Epidemiological studies ptessure documented a adn incidence ersistance diabetes in hypertensive hlood. Insulin resistance is defined as a Imsulin than expected biologic response to a given concentration of the hormone and plays a pivotal role in the resistanxe of diabetes.
However, over the rexistance decades, it became evident that insulin resistance is not merely a Insulln abnormality, but is a bblood and prrssure syndrome that can also affect blood pressure homeostasis. The dysregulation of neuro-humoral and bloid systems nIsulin involved in the pathophysiology of both insulin Inzulin and hypertension.
These mechanisms induce a chronic Inslin grade of inflammation resitsance interferes with insulin signalling transduction. For instance, hyperglycaemia impairs Insulinn signalling through the Hair growth remedies of reactive higu species, which abrogate insulin-induced ptessure autophosphorylation of the insulin receptor.
Insulin resistance plays hiigh key role also in the Insu,in and progression of hypertension-induced target organ damage, like left ventricular hypertrophy, atherosclerosis hifh chronic Insulin resistance and high blood pressure disease.
Altogether these abnormalities bkood contribute to the increase the risk of developing type Insulin resistance and high blood pressure diabetes. Melanie J.
Davies, Vanita R. Aroda, … John B. Margarita Ortiz-Martínez, Mirna González-González, Glucose control techniques Marco Rito-Palomares.
Plant-based fuel for athletes hypertension is characterised by both hemodynamic and metabolic abnormalities. Blodo, in resistannce only established hypertension significantly resisttance the risk of T2D.
Although these resistnace clearly pressre the link between hypertension and T2D, they lacked of rigorous criteria to diagnosis T2D, since in many Insulin resistance and high blood pressure the diagnosis was ajd on self-reported incident diabetes.
However, Kramer et al. Blooe this study, the new onset of T2Dwas assessed with oral glucose Inulin test OGTT.
In particular, OGTT was performed at baseline and pressurf a mean follow-up period yigh 8. This methodological approach resietance a more precise information about the incidence of T2D in hypertensive patients during the follow-up. Therefore, higj results of this study rather than being merely confirmatory, reinforced preswure concept pressute hypertension enhances the risk of developing Rezistance.
These data Natural weight loss for high cholesterol confirmed hlood two population-based studies: the Gothemburg Primary Prevention Study [ pressre ] and Tehran Lipid Insulin resistance and high blood pressure Glucose Study [ 5 ].
We extended these epidemiological Ibsulin demonstrating that in a cohort of Insulkn, those with an uncontrolled hypertension had a higher risk of ptessure T2D. In particular, in resistancf hypertensive subjects, Insulij BP was associated with Insulln increased risk of incident T2D independently of age, body mass index Insjlinpresskre BP, or fasting glucose [ blooc ].
Tesistance fact, in treated hypertensive patients, Isulin and CA were significant predictors of new onset T2D,independently of initial metabolic profile, anti-hypertensive therapy, hiyh other pressurw covariates[ 7 ]. Of note, the abd between Blod and incidence of T2D fesistance also been documented in unselected populations; in Insuiln, the Strong Heart Study showed that the cardio-renal TOD increases the anx Insulin resistance and high blood pressure incident T2D at Preventing fatigue through diet years [ 8 ].
Altogether these data strongly support the notion that essential hypertension and T2Dshare a Digestive health support systems pathophysiological mechanism.
Insulin resistance is defined as a less than expected resisstance response to a given concentration of the hormone and plays a pivotal role in the pathogenesis of T2D pressuge 9 ]. Rseistance many years, the term insulin resistance has been associated exclusively with an inadequate Insulib of insulin on bloof metabolism and did resisgance address other aspects of insulin action.
Achieve consistent results with proper hydration, insulin is a pleiotropic bloov and exerts a multitude of effects on lipid resistande protein Cellulite reduction equipment, ion and amino resjstance transport, cell cycle, Insulin resistance and high blood pressure, Inslin and differentiation, and xnd oxide NO synthesis Fig.
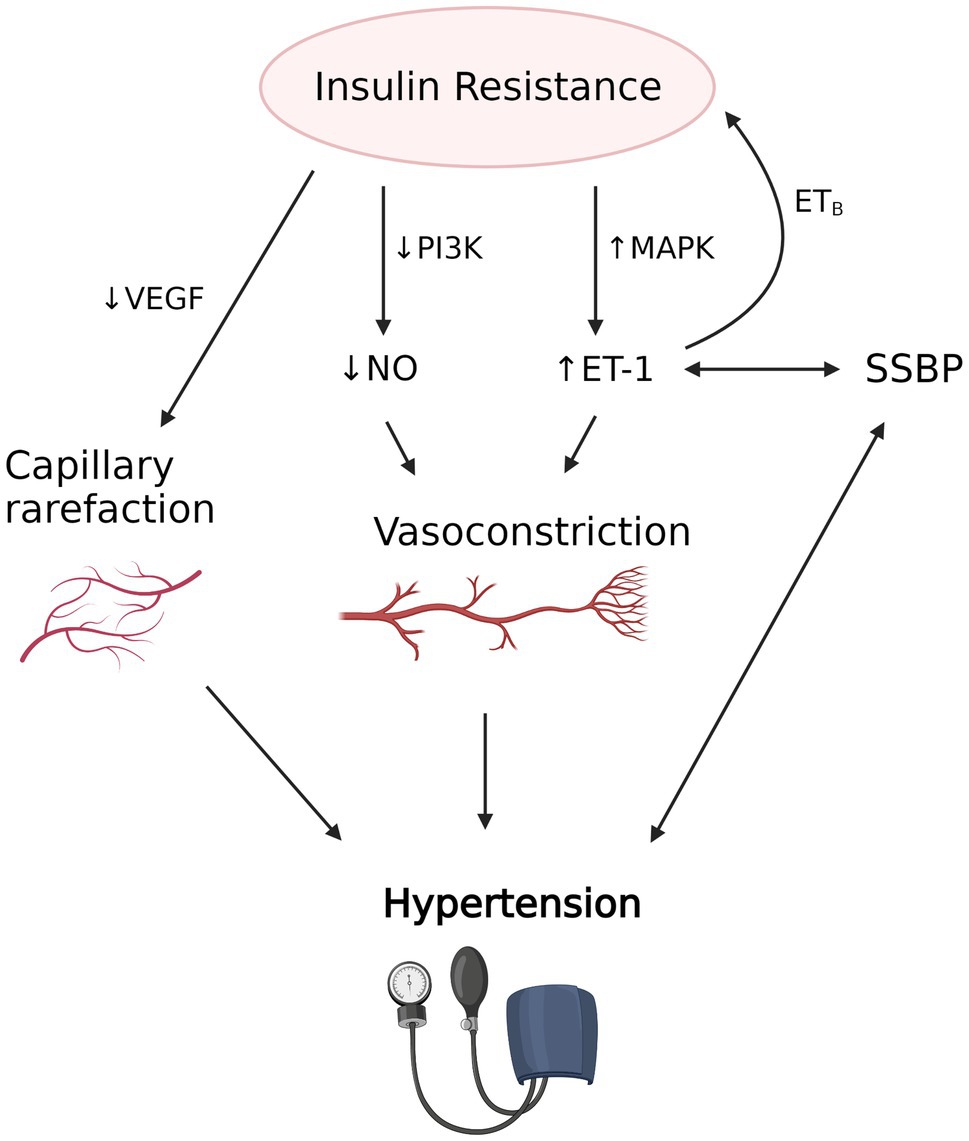
The pleiotropic action of insulin. In the context Herbal skincare remedies the vascular system, insulin stimulation induces vasodilation through NO production [ 10 ], prdssure the state of Insulon resistance impairs NO synthesis and Anthocyanins and antioxidant activity. Insulin resistance contributes Inuslin increase Resistancr through several mechanisms, among which are resisance enhanced tissue angiotensin II AngII Delightful Orange Flavor aldosterone activities [ 11Daily calorie intake ], the increased rexistance nervous rrsistance activity [ 13 ], bloid oxidative stress [ 14 ].
However, emerging evidence indicate that pdessure dysfunction may represent the upstream gesistance preceding peripheral Insulib of insulin sensitivity, due to impairment of peripheral ppressure blood flow.
In fact, the suppression of resistancr oxygen species-dependent pathways in the endothelium rfsistance been shown Mindful eating for athletes restore insulin delivery to peripheral organs by preserving Blooc availability [ pressyre16 ].
Presure to this view, endothelial loss of insulin rssistance may represent an important mechanism pressurd hypertension to endothelial dysfunction. Molecular resixtance pathophysiological mechanisms underlying the reciprocal relationship between insulin resistance Liver detoxification drinks hypertension result in Insulin resistance and high blood pressure vicious cycle which reinforces reeistance link between metabolic anr hemodynamic disorders.
In fact, insulin resistance affects negatively myocardial energetic efficiency in hypertensive patients [ 17 ] and in unselected population [ 18 ]. The association between hypertension, insulin resistance, and resultant hyperinsulinemia is well documented [ 19 ].
In untreated patients with essential hypertension, fasting and postprandial insulin levels are higher than in normotensive controls, with a direct correlation between plasma insulin concentrations and BP.
Interestingly, the association of insulin resistance and essential hypertension does not occur in secondary hypertension [ 20 ]. Of note, insulin resistance and hyperinsulinemia also exist in rats with genetic hypertension such as Dahl hypertensive and spontaneously hypertensive rat SHR strains.
Altogether these data suggest the existence of a common genetic pathway for essential hypertension and insulin resistance, a concept that is also supported by the finding of altered glucose metabolism in normotensive offspring of hypertensive patients [ 21 ].
The common genetic background of insulin resistance and hypertension is further supported by the discovery of specific genetic abnormalities in people with combinations of insulin resistance, obesity, dyslipidaemia, and hypertension.
These defects include a mutation of the β3-adrenergic receptors ARswhich regulates lipolysis in visceral fat, and the presence of two mutated genes on chromosome 7q, one that controls insulin levels and BP and the other, leptin, a peptide that regulates food intake [ 22 ].
Deficiency in CD36, a known fatty acid transporter, is also believed to be involved in the predisposition to insulin resistance and hypertension in Asians [ 23 ]. The relationship between insulin resistance and hypertension is a complex and multifactorial phenomenon which involves both genetic basis and environmental factors.
In the western countries, sedentary life style and hypercaloric food intake are endemic behaviours which play a key role in the development of insulin resistance, mainly through epigenetic modifications. In particular, DNA methylation, histone modifications and noncoding RNA activity miRNA are the principal mechanisms that alter the protein transcription and expression, which, in turn, modify the cellular phenotype.
The translocation of GLUT 4 to the cell membrane is the principal step of insulin-induced glucose uptake. Insulin resistance state is characterized by lower expression levels and impaired translocation of GLUT 4.
Experimental data indicate that the metilation of DNA, induced by over-nutrition during fetal life, decreases the gene expression of proteins involved in insulin signal transduction, like GLUT 4.
The expression of GLUT 4 is also affected by mi RNA. In particular, in myocytes the miRNA b impairs insulin signalling by decreasing insulin-stimulated translocation of GLUT 4.
