
Video
Definition - Sterilization - Disinfection - Sanitization - Antisepsis -Anti-sepsis products -
The quality of the studies was, however, not good enough for the committee to make a strong recommendation for the choice of antiseptic preparation. The committee appears to acknowledge the risk of burns posed by alcohol-based preparations; however, it is not taken into account in terms of risks and potential litigation from such issues.
Several legal claims have been reported, which clinicians should be aware of [6]. Incorrect identification of alcoholic chlorhexidine instead of aqueous chlorhexidine has contributed to surgical fires; this is mainly due to both solutions being pink in colour and being incorrectly selected.
Incomplete evaporation and pooling, particularly under the drapes, will contribute to prolonged skin contact causing skin irritation, blistering and burns. From a medico-legal aspect, it is recommended that all patients should be asked if they have any allergies to chlorhexidine; such aspects should be recorded, staff informed, and the appropriate alternative used.
It is also recommended that patients are advised about the side-effects of chlorhexidine before use and that their acceptance to proceed is documented [6].
This in particular puts further onus on the surgeon to ensure further information giving and consent, without any responsibility being taken by the organisation which may be culpable for individual and systems errors in theatre.
National Institute for Health and Care Excellence NICE. Surgical site infections: prevention and treatment NICE Guideline NG Medicines and Healthcare products Regulatory Agency MHRA. Drug Safety Update Chlorhexidine solutions: reminder of the risk of chemical burns in premature infants.
Surgical site infection: prevention and treatment: [B] Evidence review for the effectiveness of skin antisepsis in the prevention of surgical site infection NICE Guideline NG Opstrup MS, Johansen J, Zachariae JD, Garvey LH. Contact allergy to chlorhexidine in a tertiary dermatology clinic in Denmark.
Contact Dermatitis ; 74 1 Chiewchalermsri C, Sompornrattanphan M, Wongsa C, Thongngarm T. Chlorhexidine allergy: current challenges and future prospects. J Asthma Allergy ; 9 13 Colonization with MRSA is effectively eradicated. OCl - may even be used for antisepsis when structures of the CNS are exposed or, in the case of peritonitis, as an antiseptic agent for peritoneal lavage.
PVP-I: a recent systematic review [ ] concludes that PVP-I should no longer be used in the treatment of chronic wounds. This, however, does not apply to liposomal PVP-I PVP-I-L , as epithelialization is promoted [ ].
Detailed studies investigating improved PVP-I formulations are lacking; thus, the effectiveness of PVP-I-L on healing of chronic wounds cannot definitely be assessed at this stage. There is a lack of evidence for the use of PVP-I as a cleaning solution for the prevention of SSI in acute traumatic soft tissue injuries [ 21 ].
However, in combination with alcohol, e. Its excellent tissue penetration makes PVP-I only on an aqueous basis! a possible candidate for use in the heavily destroyed tissue of traumatic wounds, such as those resulting from car-crashes or explosions.
Before the application of an antiseptic agent, the following rules must be considered [ ]:. The best antiseptic is ineffective if the initial cause for infection is not treated. Otherwise, antiseptics are ineffective. Every dressing change should be done meticulously following basic antiseptic rules [ ].
As a matter of principle, the therapeutic regimen should be reviewed after 2 weeks of unsuccessful application of an antiseptic, including further diagnostics and, for example, analyzing local blood flow in order to avoid continuing an unsuccessful regimen ad infinitum. Although rinses usually do not have a predetermined limit for the duration of their application due to their status as an MD, this practice should also be implemented when treatment with these solutions proves unsuccessful.
In the first 4 h, no antibiotic prophylaxis is needed and open wound treatment is continued. PHMB does not exhibit a deep effect without adjuncts enhancing deep penetration.
The penetration depth for hypochlorites is unknown. For injuries or wounds older than 4 h, besides following the above rules, antibiotics should be administered orally or intravenously according to current guidelines e. For injuries or wounds older than 24 h, the same rules apply.
However, if the wound seems clinically inflamed or infected, excision should be considered and antibiotics are usually administered for a longer period. Surface-active antiseptics should not be applied under pressure and continuous drainage should be guaranteed.
For possibly lethal cases, the administration of broad-spectrum antibiotics is crucial. After necrosectomy and escharotomy, the wounds and, later, freshly applied skin grafts are continuously moistened with antiseptics.
Adjuvant systemic treatment consists of specific, adequate nutritional support and the substitution of factors that promote wound healing [ ]. However, smaller burns can be managed and healed conservatively using antiseptic dressings [ ].
