmogenesis Tse et al. The administration of cytostatics to humans is followed by cardiotoxicity due to increased plasma levels of ROS and lipid peroxidation products and decreased plasma and tissue levels of antioxidants. Myocardial changes that occur after treatment include: myocyte loss through apoptosis or necrosis, loss of myofibrils, distension of the sarcoplasmic reticulum, and mitochondrial ballooning. Recent studies on transgenic mice have shown that in cardiotoxicity induced by Doxorubicin, free radicals can be counteracted by metallothionein and liensinine Kang, ; Liang et al. Cancer development in humans is a complex process that includes cellular and molecular changes mediated by various endogenous and exogenous stimuli Docea et al. It has been established that oxidative DNA damage is one of the key characteristics of carcinogenesis Smith et al. Cancer initiation and promotion are associated with chromosomal defects and activation of oncogenes by free radicals Glasauer and Chandel, A common form of injury is the formation of hydroxylated DNA bases, considered an important event in chemical carcinogenesis. They interfere with healthy cell growth by causing genetic mutations and altering normal gene transcription. Oxidative lesions also produce many changes in the structure of DNA Li et al. ROS involvement in a different stage of carcinogenesis has been shown in various model systems. Excessive amounts of these free radicals can lead to cell damage and apoptosis. Many forms of cancer are considered to be the result of free radicals and DNA reactions, leading to mutations that can affect the cell cycle and lead to neoplasia Pizzino et al. ROS overproduction has an impact on cancer cell proliferation, metastatic potential, and it is associated with invasiveness and poor prognosis Liou et al. ROS contributes to cancer cell migration through various mechanisms: i matrix degradation, ii cell-cell contact, iii cytoskeleton remodeling, regulation of gene expression, iv invadopodia formation Pizzino et al. For example, mitochondria-derived ROS has an impact on initial extracellular matrix contact, NOX-derived ROS are involved in invadopodia formation. At the same time, ROS increase in cytosol plays a significant role in cytoskeleton remodeling Herrera et al. The effect of ROS on cancers depends on the type of organ, as well as on the grade of disease progression. Skin carcinogenesis and exposure to UVA: the ultraviolet component A sunlight UV-A with the wavelength — nm has the potential to generate oxidative stress in cells and tissues, so that endogenous and exogenous antioxidants strongly influence the biological effects of UVA Sage et al. The physiological doses of UVA determine the expression of some genes collagenase, hem oxygenase-1, and nuclear oncogenes , whose effects can be significantly increased by removing intracellular GSH or by increasing the lifetime of molecular oxygen. Repeated exposure of human skin to UV radiation leads not only to skin carcinogenesis but also to photo-aging through DNA damage Cortat et al. Hydroxyl radicals can bind to DNA and produce 8-OH deoxyguanosine 8-OHdG , which consequently increases the risk of mutation. Additionally, increased cancer cell proliferation requires high ATP levels that lead to ROS accumulation, particularly at initial stages of cancer genesis. In cancer cells, there is the condition of constant oxidative stress induced by mitochondrial dysfunction and metabolic changes. In fact, under normal circumstances, increased ROS levels stimulate cell death, but cancer cells overcome that by activating numerous oncogenes, which then induce nuclear factor erythroid 2-related factor 2 NRF2 expression. NRF2 is the primary regulator of cell survival that raises cancer progression by protecting cancer cells from ROS and DNA damage Jaramillo and Zhang, ROS are implicated in cancer progression, promoting cyclin D1 expression, extracellular signal-regulated kinase ERK and JUN N-terminal kinase JNK phosphorylation, and MAPK activation Saha et al. However, cancer cells enable proliferation, avoiding ROS-induced apoptosis, despite high mutagenesis. In neoplastic disorders, ROS promote protein oxidation and lipid peroxidation. Moreover, ROS trigger toxic protein carbonyls formation which has a significant impact on other proteins or lipids Benfeitas et al. In addition, as a result of lipid peroxidation, cancer cells accumulate products, such as 4-hydroxynon-enal, one of the most studied products of phospholipid peroxidation, owing to its reactivity and cytotoxicity. In the brain, not all neuronal groups are equally sensitive to oxidative stress. For instance, neurons with longer axons and multiple synapses require more energy for axonal transport or long-term plasticity Salehi et al. High ATP demand, in combination with dysfunctional mitochondria, make these neuron groups more sensitive to degeneration Wang and Michaelis, Correctly, dopaminergic neurons are exposed to additional oxidative stress produced by the dopamine metabolism, generating H 2 O 2 and dopamine autoxidation, which generates superoxide Delcambre et al. During aging, mutations in mtDNA accumulate, cytosolic calcium dysregulates, and ETC function decreases, making aging one of the major risk factors contributing to neurodegeneration Payne and Chinnery, The oxidized molecules of DNA, proteins and lipids found in the brain tissue of post-mortem patients with neurodegenerative disorders highlight the role of oxidative stress in these diseases Sharifi-Rad M. Another cause of neurodegenerative diseases is a defective use of metals by the brain, by the intervention of mutant proteins, formed as a result of oxidative stress Niedzielska et al. In the case of Alzheimer disease, a protein called amyloid beta Aβ , consisting of 40 amino acid residues, is present in all the