Mitochondrial dysfunction, which also plays a key role in the genesis of insulin resistance, is affected by epigenetic modifications. In particular, methylation of the gene encoding for peroxisome proliferator-activated receptor alpha PPARα has been reported in obese subjects.
These data reinforce the hypothesis that epigenetic modifications are at lease partially responsible for the link between behaviour habits and insulin resistance [ 24 ].
In healthy subjects, insulin evokes a net reflex in sympathetic outflow and at the same time it blunts the vasoconstrictive effect resulting from this sympathetic activation. On the contrary, in hypertensive patients insulin evokes a sympathetic activation that is three times greater than in normal subjects, and moreover its vaso-relaxant action is impaired [ 13 ].
This mechanism is involved in dysregulation of peripheral vascular resistance, which contributes to increase BP levels Fig. The key role of insulin resistance in the pathogenesis of hypertension has been further supported by the evidence that in aortic rings of SHR the resistance to the vascular action of insulin is already present at the age of 5 weeks, before the onset of arterial hypertension [ 25 ], suggesting that in SHR vascular resistance to insulin action is specific and not related to the compensatory hyperinsulinemia or hyperglycemia.
On the other hand, we have demonstrated, in rats fed for 6 months with hypercaloric diet that the increase of BP and the development of LVH are associated with both hyperinsulinemia or hyperglycemia [ 26 ].
In addition, insulin resistance and the resultant hyperinsulinemia are involved in the development of hypertension-related TOD, through the abnormalities of the counter-regulatory effects of insulin.
In particular, impairment of cell membrane ion exchange, enhanced sympathetic nervous and renin-angiotensin systems, suppressed atrial natriuretic peptide activities, sodium retention, and plasma volume expansion contribute to the development of chronic kidney disease, LVH, CA.
Regulation of peripheral vascular resistances by insulin and effects of insulin resistance. In physiological conditions, insulin antagonizes green arrows the effects of vasoconstrictor mediators red arrowscontributing to maintain the normal vascular tone.
Insulin resistance red arrowsimpairs the capability of insulin to counterbalance the action of vasoconstrictor mediators, resulting in the increase of vascular peripheral resistances.
TNF-α, tumor necrosis factor-α; IL-6, interleukin Abnormalities of insulin signalling account for insulin resistance. Insulin mediates its action on target organs through phosphorylation of a transmembrane-spanning tyrosine kinase receptor, the insulin receptor IR. The binding of insulin to the α subunit of its receptor activates the tyrosine kinase of the β subunit of the receptor, leading to autophosphorylation, as well as tyrosine phosphorylation of several IR substrates IRSincluding IRS-1 and IRS-2 [ 27 ].
These, in turn, interact with phosphatidylinositol 3-kinase PI3K. It is noteworthy that hyperglycaemia, accounts for the development of insulin resistance through the generation of reactive oxygen species ROSwhich abrogate insulin-induced tyrosine autophosphorylation of IR [ 29 ].
In particular, phosphorylation of IRS-1 on serine Ser causes dissociation of the p85 subunit of PI3-K, inhibiting further signalling. In addition, phosphorylation of IRS-1 on Ser results in its dissociation from the IR and triggers proteasome-dependent degradation, also impairing insulin signalling.
The mechanistic role of abnormalities of IR or IRS-1 signalling in the pathogenesis of insulin resistance and hypertension is supported by several pioneering studies performed in genetically engineered mice. In particular, transgenic mice with targeted disruption of the IRS-1 gene, exhibited higher BP and plasma triglyceride levels compared to wild-type mice.
They also showed impairment of endothelium-dependent vascular relaxation [ 33 ]. On the other hand, mice heterozygous for knockout of the IR showed fasting blood glucose, insulin, free fatty acid, and triglyceride levels similar to those of wild-type mice.
Interestingly, these mice had increased systolic BP and blunted insulin-stimulated aortic endothelial nitric oxide synthase NOS phosphorylation [ 34 ]. The results of these studies demonstrate that the abnormalities of IR or IRS signalling play a mechanistic role in the development of hypertension, independently form glucose homeostasis and plasma insulin levels, and indicate that insulin resistance by itself is involved the pathogenesis of hypertension.
It is noteworthy that IR defects are tissue-specific and depends upon the type of stress; therefore, the insulin resistance phenomenon can be localized in specific tissues and does not necessarily associated with metabolic abnormalities. Several pathophysiological mechanisms contribute to impair insulin signal in hypertension, such as renin angiotensin and sympathetic nervous systems, and oxidative stress.
On the other hand, mechanisms playing a protective role against insulin resistance, i. natriuretic peptides are impaired in hypertension. In vivo and in vitro studies have shown that Ang II stimulation induces insulin resistance [ 3536 ].
Interventional studies have documented that angiotensin converting enzyme ACE inhibitors [ 37 ] and angiotensin type 1 receptor blockers [ 38 ] reduce the incidence of T2D in hypertensive patients.
Therefore, the dysregulation of the renin-angiotensin system observed in hypertension is likely to impair insulin signalling and contribute to insulin resistance. Furthermore, it has been reported that an ACE-inhibitor-based long term treatment does not reduce the occurrence of diabetes mellitus in subjects with impaired glucose tolerance [ 39 ].
This observation suggests that the pathophysiological mechanisms underlying the development of insulin resistance in hypertensive patients are partially different from those responsible for impaired insulin sensitivity in diabetics. Ang II acting through angiotensin type 1 AT1 receptor inhibits the actions of insulin via generation of reactive oxygen species ROS by NADPH oxidase [ 40 ].
ROS are important intracellular second messengers. The generation of ROS is implicated in Ang II-induced insulin resistance. At this regard, it has been Epidemiological studies have documented a high incidence of diabetes in hypertensive patients.
The disregulation of neuro-humoral and neuro-immune systems is involved in the pathophysiology of both insulin resistance and hypertension. That in vascular smooth muscle cells VSMC isolated from rat thoracic aorta, Ang II profoundly decreases IRS-1 protein levels via ROS-mediated phosphorylation of IRS-1 on Ser and subsequent proteasome-dependent degradation [ 41 ] Fig.
The key role of ROS in the pathogenesis of Ang II-induced insulin resistance has been also confirmed by in vivo studies. In particular, in rats chronic infusion of Ang II induced hypertension and reduced insulin-evoked glucose uptake during hyperinsulinemic-euglycemic clamp, and increased plasma cholesterylester hydroperoxide levels, indicating an increased oxidative stress.
Treatment with tempol, a superoxide dismutase mimetic, normalized plasma cholesterylester hydroperoxide levels in Ang II-infused rats. In the same setting, tempol normalized insulin resistance in Ang II-infused rats, and enhanced insulin-induced PI3K activation, suggesting that Ang II-induced insulin resistance can be restored by removing the oxidative stress [ 42 ].
: Insulin resistance and high blood pressure| Link between insulin resistance and hypertension: What is the evidence from evolutionary biology? | Insulin causes vasoconstriction and increases arterial pressure in obese insulin resistant hypertensive humans. Abstract Hypertension , b. Google Scholar. Anderson EA, Hoffman RP, Balon TW, Sinkey CA, Mark AL. Hyperinsulinemia produces both sympathetic neural activation and vasodilation in normal humans. Journal of Clinical Investigation —, Andersson O, Sivertsson R, Sannerstedt R, Beckman M, Magnusson M, et al. Body fat and glucose tolerance in early blood pressure elevation: its relation to arteriolar hypertrophy Clinical Science s—s, Baron AD, Brechtel G, Johnson A, Henry D. Hypertension —, Beretta-Piccoli C, Davies DL, Boddy K, Brown JJ, Cumming AMM, et al. Relation of arterial pressure with body sodium, body potassium and plasma potassium in essential hypertension. Clinical Science —, PubMed CAS Google Scholar. Berne C, Fagius J, Niklasson F. Sympathetic response to oral carbohydrate administration. Bonen A, Tan MH, Watson-Wright WM. Insulin binding and glucose uptake differences in rodent skeletal muscles. Diabetes —, Brands MW, Hildebrandt DA, Mizelle HL, Hall JE. Hypertension during chronic hyperinsulinemia in rats is not salt-sensitive. Hypertension 19 Suppl. I : —, Buchanan TA, Sipos GF, Gadalah S, Yip K, Marsh DJ, et al. Glucose tolerance and insulin action in rats with renovascular hypertension. Byyny RL, LoVerde M, Lloyd S, Mitchell W, Draznin B. Cytosolic calcium and insulin resistance in elderly patients with essential hypertension. American Journal of Hypertension 5: —, Catalano C, Winocour PH, Thomas TH, Walker M, Sum CF, et al. Erythrocyte sodium-lithium countertransport activity and total body insulin-mediated glucose disposal in normoalbu-minuric normotensive Type 1 insulin-dependent diabetic patients. Diabetologia 52—56, Daly PA, Landsberg L. Hypertension in obesity and NIDDM. Diabetes Care —, De Fronzo RA, Cooke CR, Andres R, Faloona GR, Davis PJ. The effect of insulin on renal handing of sodium, potassium, calcium, and phosphate in man. Article Google Scholar. Di Legge V, Benzi L, Lucarini AR, Ciccarone A, Lenzi M, et al. Insulin resistance and cardiovascular structural changes in essential hypertensives. Abstract American Journal of Hypertension 4: A, Doria A, Fioretto P, Avogaro A, Carraro A, Morocutti A, et al. Insulin resistance is associated with high sodium-lithium countertransport in essential hypertension. American Journal of Physiology Endocrinology and Metabolism 24 : E—E, Draznin B. Insulin resistance and cytosolic free calcium concentrations. Presented at 2nd European Nitrendipine Symposium: renal and metabolic considerations in hypertension, recent advances, pp. Draznin B, Sussman KE, Eckel RH, Kao M, Yost T, et al. Possible role of cytosolic free calcium concentrations in mediating insulin resistance of obesity and hyperinsulinemia. Ferrannini E. Metabolic abnormalities of hypertension. A lesson in complexity. The insulin resistance syndrome. Current Opinion in Nephrology and Hypertension 1: —, Insulin resistance syndrome Cardiovascular Risk Factors 3: 1, Ferrannini E, Buzzigoli G, Bonadonna R, Giorico MA, Oleggini M, et al. Insulin resistance in essential hypertension. New England Journal of Medicine —, Ferrari P, Weildmann P, Shaw S, Giachino D, Riesen W, et al. Altered insulin sensitivity, hyperinsulinemia, and dyslipidemia in individuals with a hypertensive parent. American Journal of Medicine —, Folkow B. Structural factors: the vascular wall. Consequences of