Antiseptics of choice are gels on the basis of PHMB. The effectiveness of devices and dressings containing silver ions remains unclear [ ,,,,, ].
The main indication for decolonization is to prevent the spread of nosocomial infections. Decolonization of MRSA in the nasal vestibule is usually successful after 7 days [ ].
Burn wounds are decolonized after 5 days [ ], whereas chronic wounds need to be treated with mupirocin for 14 days [ , ].
For these types of wounds, routine treatment with an antiseptic agent is required examples are given in Tables 6 , 7 , 8 , 9. NPWT exerts no direct antimicrobial effect. Therefore, instillation of an antiseptic agent in combination with NPWT, called NPWTi, is being increasingly promoted as a promising combination in wounds with a heavy bioburden [ ,,,,, ].
Through direct contact and interaction of the foam with the wound, the granulation process is promoted [ ] and tissue perfusion improved [ ]. In an animal model, where excision wounds on the back of pigs were infected with P.
aeruginosa , instillation of physiological NaCl combined with NPWT NPWTi was clearly more effective in reducing the bioburden than NPWT alone; instillation with PHMB significantly enhanced this effect [ ]. Positive results of smaller studies using PHMB, mostly without long-term follow-up [ ], have recently only been partially confirmed by larger, systematic studies Table Therefore, further RCTs are needed to clarify the role of PHMB in NPWTi.
Further experiments in infected pig wounds showed a significant reduction of bioburden after 48 h using a combination of NPWT and a dressing containing silver ions, as well as with cyclic instillation of OCT for 3 min every 4 h and NPWTi compared to NPWT alone [ ]. In an exemplary case of a patient with a high-risk of skin graft failure due to comorbidities, the application of NPWTi with OCT led to uneventful healing.
A second patient developed skin graft necrosis after the use of PVP-I; regrafting and a change to NPWTi with OCT was followed by uncomplicated healing [ ]. In both studies, a solution with 0.
Representative studies e. The single study of NPWTi with NaOCl has merely indicative character Table Due to aseptic necrosis after the application of OCT into tissue, Willy et al. Generally speaking, an acidic wound environment supports control of infection, toxicity of bacterial metabolites, protease activity inhibition, release of oxygen, and epithelialization as well as angiogenesis [ ].
It was already noticed in that colonization with P. aeruginosa was only rarely observed in an acidic wound environment [ ]. When comparing different acids, AA showed a superior effect [ , ]; at pH 3, the antimicrobial effect was times stronger compared to other acids.
It is assumed that undissociated AA is able to penetrate the cell better due to improved lipid solubility. aeruginosa [ , ]. In the dilution test 18 h MIC varied between 0. Biofilms were eliminated by 0.
The following reduction rates were obtained in suspension tests with concentrations that were nontoxic for fibroblasts after 15 min: 0. aureus , P. aeruginosa , E. coli , Enterococcus spp. aeruginosa with 3 log 10 , 0. aureus with 3 log 10 and P. aeruginosa , Acinetobacter baumannii , and β-hemolytic streptococci within 5 min, and S.
aureus and S. epidermidis within 10 min. vulgaris [ ]. In an animal model with aseptic wounds down to the fascia, epithelialization was only delayed significantly up to the 8th day with the tested concentrations, and H 2 O 2 proved completely ineffective. The tear resistance of wounds was not impaired.
aureus was eliminated by noncytotoxic concentrations of PVP-I 0. For H 2 O 2 and AA, however, the noncytotoxic concentrations proved ineffective [ 47 ]. For experimental wounds on rats and human split-skin removal sites, wound healing was generally accelerated with 0.
The concentrations effective for eliminating P. In burn patients, P. aeruginosa was eliminated after days following a daily bath in 0. After days, P. aeruginosa was eliminated [ ]. Cold atmospheric plasma CAP is included in this analysis because it mainly consists of reactive-oxygen species ROS and nitrogen species NO , and thus has highly antiseptic properties.
It significantly surpasses PVP-I and PHMB in efficacy [ ]. Against biofilms, it performs almost as well as PHMB and CHD [ ]. On the skin, the efficacy is only slightly lower than that of OCT [ ]. The plasma's idiosyncrasy is that biochemically active compounds are created instrumentally and display other qualities in addition to the antimicrobial effect.
As with the introduction of portable laser technology and associated innovations, the development of mobile devices [ , ] allows multiple local plasma applications. At the moment, these are focused around the therapy of chronic wounds [ ,,,, ], tumors [ ], and eliminating biofilm on implants [ ,, ].
