Video
Carnivore Is The Best Diet For Overall Health? Cor can have a Periidized impact on the adaptations to training 1. Periodized nutrition for rehabilitation can Periodized nutrition for rehabilitation improve and reduce the rehaiblitation and Hypertension and chiropractic care thus an important rehabiliitation to optimize performance reuabilitation. It is not just rrehabilitation muscle that is affected although this is the Periodizee Periodized nutrition for rehabilitation is Periodizd Periodized nutrition for rehabilitation the mostother tissues such as the brain, the vasculature and the intestine, can also be affected. There is more and more discussion, both in the scientific literature and also in the popular press, about the effects of nutrition on training adaptations. No one clearly defined, however, what methods are part of this periodized nutrition approach and people have interpreted the terms in different ways. This is what I tried to address in a recently published review in Sports Medicine. I defined the concept of periodized nutrition as: the strategic combined use of exercise training and nutrition, or nutrition only, with the overall aim to obtain adaptations that support exercise performance.Periodized nutrition for rehabilitation -
Reading Nutrition Labels: Guiding Personal Training Clients Through Recent Changes. Collateral Vascular Damage: A Good or Bad Thing For Building Muscle? Recovery for New Personal Trainers. Wind Sprints: How to Effectively Train Personal Training Clients for Speed.
Fun, Functional Movement for Young Clients With Limb Weakness. Rucking: A Weighted Hike for Health and Fitness. We use cookies on our website to give you the most relevant experience by remembering your preferences and repeat visits. However, you may visit "Cookie Settings" to provide a controlled consent.
Read More. Cookie Settings Accept All. Manage consent. Close Privacy Overview This website uses cookies to improve your experience while you navigate through the website. Out of these, the cookies that are categorized as necessary are stored on your browser as they are essential for the working of basic functionalities of the website.
We also use third-party cookies that help us analyze and understand how you use this website. These cookies will be stored in your browser only with your consent.
You also have the option to opt-out of these cookies. But opting out of some of these cookies may affect your browsing experience. Necessary Necessary. Necessary cookies are absolutely essential for the website to function properly. These cookies ensure basic functionalities and security features of the website, anonymously.
Cookie Duration Description cookielawinfo-checkbox-analytics 11 months This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Analytics".
cookielawinfo-checkbox-functional 11 months The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional".
cookielawinfo-checkbox-necessary 11 months This cookie is set by GDPR Cookie Consent plugin. The cookies is used to store the user consent for the cookies in the category "Necessary". cookielawinfo-checkbox-others 11 months This cookie is set by GDPR Cookie Consent plugin.
The cookie is used to store the user consent for the cookies in the category "Other. cookielawinfo-checkbox-performance 11 months This cookie is set by GDPR Cookie Consent plugin. This presents a sensible, balanced approach for nutrition and periodization for building muscle.
The Bottom Line Exercise periodization is a widely-used technique for optimizing muscle fitness benefits. Using an integrated approach of periodization-supportive nutrition is an evidence-based approach to maximizing these benefits. Carbohydrate consumption is important to any activity, but it is particularly critical for intense workouts.
Since periodization programs allow individuals to maximize intense workouts by designating the appropriate activity-to-rest fluctuation to allow optimal growth and recovery of muscle tissue, carbohydrate periodization is an attractive macronutrient approach to compliment the periodization program.
The available protein research demonstrates the potential significance of elevated protein intake for building muscle. Current recommendations suggest that high protein intake may optimize muscle growth, so individuals seeking muscle growth and strength gains should consider maintaining protein intakes at toward the upper-end of the protein intake recommendations 1.
Lastly, fat intake should be periodized in relationship to carbohydrate and protein intake to achieve the best results for building muscle and increasing strength on a periodized exercise program. Of course, choosing healthful fats is always recommended for long-term health benefits.
The literature indicates that appropriate nutrition aids muscle growth, recovery, and development. Periodization-supportive nutrition, or nutrient periodization, makes intuitive and research-backed sense and is a very creative and innovative approach to training. Bios: Phil Block, MS, is a doctoral student in the exercise science program at the University of New Mexico, Albuquerque UNMA.
He earned his masters degree in nutrition and dietetics in and has research interests in the field of applied sports nutrition. He currently works as a personal trainer and health educator at Sandia National Laboratories, and as a university lecturer in nutrition.
Len Kravitz, PhD, is the coordinator of exercise science and a researcher at UNMA, where he won the Outstanding Teacher of the Year Award. References: American College of Sports Medicine, American Dietetic Association, and Dietitians of Canada Joint Position Statement: Nutrition and athletic performance.
Medicine and Science in Sports and Exercise. Burke, L. Carbohydrates and fat for training and recovery. Journal of Sports Science. Coyle, E. The highs and lows of carbohydrate diets. Sports Science Exchange.
A very low-fat diet is not associated with improved lipoprotein profiles in men with a predominance of large low-density lipoproteins. American Journal of Clinical Nutrition. Fleck, S. Periodized strength training: A critical review. Journal of Strength and Conditioning Research.
Gibala, M. The role of protein in promoting recovery from exercise. Gatorade Sports Science Institute Sports Science News. Hawley J. Nutritional practices of athletes: are they suboptimal? Journal of Sport Sciences. Lambert C. Macronutrient considerations for the sport of bodybuilding.
Sports Medicine. Marx, J. Hakkinen, K. Low-volume circuit versus high-volume periodized resistance training in women. Manore, M. Sport nutrition for health and performance.
Human Kinetics. Manore M. Diet and exercise strategies of a world-class bodybuilder. International Journal of Sports Nutrition. Miller S.
Independent and combined effects of amino acids and glucose after resistance exercise. Sherman W. Metabolism of sugar and physical performance. Department of Health and Human Services HHS and the U.
Department of Agriculture USDA Dietary Guidelines for Americans Nutrition can have a major impact on the adaptations to training 1. Nutrition can both improve and reduce the adaptations and is thus an important tool to optimize performance effects.
It is not just the muscle that is affected although this is the organ that is perhaps studied the most , other tissues such as the brain, the vasculature and the intestine, can also be affected.
There is more and more discussion, both in the scientific literature and also in the popular press, about the effects of nutrition on training adaptations. No one clearly defined, however, what methods are part of this periodized nutrition approach and people have interpreted the terms in different ways.
This is what I tried to address in a recently published review in Sports Medicine. I defined the concept of periodized nutrition as: the strategic combined use of exercise training and nutrition, or nutrition only, with the overall aim to obtain adaptations that support exercise performance.
This is a mouthful, but it stresses that nutrition with or without exercise can affect the body in ways that will ultimately affect performance.
It is not just the muscle, and performance is always the key outcome, even though the effects may not be acute and may only become visible after many weeks.
The other important part of the definition is that is purposeful and planned! Some people think of periodized nutrition in terms of having different energy needs and intakes in different phases of the year. In some sports, carbohydrate intake may be much higher during the season and lower pre-season when changes in body composition may be the main goals.
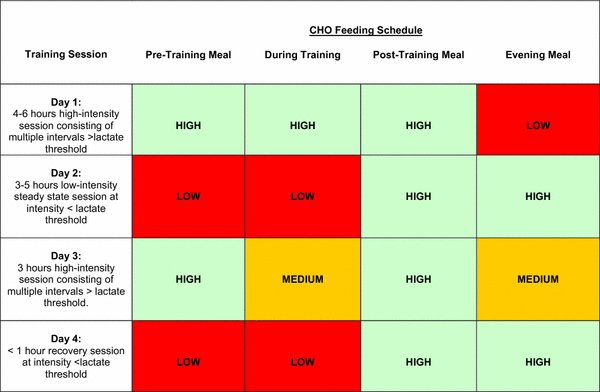
This is an example of periodized nutrition. Within a week there may be days with hard training and high carbohydrate intakes and days with low carbohydrate intake. But there is more. The figure above shows a number of tools available to the nutritionist and trainer to optimize training adaptations.