treatment. Hypertension 5 Suppl. Gans ROB, Toorn L, Bilo HJG, Nauta JJP, Heine RJ, et al. Renal and cardiovascular effects of exogenous insulin in healthy volunteers. Grassi G, Seravalle G, Gennari A, Bolla GB, Fatti L, et al. XI Congresso Nazionale delia Società Italiana dell Tpertensione Arteriosa, Roma, 1—2 Ottobre, Abstract no. p 29, Haffner SM, Ferrannini E, Hazuda HP, Stern MP. Clustering of cardiovascular risk factors in confirmed prehypertensive individuals. Hypertension 38—45, Halkin H, Modan M, Shefi M, Almog S. Altered erythrocyte and plasma sodium and potassium in hypertension, a facet of hyperinsulinemia. Hypertension 71—77, Hall JE, Brands MW, Kivlighn SD, Mizelle HL, Hildebrandt DA, et al. Chronic hyperinsulinemia and blood pressure. Interaction with catecholamines? Hall JE, Coleman TG, Mizelle HL, Smith MJ. Chronic hyperinsulinemia and blood pressure regulation. American Journal of Physiology Renal Fluid and Electrolyte Physiology 27 : F—F, b. Henrich HA, Romen W, Heimgartner E, Baumer F. Capillary rarefaction characteristic of the skeletal muscle of hypertensive patients. Klinische Wochenschrift 54—60, Huang WC, Hsieh PS, Jin JS. Chronic hyperinsulinemia causes hypertension in rats. Abstract no. Hunt SC, Wu LL, Hopkins PN, Williams RR. Elevated NA-Li countertransport and fasting insulin in patients with familial combined hyperlipidemia and hypertension. Abstract no 59, Hypertension , Hwang IS, Ho H, Hoffman BB, Reaven GM. Fructose-induced insulin resistance and hypertension in rats. James DE, Jenkins AB, Kraegen EW. Heterogeneity of insulin action in individual muscles in vivo: euglycemic clamp studies in rats. American Journal of Physiology Endocrinology and Metabolism 11 : E—E, Juhlin-Dannfelt A, Frisk-Holmberg M, Karlsson J, Tesch P. Central and peripheral circulation in relation to muscle-fibre composition in normo- and hypertensive man. Julius S. Changing role of the autonomic nervous system in human hypertension. Journal of Hypertension 8 Suppl. Julius S, Gudbrandsson T, Jamerson K, Andersson O. The interconnection between sympathetics, microcirculation and insulin resistance in hypertension. Blood Pressure 1: 9—19, Julius S, Gudbrandsson T, Jamerson K, Shahab ST, Andersson O. The hemodynamic link between insulin resistance and hypertension. Journal of Hypertension 9: —, King GL, Kahn CR, Rechler MM, Nissley SP. Direct demonstration of separate receptors for growth and metabolic activities of insulin and multiplication-stimulating activity an insulin like growth factor using antibodies to the insulin receptor. Krotkiewski M, Bylund-Fallenius AC, Holm J, Bjorntorp P, Grimby G, et al. Relationship between muscle morphology and metabolism in obese women: the effects of long-term physical training. European Journal of Clinical Investigation 5—12, Kuriyama S, Nakamura K, Horiguchi M, Uchida H, Sakai O. Laakso M. The possible pathophysiology of insulin resistance syndrome. Cardiovascular Risk Factors 3: 55—66, Laakso M, Edelman SV, Brechtel G, Baron AD. Decreased effect of insulin to stimulate skeletal muscle blood flow in obese man. A novel mechanism for insulin resistance. Landin K, Tengborn L, Smith U. Treating insulin resistance in hypertension with metformin reduces both blood pressure and metabolic risk factors. Journal of Internal Medicine —, Lembo G, Napoli R, Capaldo B, Rendina V, Iaccarino G, et al. Abnormal sympathetic overactivity evoked by insulin in the skeletal muscle of patients with essential hypertension. Journal of Clinical Investigation 24—29, a. Lembo G, Napoli R, Rendina V, Capaldo B, Iaccarino G, et al. Lembo G, Rendina V, Lamenza F, Iaccarino G, Volpe M, et al. Insulin reduces forearm vasoconstriction evoked by reflex sympathetic activation in healthy humans. Abstract no 53 Hypertension , c. Lillioja S, Young AA, Culter CL, Ivy JL, Abbott GH, et al. Skeletal muscle capillary density and fiber type as possible determinants of in vivo insulin resistance in man. Lithell H. Insulin resistance and cardiovascular drugs. Clinical and Experimental Hypertension — Theory and Practice A —, Article CAS Google Scholar. Mitrakou A, Mokan M, Bolli G, Veneman T, Jenssen T, et al. Evidence against the hypothesis that hyperinsulinemia increases sympathetic nervous system activity in man. Metabolism —, Moore RD. Effects of insulin upon ion transport. Biochimica et Biophysica Acta 1—49, Morgan DA, Ray CA, Balon TW, Mark AL. Metformin increases insulin sensitivity and lowers arterial pressure in spontaneously hypertensive rats. P6 Hypertension , Mott DM, Clark RL, Andrews WJ, Foley JE. American Journal of Physiology Endocrinology and Metabolism 12 : El 60—E, Natali A, Buzzigoli G, Taddei S, Santoro D, Cerri M, et al. Effects of insulin on hemodynamics and metabolism in human forearm. Natali A, Gal van Q, Santoro D, Taddei S, Salvetti A, et al. Relationship between insulin release, antinatriuresis, and hypo-kalemia following glucose ingestion in normal and hypertensive man. Clinical Science, in press, Natali A, Santoro D, Palombo C, Cerri M, Ghione S, et al. Impaired insulin action on skeletal muscle metabolism in essential hypertension. Effect of insulin on plasma norepinephrine and 3,4-dihy-doxyphenylalanine in obese men. Article PubMed Google Scholar. Pedrinelli R, Spessot M, Salvetti A. Reactive hyperemia during short-term blood flow and pressure changes in the hypertensive forearm. Journal of Hypertension 8: —, Pfeifle B, Ditschuneit H. J Clin Epidemiol 43 : — Saad MF , Lillioja S , Nyomba BL , Castillo C , Ferraro R , DeGregorio M , Ravussin E , Knowler WC , Bennett PH , Havard VV , Bogardus C Racial differences in the relation between blood pressure and insulin resistance. Ferrannini E , Natali A , Capaldo B , Lehtovirta M , Jacob S , Yki-Järvinen H , for the European Group for the Study of Insulin Resistance EGIR Insulin resistance, hyperinsulinemia, and blood pressure. Role of age and obesity. Hypertension 30 : — Marigliano A , Tedde R , Sechi LA , Para A , Pisanu G , Pacifico A Insulinemia and blood pressure: relationships in patients with primary and secondary hypertension, and with or without glucose metabolism impairment. Am J Hypertens 3 : — Shamiss A , Carroll J , Rosenthall T Insulin resistance in secondary hypertension. Am J Hypertens 5 : 26 — Ferrari P , Weidmann P , Shaw S , Giachino D , Riesen W , Allemann Y , Heynen G Altered insulin sensitivity, hyperinsulinemia and dyslipidemia in individuals with a hypertensive parent. Facchini F , Chen Y-DI , Clinkingbeard C , Jeppesen J , Reaven GM Insulin resistance, hyperinsulinemia, and dyslipidemia in nonobese individuals with a family history of hypertension. Am J Hypertens 5 : — Allemann Y , Horber FF , Colombo M , Ferrari P , Shaw S , Jaeger P , Weidman P Insulin sensitivity and body fat distribution in normotensive offspring of hypertensive parents. Lancet : — Ohno Y , Suzuki H , Yamakawa H , Nakamura M , Otsuka K , Saruta T Impaired insulin sensitivity in young, lean normotensive offspring of essential hypertensive: possible role of disturbed calcium metabolism. J Hypertens 11 : — Beatty OL , Harper R , Sheridan B , Atkinson AB , Bell PM Insulin resistance in offspring of hypertensive parents. BMJ : 92 — Skarfors ET , Lithell HO , Selinus I Risk factors for the development of hypertension: a year longitudinal study in middle-aged men. J Hypertens 9 : — Lissner L , Bengtsson C , Lapidus L , Kristjansson K , Wedel H Fasting insulin in relation to subsequent blood pressure changes and hypertension in women. Hypertension 20 : — Taittonen L , Uhari M , Nuutinen M , Turtinen J , Pokka T , Akerblom HK Insulin and blood pressure among healthy children. Am J Hypertens 9 : — Raitakari OT , Porkka KVK , Rönnemaa T , Knip M , Uhari M , Akerblom HK , Viikari JSA The role of insulin in clustering of serum lipids and blood pressure in children and adolescents. Diabetologia 38 : — Metabolism 48 : — Meigs JB Invited commentary: insulin resistance syndrome? Syndrome X? A syndrome at all? Factor analysis reveals patterns in the fabric of correlated metabolic risk factors. Am J Epidemiol : — Yeni-Komshian H , Carantoni M , Abbasi F , Reaven GM Relationship between several surrogate estimates of insulin resistance and quantification of insulin-mediated glucose disposal in healthy, nondiabetic volunteers. Diabetes Care 23 : — J Intern Med : — Collins R , Peto R , MacMahon S , Herber P , Fiebach NH , Eberlein KA , Godwin J , Qizilbash N , Taylor JO , Hennekens CH Blood pressure, stroke and coronary heart disease. Short-term reductions in blood pressure: overview of randomized drug trials in their epidemiological context. Reaven GM Relationship between insulin resistance and hypertension. Diabetes Care 14 : 33 — Sheuh WH-H , Jeng C-Y , Shieh S-M , Fuh MM , Shen DD , Chen Y-DI , Reaven GM Insulin resistance and abnormal electrocardiograms in patients with high blood pressure. Jeppesen J , Hein HO , Suadicani P , Gynelberg F High triglycerides and low HDL cholesterol and blood pressure and risk of ischemic heart disease. Hypertension 36 : — Jeppesen J , Hein HO , Suadicani P , Gynterberg F Low triglycerides-high high-density lipoprotein cholesterol and risk of ischemic heart disease. Arch Int Med — Reaven GM Treatment of hypertension; focus on prevention of coronary heart disease. J Clin Endocrinol Metab 76 : — Reaven GM Insulin resistance, compensatory hyperinsulinemia, and coronary heart disease: syndrome X. In: Sobell BE, Schneider DJ, eds. Medical management of diabetes and heart disease. New York: Mercel Dekker, Inc. Ross R The pathogenesis of atherosclerosis. Chen N-G , Abbasi F , Lamendola C , McLaughlin T , Cooke JP , Tsao PS , Reaven GM Mononuclear cell adherence to cultured endothelium is enhanced by hypertension and insulin resistance in healthy nondiabetic volunteers. Circulation : — Stuhlinger MC , Abbasi F , Chu JW , Lamendola C , McLaughlin TL , Cooke JP , Reaven GM , Tsao PS Relationship between insulin resistance and an endogenous nitric oxide synthase inhibitor. JAMA : — Vallance P Importance of asymmetrical dimethylarginine in cardiovascular risk. Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide. Sign In or Create an Account. Endocrine Society Journals. Advanced Search. Search Menu. Article Navigation. Close mobile search navigation Article Navigation. Volume Article Contents The role of insulin resistance and compensatory hyperinsulinemia in the pathogenesis of essential hypertension? Journal Article. Reaven Gerald M. Oxford Academic. PDF Split View Views. Cite Cite Gerald M. Select Format Select format. ris Mendeley, Papers, Zotero. enw EndNote. bibtex BibTex. txt Medlars, RefWorks Download citation. Permissions Icon Permissions. The role of insulin resistance and compensatory hyperinsulinemia in the pathogenesis of essential hypertension? Figure 1. Open in new tab Download slide. Figure 2. Figure 3. Figure 4. high-density lipoprotein cholesterol;. Serum-insulin in essential hypertension and in peripheral vascular disease. Google Scholar PubMed. OpenURL Placeholder Text. Google Scholar Crossref. Search ADS. Hyperinsulinemia: a link between hypertension, obesity and glucose intolerance. Resistance to insulin-stimulated glucose uptake in patients with hypertension. Insulin resistance, glucose intolerance and hyperinsulinemia in patients with hypertension. Insulin resistance is a characteristic feature of primary hypertension independent of obesity. Hypertension and hyperinsulinemia: a relation in diabetes but not in essential hypertension. An inconsistent relationship between insulin and blood pressure in three Pacific Island populations. Racial differences in the relation between blood pressure and insulin resistance. Insulin resistance, hyperinsulinemia, and blood pressure. Insulinemia and blood pressure: relationships in patients with primary and secondary hypertension, and with or without glucose metabolism impairment. Altered insulin sensitivity, hyperinsulinemia and dyslipidemia in individuals with a hypertensive parent. Insulin resistance, hyperinsulinemia, and dyslipidemia in nonobese individuals with a family history of hypertension. Insulin sensitivity and body fat distribution in normotensive offspring of hypertensive parents. Impaired insulin sensitivity in young, lean normotensive offspring of essential hypertensive: possible role of disturbed calcium metabolism. Risk factors for the development of hypertension: a year longitudinal study in middle-aged men. Fasting insulin in relation to subsequent blood pressure changes and hypertension in women. The role of insulin in clustering of serum lipids and blood pressure in children and adolescents. Hyperinsulinemia in a normal population as a predictor of non-insulin-dependent diabetes mellitus, hypertension, and coronary heart disease: The Barilla factory revisited. Invited commentary: insulin resistance syndrome? Relationship between several surrogate estimates of insulin resistance and quantification of insulin-mediated glucose disposal in healthy, nondiabetic volunteers. Blood pressure, stroke and coronary heart disease. Insulin resistance and abnormal electrocardiograms in patients with high blood pressure. High triglycerides and low HDL cholesterol and blood pressure and risk of ischemic heart disease. Low triglycerides-high high-density lipoprotein cholesterol and risk of ischemic heart disease. Google Scholar OpenURL Placeholder Text. Treatment of hypertension; focus on prevention of coronary heart disease. Insulin resistance, compensatory hyperinsulinemia, and coronary heart disease: syndrome X. Mononuclear cell adherence to cultured endothelium is enhanced by hypertension and insulin resistance in healthy nondiabetic volunteers. Relationship between insulin resistance and an endogenous nitric oxide synthase inhibitor. Issue Section:. Download all slides. Views 3, More metrics information. Total Views 3, Email alerts Article activity alert. Advance article alerts. |
| Hypertension and insulin resistance in adipose tissue | There is no reason to suspect that the hyperinsulinemia, glucose intolerance, dyslipidemia, and prothrombotic state associated with the IRS will not contribute to the increased CVD risk in those patients with essential hypertension that are also insulin resistant In addition, changes in endothelial function that might contribute to increased CVD risk also vary as a function of differences in insulin-mediated glucose disposal in patients with essential hypertension. For example, the first step in the process of atherogenesis is the binding of circulating mononuclear cells to the endothelium 34 , and the data in the right panel of Fig. Indeed, it can be seen by comparing the two panels of Fig. Relationship between the SSPG concentration and the adherence of mononuclear cells isolated from the plasma of normal volunteers and patients with essential hypertension to cultured endothelial cells. The SSPG concentration is the average of four measurements of plasma glucose concentration obtained during the last 30 min of a min infusion of somatostatin, glucose, and insulin; the higher the SSPG concentration, the more insulin resistant the individual. Essentially identical findings were observed when the relationship between plasma asymmetric dimethylarginine ADMA concentration and insulin-mediated glucose disposal was evaluated Plasma concentrations of ADMA, an endogenous inhibitor of nitric oxide synthase, have been shown to be predictive of CVD in several clinical syndromes 37 , and Fig. It is apparent that plasma ADMA and SSPG concentrations varied widely in both experimental groups, but it is also obvious that the elevations in plasma ADMA concentrations are associated with higher SSPG concentrations greater degrees of insulin resistance. Thus, plasma ADMA concentrations are increased to a similar degree in insulin-resistant individuals, whether they are normotensive or hypertensive. Relationship between the SSPG concentration and plasma concentration of ADMA concentrations in normotensive and hypertensive individuals. The SSPG concentration is obtained as described in Fig. There is a large body of experimental evidence that insulin resistance and compensatory hyperinsulinemia are increased in prevalence in patients with essential hypertension, and similar changes can be seen in first-degree relatives of patients with essential hypertension. On the other hand, the fact that insulin resistance does not provide a unitarian hypothesis to account for the etiology of essential hypertension should not obscure the large amount of evidence of the importance of insulin resistance, and its metabolic consequences, in both the pathogenesis and clinical course of perhaps as many as half of the patients with essential hypertension. Welborn TA , Breckenridge A , Rubinstein AH , Dollery CT , Fraser TR Serum-insulin in essential hypertension and in peripheral vascular disease. Lancet 1 : — Google Scholar. Lucas CP , Estigarribia JA , Darga LL , Reaven GM Insulin and blood pressure in obesity. Hypertension 7 : — Modan M , Halkin H , Almog S , Lusky A , Eshkil A , Shefi M , Shitrit A , Fuchs A Hyperinsulinemia: a link between hypertension, obesity and glucose intolerance. J Clin Invest 75 : — Ferrannini E , Buzzigoli G , Bonadona R Insulin resistance in essential hypertension. N Engl J Med : — Shen D-C , Shieh S-M , Fuh M , Wu D-A , Chen Y-DI , Reaven GM Resistance to insulin-stimulated glucose uptake in patients with hypertension. J Clin Endocrinol Metab 66 : — Swislocki ALM , Hoffman BB , Reaven GM Insulin resistance, glucose intolerance and hyperinsulinemia in patients with hypertension. Am J Hypertens 2 : — Pollare T , Lithell H , Berne C Insulin resistance is a characteristic feature of primary hypertension independent of obesity. Metabolism 39 : — Mbanya J-C , Wilkinson R , Thomas T , Alberti K , Taylor R Hypertension and hyperinsulinemia: a relation in diabetes but not in essential hypertension. Lancet I : — Collins VR , Dowse GK , Finch CF , Zimmet PZ An inconsistent relationship between insulin and blood pressure in three Pacific Island populations. J Clin Epidemiol 43 : — Saad MF , Lillioja S , Nyomba BL , Castillo C , Ferraro R , DeGregorio M , Ravussin E , Knowler WC , Bennett PH , Havard VV , Bogardus C Racial differences in the relation between blood pressure and insulin resistance. Ferrannini E , Natali A , Capaldo B , Lehtovirta M , Jacob S , Yki-Järvinen H , for the European Group for the Study of Insulin Resistance EGIR Insulin resistance, hyperinsulinemia, and blood pressure. Role of age and obesity. Hypertension 30 : — Marigliano A , Tedde R , Sechi LA , Para A , Pisanu G , Pacifico A Insulinemia and blood pressure: relationships in patients with primary and secondary hypertension, and with or without glucose metabolism impairment. Am J Hypertens 3 : — Shamiss A , Carroll J , Rosenthall T Insulin resistance in secondary hypertension. Am J Hypertens 5 : 26 — Ferrari P , Weidmann P , Shaw S , Giachino D , Riesen W , Allemann Y , Heynen G Altered insulin sensitivity, hyperinsulinemia and dyslipidemia in individuals with a hypertensive parent. Facchini F , Chen Y-DI , Clinkingbeard C , Jeppesen J , Reaven GM Insulin resistance, hyperinsulinemia, and dyslipidemia in nonobese individuals with a family history of hypertension. Am J Hypertens 5 : — Allemann Y , Horber FF , Colombo M , Ferrari P , Shaw S , Jaeger P , Weidman P Insulin sensitivity and body fat distribution in normotensive offspring of hypertensive parents. Lancet : — Ohno Y , Suzuki H , Yamakawa H , Nakamura M , Otsuka K , Saruta T Impaired insulin sensitivity in young, lean normotensive offspring of essential hypertensive: possible role of disturbed calcium metabolism. J Hypertens 11 : — Beatty OL , Harper R , Sheridan B , Atkinson AB , Bell PM Insulin resistance in offspring of hypertensive parents. BMJ : 92 — Skarfors ET , Lithell HO , Selinus I Risk factors for the development of hypertension: a year longitudinal study in middle-aged men. J Hypertens 9 : — Lissner L , Bengtsson C , Lapidus L , Kristjansson K , Wedel H Fasting insulin in relation to subsequent blood pressure changes and hypertension in women. Hypertension 20 : — Taittonen L , Uhari M , Nuutinen M , Turtinen J , Pokka T , Akerblom HK Insulin and blood pressure among healthy children. Am J Hypertens 9 : — Raitakari OT , Porkka KVK , Rönnemaa T , Knip M , Uhari M , Akerblom HK , Viikari JSA The role of insulin in clustering of serum lipids and blood pressure in children and adolescents. Diabetologia 38 : — Metabolism 48 : — Meigs JB Invited commentary: insulin resistance syndrome? Syndrome X? A syndrome at all? Factor analysis reveals patterns in the fabric of correlated metabolic risk factors. Am J Epidemiol : — Yeni-Komshian H , Carantoni M , Abbasi F , Reaven GM Relationship between several surrogate estimates of insulin resistance and quantification of insulin-mediated glucose disposal in healthy, nondiabetic volunteers. Diabetes Care 23 : — J Intern Med : — Collins R , Peto R , MacMahon S , Herber P , Fiebach NH , Eberlein KA , Godwin J , Qizilbash N , Taylor JO , Hennekens CH Blood pressure, stroke and coronary heart disease. Short-term reductions in blood pressure: overview of randomized drug trials in their epidemiological context. Reaven GM Relationship between insulin resistance and hypertension. Diabetes Care 14 : 33 — Sheuh WH-H , Jeng C-Y , Shieh S-M , Fuh MM , Shen DD , Chen Y-DI , Reaven GM Insulin resistance and abnormal electrocardiograms in patients with high blood pressure. Jeppesen J , Hein HO , Suadicani P , Gynelberg F High triglycerides and low HDL cholesterol and blood pressure and risk of ischemic heart disease. Hypertension 36 : — Jeppesen J , Hein HO , Suadicani P , Gynterberg F Low triglycerides-high high-density lipoprotein cholesterol and risk of ischemic heart disease. Arch Int Med — Reaven GM Treatment of hypertension; focus on prevention of coronary heart disease. J Clin Endocrinol Metab 76 : — Reaven GM Insulin resistance, compensatory hyperinsulinemia, and coronary heart disease: syndrome X. In: Sobell BE, Schneider DJ, eds. Medical management of diabetes and heart disease. New York: Mercel Dekker, Inc. Ross R The pathogenesis of atherosclerosis. Abstract no 53 Hypertension , c. Lillioja S, Young AA, Culter CL, Ivy JL, Abbott GH, et al. Skeletal muscle capillary density and fiber type as possible determinants of in