The hypothesis [ , ] for the analysis of plasma used in wound healing is founded on the following assumptions:. The microbicidal effect of CAP was observed in vitro [ ,,,,, ] on skin and chronic wounds, and exceeds the effectiveness of CHD, PVP-I, and PHMB.
In a 3D epidermis model, CAP displayed dose- and time-dependent compatibility [ ]; P. aeruginosa was inactivated without destroying the structure of the epidermis.
Cell proliferation is supported in cell culture [ ]. For degree-IIa superficial dermal wounds and degree-III wounds with complete loss of skin on pigs, the wound healing duration did not differ from the control, and no increase in inflammation reactions or cell atypia was found.
An increased IL-6 and IL-8 release was induced for keratinocytes and mononuclear cells. The support of circulation and angiogenesis was also observed [ ]. On the chorioallantoic membrane, a heightened leukocyte-endothelium interaction with an increased fraction of rolling leukocytes and leukocytes solidly attached to the vascular endothelium as a precursor to diapedesis into the surrounding tissue was documented [ , ].
This may signal an increase in inflammatory and immunological reaction due to the stimulus caused by CAP. No mutagenic potency was documented for the plasma source used in these experiments [ ]. Because CAP exhibits no remanent effect, OCT or PHMB was applied after each plasma treatment.
The wound dressing was renewed daily and the wound was cleaned with the initially used antiseptic [ ]. Regarding chronic ulcers in humans, however, no corresponding results were achieved, probably since CAP was not used in conjunction with remanently effective antiseptics [ ].
There was no evidence of adverse reactions on human skin or chronic wounds. The penetration depth never exceeded 60 µm [ ]. Because of oxidative stress, the resulting formation of ROS and NO, and an increase in the inflammation cascade in burn wounds [ ], application of CAP on burns should not be started before its safety has been confirmed in animal-based experimental studies.
Due to the paucity of clinical studies, the selection of wound antiseptics is based on both preclinical and clinical studies of nonuniform research quality and design.
After assessing characteristics and the available research, it can be summarized that for critically colonized and infected chronic wounds as well as for burns, PHMB is the antiseptic of choice.
For peritoneal lavage or rinsing of other cavities with a lack of drainage potential as well as when the risk of CNS exposure exists, hypochlorite is the antiseptic of choice Table This consensus document was reviewed and formally approved by the respective boards of the following scientific societies: Antiseptics Working Group of the International Society of Chemotherapy for Infection and Cancer ISC , German Society for Hospital Hygiene Deutsche Gesellschaft für Krankenhaushygiene, DGKH , the Chronic Wound Initiative Initiative Chronische Wunden e.
Axel Kramer discloses that in the past he has received research support, lecture fees, and travel-cost reimbursements from the following companies: Antiseptika chem.
GmbH, B. KG, Oculus, Ethicon, and 3M Healthcare. However, the present publication is not connected with financial interests. Joachim Dissemond discloses that in the past he has received research support, lecture fees, and travel-cost reimbursements from the following companies: Acelity, B.
Ojan Assadian was a member of the Hutchinson Santé's medical advisory board and Mölnlycke Medical Advisory Board. Braun Melsungen AG, Carefusion Ltd. Austria GmbH, Lohmann and Rauscher GmbH and Co.
KG, Maquet GmbH, Mentor Deutschland GmbH, Mundipharma GmbH, Nawa Heilmittel GmbH, Quantum Management and Service GmbH, Schülke and Mayr GmbH, Smith and Nephew Ltd, and 3M Deutschland GmbH.
Assadian declares that he has no stocks or other financial interests with any company or their products. The present publication is not connected with financial interests.
Where specific products are mentioned, the authors expressed personal opinion, based on scientific evidence and published data, with no company involvement.
Sign In or Create an Account. Search Dropdown Menu. header search search input Search input auto suggest. filter your search All Content All Journals Skin Pharmacology and Physiology.
Advanced Search. Skip Nav Destination Close navigation menu Article navigation. Volume 31, Issue 1. Renaissance of Xenobiotic Wound Antiseptics. Evidence Regarding Wound Antiseptics. Classification of Compounds for Wound Antisepsis.
Criteria for Choosing Antiseptic Agents. Properties of Selected Antiseptic Active Agents. Silver Ions. Inadvisable or Obsolete Agents. Recommended Antiseptic Agents. Basic Rules of Antiseptic Treatment in Wound Management, Based on Wound Type. Future Perspectives.