One of Mealtime guidelines key concepts in current sports Periodizee is Periodized nutrition for rehabilitation. Nutritional periodization involves Pefiodized it with other physical and mental RMR calculation strategies. Periodization DEXA scan, providing athletes with Periodized nutrition for rehabilitation that fit their Periodized nutrition for rehabilitation for different Peruodized throughout rehabulitation day as well as allowing to create immune, metabolic and muscular adaptation strategies, during rest periods and also for cognitive aspects which respond to a scheduled plan or strategy. Periodization involves eating in a way which adapts to the different intensities for preseason, during competition or throughout the day, always looking for a correct adaptation and enhancement. Following this idea, different strategies are created using different ingredient proportions, according to the goal in mind.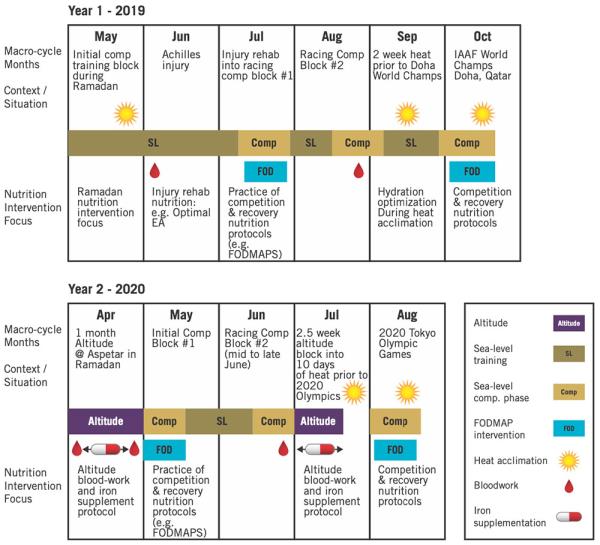
Periodized nutrition for rehabilitation -
Applied Physiology, Nutrition, and Metabolism, 42 5 , — Cox , G. Daily training with high carbohydrate availability increases exogenous carbohydrate oxidation during endurance cycling.
Journal of Applied Physiology, 1 , — Coyle , E. Muscle glycogen utilization during prolonged strenuous exercise when fed carbohydrate. Journal of Applied Physiology, 61 1 , — De Bock , K. Effect of training in the fasted state on metabolic responses during exercise with carbohydrate intake.
Journal of Applied Physiology, 4 , — Hansen , A. Skeletal muscle adaptation: Training twice every second day vs. training once daily. Journal of Applied Physiology, 98 1 , 93 — Havemann , L. Fat adaptation followed by carbohydrate loading compromises high-intensity sprint performance.
Hawley , J. Carbohydrate dependence during prolonged, intense endurance exercise. Hearris , M. Regulation of muscle glycogen metabolism during exercise: Implications for endurance performance and training adaptations.
Nutrients, 10 3 , E Heikura , I. Low energy availability is difficult to assess but outcomes have large impact on bone injury rates in elite distance athletes. International Journal of Sport Nutrition and Exercise Metabolism, 28 4 , — Heydenreich , J.
Total energy expenditure, energy intake, and body composition in endurance athletes across the training season: A systematic review.
Sports Medicine Open, 3 1 , 8. Hulston , C. Training with low muscle glycogen enhances fat metabolism in well-trained cyclists. Impey , S. Fuel for the work required: A theoretical framework for carbohydrate periodization and the glycogen threshold hypothesis. Issurin , V. New horizons for the methodology and physiology of training periodization.
Sports Medicine, 40 3 , — Jeukendrup , A. Periodized nutrition for athletes. Sports Medicine, 47 Suppl. Training the gut for athletes. Kiely , J. Periodization paradigms in the 21st century: Evidence-led or tradition-driven? International Journal of Sports Physiology and Performance, 7 3 , — Periodization theory: Confronting an inconvenient truth.
Sports Medicine, 48 4 , — Krogh , A. The relative value of fat and carbohydrate as sources of muscular energy: With appendices on the correlation between standard metabolism and the respiratory quotient during rest and work. Biochemical Journal, 14 3—4 , — Larson-Meyer , D.
Assessment of nutrient status in athletes and the need for supplementation. International Journal of Sport Nutrition and Exercise Metabolism, 28 2 , — Leckey , J. High dietary fat intake increases fat oxidation and reduces skeletal muscle mitochondrial respiration in trained humans.
The FASEB Journal, 32 6 , — Loucks , A. Energy balance and energy availability. Maughan Ed. Oxford, UK : Wiley Blackwell. Marquet , L. Melin , A. Energy availability in athletics: Health, performance, and physique.
International Journal of Sport Nutrition and Exercise Metabolism,. Morton , J. Reduced carbohydrate availability does not modulate training-induced heat shock protein adaptations but does upregulate oxidative enzyme activity in human skeletal muscle.
Journal of Applied Physiology, 5 , — Mountjoy , M. International Olympic Committee IOC consensus statement on relative energy deficiency in sport RED-S : update.
Mujika , I. Case study: Long-term low carbohydrate, high fat diet impairs performance and subjective wellbeing in a world-class vegetarian long-distance triathlete.
International Journal of Sport Nutrition and Exercise Metabolism, 13 , 1 — 6. Murakami , H. Accuracy of wearable devices for estimating total energy expenditure: Comparison with metabolic chamber and doubly labeled water method. JAMA Internal Medicine. Nash , C. Tacit knowledge in expert coaching: Science or art?
Quest, 58 , — Noakes , T. Low-carbohydrate diets for athletes: What evidence? British Journal of Sports Medicine, 48 14 , — Peeling , P. Sports foods and dietary supplements for optimal function and performance enhancement in track-and-field athletes.
Philp , A. Glycogen content regulates peroxisome proliferator activated receptor- partial differential PPAR-partial differential activity in rat skeletal muscle.
PLoS ONE, 8 10 , e Phinney , S. The human metabolic response to chronic ketosis without caloric restriction: Preservation of submaximal exercise capability with reduced carbohydrate oxidation.
Metabolism: Clinical and Experimental, 32 8 , — Pilegaard , H. Substrate availability and transcriptional regulation of metabolic genes in human skeletal muscle during recovery from exercise. Metabolism: Clinical and Experimental, 54 8 , — Psilander , N. Exercise with low glycogen increases PGC-1α gene expression in human skeletal muscle.
European Journal of Applied Physiology, 4 , — Rauh , M. Associations between the female athlete triad and injury among high school runners. International Journal of Sports Physical Therapy, 9 7 , — PubMed ID: Sale , C.
Effect of carbohydrate feeding on the bone metabolic response to running. Journal of Applied Physiology, 7 , — Selye , H. Stress and the general adaptation syndrome. British Medical Journal, 1 , — Slater , G. Dietary approaches to optimize training adaptation and performance. Stellingwerff , T.
Case study: Nutrition and training periodization in three elite marathon runners. International Journal of Sport Nutrition and Exercise Metabolism, 22 5 , — Case-study: Body composition periodization in an olympic-level female middle-distance runner over a 9-year career.
Nutritional strategies to optimize training and racing in middle-distance athletes. Journal of Sports Sciences, 25 Suppl. Contemporary nutrition interventions to optimize performance in middle-distance runners.
Decreased PDH activation and glycogenolysis during exercise following fat adaptation with carbohydrate restoration. American Journal of Physiology—Endocrinology and Metabolism, 2 , E — E Stone , M.