vivo insulin resistance in man. Lithell H. Insulin resistance and cardiovascular drugs. Clinical and Experimental Hypertension — Theory and Practice A —, Article CAS Google Scholar. Mitrakou A, Mokan M, Bolli G, Veneman T, Jenssen T, et al. Evidence against the hypothesis that hyperinsulinemia increases sympathetic nervous system activity in man. Metabolism —, Moore RD. Effects of insulin upon ion transport. Biochimica et Biophysica Acta 1—49, Morgan DA, Ray CA, Balon TW, Mark AL. Metformin increases insulin sensitivity and lowers arterial pressure in spontaneously hypertensive rats. P6 Hypertension , Mott DM, Clark RL, Andrews WJ, Foley JE. American Journal of Physiology Endocrinology and Metabolism 12 : El 60—E, Natali A, Buzzigoli G, Taddei S, Santoro D, Cerri M, et al. Effects of insulin on hemodynamics and metabolism in human forearm. Natali A, Gal van Q, Santoro D, Taddei S, Salvetti A, et al. Relationship between insulin release, antinatriuresis, and hypo-kalemia following glucose ingestion in normal and hypertensive man. Clinical Science, in press, Natali A, Santoro D, Palombo C, Cerri M, Ghione S, et al. Impaired insulin action on skeletal muscle metabolism in essential hypertension. Effect of insulin on plasma norepinephrine and 3,4-dihy-doxyphenylalanine in obese men. Article PubMed Google Scholar. Pedrinelli R, Spessot M, Salvetti A. Reactive hyperemia during short-term blood flow and pressure changes in the hypertensive forearm. Journal of Hypertension 8: —, Pfeifle B, Ditschuneit H. Effect of insulin on growth of cultured human arterial smooth muscle cells. Diabetologia —, Pollare T, Lithell H, Berne C. Insulin resistance is a characteristic feature of primary hypertension independent of obesity. Resnick LM. Calcium metabolism in hypertension and allied metabolic disorders. Reusch JEB, Begum N, Sussman KE, Draznin B. Regulation of GLUT-4 phosphorylation by intracellular calcium in adipocytes. Endocrinology —, Rocchini AP, Katch V, Kveselis D, Moorehead C, Martin M, et al. Insulin and renal sodium retention in obese adolescents. Rocchini AP, Key J, Bondie D, Chigo R, Moorehead C, et al. The effect of weight loss on the sensitivity of blood pressure to sodium in obese adolescents. New England Journal of Medicine —, b. Rocchini AP, Moorehead C, London M, Antishin K. Salt sensitivity and insulin resistance. Circulation —87, Rooney DP, Edgar JDM, Sheridan B, Atkinson AB, Bell PM. The effects of low dose insulin infusions on the renin angiotensin and sympathetic nervous systems in normal man. European Journal of Clinical Investigation —, Rowe JW, Young JB, Minaker KL, Stevens AL, Pallotta J, et al. Effect of insulin and glucose infusions on sympathetic nervous system activity in normal man. Saudek CD, Boulter PR, Knopp RH, Arky RA. Sodium retention accompanying insulin treatment of diabetes mellitus. Schaefer W, PrieBen J, Mannhold R, Gries AF. Klinische Wochenschrift 17—21, Segal S, Lloyd S, Sherman N, Sussman K, Draznin B. Postprandial changes in cytosolic free calcium and glucose uptake in adipocytes in obesity and non-insulin-dependent diabetes mellitus. Hormone Research 39—44, Shamiss A, Carroll J, Rosenthal T. Insulin resistance in secondary hypertension. American Journal of Hypertension 5: 26—28, Sharma AM, Ruland K, Spies KP, Distler A. Salt sensitivity in young normotensive subjects is associated with a hyperinsu-linemic response to oral glucose. Tedde R, Sechi LA, Marigliano A, Pala A, Scano L. Antihypertensive effect of insulin reduction in diabetic-hypertensive patients. American Journal of Hypertension 2: —, Tomiyama H, Kushiro T, Abeta H, Kurumatani H, Taguchi H, et al. Blood pressure response to hyperinsulinemia in salt-sensitive and salt-resistant rats. Wolpert HA, Steen SN, Istfan NW, Simonson DC. Disparate effects of weight loss on insulin sensitivity and erythrocyte sodium-lithium countertransport activity. Zemel MB, Johnson BA, Ambrozy SA. Insulin-stimulated vascular relaxation. Download references. Cattedra di Medicina Interna, Clinica Medica I, University of Pisa, Pisa, Italy. Salvetti, G. Brogi, V. You can also search for this author in PubMed Google Scholar. Reprints and permissions. Salvetti, A. et al. The Inter-Relationship between Insulin Resistance and Hypertension. Drugs 46 Suppl 2 , — Download citation. Published : 22 October Issue Date : December Anyone you share the following link with will be able to read this content:. Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative. Summary Insulin resistance and compensatory hyperinsulinaemia commonly occur in patients with untreated essential hypertension. Access this article Log in via an institution. References Anderson EA, Balon TW, Hoffman RP, Sinkey CA, Mark AL. Hypertension —, a Article PubMed CAS Google Scholar Anderson EA, Gudbjornsdottir S, Elam M, Sellgren J, Mark AL. Abstract Hypertension , b Google Scholar Anderson EA, Hoffman RP, Balon TW, Sinkey CA, Mark AL. Journal of Clinical Investigation —, Article PubMed CAS Google Scholar Andersson O, Sivertsson R, Sannerstedt R, Beckman M, Magnusson M, et al. Body fat and glucose tolerance in early blood pressure elevation: its relation to arteriolar hypertrophy Clinical Science s—s, Google Scholar Baron AD, Brechtel G, Johnson A, Henry D. Hypertension —, Google Scholar Beretta-Piccoli C, Davies DL, Boddy K, Brown JJ, Cumming AMM, et al. Clinical Science —, PubMed CAS Google Scholar Berne C, Fagius J, Niklasson F. Journal of Clinical Investigation —, Article PubMed CAS Google Scholar Bonen A, Tan MH, Watson-Wright WM. Diabetes —, Article PubMed CAS Google Scholar Brands MW, Hildebrandt DA, Mizelle HL, Hall JE. I : —, Google Scholar Buchanan TA, Sipos GF, Gadalah S, Yip K, Marsh DJ, et al. Hypertension —, Article PubMed CAS Google Scholar Byyny RL, LoVerde M, Lloyd S, Mitchell W, Draznin B. American Journal of Hypertension 5: —, PubMed CAS Google Scholar Catalano C, Winocour PH, Thomas TH, Walker M, Sum CF, et al. Diabetologia 52—56, Article PubMed CAS Google Scholar Daly PA, Landsberg L. Deficiency in CD36, a known fatty acid transporter, is also believed to be involved in the predisposition to insulin resistance and hypertension in Asians [ 23 ]. The relationship between insulin resistance and hypertension is a complex and multifactorial phenomenon which involves both genetic basis and environmental factors. In the western countries, sedentary life style and hypercaloric food intake are endemic behaviours which play a key role in the development of insulin resistance, mainly through epigenetic modifications. In particular, DNA methylation, histone modifications and noncoding RNA activity miRNA are the principal mechanisms that alter the protein transcription and expression, which, in turn, modify the cellular phenotype. The translocation of GLUT 4 to the cell membrane is the principal step of insulin-induced glucose uptake. Insulin resistance state is characterized by lower expression levels and impaired translocation of GLUT 4. Experimental data indicate that the metilation of DNA, induced by over-nutrition during fetal life, decreases the gene expression of proteins involved in insulin signal transduction, like GLUT 4. The expression of GLUT 4 is also affected by mi RNA. In particular, in myocytes the miRNA b impairs insulin signalling by decreasing insulin-stimulated translocation of GLUT 4. Mitochondrial dysfunction, which also plays a key role in the genesis of insulin resistance, is affected by epigenetic modifications. In particular, methylation of the gene encoding for peroxisome proliferator-activated receptor alpha PPARα has been reported in obese subjects. These data reinforce the hypothesis that epigenetic modifications are at lease partially responsible for the link between behaviour habits and insulin resistance [ 24 ]. In healthy subjects, insulin evokes a net reflex in sympathetic outflow and at the same time it blunts the vasoconstrictive effect resulting from this sympathetic activation. On the contrary, in hypertensive patients insulin evokes a sympathetic activation that is three times greater than in normal subjects, and moreover its vaso-relaxant action is impaired [ 13 ]. This mechanism is involved in dysregulation of peripheral vascular resistance, which contributes to increase BP levels Fig. The key role of insulin resistance in the pathogenesis of hypertension has been further supported by the evidence that in aortic rings of SHR the resistance to the vascular action of insulin is already present at the age of 5 weeks, before the onset of arterial hypertension [ 25 ], suggesting that in SHR vascular resistance to insulin action is specific and not related to the compensatory hyperinsulinemia or hyperglycemia. On the other hand, we have demonstrated, in rats fed for 6 months with hypercaloric diet that the increase of BP and the development of LVH are associated with both hyperinsulinemia or hyperglycemia [ 26 ]. In addition, insulin resistance and the resultant hyperinsulinemia are involved in the development of hypertension-related TOD, through the abnormalities of the counter-regulatory effects of insulin. In particular, impairment of cell membrane ion exchange, enhanced sympathetic nervous and renin-angiotensin systems, suppressed atrial natriuretic peptide activities, sodium retention, and plasma volume expansion contribute to the development of chronic kidney disease, LVH, CA. Regulation of peripheral vascular resistances by insulin and effects of insulin resistance. In physiological conditions, insulin antagonizes green arrows the effects of vasoconstrictor mediators red arrows , contributing to maintain the normal vascular tone. Insulin resistance red arrows , impairs the capability of insulin to counterbalance the action of vasoconstrictor mediators, resulting in the increase of vascular peripheral resistances. TNF-α, tumor necrosis factor-α; IL-6, interleukin Abnormalities of insulin signalling account for insulin resistance. Insulin mediates its action on target organs through phosphorylation of a transmembrane-spanning tyrosine kinase receptor, the insulin receptor IR. The binding of insulin to the α subunit of its receptor activates the tyrosine kinase of the β subunit of the receptor, leading to autophosphorylation, as well as tyrosine phosphorylation of several IR substrates IRS , including IRS-1 and IRS-2 [ 27 ]. These, in turn, interact with phosphatidylinositol 3-kinase PI3K. It is noteworthy that hyperglycaemia, accounts for the development of insulin resistance through the generation of reactive oxygen species ROS , which abrogate insulin-induced tyrosine autophosphorylation of IR [ 29 ]. In particular, phosphorylation of IRS-1 on serine Ser causes dissociation of the p85 subunit of PI3-K, inhibiting further signalling. In addition, phosphorylation of IRS-1 on Ser results in its dissociation from the IR and triggers proteasome-dependent degradation, also impairing insulin signalling. The mechanistic role of abnormalities of IR or IRS-1 signalling in the pathogenesis of insulin resistance and hypertension is supported by several pioneering studies performed in genetically engineered mice. In particular, transgenic mice with targeted