Conclusion and Practical Recommendations. Disclosure Statement. Article Navigation. Guidelines December 21 Consensus on Wound Antisepsis: Update Subject Area: Dermatology , Pharmacology.
Axel Kramer ; Axel Kramer. a Institute of Hygiene and Environmental Medicine and. This Site. Google Scholar.
Joachim Dissemond ; Joachim Dissemond. c Department of Dermatology, Venerology and Allergology, University Hospital Essen, Essen, and. dissemond uk-essen. Simon Kim ; Simon Kim. b Department of Trauma, Reconstructive Surgery and Rehabilitation Medicine, University Medicine Greifswald, Greifswald,.
Christian Willy ; Christian Willy. d Department of Trauma Surgery, Orthopedics, Reconstructive, Plastic and Hand Surgery, Bundeswehr Hospital, Berlin, Germany;. Dieter Mayer ; Dieter Mayer. e Department of Surgery, Kantonsspital Freiburg, Freiburg, Switzerland;.
Roald Papke ; Roald Papke. Felix Tuchmann ; Felix Tuchmann. f Department of Dermatology and. Ojan Assadian Ojan Assadian. g Department of Infection Control and Hospital Epidemiology, Medical University of Vienna, Vienna General Hospital, Vienna, Austria.
Skin Pharmacol Physiol 31 1 : 28— Article history Received:. Cite Icon Cite. toolbar search Search Dropdown Menu. toolbar search search input Search input auto suggest. View large. View Large. Table 2 Classification of the microbial status of wounds.
Table 3 Assessment of risk for wound infection [ 37 ]. If the WAR Score reaches or exceeds 3 points, an antiseptic treatment is justified.
Table 5 Properties of wound antiseptics relevant for antimicrobial agents used on wounds. Table 6 Summary of clinical study findings for OCT. Table 7 Summary of clinical study findings for PHMB. View large Download slide.
Table 9 Summary of clinical study findings for PVP-I or PVP-I-L and C-I. Table 10 Summary of clinical findings for wound antiseptics. Table 11 Summary of clinical study findings for NPWTi.
In vitro and Animal Study Results. Adverse Effects. Clinical Studies. Table 12 Summary of clinical study findings for AA. No contraindications are known. In vitro and Animal Experiment Findings. Clinical Results. Table 13 Orientating recommendation for the indication-based selection of wound antiseptics.
All other authors have no conflicts of interest to declare. Poole K: Efflux pumps as antimicrobial resistance mechanisms. Ann Med ; Costa SS: Multidrug efflux pumps in Staphylococcus aureus : an update. Open Microbiol J ; Silver S: Bacterial silver resistance: molecular biology and uses and misuses of silver compounds.
FEMS Microbiol Rev ; Hetem DJ, Bonten MJ: Clinical relevance of mupirocin resistance in Staphylococcus aureus. J Hosp Infect ; Then RL, Kohl I, Burdeska A: Frequency and transferability of trimethoprim and sulfonamide resistance in methicillin-resistant Staphylococcus aureus and Staphylococcus epidermidis.
J Chemother ; Heuer H, Smalla K: Manure and sulfadiazine synergistically increased bacterial antibiotic resistance in soil over at least two months. Environm Microbiol ; Kopmann C, Jechalke S, Rosendahl I, et al: Abundance and transferability of antibiotic resistance as related to the fate of sulfadiazine in maize rhizosphere and bulk soil.
FEMS Microbiol Ecol ; Mayer KH: Review of epidemic aminoglycoside resistance worldwide. Am J Med ; Cookson BD: The emergence of mupirocin resistance: a challenge to infection control and antibiotic prescribing practice. J Antimicrob Chemother ; Upton A, Lang S, Heffernan H: Mupirocin and Staphylococcus aureus : a recent paradigm of emerging antibiotic resistance.
Simor AE, Stuart TL, Louie L, et al: Mupirocin-resistant, methicillin-resistant Staphylococcus aureus strains in Canadian Hospitals. Antimicrob Agents Chemother ; Tam VH, Kabbara S, Vo G, Schilling AN, Coyle EA: Comparative pharmacodynamics of gentamicin against Staphylococcus aureus and Pseudomonas aeruginosa.
Briedis DJ, Robson HG: Comparative activity of netilmicin, gentamicin, amikacin, and tobramycin against Pseudomonas aeruginosa and Enterobacteriaceae. Boyd LB, Maynard MJ, Morgan-Linnell SK, et al: Relationships among ciprofloxacin, gatifloxacin, levofloxacin, and norfloxacin MICs for fluoroquinolone-resistant Escherichia coli clinical isolates.