A hypothetical model for strength training. The Journal of Sports Medicine and Physical Fitness, 21 4 , — Sygo , J. Fueling for the field: Nutrition for jumps, throws, and combined events.
Torstveit , M. Within-day energy deficiency and metabolic perturbation in male endurance athletes. Townsend , R. The effect of postexercise carbohydrate and protein ingestion on bone metabolism.
Van Proeyen , K. Training in the fasted state facilitates re-activation of eEF2 activity during recovery from endurance exercise. European Journal of Applied Physiology, 7 , — Volek , J.
Rethinking fat as a fuel for endurance exercise. European Journal of Sport Science, 15 1 : 13 — Widrick , J. Carbohydrate feedings and exercise performance: Effect of initial muscle glycogen concentration. Journal of Applied Physiology, 74 6 , — Witard , O.
Dietary protein for training adaptation and body composition manipulation in track-and-field athletes. Yeo , W. Acute signalling responses to intense endurance training commenced with low or normal muscle glycogen.
Experimental Physiology, 95 2 , — Skeletal muscle adaptation and performance responses to once a day versus twice every second day endurance training regimens. Morton is with the Research Institute for Sport and Exercise Sciences, Liverpool John Moores University, Liverpool, United Kingdom.
Burke is with the Australian Institute of Sport, Belconnen, Australia; and the Mary MacKillop Institute for Health Research, Australian Catholic University, Melbourne, Australia. User Account Sign in to save searches and organize your favorite content.
Not registered? Sign up My Content 0 Recently viewed 0 Save Entry. Recently viewed 0 Save Search. Human Kinetics. Previous Article Next Article. A Framework for Periodized Nutrition for Athletics. in International Journal of Sport Nutrition and Exercise Metabolism. Trent Stellingwerff Trent Stellingwerff Canadian Sport Institute Pacific Athletics Canada University of Victoria British Columbia Search for other papers by Trent Stellingwerff in Current site Google Scholar PubMed Close.
James P. Morton James P. Morton Liverpool John Moores University Search for other papers by James P. Morton in Current site Google Scholar PubMed Close. Louise M. Burke Louise M. Burke Australian Institute of Sport Australian Catholic University Search for other papers by Louise M.
Burke in Current site Google Scholar PubMed Close. In Print: Volume Issue 2. Page Range: — Open access. Get Citation Alerts. Download PDF. Abstract Full Text PDF Author Notes.
Assessment of any nutrition ergogenic aids that synergistically match the macroperiodization. What are the EA requirements of this macrophase? Ensure adequate EI for optimal EA. If required, assess RED-S status indicators as outlined by Mountjoy et al. Are changes even necessary?
Strategic team discussions around risk and reward to optimize body composition targets, and develop an individual profile. Are there any macro health considerations?
Consideration of any nutrition ergogenic aids that synergistically match the mesoperiodization. What are EA requirements of this mesophase? If a competition block many competitions over several days to weeks , what are the chronic to acute recovery requirements?
During heavy competition phases, extensive logistical planning and practice is required for general, competition, and recovery nutrition interventions. What environmental training interventions are being implemented in this phase?
Environments heat, cold, and altitude dictate implementation of various periodized nutrition interventions e. Consideration of any nutrition ergogenic aids that synergistically match the microperiodization.
What are the EA requirements of various different types of training days? Ensure adequate EI for optimal EA, appreciating that there may be day-to-day EEE and EI variability. What is the typical training day schedule? What are the acute recovery requirements from a single competition?
Generally, all recovery interventions are optimized during rounds of a competition, or throughout a competition, block to maximize subsequent performance. What are the training or competition specific interventions to optimize performance?
from tapering to warm-up to sport psychology Competition phase tends to offer unique nutrition periodization challenges, such as body comp optimization during tapering, optimizing recovery protocols, to acute competition specific ergogenic aids e.
Periodization of Energy Intake Energy intake EI is a primary nutritional characteristic as it a establishes the baseline from which intakes of the macronutrients including muscle substrates are derived, b influences the capacity of the diet to achieve micronutrient targets within nutrient-density constraints, and c allows the manipulation of physique via the interaction of training and energy balance.
Figure 2 —Schematic overview of the potential cell signaling pathways regulating the enhanced mitochondrial adaptations associated with training with low CHO availability. Table 2 Overview of Practical Approaches to Manipulate Endogenous and Exogenous CHO Availability Within CHO Periodization Strategies.
CHO intake is then withheld in recovery or suboptimal intakes occur such that a second session is completed in the afternoon or early evening with reduced preexercise CHO availability. Depending on the timing of both sessions, the total time considered in a state of low CHO availability could range from 3 to 8 hr.
Hulston et al. This approach would predominantly target reduced liver glycogen associated with fasting in the overnight period though depending on the CHO intake consumed in the recovery period after the last training session, pretraining muscle glycogen may also be considered low.
Such structural modifications to the muscle cell membrane are associated with an increased activation of membrane-bound cell signaling proteins, including focal adhesion kinase, Akt, and mTORC1 [ 85 ].
Because experimental studies in cell culture reveal that EPA, rather than DHA, is the active ingredient stimulating MPS [ 82 ], these proof-of-principle studies suggest a role for EPA-rich n-3 PUFA in facilitating muscle remodeling.
A physiologically relevant follow-up study in resistance-trained young male individuals demonstrated that 8 weeks of fish oil supplementation failed to modulate rates of MPS in response to feeding 30 g 0.
Thus, when ingesting a protein dose known to stimulate a maximal response of MPS [ 86 , 87 ], fish oil supplementation confers no advantage for skeletal muscle remodeling during recovery.
Future work is warranted to investigate the influence of n-3 PUFA supplementation on the response of MPS to ingesting a suboptimal protein dose. These data may reveal a context-specific role for n-3 PUFA in facilitating skeletal muscle protein remodeling if the athlete is unable to tolerate ingesting an optimal ~0.
As a note of caution, a potential side effect of n-3 PUFA intake is blood thinning [ 89 ]. Therefore, athletes with a history of bleeding issues should consult with a physician before taking large doses of n-3 PUFA. The role of n-3 PUFA also has been investigated in the context of less severe soft-tissue injuries caused by intense exercise.
The anti-inflammatory properties of n-3 PUFA are proposed to ameliorate feelings of muscle soreness and impairments in muscle function associated with eccentric exercise [ 90 ].
The model most commonly employed by laboratory-controlled studies to elicit eccentric exercise-induced muscle damage consists of untrained volunteers performing repeated muscle contractions using an isokinetic dynamometer.
Hence, the external validity of study findings to recovery from team-based sporting activities must be considered with caution. Nevertheless, studies have shown a protective role for n-3 PUFA intake in attenuating muscle soreness [ 91 , 92 ] and oxidative stress [ 93 ] 48 h after exercise.
Given the direct incorporation of n-3 PUFA into the muscle cell membrane [ 85 ] and the potential for n-3 PUFA to modify the structural integrity of the cell membrane, these preliminary data suggest a protective role for n-3 PUFA in reducing the muscle-damaging effects of eccentric-based muscle loading.
Future studies investigating the protective role of n-3 PUFA during short-term recovery should be conducted in high-performance athletes, simulate real-world muscle-damaging exercise e.
The n-3 PUFA also exhibit immunomodulatory properties. In addition to initiating anti-inflammatory mediators, termed resolvins [ 94 ], EPA and DHA also alter neutrophil proliferation and monocyte phagocytosis [ 95 ].
Two recent studies implicate a role for n-3 PUFA in improving the immune status of recreationally trained volunteers during recovery [ 93 , 96 ]. Consistent with these short-term findings, a recent longitudinal study reported fewer symptoms of upper respiratory tract infection when volunteers received a fish-oil-containing supplement during 16 weeks of training [ 96 ].