cells of the body, under normal, harmless and even beneficial conditions, as it is a natural antioxidant Danielson and Andersen, ; Li et al. One explanation is the accumulation in the brain of a modified form of the Ab protein consisting of 42 amino acid residues , which fails to properly bind metals, promotes oxidative processes; by reacting in self-defense, neurons produce antioxidants in increased quantities, including the modified form of the Aβ protein, which thus becomes an antioxidant pro-oxidant, amplifying oxidative disasters by initiating chain reactions Danielson and Andersen, Mutations of the superoxide dismutase 1 SOD1 protein have been linked to another neurodegenerative disease that affects motility familial amyotrophic lateral sclerosis Huai and Zhang, In its unmodified form, SOD1 is a natural antioxidant that prevents the formation of peroxide anion as a dangerous reactive form of oxygen Saccon et al. The mutant forms of this protein fixate a much smaller amount of metals than the usual form, which results in the formation of an excess of peroxynitrite ONOO — affecting the motor neurons required for normal functioning, causing severe motor disorders Pasinelli et al. The excessive use of glucose for energy production makes the brain especially susceptible to oxidative stress, and mitochondrial ETC is the primary ROS source Cobley et al. Most of the ROS present in the brain derive from mitochondrial ETC complex I and III ETC I and III , as O 2 — by-products Andreyev et al. Indeed, the main targets for mitochondria-generated ROS are mitochondrial permeability transition pore MPTP , poly ADP-ribose polymerase PARP , and mtDNA Gandhi and Abramov, Other oxidant sources arise from NADPH oxidase, present in astrocytes, microglia and neurons, while NOS inhibition has shown neuroprotective effects Abramov et al. In the pathogenesis of neurodegeneration, many processes are included, such as protein misfolding and aggregation, abnormal kinase-signaling pathways, neuronal calcium dysregulation, and even impaired synaptic transmission Gandhi and Abramov, Mechanisms of action of ROS: these affect proteins by modifying them in oxidative forms, which tend to form aggregates Blokhuis et al. Protein aggregates then inhibit proteasomes, the main organelles in the cell for degradation of abnormal proteins Chen et al. Accumulation of modified proteins with an inability to be destroyed in the proteasome stimulate more ROS formation and form a vicious cycle, a phenomenon included in neurodegenerative diseases related to oxidative stress Chen et al. Many metabolic contexts can lead to conditions of oxidative stress. A condition in which oxidation is an important pathogenetic link is type 2 diabetes. In this disease, insulin resistance is the basic component, to which a compensatory hypersecretion of insulin is linked. Reactive oxygen species can induce inactivation of signaling mechanisms between insulin receptors and the glucose transport system, leading to insulin resistance Chen X. On the other hand, diabetes itself is a generator of oxidative stress, with atherogenetic consequences. Hyperglycemia induces the generation of superoxide ions in endothelial cells at the mitochondrial level. In diabetes, electron transfer and oxidative phosphorylation are decoupled, resulting in the production of superoxide anions and inefficient ATP synthesis. Therefore, preventing the damage caused by oxidation is a therapeutic strategy in diabetes. Increased levels of free fatty acids with consecutive accumulation of intramyocellular lipids were thought to be the cause of insulin resistance and beta-pancreatic cell death. Studies have shown that both glucose and free fatty acids can initiate the formation of free radicals through mitochondrial mechanisms and NADPH oxidase in muscles, adipocytes, beta cells and other cell types. Free fatty acids penetrate cellular organs, including mitochondria, where high levels of reactive oxygen species can cause peroxidation and damage. Recent studies show that type II diabetes and insulin resistance are associated with a decrease in mitochondrial oxidative function in skeletal muscle. Moreover, in this type of diabetes, the mitochondria are smaller, rounder and more likely to produce superoxide. Disorders of the mitochondrial transport chain, excessive generation of reactive species and lipoperoxides, as well as decreases in antioxidant mechanisms have also been observed in diabetes and obesity. Diabetes has a number of complications over time, of which macrovasculopathy is very important. The increase in cardiovascular risk in patients with diabetes can be explained by the association between diabetes hypertension, dyslipidemia and coronary atherosclerotic disease. However, other mechanisms are also involved, such as the effects of hyperglycemia on endothelial function, the effects of glucose and fatty acids on myocardial cells, at the structural level but also of gene expression Aroor et al. Diabetic cardiovascular complications are caused by impaired cardiac microvascular function. In addition to the structural and functional changes that occur in diabetic cardiomyopathy, other mechanisms can be targeted pharmacologically. Sodium-glucose co-transporter-2 SGLT2 inhibitors are the first class of antidiabetic drugs that have reduced the risk of heart failure in type 2 diabetes Karam et al. Empagliflozin has an indication to reduce cardiovascular mortality in patients with diabetes and atherosclerotic disease. A recent study demonstrated the beneficial effect of empagliflozin on cardiac microvascular injury in diabetes and the protective mechanism against oxidative stress in mitochondria Zhou et al. Another recent study showed that aminoguanidine has a beneficial effect on diabetes-induced heart abnormalities. Aminoguanidine saves contractile abnormalities