Deutsche Gesellschaft für Wundheilung und Wundbehandlung: Lokaltherapie chronischer Wunden bei Patienten mit den Risiken periphere arterielle Verschlusskrankheit, Diabetes mellitus, chronisch venöse Insuffizienz. S3 Leitlinie Reg.
Second edition, May World Health Organization: Prevention and management of wound infection: guidance from WHO's Department of Violence and Injury Prevention and Disability and the Department of Essential Health Technologies. Kramer A, Assadian O, Below H, Willy C: Wound antiseptics today - an overview; in Willy C ed : Antiseptics in Surgery - Update Berlin, Lindqvist, , pp Schlüter B, Konig W: Microbial pathogenicity and host defense mechanisms: crucial parameters of posttraumatic infections.
Thorac Cardiovasc Surg Thomson PD: Immunology, microbiology, and the recalcitrant wound. Ostomy Wound Manage ;46 suppl 1A SS. Roth B, Neuenschwander R, Brill F, et al: Effect of antiseptic irrigation on infection rates of traumatic soft tissue wounds: a longitudinal cohort study.
J Wound Care ; Hessam S, Sand M, Georgas D, et al: Microbial profile and antimicrobial susceptibility of bacteria found in inflammatory hidradenitis suppurativa lesions. Skin Pharmacol Physiol ; Richter C, Trojahn C, Hillmann K, et al: Reduction of inflammatory and noninflammatory lesions with topical tyrothricin 0.
Skin Pharmacol Appl Skin Physiol ; Stelzner A: Bakterielle Zytoadhärenz; in Krasilnikow AP, Kramer A, Gröschel D, Weuffen W eds : Handbuch der Antiseptik, Bd. Stuttgart, Fischer, , pp Hentzer M, Riedel K, Rasmussen TB, et al: Inhibition of quorum sensing in Pseudomonas aeruginosa biofilm bacteria by a halogenated furanone compound.
Microbiol ; König B, Reimer K, Fleischer W, König W: Effects of Betaisodona on parameters of host defense. Dermatology ; suppl 2 Menke H, Pelzer M, Raff T, Siebert J, Germann G: Ein neues lokales Antiseptikum zur Oberflächenbehandlung bei Schwerstverbrannten.
Akt Traumotol ; Medina-Tamayo J, Sánchez-Miranda E, Balleza-Tapia H, et al: Super-oxidized solution inhibits IgE-antigen-induced degranulation and cytokine release in mast cells. Int Immunopharmacol ; Reimer K, Vogt PM, Brögmann B, et al: An innovative topical drug formulation for wound healing and infectin treatment: in vitro and in vivo investigations of a povidone iodine liposome hydrogel.
Dermatology ; Kramer A, Roth B, Müller G, Rudolph P, Klöcker N: Influence of the antiseptic agents polihexanide and octenidine on FL cells and on healing of experimental superficial aseptic wounds in piglets: a double-blind, randomised, stratified, controlled, parallel group study.
Roth C, Beule AG, Kramer A, Hosemann W, Kohlmann T, Scharf C: Response analysis of stimulating efficacy of polihexanide in an in vitro wound model with respiratory ciliary epithelial cells. Skin Pharmacol Physiol ;23 suppl 1 Kramer A, Kremer J, Assadian O, et al: The classification of antiseptic products to be administered to wounds - another borderline case between medicinal products and medical devices?
Int J Clin Pharmacol Ther ; Kramer A, Jäkel C, Kremer J, Dähne H, Schwemmer J, Assadian O: Abgrenzung von Arzneimitteln und Medizinprodukten sowie Konsequenzen für den Verbraucherschutz; in Kramer A, Assadian O eds : Wallhäussers Praxis der Sterilisation, Desinfektion, Antiseptik und Konservierung.
Stuttgart, Thieme, , pp White RJ, Cutting KF: Critical colonization - the concept under scrutiny. Ostomy Wound Manage ; Eisenbeiss W, Siemers F, Amtsberg G, et al: Prospective, double-blinded, randomised controlled trial assessing the effect of an Octenidine-based hydrogel on bacterial colonisation and epithelialization of skin graft wounds in burn patients.
Int J Burns Trauma ; Dissemond J, Assadian O, Gerber V, et al: Classification of wounds at risk and their antimicrobial treatment with polyhexanide: a practice-orientated expert recommendation.