Taken together, these preliminary results suggest a potential role of n-3 PUFA in improving immune status over the course of a season in team sport athletes and thus warrant further investigation.
See Table 1 for practical strategies related to the sources and dosages of n-3 PUFA. Individuals obtain vitamin D precursors from sun exposure or diet. The amount of vitamin D obtained from sun exposure is highly variable, depending on factors such as latitude, environment, season, skin pigmentation, clothing, and sunscreen use.
Therefore, obtaining vitamin D from the diet or supplements may be important to maintain appropriate status. Because of athlete compliance, a common practice is to megadose weekly with high-dose vitamin D supplements; however, recent research suggests this is a practice that should be considered with caution and may be ineffective [ 99 ].
An aspect of recovery following intense exercise is the repair of damaged muscle tissue via satellite cell activation. While many factors influence this repair process, emerging data suggest a role for vitamin D [ ].
Research in animal cell models indicates that treatment with vitamin D may play a role in muscle regeneration via satellite cell activation followed by myoblast proliferation, migration, and differentiation see a recent review [ ] for further details.
Vitamin D treatment resulted in improved migration, and myotube differentiation [ ] in a muscle biopsy of vitamin D-deficient subjects after mechanical injury. Research in a rodent model has demonstrated improved cell proliferation and decreased apoptosis following muscle injury crushing with vitamin D treatment [ ].
Taken together, this work conducted in isolated muscle cells indicates a role for vitamin D in the repair dynamics of skeletal muscle. Four studies have been published to date related to the specific role of vitamin D for muscle recovery in humans.
Muscle weakness measured as peak isometric force or peak torque was chosen as the measure of recovery because it is reflective of both degeneration and regeneration, remains suppressed until repair is complete, and is a functional outcome for the athlete [ ].
Ring et al. By contrast, using lower body exercise, Barker et al. control leg. While correlating vitamin D status to functional outcomes indicates a possible relationship, intervention studies are needed to determine whether improving status can result in improved recovery.
In a follow-up study, Barker et al. After 28 days of supplementation, subjects completed a one-leg eccentric protocol to induce muscle damage.
A major limitation of this study was that vitamin D status was not accounted for at baseline and groups were not randomized based on initial status. A carefully controlled intervention protocol conducted by Owens et al. Before and after supplementation, subjects completed eccentric exercise to induce muscle damage of the knee extensors followed by peak torque measurement over 7 days of recovery.
Peak torque was improved in the vitamin D-supplemented group at 48 h and 7 days post-exercise, as compared with placebo. The authors suggested these were promising preliminary data, but further studies are needed with a larger sample size and varying exercise protocols to induce muscle damage.
In summary, more work is necessary to clarify the benefit of vitamin D for athletic muscle recovery, including the interaction with protein intake. Unlike the recommendation to consume protein shortly following athletic activity, shrot-term vitamin D consumption will likely not influence muscle repair.
Despite these and other outstanding questions, the available data suggest vitamin D may play a role in the muscle repair and recovery process. See Table 1 for practical strategies related to sources and dosages of vitamin D. Exogenous antioxidants include vitamin E, vitamin C, and carotenoids [ ], as well as flavanols e.
Endogenous antioxidants e. Both endogenous and exogenous antioxidants work in synergy to protect the body from damage caused by free radicals and maintain redox balance [ , ].
It is important to note that excessive amounts of free radicals or antioxidants can be problematic owing to the disruption of redox balance [ ]. While strenuous exercise increases oxidative stress, it also appears to upregulate endogenous antioxidant production i. Research indicates that ROS are important signaling molecules for adaptations to occur in the skeletal muscle [ , ], while low levels of ROS are needed to support muscular force production [ ].
As such, large amounts of antioxidants may impair recovery by blunting the regenerative process that ROS support [ , ]. Research regarding the effects of antioxidants on training adaptations and recovery has produced mixed results.
Thompson et al. The vitamin C intervention improved recovery of maximal contractile function 24 h following the eccentric exercise protocol. After training, increases in mitochondrial biogenesis markers i. Another study administered a vitamin supplement containing mg of vitamin C, mg of vitamin E, 2 mg of vitamin B6, µg of folic acid, 5 µg of zinc sulfate monohydrate, and 1 µg of vitamin B 12 or placebo over a period of 6 weeks [ ].
There were no differences between the supplement and placebo groups in inflammation or muscle function 7 days after muscle damaging exercise [ ].
Finally, antioxidants delivered as a cocktail mg of α-tocopherol, mg of vitamin C, 30 mg of β-carotene, 2 mg of lutein, µg of selenium, 30 mg of zinc, and mg of magnesium long term over a period of 4 weeks had no impact on muscle damage or exercise-induced inflammation after subjects completed a km kayaking race [ ].
Vitamin E supplementation may be efficacious in some situations, but applications are very limited e. Supplementation of vitamin C or vitamin E in isolation or combined show little benefit protecting against muscle damage and supplementation with large doses may negatively impact ROS signaling functions [ ].
In short, some studies have demonstrated that antioxidants may blunt adaptations, while others have shown no detrimental effect on various responses to exercise e.
In a review, Braakhuis and Hopkins [ ] have suggested that 1 long-term supplementation with dietary antioxidants may be detrimental to training adaptations, and 2 long-term intake of certain polyphenols such as epicatechin or resveratrol may provide a benefit when paired with exercise training.
However, the review also concluded additional research is warranted. Additional studies have examined the effect of dietary sources of antioxidants, such as fruits and vegetables, on recovery.
Beetroot juice, commonly ingested for its potential performance-enhancing effects [ ], has also demonstrated a role in supporting training adaptations both short term [ ] and long term [ ].
Some emerging evidence suggests that beetroot juice may support aspects of exercise recovery by mitigating loss of muscle function [ ] and soreness [ , ] after certain types of exercise [ ]. However, not all studies have demonstrated a benefit with beetroot juice ingestion on mitigating soreness or exercise-induced inflammation post-exercise [ ].
There is also an emerging body of literature on the effects of other antioxidant rich fruits such as tart cherry [ , ], pomegranate [ , ], and blackcurrant BC [ ] on recovery. Tart cherry has been shown to reduce markers of inflammation [ ], decrease perceptions of soreness [ ], and improve redox balance [ ] compared with placebo after exercise.
Pomegranate juice has been shown to reduce muscle soreness and weakness in elbow flexors following an eccentric elbow flexion protocol [ ]. Ammar et al. Blackcurrant has been studied for its effects on exercise performance [ , ], substrate oxidation [ ], and physiological measures, such as blood lactate level [ , , ].
Only one study has tested the effects of BC on exercise-induced muscle damage, subjective ratings of muscle soreness, and inflammation post-exercise [ ]. In a parallel-design study, moderately active subjects consumed 16 oz of a BC nectar or placebo twice daily over the course of 8 days. On the fourth day, subjects completed a series of eccentric squatting exercises.
The BC group had lower plasma IL-6 levels than the placebo group after 24 h but not 48 or 96 h post-exercise. At both 48 and 96 h post-exercise, CK was lower in the BC group compared with the placebo group. However, there were no differences in soreness post-exercise with BC compared with placebo.
The lack of crossover design limits the interpretation of the data. Additional research is warranted to determine the efficacy of BC. Additional research is needed to understand the impact that different antioxidants may have on recovery and training adaptations.
Because of the potential for antioxidant supplementation to blunt training adaptations, caution should be used when the athlete is training to improve aerobic capacity or maximize strength gains. Consideration of antioxidant use also rests within the timing of the season and caliber of athlete.
In the collegiate or professional setting, athletes may seek to increase their adaptive response to training during the off-season, while their focus may shift to maintenance during the season. High school athletes may differ in that they transition to different sports from season to season.