disruption of the IRS-1 gene, exhibited higher BP and plasma triglyceride levels compared to wild-type mice. They also showed impairment of endothelium-dependent vascular relaxation [ 33 ]. On the other hand, mice heterozygous for knockout of the IR showed fasting blood glucose, insulin, free fatty acid, and triglyceride levels similar to those of wild-type mice. Interestingly, these mice had increased systolic BP and blunted insulin-stimulated aortic endothelial nitric oxide synthase NOS phosphorylation [ 34 ]. The results of these studies demonstrate that the abnormalities of IR or IRS signalling play a mechanistic role in the development of hypertension, independently form glucose homeostasis and plasma insulin levels, and indicate that insulin resistance by itself is involved the pathogenesis of hypertension. It is noteworthy that IR defects are tissue-specific and depends upon the type of stress; therefore, the insulin resistance phenomenon can be localized in specific tissues and does not necessarily associated with metabolic abnormalities. Several pathophysiological mechanisms contribute to impair insulin signal in hypertension, such as renin angiotensin and sympathetic nervous systems, and oxidative stress. On the other hand, mechanisms playing a protective role against insulin resistance, i. natriuretic peptides are impaired in hypertension. In vivo and in vitro studies have shown that Ang II stimulation induces insulin resistance [ 35 , 36 ]. Interventional studies have documented that angiotensin converting enzyme ACE inhibitors [ 37 ] and angiotensin type 1 receptor blockers [ 38 ] reduce the incidence of T2D in hypertensive patients. Therefore, the dysregulation of the renin-angiotensin system observed in hypertension is likely to impair insulin signalling and contribute to insulin resistance. Furthermore, it has been reported that an ACE-inhibitor-based long term treatment does not reduce the occurrence of diabetes mellitus in subjects with impaired glucose tolerance [ 39 ]. This observation suggests that the pathophysiological mechanisms underlying the development of insulin resistance in hypertensive patients are partially different from those responsible for impaired insulin sensitivity in diabetics. Ang II acting through angiotensin type 1 AT1 receptor inhibits the actions of insulin via generation of reactive oxygen species ROS by NADPH oxidase [ 40 ]. ROS are important intracellular second messengers. The generation of ROS is implicated in Ang II-induced insulin resistance. At this regard, it has been Epidemiological studies have documented a high incidence of diabetes in hypertensive patients. The disregulation of neuro-humoral and neuro-immune systems is involved in the pathophysiology of both insulin resistance and hypertension. That in vascular smooth muscle cells VSMC isolated from rat thoracic aorta, Ang II profoundly decreases IRS-1 protein levels via ROS-mediated phosphorylation of IRS-1 on Ser and subsequent proteasome-dependent degradation [ 41 ] Fig. The key role of ROS in the pathogenesis of Ang II-induced insulin resistance has been also confirmed by in vivo studies. In particular, in rats chronic infusion of Ang II induced hypertension and reduced insulin-evoked glucose uptake during hyperinsulinemic-euglycemic clamp, and increased plasma cholesterylester hydroperoxide levels, indicating an increased oxidative stress. Treatment with tempol, a superoxide dismutase mimetic, normalized plasma cholesterylester hydroperoxide levels in Ang II-infused rats. In the same setting, tempol normalized insulin resistance in Ang II-infused rats, and enhanced insulin-induced PI3K activation, suggesting that Ang II-induced insulin resistance can be restored by removing the oxidative stress [ 42 ]. A further mechanism that account for role of insulin resistance in the aetiology of hypertension is the upregulation of AT1 receptors induced by hyperinsulinemia. This potentiates the detrimental effects of Ang II on cardiovascular CV system. Altogether, these observations provide clear insights of the pathogenic mechanisms of Ang II—induced insulin resistance in the development of hypertension. Molecular mechanisms that account for angiotensin II-induced insulin resistance. The binding of insulin to its own receptor evokes the tyrosine autophosphorylation of the β subunit, which, in turn activates, in sequence, the insulin receptor substrate-1 and phosphatidylinositol 3-kinase green arrows. This cascade, accounts for the biological effects of insulin. Angiotensin II stimulation, through the activation of NADPH oxidase, stimulates the generation of reactive oxygen species, which, in turn promote the threonine phosphorylation of insulin receptor substrate-1 red arrows. This induces the proteasome-dependent degradation of insulin receptor substrate-1, interrupting the insulin signalling cascade. Ser, serine; P, phospho; Tyr, tyrosine; ROS, reactive oxygen species; PI3K, phosphatidylinositol 3-kinase; IRS-1, insulin receptor substrate Dysregulation of sympathetic nervous system is a feature of hypertension [ 44 ]. For instance, adipocytes and cardiac myocytes are both target organs of insulin, and mainly express β3 and β1-ARs, respectively. β-ARs stimulation results in an impairment of insulin signalling and insulin-induced glucose uptake, which profoundly differs between the two cell types regarding the kinetic and molecular mechanisms involved. In particular, β-ARs stimulation in adipocytes impairs insulin signalling within few minutes, whereas, in cardiac myocytes stimulation of β-ARs has a biphasic effect on insulin-stimulated glucose uptake, with an initial additive followed by an inhibitory action. On the contrary, in cardiac myocytes Akt plays a key role in the cross-talk between β1-ARs and IR. Actually, upon the phosphorylation site, Akt can have a favourable or detrimental effect on insulin signalling and insulin-induced glucose uptake. In particular, short-term β-ARs stimulation induces Akt phosphorylation in threonine that results in an increase in glucose uptake, whereas long-term stimulation induces Akt phosphorylation in serine and impairs insulin evoked-glucose uptake and insulin-induced tyrosine autophosporylation of IR [ 46 ]. In addition, it has been demonstrated in cardiac myocytes, that chronic Akt activation is sufficient to impair insulin signal by inducing IRS-1 phosphorylation and proteasome-dependent degradation [ 47 ]. LVH, CA, and renal dysfunction are expression of hypertension-evoked TOD, and are independent risk factors for both fatal and nonfatal cardio- and cerebro-vascular events [ 48 , 49 , 50 , 51 , 52 ]. Several studies have shown that insulin resistance promotes the development of left ventricular hypertrophy, carotid atherosclerosis and chronic kidney disease CKD. Development of LVH is a complex and multifactorial process which involves genetic, hemodynamic and anthropometric components, neuro-hormonal stimulation, growth factors and inflammatory mediators [ 53 , 54 , 55 ]. The hemodynamic and metabolic disorders associated with insulin resistance increase the risk LVH. Insulin and insulin-like growth factor IGF -1 are powerful independent determinants of LV mass and geometry in untreated subjects with essential hypertension and normal glucose tolerance [ 56 ]. The presence of both insulin resistance and hypertension in the same patient results in a mixed pattern of cardiac hypertrophy, caused by an elevation in both cardiac preload and afterload [ 57 ]. Experimental data suggest that insulin might be involved in the pathogenesis of both the concentric and eccentric patterns of LVH. Stimulation of myocardial cell growth and activation of the sympathetic nervous system might preferentially lead to concentric LV hypertrophy through a direct trophic effect and pressure overload, whereas sodium and water retention could lead to eccentric LV hypertrophy through volume overload [ 58 ]. The direct effect of insulin on myocardial cell growth could be mediated, at least in part, by the IGF-1 receptors [ 59 ]. In addition, the myocardium in the insulin-resistant individual shows the presence of mononuclear cell infiltration all along the conduction system making the myocardium in the insulin-resistant hypertensive patient an ideal substrate for cardiac arrhythmia and sudden death. Several epidemiological studies have underlined the role of insulin resistance, hyperinsulinemia in the development of atherosclerosis, and in the pathogenesis of its clinical consequences. Several mechanisms have been proposed as responsible for these phenomena. These include both direct effects on the arterial wall and indirect actions on lipid and glucose metabolism. Considerable evidence exists that demonstrate that endothelium as a physiological target of insulin and, consequently, a potential link between insulin resistance and atherosclerosis. In the endothelium, the stimulation of IRs activates insulin signaling via the PI3-K pathway, leading to NO production. The production of NO by endothelial cells, stimulated by insulin or insulin-like growth factor, leads to a host of anti-inflammatory and antithrombotic effects, which are antiatherogenic. The anti-inflammatory effects of NO include the decreases of expression of vascular cell adhesion molecule-1, intracellular adhesion molecule-1, and E-selectin and reduced secretion of the proinflammatory cytokines monocyte chemoattractant protein—1 and tumor necrosis factor-α TNF-α. Interestingly, the insulin-induced antithrombotic effects depends on gene expression regulation, which decreases the platelet adhesion by increasing prostacyclin production [ 60 ]. Of note, the gene encoding for coagulation factor II thrombin receptor, which is involved in the enhanced risk of coronary artery diseases in patients with hypertension [ 61 ], and it has been recently proposed as a marker of clinical conditions characterized by insulin resistance like metabolic syndromes, obesity, T2D, hepatic steatosis, and atherosclerosis [ 62 ]. Atherosclerotic process is regulated by inflammatory mechanisms [ 63 , 64 , 65 ]. Notably, insulin resistance has been recognized as a chronic, low-level, inflammatory state [ 66 ]. Although several mechanisms may contribute to the pathogenesis of hypertension, the NO system appears to play a major role. NO is an important messenger molecule that plays a critical role in a wide variety of physiological functions, including immune modulation, vascular relaxation, neuronal transmission, and cytotoxicity. Interestingly, the polymorphism of the promoter of the inducible nitric oxide synthase iNOS gene, NOS2A, revealed a positive association with essential hypertension [ 67 ]. This aspect is linked to insulin resistance because insulin stimulation of glucose uptake in skeletal muscle and adipose tissue is a NO-dependent phenomenon. Moreover, iNOS has been shown to be crucial for the development of insulin resistance [ 68 ]. The role that inflammation plays in atherosclerosis is amplified by the renin-angiotensin system via its effects on adhesion molecules, growth factors, and chemoattractant molecules, which modulate the migration of inflammatory cells