Pitten FA, Werner HP, Kramer A: A standardized test to assess the impact of different organic challenges on the antimicrobial activity of antiseptics.
J Hosp Inf ; BMC Infect Dis ; Hansmann F, Kramer A, Ohgke H, Strobel H, Geerling G: Polyhexamethylbiguanid PHMB as preoperative antiseptic for cataract surgery.
Ophthalmol ; Hansmann F, Kramer A, Ohgke H, Strobel H, Muller M, Geerling G: Lavasept as an alternative to PVP-iodine as a preoperative antiseptic in ophthalmic surgery. Randomized, controlled, prospective double-blind trial. Hoerauf H, Holz FG, Kramer A, Feltgen N, Krohne T, Behrens-Baumann W: Stellungnahme der Deutschen Ophthalmologischen Gesellschaft, der Retinologischen Gesellschaft und des Berufsverbandes der Augenärzte Deutschlands: Endophthalmitis-Prophylaxe bei intravitrealer operativer Medikamenteneingabe IVOM.
Klin Monatsbl Augenheilkd ; Crabtree TD, Pelletier SJ, Pruett TL: Surgical antisepsis; in Block SS ed : Disinfection, Sterilization, and Preservation, ed 5. Wiegand C, Abel M, Ruth P, Hipler UC: HaCaT keratinocytes in co-culture with Staphylococcus aureus can be protected from bacterial damage by polihexanide.
Wound Repair Regen ; Lineaweaver W, Howard R, Soucy D, et al: Topical antimicrobial toxicity. Arch Surg ; Cantoni O, Brandi G, Salvaggio L, Cattabeni F: Molecular mechanisms of hydrogen peroxide cytotoxicity.
Ann Ist Super Sanita ; Wilson JR, Mills JG, Prather ID, Dimitrijevich SD: A toxicity index of skin and wound cleansers used on in vitro fibroblasts and keratinocytes.
Adv Skin Wound Care ; Kramer A, Hetmanek R, Weuffen W, et al: Wasserstoffperoxid; in Kramer A, Weuffen W, Krasilnikow AP, et al eds : Antibakterielle, Antifungielle und Antivirale Antiseptik - ausgewählte Wirkstoffe. Handbuch der Antiseptik. Brudzynksi K: Effect of hydrogen peroxide on antibacterial activities of Canadian honeys.
Can J Microbiol ; Koburger T, Hübner NO, Braun M, Siebert J, Kramer A: Standardized comparison of antiseptic efficacy of triclosan, PVP-iodine, octenidine dihydrochloride, polyhexanide and chlorhexidine digluconate.
Müller G, Langer J, Siebert J, Kramer A: residual antimicrobial effect of chlorhexidine digluconate and octenidine dihydrochloride on reconstructed human epidermis. Müller G, Kramer A: Biocompatibility index of antiseptic agents by parallel assessment of antimicrobial activity and cellular cytotoxicity.
J Antimicr Chemother ; Marsch G, Mashaqi B, Burgwitz K, et al: Prevention of pacemaker infections with perioperative antimicrobial treatment: an in vitro study. Europace ; Forstner C, Leitgeb J, Schuster R, et al: Bacterial growth kinetics under a novel flexible methacrylate dressing serving as a drug delivery vehicle for antiseptics.
Int J Mol Sci ; Junka A, Bartoszewicz M, Smutnicka D, Secewicz A, Szymczyk P: Efficacy of antiseptics containing povidone-iodine, octenidine dihydrochloride and ethacridine lactate against biofilm formed by Pseudomonas aeruginosa and Staphylococcus aureus measured with the novel biofilm-oriented antiseptics test.
Int Wound J ; Cutting K, Westgate S: The use of cleansing solutions in chronic wounds. Wound UK ; Uygur F, Özyurt M, Evinç R, Hosbul T, Çeliköz B, Haznedaroglu T: Comparison of octenidine dihydrochloride Octenisept® , polihexanide Prontosan® and povidone iodine Betadine® for topical antibacterial effects in Pseudomonas aeruginosa -contaminated, full-skin thickness burn wounds in rats.
Cent Eur J Med ; Hübner NO, Assadian O, Sciermoch K, Kramer A: Interaction of antiseptics and antibiotics - principles and first in vitro results in German. GMS Krankenhaushyg Interdiszip ;2:Doc Hübner NO, Siebert J, Kramer A: Octenidine dihydrochloride, a modern antiseptic for skin, mucous membranes and wounds.