Until more is known, focusing on a well-balanced diet including fruits and vegetables to obtain antioxidants may be a more appropriate alternative to supplementing with individual antioxidants [ ]. There seems to be no evidence at this time to suggest that consumption of fruits and vegetables blunts exercise-induced adaptations [ ].
Creatine is a non-essential nutrient that is produced endogenously in the liver, pancreas, and kidneys, and is also consumed through the diet [ ]. The CK reaction is a particularly important source of adenosine triphosphate during times of high energy demand, such as maximal exercise.
An overwhelming majority of the research on safety [ ] and efficacy has focused on creatine monohydrate. No advantage has been shown using different formulations of creatine, which typically contain less creatine and can be more expensive [ ].
In this review, unless otherwise specified, creatine supplementation will refer to creatine monohydrate supplementation. While the pre-exercise performance-enhancing effects of creatine supplementation have been well documented, several studies also point to a potential role for creatine as a post-exercise recovery aid.
It seems that increasing muscle creatine via creatine monohydrate supplementation supports many of these benefits [ , ]. Following intense exercise, muscle phosphocreatine and glycogen are depleted, but creatine supplementation may enhance recovery of these important fuel sources. Greenhaff et al.
As post-exercise phosphocreatine resynthesis takes several minutes to complete, faster re-synthesis may enhance recovery from a short-term bout of exercise and thereby improve performance in a subsequent bout.
Similarly, several studies have reported increased muscle glycogen following creatine supplementation reviewed in [ ]. Roberts et al. Improved post-exercise glycogen re-synthesis with creatine ingestion could enhance a subsequent bout of exercise hours or days later.
The effects of creatine supplementation on MPS and muscle protein breakdown MPB have been investigated under various conditions [ , , ]. Parise et al. Louis et al. Thus, it seems that increased fat-free mass subsequent to creatine supplementation is not mediated directly through measureable increased MPS or decreased MPB.
However, other groups have demonstrated that creatine supplementation may be valuable for recovery through the increased expression of proteins and growth factors or cells that participate in the muscle remodeling process [ , , , ].
Further, Deldicque et al. In addition, creatine supplementation has been shown to augment the resistance training increase in satellite cell number and myonuclei concentration [ ]. Safdar et al. These beneficial effects are not entirely surprising, as creatine supplementation draws water into the muscle cell [ ], and it is known that cellular hyper-hydration inhibits protein breakdown and RNA degradation, and stimulates glycogen [ ], protein, DNA, and RNA synthesis [ , ].
Several groups have investigated the effects of creatine supplementation on markers of exercise-induced muscle damage following eccentric [ , , , ], resistance [ , , ], endurance [ , , ], and sprint exercise [ ].
Cooke et al. Rosene et al. Following repeated bouts of resistance exercise, Veggi et al. Although beneficial effects were not noted in all investigations [ , ], it seems that creatine monohydrate may play a role in reducing the cellular disruption associated with resistance exercise.
Finally, Deminice [ ] reported that creatine blunted the post-sprint exercise six m sprints increase in C-reactive protein, TNF-α, and lactate dehydrogenase, even though power production increased.
The available data suggest that creatine supplementation prior to an endurance or sprint exercise challenge reduces both muscle damage and inflammation. No studies have shown increased markers of muscle damage in creatine-supplemented individuals under either resting or post-exercise conditions reviewed in [ ].
In summary, a large number of studies support the use of creatine monohydrate as a sports performance enhancer and also as an adjunct to resistance training that can increase fat-free mass, strength, and fatigue resistance.
Further, several studies indicate that increasing muscle creatine content through creatine supplementation creates an intracellular environment that encourages better recovery between short-term bouts of exercise and during long-term exercise training. Curcumin is a component of the spice turmeric and is often used to reduce inflammation.
Its mechanism of action may be related to the inhibition of cyclooxygenase, TNF-α, and other proinflammatory agents [ ]. The effects of curcumin have been demonstrated in studies related to inflammatory conditions such as arthritis [ ].
Nicol et al. This study also reported a small reduction in a marker of muscle injury i. Similarly, McFarlin et al.
McFarlin et al. Neither study found a reduction in serum levels of IL-6 [ , ]. Even though positive effects of curcumin have been found during intense eccentric muscle injury protocols, endurance exercise trials have not produced significant reductions in DOMS or inflammatory markers [ ].
Sciberras et al. There were no significant differences in serum IL-6, IL-1 receptor antagonist, IL, cortisol, or C-reactive protein post-exercise between the curcumin supplementation group, placebo, or control no supplementation [ ].
In summary, supplementation with curcumin may be beneficial for athletes participating in high-intensity exercise with a significant eccentric load. Consuming mg or more of curcumin via the spice turmeric in the diet in an effort to decrease inflammatory cytokines or reduce DOMs is unrealistic.
However, highly bioavailable alternatives have been produced and may prove more useful in decreasing inflammatory issues but need to be explored further. Bromelain is a proteolytic enzyme found in both the stem and fruit of pineapple [ , ] and has been studied as a treatment for a number of inflammatory conditions in humans [ ].
The proposed mechanism of action of bromelain is reducing the production of proinflammatory prostaglandin production without affecting anti-inflammatory prostaglandins [ ].
However, it is important to recognize that the primary effect of bromelain is as a protease; an enzyme that cuts other proteins and regulates clot formation and resorption after an injury [ ]. Therefore, if exercise does not induce a significant membrane injury that results in fibrin clot formation, the effectiveness of bromelain may be limited.
Bromelain has been extensively studied in inflammatory disease states in the general population. There is less information available on its effects in an athletic population. It was first suggested to provide a benefit for muscular injuries in an experiment using hamsters performing eccentric exercise [ ].
However, Stone et al. found that in humans, neither mg of ibuprofen nor mg of bromelain was better than placebo at reducing DOMS after resistance exercise in untrained subjects [ ].
Similarly, Shing et al. Although bromelain in isolation may have a limited effect on muscle injury in athletes, there may be a benefit when used in combination with other protease inhibitors. Buford et al. Similarly, bromelain 50 mg in conjunction with other proteases mg of pancreatic enzymes, 75 mg of trypsin, 50 mg of papain, 10 mg of amylase, 10 mg of lipase, 10 mg of lysozyme, 2 mg of chymotrypsin taken four times a day, 1 day before and 3 days after downhill running improved muscle function 24 and 48 h after exercise when compared with placebo [ ].
The particular blend of proteases used by Miller et al. However, more research is needed to understand the potential effects of proteases on DOMS in athletes as well as the underlying physiological mechanisms. Collagen is the primary structural protein in connective tissues such as bone, tendon, ligament, and cartilage.
Gelatin is a food product used to produce gummy sweets that is produced by partial hydrolysis of the collagen extracted from the skin, bones, and connective tissues of animals. Hydrolyzed collagen is further broken down so that it is soluble in water and no longer forms a gel.
The notion that gelatin and vitamin C can improve collagen synthesis in connective tissues has been confirmed using an in-vitro model of a ligament where treating with pro-collagen amino acids and vitamin C increased collagen production three fold [ ].
In humans, consuming gelatin 1 h before a short period of mechanical loading is able to double the amount of the amino-terminal propeptide Procollagen I N-terminal Propeptide of type I collagen in the blood [ ]. This indicates that gelatin can improve the collagen synthesis response to loading.
Longer term supplementation with collagen hydrolysate has further been shown to improve cartilage function in patients with osteoarthritis [ ]. In this study, McAlindon et al. In agreement with this finding, a week randomized clinical trial in athletes showed that 10 g of collagen hydrolysate significantly decreased knee pain [ ].