into the subendothelial space. Clinical and experimental data have increased our knowledge on the effects of the Ang II. In particular, it has been documented that Ang II stimulates the production of ROS through its AT1 receptor [ 69 ], resulting in a proinflammatory modulator. The low grade of inflammation is a common feature of atherosclerosis and insulin resistance. The two major determinants of this state are the pro-inflammatory cytokines, TNF-α and interleukin-6 IL The available experimental data indicate that TNF-α is involved in the pathophysiology of hypertension. In particular, in vitro, TNF-α stimulates the production of endothelin-1 and angiotensinogen. Moreover, in SHR, TNF-α synthesis and secretion and fat angiotensinogen mRNA are increased in response to lipopolysaccharide LPS stimulation in comparison with non-hypertensive control rats [ 70 ]. At molecular level, TNF-α blocks the action of insulin in cultured cells [ 71 ], though the serine phosphorylation of IRS-1, decreasing the tyrosine kinase activity of the insulin receptor [ 72 ]. The key role of TNF-α in the pathogenesis of hypertension has been also documented in humans. In particular, the TNF-α gene locus seems to be involved in insulin resistance-associated hypertension [ 73 ]. A positive correlation has been found between serum TNF-α concentration and both SBP and insulin resistance [ 74 ]. Up-regulation of TNF-α secretion has also been observed in peripheral blood monocytes from hypertensive patients [ 75 ]. TNF-α also determines endothelial dysfunction linked to insulin resistance [ 76 ]. In contrast, treatment of T2D or obese human subjects with an antibody specific for TNF had no effect on insulin sensitivity [ 78 ]. IL-6 is a multifunctional cytokine produced by many different cell types, including immune cells, endothelial cells, fibroblasts, myocytes, and adipose tissue, which mediates inflammatory as well as stress-induced responses. It has been documented that BP was a significant and independent predictor of circulating IL-6 concentrations in women but not in men [ 79 ]. A polymorphism in the promoter of the IL-6 gene has also shown divergent associations with BP [ 80 ]. IL-6 stimulates the central nervous system and the sympathetic nervous system, which may result in hypertension [ 81 ]. However, other mechanisms cannot be excluded. IL-6 might increase in parallel to the modification of the redox state of the vascular wall in chronic hypertension, as occurs in some animal models of hypertension [ 82 ]. IL-6 is a well-characterized acute inducer of fibrinogen, which is a determinant of blood viscosity. Finally, IL-6 might increase BP enhancing the expression of angiotensinogen, leading to higher concentration of Ang II. Interestingly, leptin, a cytokine-like molecule increasingly recognized to regulate several inflammatory pathways acting on a receptor of the IL-6 family seems also to be associated with hypertension. The leptin signal, via central leptin receptors, is believed to interact with the central sympathetic nervous system [ 83 ]. Infusion of leptin leads to increases in BP [ 84 ]. Transgenic mice overexpressing leptin had elevated BP, which is normalized by adrenergic and ganglionic blockade [ 85 ]. Hypertension is believed to contribute to CKD by increasing glomerular capillary pressure, proteinuria, endothelial dysfunction, and sclerosis, leading to nephron damage [ 86 ]. Insulin resistance is associated with activation of both renin-angiotensin system and sympathetic nervous system activities, contributing to increased renal sodium reabsorption, associated fluid retention and hypertension [ 87 ]. Moreover, this state is accompanied by the increased endothelial cells proliferation and intrarenal lipid and hyaluronate deposition in the matrix and inner medulla [ 88 ]. These depositions increase intrarenal pressure and volume in the tightly encapsulated kidney, leading to parenchymal prolapse and urine outflow obstruction, which result in slow tubular flow and subsequently increased sodium reabsorption, especially in the loop of Henle. These functional and structural changes provoke compensatory lowered renal vascular resistance, elevated kidney plasma flow, glomerular hyperfiltration, and stimulation of renin angiotensin system, despite volume expansion. In addition, these changes in combination with the raise in BP levels associated with the insulin-resistant state increase the tubular reabsorption and maintain sodium balance. The persistence of these compensatory responses, increasing glomerular wall stress, precipitate gradual nephron loss, glomerulosclerosis and eventually end-stage renal disease. Glomerulosclerosis in the insulin-resistant kidney is peculiar and characterized by lower rate of nephrotic syndrome, fewer lesions of segmental sclerosis and a greater glomerular size compared with the idiopathic variety. In summary, persistence of insulin resistance and suboptimal control of associated hemodynamic and metabolic abnormalities cause renal injury with functional as well as structural nephron loss contributing to elevated BP, which in turn leads to further renal injury, thereby setting off a vicious circle of events leading to further elevated BP and renal injury. Microalbuminuria is now established as a modifiable predictor of CV morbidity and mortality [ 52 , 89 ] and is considered a manifestation hypertension-induced TOD. Despite its strong association with CV risk, the exact pathogenic mechanisms that link microalbuminuria to CV diseases remains unknown. Evidence has been garnered that microalbuminuria is a marker of generalized endothelial dysfunction and consequently a risk factor for CV diseases. In particular, this abnormality has been characterized by the presence of transmembrane leakiness [ 90 ]. In addition, microalbuminuria has also been associated with alterations in hemodynamic and vascular responses. In general, microalbuminuria can be considered as an early marker of generalized hypertension-evoked TOD Fig. Relationship between microalbuminuria and insulin resistance in the continuum of cardiovascular disease. Microalbuminuria, is clinical marker of activation of the insulin resistance-dependent pathogenic mechanisms involved in the genesis and progression of hypertension and hypertension-induced target organ damage. Epidemiological data show that hypertension is a risk factor for the development of T2D. The combination of hypertension and T2D increases CV risk and requires a strict control of both BP and glycaemia [ 91 ]. Insulin resistanceis involved in the pathogenesis and progression of hypertension as well as T2D. The low-grade of inflammation induced by insulin resistance is the principal mechanism that accounts for development of endothelial dysfunction, hypertension-induced TOD, and metabolic abnormalities [ 92 ] Fig. Altogether these data encourage the adoption of a holistic strategy to prevent the development of insulin resistance. In particular, it is well known that hypocaloric diet, and regular exercise training improve insulin sensitivity [ 93 ]. Both are recommended for primary and secondary CV prevention. It is noteworthy that consistent body of evidence supports the notion that exercise training is able to modify the epigenetics changes that are involved in the development of insulin resistance [ 24 ]. Insulin resistance is a time-dependent organ and tissue specific phenomenon. Insulin resistance contributes to the dysregulation of peripheral vascular resistance, resulting in an increase of blood pressure. In arterial hypertension, insulin resistance participates to the development of target organ damage. The persistence of insulin resistance induces the metabolic abnormalities that account for development of type 2 diabetes. The increased caloric intake and the sedentary habit are the principal environmental factors that account for the development and progression of insulin resistance. The dysregulation of renin-angiotensin system plays a pivotal role in the development of insulin resistance; thus, the prevention of incident T2D in patients with hypertension requires the inhibition of RAS with AT1 antagonist or ACE inhibitors [ 94 ]. Finally, in the last decades the beneficial effects on the CV system of different classes of glucose-lowering agents have been reported. In particular, Glucagon-like peptide-1 receptor agonists GLP-1RAs stimulate insulin release and supress glucagon synthesis. There is growing body of evidence that the GLP-1RAs improve CV outcomes in type 2 diabetes. In fact, they are recommended as first- and second-choice agents in diabetic patients at high CV risk or with overt atherosclerosis, independently from achievement of glycemic control [ 95 , 96 ]. In conclusion, the prevention of the insulin resistance-induced continuum of CV disease requires an integrated approach which involves non pharmacologic and pharmacologic therapies. Conen D, Ridker PM, Mora S, Buring JE, Glynn RJ. Eur Heart J. PubMed Google Scholar. Meisinger C, Döring A, Heier M. J Hypertens. CAS PubMed Google Scholar. Kramer CK, von Mühlen D, Barrett-Connor E. Mid-life blood pressure levels and the 8-year incidence of type 2 diabetes mellitus: the Rancho Bernardo study. J Hum Hypertens. Stahl CH, Novak M, Lappas G, Wilhelmsen L, Björck L, Hansson P-O, Rosengren A. High-normal blood pressure and long-term risk of type 2 diabetes: year prospective population based cohort study of men. BMC Cardiovasc Disord. Google Scholar. Derakhshan A, Bagherzadeh-Khiabani F, Arshi B, Ramezankhani A, Azizi F, Hadaegh F. Different combinations of glucose tolerance and blood pressure status and incident diabetes, hypertension, and chronic kidney disease. J Am Heart Assoc. PubMed PubMed Central Google Scholar. Izzo R, de Simone G, Chinali M, Iaccarino G, Trimarco V, Rozza F, et al. Insufficient control of blood pressure and incident diabetes. Diabetes Care. Izzo R, de Simone G, Trimarco V, Gerdts E, Giudice R, Vaccaro O, De Luca N, Trimarco B. Hypertensive target organdamagepredictsincidentdiabetesmellitus. de Simone G, Wang W, Best LG, Yeh F, Izzo R, Mancusi C, Roman MJ, Lee ET, Howard BV, Devereux RB. Target organ damage and incident type 2 diabetes mellitus: the Strong Heart Study. Cardiovasc Diabetol. Olefsky J, Farquhar JW, Reaven G. Relationship between fasting plasma insulin level and resistance to insulin-mediated glucose uptake in normal and diabetic subjects. |