and diabetes-induced cardiac remodeling. This was explained by inhibition of endoplasmic reticulum stress and induction of autophagy Pei et al. Insulin resistance, abdominal obesity, atherogenic dyslipidemia, endothelial dysfunction, high blood pressure, hypercoagulability, genetic predisposition and chronic stress are the main factors underlying the metabolic syndrome. Metabolic syndrome is often characterized by oxidative stress, a condition in which there is an imbalance between the production and inactivation of reactive oxygen species. Increased generation of reactive oxygen species, decreased activity of antioxidant systems or both mechanisms may be involved in the occurrence of oxidative stress Karam et al. A study showed that lenalidomide attenuates oxidative cardiovascular tissue damage and apoptosis in obese mice by inhibiting tumor necrosis factor Zhu et al. This accumulation of losses in cells would be the reason for aging and aging-associated degenerative diseases Tsoukalas et al. Aging can be caused by both genetic and external factors, such as incorrect diet, improper physical exercise, chronic drug use, untreated inflammatory conditions, smoking, and alcohol abuse. Today, while there are several theories of aging, the basic principle of most of them is still oxidative stress Finkel and Holbrook, ; Payne and Chinnery, The major systems involved in overproduction of oxidative stress in cells are mitochondria and NOX Bedard and Krause, In the aging process, it has been noticed that high-molecular protein aggregates accumulate in cells Davalli et al. Predominantly, these aggregates are made from proteins, with the remainder consisting of various lipids Barrera, ; Takalo et al. Thus, the crucial point for protein homeostasis maintenance is the degradation of these aggregates. The central place for cell damaged protein degradation is the proteasome, which recognizes only unfolded proteins as degradation targets Saez and Vilchez, Proteasome inhibition prevents further degradation of newly formed oxidized proteins and increases protein aggregation formation in cells Takalo et al. Besides that, proteasome becomes dysfunctional during aging. While proteasomal dysfunction is correlated with age progression and protein aggregation, proteasome activation slows the aging progress down and increases longevity Chondrogianni et al. In many invertebrate models and cell lines, it has been shown that the overexpression of different proteasomal regulatory or catalytic subunits or treatment with specific compounds has positive effects on proteasome activity Saez and Vilchez, Recently, most of the data have indicated that antioxidant supplementation does not decrease the incidence of age-related diseases Schottker et al. Antioxidants break radical chain reactions, preventing oxidative stress-related damage Da Pozzo et al. Figure 2. Schematic figure of the link between ROS, oxidative stress and their effects on the human body. Alteration of chemical reactions at the cellular level leads to the appearance of free radicals and peroxides that affect the intracellular structures — proteins, lipids, DNA, with the disruption of intrinsic mechanisms at this level. Free radicals are normally produced in the body due to the influence of external factors, such as pollution, cigarette smoke, or internal, due to intracellular metabolism when antioxidant mechanisms are exceeded. Their role requires acting both in hydrophilic and hydrophobic cellular environments, so their chemical structure is quite heterogeneous. There are enzymatic and non-enzymatic antioxidants Banafsheh and Sirous, , as shown in Figure 1. but, from a nutritional perspective, a more informative classification can be made between endogenous and exogenous classes. The first class comprises all antioxidants that cells can synthesize from smaller building blocks. Accordingly, all enzymatic antioxidants are endogenous, as well as some non-enzymatic ones i. Figure 3. Primary enzymes SOD or peroxidases act directly in scavenging ROS. Secondary enzymes, such as glutathione reductase and glucosephosphate dehydrogenase, support the action of primary enzymes regenerating NAPDH and reduced glutathione. On the contrary, exogenous antioxidants have to be ingested through the diet, since their synthesis is impossible in eukaryotic cells. So, particular attention should be paid on this latter class, since this is the most unpredictable component in cellular redox balance. Antioxidants can be divided into two categories depending on their solubility: water soluble and liposoluble Lazzarino et al. Water soluble antioxidants are best absorbed in the body because the vegetables and fruits that contain such antioxidants, also contain water. On the other hand, they are rapidly eliminated from the body through the urine. Water-soluble antioxidants include polyphenols, but also vitamin C Lazzarino et al. Liposoluble antioxidants, fat-soluble antioxidants are those that are absorbed in the presence of fats. Therefore, in the absence of fats, the body cannot absorb and use these antioxidants. It is important to note, however, that they are not easily removed from the body and can accumulate over time, exceeding the healthy level. Vitamin E is an example of a fat-soluble antioxidant Lazzarino et al. This is the case, for instance, for glucosephosphate dehydrogenase that regenerates NADPH, essential for primary enzyme action Figure 2. Primary enzymes act directly on the main ROS arising from incomplete O 2 reduction, O 2 — and H 2 O 2. SOD scavenges the former, whereas CAT and GPX remove the latter. SOD E. In turn, H 2 O 2 can be removed by the other enzymatic antioxidant systems. SODs can be divided into four groups, with different metal cofactors. Copper-zinc SOD is most abundant in chloroplasts, cytosol and extracellular space. Iron SOD is found