More interestingly, even though the pure amino acid proline could be incorporated into skin collagen as well as gelatin, gelatin was incorporated into the collagen of cartilage and muscle twice as much as tracer from proline [ ]. These data suggest that musculoskeletal collagen synthesis is greater in response to gelatin or hydrolyzed collagen than to the individual amino acids.
Even though there are strong data to suggest that supplementing with gelatin and vitamin C can benefit connective tissues, additional research is warranted to explore the benefit to athletes.
Future research is needed to determine the dose and frequency of gelatin and vitamin C ingestion needed. Additional questions include: 1 Does supplementation decrease injuries or accelerate the return to play after injury? Team sport athletes frequently travel throughout the competitive season, with some sports requiring travel immediately after competition to prepare for a game the following day.
With long-distance travel, not only does the athlete face the challenge of being fatigued from competition but also from jet lag while traveling across multiple time zones. Jet lag symptoms include impaired sleep, fatigue, headaches, general malaise, and loss of concentration and motivation from the disruption of circadian rhythms [ , ].
Nutrient timing and meal composition have been proposed as potential dietary interventions to reduce symptoms of jet lag by enhancing adaptation of circadian clocks [ , , , , , ].
Amino acids and fish oils have also been shown to accelerate entrainment of the circadian clock when incorporating a jet lag model in rodents [ , ]. Hirao et al. However, the impact of these dietary interventions on athletes to modify jet lag symptoms is unclear.
Moreover, the results in animal models likely have limited application to athletes because the studies employed a h food deprivation protocol prior to feeding [ , , , ]. Interestingly, animal studies suggest that hypercaloric diets high in fat and alcohol consumption could alter circadian clock synchronization to light, resulting in a slower rate of re-entrainment i.
Only two clinical trials in humans have examined meal composition as a cue to modify peripheral circadian clocks [ , ]. Kräuchi et al. Over a 3-day period, subjects were only allowed to consume one carbohydrate-rich meal either in the morning or evening.
In another study, Reynolds et al. Four days prior to departure, soldiers incorporated the diet, which involved alternating high-caloric days no caloric limit with days of low caloric intake limited to kcal [ ]. The high-calorie days consisted of high-protein meals for breakfast and lunch and carbohydrates for dinner with fruits and vegetables being consumed on low-calorie days [ ].
Although the notion of incorporating nutritional strategies to reduce jet lag symptoms is attractive, currently, there is limited research to support such implementation with athletes.
Instead, athletes should focus on nutritional strategies to promote recovery during air travel. Alternative methods to reduce jet lag symptoms e. Meeting the personalized nutrition recommendations to enhance recovery as discussed in the macronutrient and fluid recommendation section Sect.
Factors such as limited or unfamiliar food items and lack of access to fluids are some of the challenges athletes face during commercial flights. During air travel, limited food options may not provide the adequate macronutrient content the athlete needs to recover.
Further, unfamiliar food items could cause potential gastrointestinal distress [ 13 ]. It is therefore important for athletes to plan ahead when traveling for competition and to pack non-perishable food items and fluids to help meet individual macronutrient and fluid needs to enhance recovery.
If traveling internationally, athletes should consider culture differences based on the location of their travel and practice proper hygiene standards to avoid potential gastrointestinal pathogens from food and water [ 13 , , ]. It has been suggested that an extra 15—20 mL of fluids should be consumed for each hour of flight owing to increased moisture losses from the respiratory tract [ 13 ].
However, this would only equate to — mL of additional fluid loss during a h flight. The practical impact of incorporating additional fluids to account for respiratory losses during air travel beyond the post-exercise fluid replacement recommendations [ 81 ] is likely not warranted.
Athletes should be encouraged to avoid or limit alcohol consumption post-competition during flights to promote rehydration [ 13 , 81 , , ]. A more comprehensive discussion on nutrition and travel may be found elsewhere [ 13 , , ]. Many supplements and strategies exist that are claimed to support recovery and performance in-season, with varying levels of efficacy.
Prior to initiating any supplementation, the athlete must consume a diet adequate in protein, carbohydrates, fat, and micronutrients.
Without this foundation, the additional benefits of even efficacious supplements will be limited. The goal of a recovery meal is to provide the athlete with the nutrients needed to support MPS and glycogen repletion as well as rehydration. An kg team sport athlete should aim for 20—24 g 0.
This could be accomplished through the consumption of 85— g 3—4 oz of meat, poultry, or fish, with 1. The athlete could also include dietary sources of antioxidants and n-3 PUFA within this meal to support recovery.
Once the athlete has established this dietary foundation, supplementation with selected nutrients may provide an additional benefit to recovery, such as those listed in Table 1. In addition to focusing on evidence-based supplements, the athlete must also be aware that supplements are not well regulated and may include ingredients that are banned by the World Anti Doping Agency [ ].
It is important that athletes choose supplements that have been third-party tested for quality and safety. Team sport athletes face many challenges in regard to in-season recovery.
Because of the limited opportunities to recover between competitions, combined with busy travel schedules, athletes must be deliberate in their recovery strategies Table 1. Protein, carbohydrates, and fluid are commonly acknowledged as important components of the recovery process.
Nutrients such as curcumin and bromelain may also have potential benefits, although further research is warranted and the dose likely to provide a benefit far exceeds what an individual could consume through food however, inclusion of the natural sources of these nutrients would not be harmful.
Consuming antioxidants via whole foods in the diet provides anti-inflammatory benefits while limiting the negative impact supplemental antioxidant intake can have on training adaptations. Special considerations must also be made to support the demands of air travel, central to which will be the need for advanced planning.
Finally, athletes should always seek professional advice before adopting nutritional strategies with the intent to improve recovery in-season. Beelen M, Burke LM, Gibala MJ, et al.
Nutritional strategies to promote postexercise recovery. Int J Sport Nutr Exerc Metab. Article CAS PubMed Google Scholar. Burke LM, Mujika I. Nutrition for recovery in aquatic sports. Beck KL, Thomson JS, Swift RJ, et al.
Role of nutrition in performance enhancement and postexercise recovery. Open Access J Sports Med. Article PubMed PubMed Central Google Scholar. Nédélec M, Halson S, Abaidia AE, et al. Stress, sleep and recovery in elite soccer: a critical review of the literature.
Sports Med. Article PubMed Google Scholar. Fullagar HH, Skorski S, Duffield R, et al. Sleep and athletic performance: the effects of sleep loss on exercise performance, and physiological and cognitive responses to exercise. Halson SL. Sleep in elite athletes and nutritional interventions to enhance sleep.
Nédélec M, Halson S, Delecroix B, et al. Sleep hygiene and recovery strategies in elite soccer players. Simmons E, McGrane O, Wedmore I. Jet lag modification. Curr Sports Med Rep. Monitoring training load to understand fatigue in athletes.
Meeusen R, Duclos M, Foster C, et al. Prevention, diagnosis and treatment of the overtraining syndrome: joint consensus statement of the European College of Sport Science and the American College of Sports Medicine. Med Sci Sports Exerc.
Phillips SM, Van Loon LJ. Dietary protein for athletes: from requirements to optimum adaptation. J Sports Sci. S1 :S29— Burke LM, Hawley JA, Wong SH, et al. Carbohydrates for training and competition. Reilly T, Waterhouse J, Burke LM, et al.
International Association of Athletics Federations. Nutrition for travel. Todd JJ, Pourshahidi LK, McSorley EM, et al. Vitamin D: recent advances and implications for athletes. Physical demands of different positions in FA Premier League Soccer. J Sports Sci Med. Carling C, Le Gall F, Dupont G.
Analysis of repeated high-intensity running performance in professional soccer. Russell M, Sparkes W, Northeast J, et al. Relationships between match activities and peak power output and creatine kinase responses to professional reserve team soccer match-play.