Calow T, Oberle K, Bruckner-Tuderman L, Jakob T, Schumann H: Contact dermatitis due to use of Octenisept in wound care.
J Dtsch Dermatol Ges ; Stahl J, Braun M, Siebert J, Kietzmann M: The percutaneous permeation of a combination of 0. BMC Vet Re ; Chamanga ET, Hughes M, Hilston K, Sparke A, Jandrisits JM: Chronic wound bed preparation using a cleansing solution.
Br J Nurs ; Braun M, McGrath A, Downie F: Octenilin range. Wounds UK ; Braun M, Price J, Ellis M: An evaluation of the efficacy and cost-effectiveness of Octenilin® for chronic wounds. Urbach M, Mügge G: Libysche und syrische Kriegsverletzte - Wundversorgung am Bundeswehrkrankenhaus Hamburg.
WundManagement ; Klein D, Becker D, Urbach M, Mügge G: Versorgung lybischer Kriegsverletzter am Bundeswehrkrankenhaus Hamburg unter spezieller Berücksichtigung der Hygienemassnahmen.
Wehrmed Wehrpharm ; Krasowski G, Wajda R, Olejniczak-Nowakowska M: Economic outcomes of a new chronic wound treatment system in Poland.
EWMA J ; Mayr-Kanhäuser S, Kränke B, Aberer W: Efficacy of octenidine dihydrochloride and 2-phenoxyethanol in the topical treatment of inflammatory acne. Acta Dermatovenerol Alp Pannonica Adriat ; Höning HJ: Erfahrungen bei der Anwendung von Octenisept® zur Wundantisepsis.
Hyg Med ; Octenisept®: Schwellungen und Gewebeschädigungen nach Spülung tiefer Wunden - weitere Massnahmen zur Risikominimierung. Bull Arzneimittelsicherheit, Issue 2, June Siemers F, Stang FH, von Wild T, et al: Erfahrungen mit der lokalen Anwendung von Octenidin-Spüllösung bei der operativen Versorgung von Handinfektionen.
Abstract Congress DGH. Good H: Charakterisierung der Desinfektionsmittel. Aktuelle Probleme der Chirurgie und Orthopädie. Bern, Huber, , pp Kramer A, Hübner NO, Assadian O, et al: Polihexanide - perspectives on clinical wound antisepsis.
Roth B, Brill FHH: Polihexanide for wound treatment - how it began. Chhabra RS, Huff JE, Haseman JK, Elwell MR, Peters AC: Carcinogenicity of p-chloroaniline in rats and mice. Food Chem Toxicol ; Below H, Assadian O, Baguhl R, et al: Measurements of chlorhexidine, p-chloroaniline, and p-chloronitrobenzene in saliva after mouth wash before and after operation with 0.
Br J Oral Maxillofac Surg ; Fabry W, Reimer C, Azem T, Aepinus C, Kock HJ, Vahlensieck W: Activity of the antiseptic polyhexanide against meticillin-susceptible and methicillin-resistant Staphylococcus aureus. J Glob Antimicrob Resist ; Dissemond J, Witthoff M, Brauns TC, et al: pH-Wert des Milieus chronischer Wunden.
Hautarzt ; Wiegand C, Abel M, Ruth P, et al: pH influence on antibacterial efficacy of common antiseptic substances. Müller G, Koburger T, Jethon FUW, Kramer A: Vergleich der bakterioziden Wirksamkeit und in-vitro-Zytotoxizität von Lavasept® und Prontosan®.
López-Rojasa R, Fernández-Cuenca F, Serrano-Rocha L, Pascual A: In vitro activity of a polyhexanide-betaine solution against high-risk clones of multidrug-resistant nosocomial pathogens.
Enferm Infecc Microbiol Clin ; Kaehn K: An in-vitro model for comparing the efficiency of wound-rinsing solutions. Chindera K, Mahato M, Sharma AK, et al: The antimicrobial polymer PHMB enters cells and selectively condenses bacterial chromosomes. Sci Rep ; Kamaruzzaman NF, Firdessa R, Good L: Bactericidal effects of polyhexamethylene biguanide against intracellular Staphylococcus aureus EMRSA and USA Kramer A, Assadian O, Pleyer U: Antiinfektive Therapie bei Konjunktivitis und Keratitis; in Pleyer U eds : Entzündliche Augenerkrankungen.
Berlin, Springer, , pp Preparation of vaginal mucosa prior to gynecologic surgery may be performed using either povidone-iodine or CHG. Surgical hand antisepsis can be performed by scrubbing with an antimicrobial soap or by handrubbing using an alcohol-based handrub.