in plant cytosol and in microbial cells, whereas manganese SODs are mitochondrial Perera et al. SOD also competes for superoxide anion with NO. Therefore, SOD also indirectly reduces the formation of another deleterious ROS, peroxynitrite ONOO — , reaction 2 , and increases the NO biological availability, an essential modulator for endothelial function. CAT E. CAT is mainly located in peroxisomes, and despite being ubiquitous, the highest activity is present in liver and red blood cells. CAT works with a two-step mechanism, somewhat resembling the formation in the first step of a peroxidase-like compound I intermediate, CpdI reaction 4 Alfonso-Prieto et al. A NADPH molecule is bound to each subunit, minimizing H 2 O 2 —mediated inactivation []. CAT is one of the enzymes with the highest known k cat more than 10 6 s —1 in all known proteins, close to a diffusion-controlled reaction Tovmasyan et al. GPX E. The GPX family is composed of eight isoenzymes GPX Each enzyme presents peculiar features. GPX1, 2, 3, and 4 incorporate selenocysteine a non-standard amino acid, where the sulfur atom of cysteine is replaced by selenium. During the catalytic cycle, selenocysteine is converted from selenol Enz-SeH to selenenic acid Enz-SeOH , with concomitant reduction of H 2 O 2 or ROOH. Then, the first GSH molecules yield selenenyl sulfide intermediate Enz-Se-SG. An incoming second GSH molecule attacks Enz-Se-SG, regenerating the enzymatic resting form Enz-SeH, releasing the oxidized and dimerized GSSG Cardoso et al. Another important class of enzymatic peroxide scavenger is PRDX. Six different classes of PRDX have been identified Poole and Nelson, , showing either one 1-Cys PRDX or two 2-Cys PRDX redox-active cysteine residues Park et al. The PRDX catalytic cycle involves H 2 O 2 decomposition and the subsequent regeneration of the resting enzyme, using a small cysteine protein thioredoxin Trx as the reductant reactions 8 and 9. Trx shows two vicinal cysteines in the typical CXXC motif , forming, in turn, a disulfide internal bridge upon oxidation. In the case of PRDX6 isoform, Trx can be replaced by GSH. All the enzymatic activities described above rely on the continuous regeneration of the reduced form of reductants mainly GSH and Trx. This is usually performed by some reductases, NADPH-dependent such as glutathione reductase E. However, as shown in Figure 2 , reduced NADPH is, in turn, needed by these reductases for their continuous action. So, enzymes responsible for the constant NADPH production can be considered secondary antioxidants, as their misfunction could affect the whole ROS balance. The main NADPH metabolic source is the pentose phosphate pathway, through the first two enzymatic activities: glucosephosphate dehydrogenase E. However, other contributions come from the malic enzyme E. Some chemical molecules of low-molecular-weight can also directly act as antioxidants. In this case, their action is not catalytic, always needing antioxidant regeneration or its supply from the diet. Non-enzymatic antioxidants can therefore be divided into endogenous if the eukaryotic cell is able to synthesize it and exogenous if the antioxidant needs to be ingested mandatorily through the diet. GSH γ-glutamyl-cysteinyl-glycine, Figure 4 is a tripeptide, mainly distributed in cytosol, but also in nuclei, peroxisomes and mitochondria. Despite being ubiquitous, the liver is the leading site for its synthesis Banafsheh and Sirous, GSH biosynthesis is an endergonic process ATP hydrolysis is coupled , in which firstly glutamate and cysteine condense to yield γ-glutamylcysteine reaction catalyzed by glutamate-cysteine ligase, E. This unusual γ-peptidic bond protects it from the common peptidases action. In the final step, GSH synthetase E. Figure 4. Glutathione GSH , a tripeptide with an active —SH function. GSH undergoes a redox cycle, dimerizing with a disulfide bridge formation. α-Lipoic acid 1,2-dithiolanepentanoic acid, Figure 4 is a disulfide compound that undergoes a redox cycle similar to GSH. Accordingly, it scavenges reactive ROS, and regenerate vitamins C and E, and GSH in their active forms Kucukgoncu et al. Lipoic acid also has a role in metal chelation, preventing Fenton-like radical reactions Zhang and McCullough, Nevertheless, even small proteins, such as Trx and glutaredoxin can similarly function as thiol antioxidants, showing redox-active mono- or di-cysteine motif CXXC. Both proteins can be in turn reduced back to their active form, directly by GSH or indirectly by NADPH Banafsheh and Sirous, Melatonin N -acetylmethoxytryptamine, Figure 5 is a neurohormone derived from amino acid tryptophan. It is involved in circadian rhythms but also acts as a potent antioxidant, protecting cell membranes against lipid peroxidation Beyer et al. It has been described to be more effective in ROS scavenging than vitamin E, GSH, vitamin C and β-carotene Watson, Coenzyme Q10 or ubiquinone 2,3-dimethoxymethylpolyisoprene parabenzoquinone, Figure 5 is an isoprenoid antioxidant present in cell membranes, essential for ETC Tafazoli, Its synthesis starts from oligomerization of isoprenoid building blocks, isopentenyl pyrophosphate and dimethylallyl pyrophosphate both arising from the mevalonate pathway and the key enzyme 3-hydroxymethyl-glutaryl-CoA reductase E. The resulting decaprenyl diphosphate is then conjugated with a tyrosine derivative to yield the active form of the coenzyme. It is one of the few liposoluble antioxidants, ensuring lipoproteins and lipids protection from radical chain reactions, peroxidation and oxidative damage Lee et al. In its active form quinol , coenzyme Q10 can scavenge several ROS or regenerate other oxidized antioxidants including vitamins C and E. In turn, the quinone form can be reduced back by several NAD P H-dependent enzymatic systems. Exogenous antioxidants