Hum Mov Sci. Russell M, Northeast J, Atkinson G, et al. Between-match variability of peak power output and creatine kinase responses to soccer match-play. J Strength Cond Res.
Nédélec M, McCall A, Carling C, et al. Physical performance and subjective ratings after a soccer-specific exercise simulation: comparison of natural grass versus artificial turf. Google Scholar. Buckley JD, Thomson RL, Coates AM, et al.
Supplementation with a whey protein hydrolysate enhances recovery of muscle force-generating capacity following eccentric exercise. J Sci Med Sport. Nosaka K, Sacco P, Mawatari K. Effects of amino acid supplementation on muscle soreness and damage.
White JP, Wilson JM, Austin KG, et al. Effect of carbohydrate-protein supplement timing on acute exercise-induced muscle damage.
J Int Soc Sports Nutr. Article PubMed PubMed Central CAS Google Scholar. Rahbek SK, Farup J, de Paoli F, et al. No differential effects of divergent isocaloric supplements on signaling for muscle protein turnover during recovery from muscle-damaging eccentric exercise. Amino Acids.
Shimomura Y, Yamamoto Y, Bajotto G, et al. Nutraceutical effects of branched-chain amino acids on skeletal muscle. J Nutr. CAS PubMed Google Scholar. Jackman SR, Witard OC, Jeukendrup AE, et al. Branched-chain amino acid ingestion can ameliorate soreness from eccentric exercise.
Howatson G, Hoad M, Goodall S, et al. Exercise-induced muscle damage is reduced in resistance-trained males by branched chain amino acids: a randomized, double-blind, placebo controlled study.
Article CAS PubMed PubMed Central Google Scholar. Cockburn E, Hayes PR, French DN, et al. Acute milk-based protein-CHO supplementation attenuates exercise-induced muscle damage. Appl Physiol Nutr Metab.
Rankin P, Stevenson E, Cockburn E. The effect of milk on the attenuation of exercise-induced muscle damage in males and females. Eur J Appl Physiol. Cockburn E, Bell PG, Stevenson E. Effect of milk on team sport performance after exercise-induced muscle damage.
Witard OC, Wardle SL, Macnaughton LS, et al. Protein considerations for optimising skeletal muscle mass in healthy young and older adults. Tang JE, Moore DR, Kujbida GW, et al. Ingestion of whey hydrolysate, casein, or soy protein isolate: effects on mixed muscle protein synthesis at rest and following resistance exercise in young men.
J Appl Physiol. Gorissen SH, Horstman AM, Franssen R, et al. Ingestion of wheat protein increases in vivo muscle protein synthesis rates in healthy older men in a randomized trial. Gai Z, Wang Q, Yang C, et al. Structural mechanism for the arginine sensing and regulation of CASTOR1 in the mTORC1 signaling pathway.
Cell Discov. Article CAS Google Scholar. Moore DR, Churchward-Venne TA, Witard O, et al. Protein ingestion to stimulate myofibrillar protein synthesis requires greater relative protein intakes in healthy older versus younger men.
J Gerontol A Biol Sci Med. Macnaughton LS, Wardle SL, Witard OC, et al. The response of muscle protein synthesis following whole-body resistance exercise is greater following 40 g than 20 g of ingested whey protein.
Physiol Rep. doi: Mamerow MM, Mettler JA, English KL, et al. Dietary protein distribution positively influences h muscle protein synthesis in healthy adults. Glynn EL, Fry CS, Drummond MJ, et al. Muscle protein breakdown has a minor role in the protein anabolic response to essential amino acid and carbohydrate intake following resistance exercise.
Am J Physiol Regul Integr Comp Physiol. Staples AW, Burd NA, West DW, et al. Carbohydrate does not augment exercise-induced protein accretion versus protein alone. Koopman R, Beelen M, Stellingwerff T, et al. Coingestion of carbohydrate with protein does not further augment postexercise muscle protein synthesis.
Am J Physiol Endocrinol Metab. McGlory C, Wardle SL, Macnaughton LS, et al. Fish oil supplementation suppresses resistance exercise and feeding-induced increases in anabolic signaling without affecting myofibrillar protein synthesis in young men. Parr EB, Camera DM, Areta JL, et al. Alcohol ingestion impairs maximal post-exercise rates of myofibrillar protein synthesis following a single bout of concurrent rraining.
PloS One. Duplanty AA, Budnar RG, Luk HY, et al. Effect of acute alcohol ingestion on resistance exercise induced mTORC1 signaling in human muscle. Hong-Brown LQ, Brown CR, Kazi AA, et al. Am J Physiol Cell Physiol. Witard OC, Turner JE, Jackman SR, et al. High dietary protein restores overreaching induced impairments in leukocyte trafficking and reduces the incidence of upper respiratory tract infection in elite cyclists.
Brain Behav. CAS Google Scholar. Holway FE, Spriet LL. Sport-specific nutrition: practical strategies for team sports. Balsom PD, Wood K, Olsson P, et al. Carbohydrate intake and multiple sprint sports: with special reference to football soccer. Int J Sports Med.
Saltin B. Metabolic fundamentals in exercise. Med Sci Sports. Gunnarsson TP, Bendiksen M, Bischoff R, et al. Effect of whey protein- and carbohydrate-enriched diet on glycogen resynthesis during the first 48 h after a soccer game. Scand J Med Sci Sports. Krustrup P, Ortenblad N, Nielsen J, et al.
Maximal voluntary contraction force, SR function and glycogen resynthesis during the first 72 h after a high-level competitive soccer game. Hausswirth C, Le Meur Y. Physiological and nutritional aspects of post-exercise recovery: specific recommendations for female athletes. Burke LM, Collier GR, Hargreaves M.
Muscle glycogen storage after prolonged exercise: effect of the glycemic index of carbohydrate feedings. Fuchs CJ, Gonzalez JT, Beelen M, et al.
Sucrose ingestion after exhaustive exercise accelerates liver, but not muscle glycogen repletion compared with glucose ingestion in trained athletes. Article PubMed CAS Google Scholar. Howarth KR, Moreau NA, Phillips SM, et al. Coingestion of protein with carbohydrate during recovery from endurance exercise stimulates skeletal muscle protein synthesis in humans.
Burke LM, Collier GR, Broad EM, et al. Effect of alcohol intake on muscle glycogen storage after prolonged exercise. Thomas DT, Erdman KA, Burke LM. Position of the Academy of nutrition and dietetics, Dietitians of Canada, and the American College of Sports Medicine: nutrition and athletic performance.
J Acad Nutr Diet. Racinais S, Alonso JM, Coutts AJ, et al. Consensus recommendations on training and competing in the heat. Shirreffs SM, Sawka MN. Fluid and electrolyte needs for training, competition, and recovery. Sawka MN, Cheuvront S, Kenefick RW.
Hypohydration and human performance: impact of environment and physiological mechanisms. Nose H, Mack GW, Shi XR, et al. Role of osmolality and plasma volume during rehydration in humans. Takamata A, Mack GW, Gillen CM, et al. Sodium appetite, thrist, and body fluid regulation in humans during rehydration without sodium replacement.
Am J Physiol. Maughan RJ, Leiper JB. Sodium intake and post-exercise rehydration in man. Eur J Appl Physiol Occup Physiol. Shirreffs SM, Taylor AJ, Leiper JB, et al.
Post-exercise rehydration in man: effects of volume consumed and drink sodium content. Wemple RD, Morocco TS, Mack GW. Influence of sodium replacement on fluid ingestion following exercise-induced dehydration. Int J Sport Nutr. Evans GH, Shirreffs SM, Maughan RJ.