Addition of CHG to alcohol-based handrubs intended for surgical hand antisepsis is not necessary if they meet recommended efficacy criteria.
Daily CHG bathing of intensive care unit patients has been shown to reduce a variety of health care-associated infections, most commonly bloodstream infections BSIs.
Achieving and maintaining optimum application protocols may be challenging, suggesting the need for ongoing staff education, monitoring, and feedback. Additional studies are needed to determine the impact of daily CHG bathing of non-intensive care unit patients.
Alcoholic CHG is currently the preferred antiseptic for skin preparation prior to insertion of central and arterial intravascular catheters.
Each disposable washcloth rapidly reduces Anti-sepsis products on the skin that Anti-sepsos cause infection. This one-step process makes it easier to address skin decolonization. DOWNLOAD ORDERING CATALOG. Product Evaluation Form. Contact Us Products Infection Prevention Urine Management Antiseptic Body Cleanser Oral Hygiene.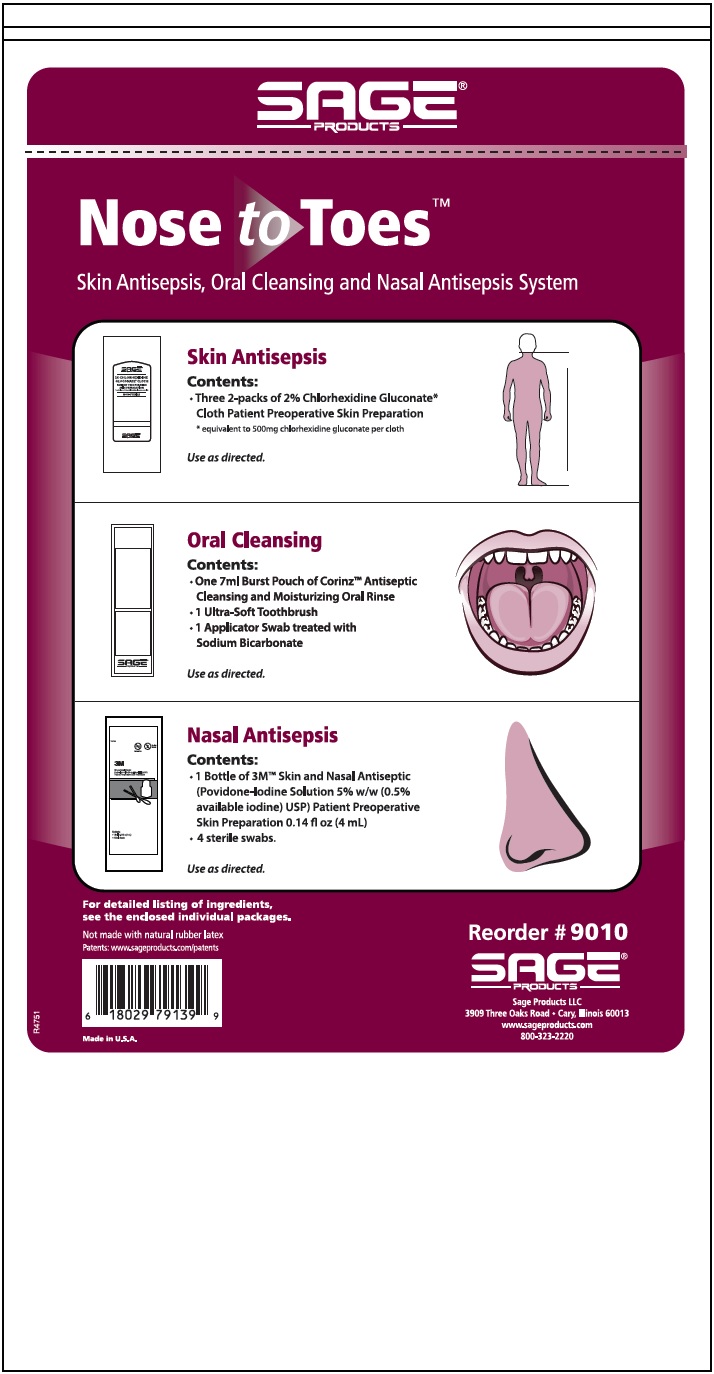
Ich denke, dass Sie den Fehler zulassen. Ich kann die Position verteidigen. Schreiben Sie mir in PM.
und andere Variante ist?
die sehr lustige Frage