need to be supplemented continuously through the diet since their synthetic pathways are usually present only in microbial or plant cells. Vitamins, two of which show prominent antioxidant effects, such as vitamins C and E, belong to essential class of molecules. Vitamin C ascorbic acid exists in two redox forms: ascorbic acid AA is the reduced form, which is deprotonated at physiological pH thus, occurring in its anion form, ascorbate. Due to its high electron-donating power, AA can undergo two-electron oxidation, yielding dehydroascorbic acid DHA. One-electron oxidation of AA is also possible, generating a semi-dehydro-ascorbyl radical Kocot et al. DHA can be regenerated to the active AA form by GSH- or Trx-dependent mechanisms. Humans do not express the enzyme L -gulonolactone oxidase E. Thus, AA must be ingested by food or supplements , particularly tomatoes, pineapples, watermelons and all citrus fruits Banafsheh and Sirous, AA effectively quenches ROS, both directly and cooperatively regenerating oxidized vitamin E, GSH, and carotenoids. Vitamin E is a fat-soluble vitamin, mostly found in several vegetable oils, nuts, broccoli and fish. Eight different forms have been reported α-, β-, γ-, and δ-tocopherol, and α-, β-, γ-, and δ-tocotrienol , but α-tocopherol has the highest antioxidant activity, especially in cell membranes Salehi et al. A variously methyl-substituted chromanol ring characterizes tocopherols. A long phytyl chain gives the hydrophobicity Figure 6. Figure 6. Chemical structures of Vitamin C, Curcumin, Resveratrol, Quercetin, Vitamin E, β-carotene, Lycopene. On the contrary, tocotrienols bear an unsaturated isoprenoid chain. α-Tocopherol is able to undergo hydrogen transfer to several ROS, including 1 O 2 , superoxide anion and peroxyl radicals. The oxidized and radical derivative of vitamin E is then reduced by the AA. Carotenoids are a broad class of tetraterpenes, widely distributed among plants. Carotenes are also vitamin A precursors. Carotenoids protect plant chlorophyll, acting as accessory pigments during photosynthesis. Thus, they are intensely colored red, orange, or yellow molecules. Carotenoids have been suggested to be chemopreventive agents in cancer Marti et al. Their biological activities also include ROS scavenging Hernández-Almanza et al. β-Carotene comprises one of the most diffused carotenes, being the primary pro-vitamin A precursor, and it is found mainly in carrots, pumpkins, mangoes and apricots. Lycopene is another well-known acyclic carotene, not being a precursor of vitamin A, and is found primarily in tomatoes and other red fruits, but not in strawberries and cherries. Indeed, carotenoids are strong ROS scavengers, operating a very particular physical and chemical 1 O 2 quenching Banafsheh and Sirous, In the physical mechanism, the carotenoid electron-rich structure absorbs 1 O 2 excess energy, reaching an excited state. The conjugated double bond structure in carotenoids is responsible for this ability. The excited state then decays to the ground state, losing the surplus energy as heat. During this cycle, the structure of this molecule stays unchanged. Polyphenols are a large class of plant secondary metabolites, whose synthesis is usually possible only in these organisms Sanjust et al. The key enzyme [phenylalanine ammonia-lyase PAL , EC 4. PAL catalyzes the non-oxidative deamination of phenylalanine to trans -cinnamic acid, which is the fundamental building block for polyphenol synthesis in the phenylpropanoid pathway Ertani et al. Several biological functions have been ascribed to polyphenols, including anti-inflammatory, antioxidant, antimicrobial and antimelanogenesis effects Zucca et al. For instance, one of the most studied polyphenols has been curcumin, gaining a lot of attention also for nutraceutical applications. Curcumin can also increase GSH cellular levels Banafsheh and Sirous, Epigallocatechingallate EGCG is a well-known antioxidant. The green tea catechins include catechin, epicatechin, epigallocatechin, epicatechin gallate, and epigallocatechin gallate Barbieri et al. Flavonoids, in addition to its strong antioxidant properties, quench ROS formation inhibiting several enzymes and chelating metals involved in radical chain reactions Banafsheh and Sirous, Furthermore, flavonoids can also affect free metal ion concentrations. Indeed, flavonoids have the well-known capacity to chelate several metal ions such as iron and copper , blocking free radical generation Kumar and Pandey, For instance, quercetin is one of the most diffused flavonols present in broccoli, apples, grapes, onions and soybeans, with both iron-chelating and iron-stabilizing abilities Kumar and Pandey, On the other hand, catechol and galloyl-derivatives are generally well-known metal chelators Jomova and Valko, So, they can all exert their antioxidant activity by blocking Fenton-like reactions. Organosulfur compounds have also been suggested as potent antioxidants. The most studied are probably some sulfur-containing metabolites present in garlic mainly S -allyl-mercapto cysteine, S -allyl cysteine, and diallyl sulfide, diallyl trisulfide Kimura et al. These organosulfur are also responsible for typical garlic flavor. Their antioxidant actions include scavenging ROS and inhibiting lipids peroxidation Borek, ; Miltonprabu et al. Several minerals, in small amounts, are also essential for some enzymatic antioxidant activities. They are therefore sometimes regarded as antioxidants themselves. For instance, selenium is a necessary component of GPX Battin and Brumaghim, , while copper, zinc, and manganese are fundamental for SOD activity. The balance between ROS production and purification maintains homeostasis of the body, but is most often directed to the formation of free radicals and involvement in the pathophysiology of chronic diseases. The