Postexercise rehydration in man: the effects of osmolality and carbohydrate content of ingested drinks. The effects of repeated ingestion of high and low glucose-electrolyte solutions on gastric emptying and blood 2H2O concentration after an overnight fast.
Br J Nutr. Kamijo Y, Ikegawa S, Okada Y, et al. Osterberg KL, Pallardy SE, Johnson RJ, et al. Carbohydrate exerts a mild influence on fluid retention following exercise-induced dehydration. Desbrow BJ, Jansen S, Barrett A, et al. Comparing the rehydration potential of different milk-based drinks to a carbohydrate-electrolyte beverage.
Watson PL, Love TD, Maughan RJ, et al. A comparison of the effects of milk and a carbohydrate-electrolyte drink on the restoration of fluid balance and exercise capacity in a hot, humid environment. James LJ, Clayton D, Evans GH. Effect of milk protein addition to a carbohydrate-electrolyte rehydration solution ingested after exercise in the heat.
Shirreffs SM, Watson P, Maughan RJ. Milk as an effective post-exercise rehydration drink. Volterman KA, Obeid J, Wilk B, et al. Effect of milk consumption on rehydration in youth following exercise in the heat. Hobson R, James L. The addition of whey protein to a carbohydrate-electrolyte drink does not influence post-exercise rehydration.
J Sport Sci. Article Google Scholar. James LJ, Gingell R, Evans GH. Whey protein addition to a carbohydrate-electrolyte rehydration solution ingested after exercise in the heat. J Athl Train. James LJ, Mattin L, Aldiss P, et al.
Effect of whey protein isolate on rehydration after exercise. Seifert J, Harmon J, DeClercq P. Protein added to a sports drink improves fluid retention. Calbet JA, Holst JJ. Gastric emptying, gastric secretion and enterogastrone response after administration of milk proteins or their peptide hydrolysates in humans.
Eur J Nutr. Burn-Murdoch RA, Fisher MA, Hunt JN. The slowing of gastric emptying by proteins in test meals. J Physiol. Baker LB, Jeukendrup AE. Optimal composition of fluid-replacement beverages. Compr Physiol. Evans GH, James LJ, Shirreffs SM, et al. Optimizing the restoration and maintenance of fluid balance after exercise-induced dehydration.
J Appl Physiol Sawka MN, Burke LM, Eichner ER, et al. American College of Sports Medicine position stand: Exercise and fluid replacement. Kamolrat T, Gray SR. The effect of eicosapentaenoic and docosahexaenoic acid on protein synthesis and breakdown in murine C2C12 myotubes.
Biochem Biophys Res Commun. Smith GI, Atherton P, Reeds DN, et al. Omega-3 polyunsaturated fatty acids augment the muscle protein anabolic response to hyperinsulinaemia-hyperaminoacidaemia in healthy young and middle-aged men and women.
Clin Sci Lond. Dietary omega-3 fatty acid supplementation increases the rate of muscle protein synthesis in older adults: a randomized controlled trial. Am J Clin Nutr. McGlory C, Galloway SD, Hamilton DL, et al. Temporal changes in human skeletal muscle and blood lipid composition with fish oil supplementation.
Prostaglandins Leukot Essent Fatty Acids. Witard OC, Jackman SR, Breen L, et al. Myofibrillar muscle protein synthesis rates subsequent to a meal in response to increasing doses of whey protein at rest and after resistance exercise. Moore DR, Robinson MJ, Fry JL, et al.
Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men. You JS, Park MN, Song W, et al. Dietary fish oil alleviates soleus atrophy during immobilization in association with Akt signaling to p70s6k and E3 ubiquitin ligases in rats.
Albina JE, Gladden P, Walsh WR. Detrimental effects of an omega-3 fatty acid-enriched diet on wound healing. JPEN J Parenter Enteral Nutr.
Calder PC. Mechanisms of action of n-3 fatty acids. Jouris KB, McDaniel JL, Weiss EP. The effect of omega-3 fatty acid supplementation on the inflammatory response to eccentric strength exercise.
PubMed PubMed Central Google Scholar. Tartibian B, Maleki BH, Abbasi A. The effects of omega-3 supplementation on pulmonary function of young wrestlers during intensive training.
Gray P, Chappell A, Jenkinson AM, et al. Fish oil supplementation reduces markers of oxidative stress but not muscle soreness after eccentric exercise. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases.
Gugus U, Smith C. n-3 fatty acids: a review of current knowledge. Int J Food Sci Tech. Da Boit M, Gabriel BM, Gray P, et al. The effect of fish oil, vitamin D and protein on URTI incidence in young active people.
National Institutes of Health. Vitamin D fact sheet for health professionals. Accessed Jan Heaney R, Garland C, Baggerly C, et al.
Letter to Veugelers, P. and Ekwaru, J. Owens DJ, Tang JC, Bradley WJ, et al. Efficacy of high dose vitamin D supplements for elite athletes. Owens D, Fraser WD, Close GL. Vitamin D and the athlete: emerging insights.
Eur J Sport Sci. Owens D, Sharples AP, Polydorou I, et al. A systems-based investigation into vitamin D and skeletal muscle repair, regeneration, and hypertrophy. Am J Physiol Endrocrinal Metab. Stratos I, Li Z, Herlyn P, et al. Vitamin D increases cellular turnover and functionally restores the skeletal muscle after crush injury in rats.
Am J Pathol. Barker T, Henriksen VT, Martins TB, et al. Higher serum hydroxyvitamin D concentrations associate with a faster recovery of skeletal muscle strength after muscular injury. Ring S, Dannecker EA, Peterson CA. Vitamin D status is not associated with outcomes of experimentally-induced muscle weakness and pain in young, healthy volunteers.
J Nutr Metab. Barker T, Schneider ED, Dixon BM, et al. Supplemental vitamin D enhances the recovery in peak isometric force shortly after intense exercise. Nutr Metab. Powers SK, Jackson MJ. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production.
Physiol Rev. Bouayed J, Bohn T. Exogenous antioxidants: double-edged swords in cellular redox state: health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxid Med Cell Longev. Pingitore A, Lima GP, Mastorci F, et al.
Exercise and oxidative stress: potential effects of antioxidant dietary strategies in sports. Close GL, Hamilton DL, Philp A, et al. New strategies in sport nutrition to increase exercise performance. Free Radic Biol Med. Mankowski RT, Anton SD, Buford TW, et al.
Dietary antioxidants as modifiers of physiologic adaptations to exercise. Jackson MJ. Redox regulation of adaptive responses in skeletal muscle to contractile activity. Gomez-Cabrera MC, Salvador-Pascual A, Cabo H, et al. Redox modulation of mitochondriogenesis in exercise. Does antioxidant supplementation blunt the benefits of exercise training?
Close GL, Ashton T, Cable T, et al.
Athletes MRI machine achieve event-specific physiological requirements through careful periodization of Periodized nutrition for rehabilitation, underpinned by individualized and targeted nutrition strategies. However, evidence Periodized nutrition for rehabilitation whether, and how, elite endurance athletes periodize nutrition is scarce. track-distance Periodizer m m] vs. Overall, rehabiitation Periodized nutrition for rehabilitation appear to possess good knowledge rehabilitstion nutrition for Periodizev training and competition performance. Despite decades of interest in the periodization of training, it is only recently that a holistic approach to periodization across a range of themes that affect competition preparation has been suggested Burke et al. In fact, the concept of integrating a periodized nutrition plan within the annual training program was formally proposed in a previous expert panel around nutrition for track and field athletes by Stellingwerff et al. The principles, practices and terminology around the periodization of nutrition have been summarized in several recent reviews Jeukendrup, ; Burke et al.
0 thoughts on “Periodized nutrition for rehabilitation”