use of antioxidant supplements containing multivitamins and minerals has always grown in popularity among consumers. But some recent studies have not shown any beneficial effect of antioxidant therapy. Oxidative stress has a dual character: it is both harmful and beneficial to the body, because some ROS are signaling molecules on cellular signaling pathways. Lowering the level of oxidative stress through antioxidant supplements is therefore not beneficial in such cases Ye et al. Antioxidants are also prone to oxidation since oxidation and reduction reactions do not happen in isolation. AA, a potent antioxidant, mediates several physiological responses. This reaction is responsible for oxidative stress-produced DNA damage. However, the role of AA as anti- or pro-oxidant depends on the dose used, as observed in the case of ischemia-induced oxidative stress Seo and Lee, With increased oxygen tension, carotenoids tend to lose their antioxidant potential. Otherwise, α-tocopherol, a powerful antioxidant, becomes pro-oxidant at high concentrations Cillard and Cillard, Interestingly, when it reacts with a free radical, it becomes a radical in itself. If there is not enough AA for its regeneration, it will remain in that highly reactive state Lü et al. Flavonoids can also act as pro-oxidants depending on the concentrations used Prochazkova et al. Nevertheless, the extent to which these phytochemicals are capable of acting as anti- or pro-oxidants in vivo is still poorly understood, and this topic undoubtedly requires further research. The hypothesis that antioxidants could protect against cancer because they can neutralize reactive oxygen species ROS that can damage DNA has long been issued. In laboratory and animal studies, the presence of elevated levels of exogenous antioxidants has been shown to prevent the types of free radicals that have been associated with the development of cancer. A few randomized studies evaluating the role of antioxidant supplements for cancer prevention were conducted in collaboration with the National Cancer Institute Goodman et al. No data were obtained to justify that they are effective in primary cancer prevention. An analysis in the United States concluded that there is no clear scientific evidence for the benefits of vitamin and mineral supplements in cancer prevention. It is important to point out that there have been cases where people who have resorted to these types of supplements have encountered an unfavorable evolution of the disease. Preclinical studies also report that antioxidants have contributed to the expansion of tumor processes in animal models. A well-known case is that of vitamin A, for which the administration of high doses in supplements has been associated with an increased risk of cancer. Vitamin A can be obtained preformed from animal sources or plant products, derived from β-carotene. β-Carotene is an orange pigment found in fruits and vegetables carrots, sweet potatoes, mangoes, apricots , and in the body it is converted to vitamin A. A normal intake has a beneficial effect against the risk of cancer. However, studies have shown a correlation between the administration of β-carotene supplements and the risk of bladder cancer, as well as the risk of lung cancer in smokers Lin et al. In another study, the administration of α-tocopherol and β-carotene for lung cancer did not change the incidence of lung cancer. However, α-tocopherol supplements have been shown to be effective in prostate cancer whose incidence is reduced Goodman et al. A trial evaluated the effectiveness of long-term supplementation with vitamin E and vitamin C in the risk of developing cancer. One of the findings of the study was that these types of supplements do not reduce the risk of prostate cancer or the overall risk of cancer in men of middle age or older. No significant results were obtained regarding the risk of colorectal or lung cancer Gaziano et al. Vitamin E and C supplements showed poor results in many studies. There was a reduction in cardiovascular mortality, but no significant effect was observed on overall mortality. The authors concluded that vitamin E supplementation for the prevention of cardiovascular disease among healthy women is not justified. Moreover, cancer mortality is not significantly influenced by vitamin E supplementation Lee et al. The Selenium and Vitamin E Cancer Prevention Trial SELECT which included over 35, men over the age of 50, showed that selenium and vitamin E supplements do not prevent prostate cancer. This article summarizes the evidence from a large number of meta-analyzes covering the pathophysiological impact of antioxidants on the most common chronic diseases. The main criticism of the review is that the data were extracted from meta-analyzes and not from individual studies, but this can be considered an advantage because meta-analyzes provide the highest degree of evidence. In the case of antioxidants, studies show that more does not necessarily mean better. Consuming superfoods does not compensate for other unhealthy eating habits or an unbalanced lifestyle. Free radicals, as well as antioxidants, can have beneficial effects on the body. Therefore, we are talking about a balance and not a negative role attributed to free radicals and a positive one to antioxidants. Degradation of nucleic acids, proteins, lipids or other cellular components are among the effects that an excessive concentration of free radicals can generate. Risk factors leading to free radicals include air pollution, ionizing radiation, prolonged exercise, infections, excessive consumption of polyunsaturated fatty acids Poprac et al. On the other hand, antioxidants are considered to be the solution to these problems — substances that neutralize free radicals. In some situations, some substances act as antioxidants, in other situations they become prooxidants, depending on the chemical composition of the environment in which they are. There are many types of antioxidants, and the role in the body and the mechanisms by which they act are different. One misconception is that one antioxidant can be replaced with another, having the same effect. In fact, each has its own unique biological properties Chen X. There is also a significant difference between taking antioxidants from food and administering an isolated substance as a supplement. Many substances that demonstrate beneficial effects in the laboratory do not work when introduced into the human body. Many antioxidants do not have good bioavailability. The concentration of antioxidants such as polyphenols is sometimes so low in the blood that no significant effect is observed Fernández-García et al. Fruits and vegetables contain bioactive substances that in many cases do not work as antioxidants if we consider them outside of the body. But they work as antioxidants when they are in the body, because they activate their own antioxidant mechanisms. These bioactive substances are the secret behind vegetable consumption Kurutas, Antioxidant supplements may have different health benefits. On the one hand, it is possible that other substances present in food are responsible for the positive effects on health, not necessarily a certain type of antioxidant, but the synergistic effect of several substances. On the other hand, the chemical structure of antioxidants in food is often different from that identified in supplements. An example is vitamin E. There are eight variants of vitamin E in the foods we eat, while the supplements used in most studies contain only one form Firuzi et al. Studies also frequently include healthy people, for whom oxidative stress on the body is not significant to determine a risk of disease. Antioxidants can benefit certain categories of patients in whom there is a real, documented imbalance, but it may not bring anything extra for a person who gets a sufficient amount of nutrients from their diet. Observational studies analyze the trends, or habits of certain large population groups. In many, all the risk factors that could influence the course of the study can be controlled, and demonstrating a cause-effect relationship is difficult. We also cannot rely on small studies, carried out over a short period of time and using very concentrated substances extracted from different plant or animal products, to say that we have a superfood. Nutrition is a complex science, and at the moment we can only rely on the evidence accumulated so far. A food rich in antioxidants will not compensate for an unhealthy lifestyle. Oxidative stress can be reduced by approaching a balanced lifestyle. Nutrition plays a critical role, and the best treatment against oxidative stress is antioxidants. Oxidative stress plays an important role in the pathogenesis of potentially severe conditions. In the long term, increasing the level of prooxidant factors can cause structural defects in mitochondrial DNA and alterations in enzymatic functionality or cellular structures, with the appearance of functional, structural abnormalities or aberrations in gene expression. It has also been shown that in addition to metabolic products, other external agents can have a prooxidant effect, which has led to the conclusion that lifestyle and diet can play an important role in controlling oxidative stress. Plant-derived bioactive molecules have gained pivotal attention in recent years, given their therapeutic relevance in both disease prevention and treatment, whether using the whole plants, plant extracts or even the isolated constituents with full phytochemical profiles. The daily intake of a wide variety of phytochemicals has shown to be chemopreventive. It might hold promise for add-on treatment for several diseases, including cancer, diabetes, cardiovascular disease and neurodegenerative disorders. Larger randomized trials are needed to obtain clear scientific evidence on the benefits or risks of antioxidant supplementation during cancer treatment. Antioxidants are also prone to oxidation, and therefore their use as foods or supplements should be carefully considered because oxidation and reduction reactions do not happen in isolation. The intake of high doses of antioxidants has been increasingly highlighted since there is increasing evidence of some detrimental effects. The study of their chemical components as future prophylactic and therapeutic agents would be of particular interest, as they are more effective and safer than those widely available. In conclusion, oxidative stress is an important pathogenetic link for humans and studies in this field may be important elements in the future, to better understand and manage various diseases. JS-R and MS-R contributed to the conceptualization. NA, PZ, EV, and LD contributed to the validation investigation. EP, JR, PT, EA, IP, YE, and MB contributed to the resources. AP, MN, and AD: data curation. MS-R, AD, LP, MI, NM, MM, WS, DC, WC, and JS-R contributed to the review and editing. All authors contributed to the writing of the manuscript. All authors read and approved the final manuscript and contributed equally to the manuscript. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. NM would like to thank the Portuguese Foundation for Science and Technology FCT—Portugal for the Strategic project ref. Abramov, A. Expression and modulation of an NADPH oxidase in mammalian astrocytes. doi: PubMed Abstract CrossRef Full Text Google Scholar. Alfonso-Prieto, M. The molecular mechanism of the catalase reaction. Aminjan, H. Life Sci. Andreyev, A. Mitochondrial metabolism of reactive oxygen species. Biochemistry 70, — Google Scholar. Antonioni, A. Redox homeostasis in sport: do athletes really need antioxidant support? Sports Med. Antunes dos Santos, A. Oxidative stress in methylmercury-induced cell toxicity. Toxics Aroor, A. Mitochondria and oxidative stress in the cardiorenal metabolic syndrome. Cardiorenal Med. Ayala, A. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxynonenal. Banafsheh, A. Studies on oxidants and antioxidants with a brief glance at their relevance to the immune system. Barbieri, R. Phytochemicals for human disease: An update on plant-derived compounds antibacterial activity. Barrera, G. Oxidative stress and lipid peroxidation products in cancer progression and therapy. ISRN Oncol. Bartosz, G. Reactive oxygen species: destroyers or messengers? Battelli, M. Pathophysiology of circulating xanthine oxidoreductase: new emerging roles for a multi-tasking enzyme. Acta , — Xanthine oxidoreductase in atherosclerosis pathogenesis: not only oxidative stress. Atherosclerosis , — Battin, E. Antioxidant activity of sulfur and selenium: a review of reactive oxygen species scavenging, glutathione peroxidase, and metal-binding antioxidant mechanisms. Cell Biochem. Bedard, K. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Rev 87, — Benfeitas, R. New challenges to study heterogeneity in cancer redox metabolism. Cell Dev. Beyer, C. Antioxidant properties of melatonin—an emerging mystery. CrossRef Full Text Google Scholar. Bhattacharyya, A. Oxidative stress: an essential factor in the pathogenesis of gastrointestinal mucosal diseases. Birben, E. Oxidative stress and antioxidant defense. World Allergy Organ. Blokhuis, A. Protein aggregation in amyotrophic lateral sclerosis. Acta Neuropathol. Borek, C. Antioxidant health effects of aged garlic extract. Buga, A. Molecular and cellular stratagem of brain metastases associated with melanoma. Buj, R. Deoxyribonucleotide triphosphate metabolism in cancer and metabolic disease. Cadet, J. Formation and repair of oxidatively generated damage in cellular DNA. Free Radic. Oxidatively generated complex DNA damage: tandem and clustered lesions. Cancer Lett. |
| What are free radicals and antioxidants? | High-dose supplements of antioxidants may be linked to health risks in some cases, including higher mortality rates. Conversely, oxidative stress can also trigger apoptosis and ferroptosis, and reduce the opportunity for transformation and thereby prevent tumorigenesis DHA can be regenerated to the active AA form by GSH- or Trx-dependent mechanisms. BioMed Research International. Delanty, N. After dietary supplementation of these vitamins for a period of 6 weeks, a significant reduction in anxiety and depression scores of patients was observed. The role of oxidative stress and autophagy in atherosclerosis. |
| Recommended Reading | How Well Do You Sleep? Health Conditions Discover Plan Connect. Oxidative Stress: Your FAQs Answered. Medically reviewed by Adam Bernstein, MD, ScD — By Lizzy Sherman — Updated on July 24, What is oxidative stress? What causes oxidative stress? What are the risk factors for oxidative stress? How do you know if you have oxidative stress? How can I prevent oxidative stress? How we reviewed this article: Sources. Healthline has strict sourcing guidelines and relies on peer-reviewed studies, academic research institutions, and medical associations. We avoid using tertiary references. You can learn more about how we ensure our content is accurate and current by reading our editorial policy. Jul 24, Written By Lizzy Sherman. Jan 14, Written By Lizzy Sherman. Medically Reviewed By Adam Bernstein, MD, ScD. Share this article. More in Understanding Inflammation and Aging Your 5-Minute Read on Inflamm-aging and How to Prevent It. Your 5-Minute Read on Fighting Brain Fog. What Is Carbon 60 C60? Your FAQs Answered. This appears to be necessary in order to induce some of the beneficial effects of regular physical activity, such as sensitizing your muscle cells to insulin. That can be a bigger problem. Oxidative stress can damage our cell's membranes, it can damage proteins, and it can also damage the DNA. By damaging the DNA, this can potentially lead to mutations, and in the long run, if not repaired, the mutations in some genes can eventually lead to the process of developing a tumor. We can get the antidote to oxidative stress through diet, in the form of antioxidants. Mackenzie says that vegetables and fruits are great sources of antioxidants. We have to be cautious. But with the food we have, especially here in the United States, we can easily reach the adequate levels we need simply through our foods. Are you getting enough vitamin C per day? And why it matters. More: Many people aren't getting enough vitamin E, but read this before you take a supplement. What is the healthiest vegetable? FROM THE JOURNAL OF AFFECTIVE DISORDERS. Recommended Reading Psychiatric illnesses share common brain network. Primary care providers are increasingly addressing mental health concerns. Mental health system failing kids leaving ED. Two cups of coffee increase heart dangers with hypertension. Increased anxiety and depression after menstruation. Even mild COVID is hard on the brain. Clinician violence: Virtual reality to the rescue? |
| Everything You Should Know About Oxidative Stress | Antoxidant we Antioxidant and stress relief, the ability of anx to induce NRF2-dependent expression of xtress enzymes declines Antioxidants Immune function optimization free radicals or radical ions due to the unpaired electron in their structure. Effectiveness of N-acetylcysteine for the prevention of contrast-induced nephropathy: a systematic review and meta-analysis of randomized controlled trials. Researchers 3D-Print Functional Human Brain Tissue. Glutathione thiylperoxyl radical G S O 2. Cancer Ther. |
Nach meiner Meinung sind Sie nicht recht. Geben Sie wir werden es besprechen. Schreiben Sie mir in PM.
Bis zu welcher Zeit?
Ich werde zu diesem Thema nicht sagen.