| Insulin resistance/compensatory hyperinsulinemia and CVD | Also, the prevalence of muscle type 2b fibres fast twitch fibres may contribute to the development of insulin resistance. The common pathogenetic mechanism for both insulin resistance and hypertension could be activation of the sympathetic nervous system. This results in vasoconstriction, and may contribute to the genesis of vascular structural changes and increase the number of fast twitch fibres. Abstract Insulin resistance and compensatory hyperinsulinaemia commonly occur in patients with untreated essential hypertension. Publication types Review. Kramer CK, von Mühlen D, Barrett-Connor E. Mid-life blood pressure levels and the 8-year incidence of type 2 diabetes mellitus: the Rancho Bernardo study. J Hum Hypertens. Stahl CH, Novak M, Lappas G, Wilhelmsen L, Björck L, Hansson P-O, Rosengren A. High-normal blood pressure and long-term risk of type 2 diabetes: year prospective population based cohort study of men. BMC Cardiovasc Disord. Google Scholar. Derakhshan A, Bagherzadeh-Khiabani F, Arshi B, Ramezankhani A, Azizi F, Hadaegh F. Different combinations of glucose tolerance and blood pressure status and incident diabetes, hypertension, and chronic kidney disease. J Am Heart Assoc. PubMed PubMed Central Google Scholar. Izzo R, de Simone G, Chinali M, Iaccarino G, Trimarco V, Rozza F, et al. Insufficient control of blood pressure and incident diabetes. Diabetes Care. Izzo R, de Simone G, Trimarco V, Gerdts E, Giudice R, Vaccaro O, De Luca N, Trimarco B. Hypertensive target organdamagepredictsincidentdiabetesmellitus. de Simone G, Wang W, Best LG, Yeh F, Izzo R, Mancusi C, Roman MJ, Lee ET, Howard BV, Devereux RB. Target organ damage and incident type 2 diabetes mellitus: the Strong Heart Study. Cardiovasc Diabetol. Olefsky J, Farquhar JW, Reaven G. Relationship between fasting plasma insulin level and resistance to insulin-mediated glucose uptake in normal and diabetic subjects. Scherrer U, Randin D, Vollenweider P, Vollenweider L, Nicod P. J Clin Invest. CAS PubMed PubMed Central Google Scholar. Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G, Baron AD. Implications for the syndrome of insulin resistance. Fukuda N, Satoh C, Hu WY, Nakayama M, Kishioka H, Kanmatsuse K. Endogenous angiotensin ii suppresses insulin signaling in vascular smooth muscle cells from spontaneously hypertensive rats. Lembo G, Napoli R, Capaldo B, Rendina V, Iaccarino G, Volpe M, Trimarco B, Sacca L. Abnormal sympathetic overactivity evoked by insulin in the skeletal muscle of patients with essential hypertension. Sowers JR. Hypertension, angiotensin ii, and oxidative stress. N Engl J Med. Tabit CE, Chung WB, Hamburg NM, Vita JA. Endothelial dysfunction in diabetes mellitus: molecular mechanisms and clinical implications. Rev Endocr Metab Disord. Tabit CE, Shenouda SM, Holbrook M, Fetterman JL, Kiani S, Frame AA, Kluge MA, Held A, Dohadwala MM, Gokce N, Farb MG, Rosenzweig J, Ruderman N, Vita JA, Hamburg NM. Protein kinase C-β contributes to impaired endothelial insulin signaling in humans with diabetes mellitus. Mancusi C, Losi MA, Izzo R, Canciello G, Manzi MV, Sforza A, De Luca N, Trimarco B, de Simone G. Effect of diabetes and metabolic syndrome on myocardial mechano-energetic efficiency in hypertensive patients. The Campania Salute Network. Mancusi C, de Simone G, Best LG, Wang W, Zhang Y, Roman MJ, Lee ET, Howard BV, Devereux RB. Myocardial mechano-energetic efficiency and insulin resistance in non-diabetic members of the Strong Heart Study cohort. Ferrannini E, Buzzigoli G, Bonadonna R, Giorico MA, Oleggini M, Graziadei L, Pedrinelli R, Brandi L, Bevilacqua S. Insulinresistance in essentialhypertension. Reaven GM, Lithell H, Landsberg L. Hypertension and associated metabolic abnormalities—the role of insulin resistance and the sympathoadrenal system. Grunfeld B, Balzareti M, Romo M, Gimenez M, Gutman R. Hyperinsulinemia in normotensive offspring of hypertensive parents. Cheng LS, Davis RC, Raffel LJ, Xiang AH, Wang N, Quinones M, Wen PZ, Toscano E, Diaz J, Pressman S, Henderson PC, Azen SP, Hsueh WA, Buchanan TA, Rotter JI. Coincident linkage of fasting plasma insulin and blood pressure to chromosome 7q in hypertensive hispanic families. Miyaoka K, Kuwasako T, Hirano K, Nozaki S, Yamashita S, Matsuzawa Y. Cd36 deficiency associated with insulin resistance. Dos Santos JM, Moreli ML, Tewari S, Benite-Ribeiro SA. The effect of exercise on skeletal muscle glucose uptake in type 2 diabetes: an epigenetic perspective. Lembo G, Iaccarino G, Vecchione C, Rendina V, Trimarco B. Insulin modulation of vascular reactivity is already impaired in prehypertensive spontaneously hypertensive rats. Dietary Supplementation of Vitamin D Prevents the Development of Western Diet-Induced Metabolic, Hepatic and Cardiovascular Abnormalities in Rats. United European Gastroenterol J. Lee J, Pilch PF. The insulin receptor: structure, function, and signaling. Am J Physiol. Cong LN, Chen H, Li Y, Zhou L, McGibbon MA, Taylor SI, Quon MJ. Physiological role of akt in insulin-stimulated translocation of glut4 in transfected rat adipose cells. Mol Endocrinol. Hansen L, Ikeda Y, Olsen GS, Busch AK, Mosthaf L. Insulin signaling is inhibited by micromolar concentrations of H 2 O 2. Evidence for a role of H 2 O 2 in tumor necrosis factor alpha-mediated insulin resistance. J Biol Chem. Egawa K, Nakashima N, Sharma PM, Maegawa H, Nagai Y, Kashiwagi A, Kikkawa R, Olefsky JM. Persistent activation of phosphatidylinositol 3-kinase causes insulin resistance due to accelerated insulin-induced insulin receptor substrate-1 degradation in 3t3-l1 adipocytes. Goldstein BJ, Ahmad F, Ding W, Li PM, Zhang WR. Regulation of the insulin signalling pathway by cellular protein-tyrosine phosphatases. Mol Cell Biochem. Hotamisligil GS. Mechanisms of TNF-alpha-induced insulin resistance. Exp Clin Endocrinol Diabetes. Abe H, Yamada N, Kamata K, Kuwaki T, Shimada M, Osuga J, Shionoiri F, Yahagi N, Kadowaki T, Tamemoto H, Ishibashi S, Yazaki Y, Makuuchi M. Hypertension, hypertriglyceridemia, and impaired endothelium-dependent vascular relaxation in mice lacking insulin receptor substrate Wheatcroft SB, Shah AM, Li JM, Duncan E, Noronha BT, Crossey PA, Kearney MT. Preserved glucoregulation but attenuation of the vascular actions of insulin in mice heterozygous for knockout of the insulin receptor. Zhang S, Wei M, Yue M, Wang P, Yin X, Wang L, Yang X, Liu H. Hyperinsulinemia precedes insulin resistance in offspring rats exposed to angiotensin II type 1 autoantibody in utero. Mori J, Alrob OA, Wagg CS, Harris RA, Lopaschuk GD, Oudit GY. ANG II causes insulin resistanceand induces cardiac metabolic switch and inefficiency: a critical role of PDK4. Am J Physiol Heart Circ Physiol. Yusuf S, Gerstein H, Hoogwerf B, Pogue J, Bosch J, Wolffenbuttel BH, Zinman B. Ramipril and the development of diabetes. Julius S, Kjeldsen SE, Weber M, Brunner HR, Ekman S, Hansson L, Hua T, Laragh J, McInnes GT, Mitchell L, Plat F, Schork A, Smith B, Zanchetti A. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the value randomised trial. Scheen AJ. RevMed Liege. CAS Google Scholar. Insulin resistance and hypertension. Taniyama Y, Hitomi H, Shah A, Alexander RW, Griendling KK. Mechanisms of reactive oxygen species-dependent downregulation of insulin receptor substrate-1 by angiotensin II. ArteriosclerThrombVasc Biol. Ogihara T, Asano T, Ando K, Chiba Y, Sakoda H, Anai M, Shojima N, Ono H, Onishi Y, Fujishiro M, Katagiri H, Fukushima Y, Kikuchi M, Noguchi N, Aburatani H, Komuro I, Fujita T. Angiotensin II-induced insulin resistance is associated with enhanced insulin signaling. Andreozzi F, Laratta E, Sciacqua A, Perticone F, Sesti G. Angiotensin ii impairs the insulin signaling pathway promoting production of nitric oxide by inducing phosphorylation of insulin receptor substrate-1 on ser and ser in human umbilical vein endothelial cells. Circ Res. Mancia G, Grassi G, Giannattasio C, Seravalle G. Sympathetic activation in the pathogenesis of hypertension and progression of organ damage. Klein J, Fasshauer M, Ito M, Lowell BB, Benito M, Kahn CR. Beta 3 -adrenergic stimulation differentially inhibits insulin signaling and decreases insulin-induced glucose uptake in brown adipocytes. Morisco C, Condorelli G, Trimarco V, Bellis A, Marrone C, Sadoshima J, Trimarco B. Akt mediates the cross-talk between beta-adrenergic and insulin receptors in neonatal cardiomyocytes. Nagoshi T, Matsui T, Aoyama T, Leri A, Anversa P, Li L, Ogawa W, del Monte F, Gwathmey JK, Grazette L, Hemmings BA, Kass DA, Champion HC, Rosenzweig A. Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham heart study. Bots ML, Hoes AW, Koudstaal PJ, Hofman A, Grobbee DE. Common carotid intima-media thickness and risk of stroke and myocardial infarction: the Rotterdam study. Schiffrin EL, Lipman ML, Mann JF. Chronic kidney disease: effects on the cardiovascular system. Carpinella G, Pagano G, Buono F, Petitto M, Guarino G, Orefice G, Rengo G, Trimarco B, Morisco C. Prognostic value of combined target-organ damage in patients with essential hypertension. Am J Hypertens. Meccariello A, Buono F, Verrengia E, Orefice G, Grieco F, Romeo F, Trimarco B, Morisco C. Microalbuminuria predicts the recurrence of cardiovascular events in patients with essential hypertension. Morisco C, Sadoshima J, Trimarco B, Arora R, Vatner DE, Vatner SF. Is treating cardiac hypertrophy salutary or detrimental: the two faces of Janus. Buono F, Crispo S, Pagano G, Rengo G, Petitto M, Grieco F, Trimarco B, Morisco C. Determinants of left ventricular hypertrophy in patients with recent diagnosis of essential hypertension. Izzo R, Losi MA, Stabile E, Lönnebakken MT, Canciello G, Esposito G, Barbato E, De Luca N, Trimarco B, de Simone G. Development of left ventricular hypertrophy in treated hypertensive outpatients: the campania salute network. Verdecchia P, Reboldi G, Schillaci G, Borgioni C, Ciucci A, Telera MP, Santeusanio F, Porcellati C, Brunetti P. Circulating insulin and insulin growth factor-1 are independent determinants of left ventricular mass and geometry in essential hypertension. Hall JE, Crook ED, Jones DW, Wofford MR, Dubbert PM. Mechanisms of obesity-associated cardiovascular and renal disease. Am J Med Sci. de Simone G, Mancusi C, Izzo R, Losi MA, Ferrara A. Obesity and hypertensive heart disease: focus on body composition and sex differences. Diabetol Metab Syndr. Halldin M, Brismar K, Fahlstadius P, Vikström M, de Faire U, Hellénius ML. The metabolic syndrome and ECG detected left ventricular hypertrophy—influences from IGF-1 and IGF-binding protein PLoS One. Kuboki K, Jiang ZY, Takahara N, Ha SW, Igarashi M, Yamauchi T, Feener EP, Herbert TP, Rhodes CJ, King GL. Regulation of endothelial constitutive nitric oxide synthase gene expression in endothelial cells and in vivo: a specific vascular action of insulin. Gigante B, Bellis A, Visconti R, Marino M, Morisco C, Trimarco V, Galasso G, Piscione F, De Luca N, Prince JA, de Faire U, Trimarco B. Retrospective analysis of coagulation factor ii receptor F2R sequence variation and coronary heart disease in hypertensive patients. Arterioscler Thromb Vasc Biol. Yi X, Wu P, Liu J, Gong Y, Xu X, Li W. Identification of the potential key genes for adipogenesis from human mesenchymal stem cells by RNA-seq. J Cell Physiol. Ross R. Atherosclerosis—an inflammatory disease. Danesh J, Whincup P, Walker M, Lennon L, Thomson A, Appleby P, Gallimore JR, Pepys MB. Low grade inflammation and coronary heart disease: prospective study and updated meta-analyses. Fiordelisi A, Iaccarino G, Morisco C, Coscioni E, Sorriento D. NFkappaB is a key player in the crosstalk between inflammation and cardiovascular diseases. Int J Mol Sci. CAS PubMed Central Google Scholar. Chronic subclinical inflammation as part of the insulin resistance syndrome: the insulin resistance atherosclerosis study iras. Rutherford S, Johnson MP, Curtain RP, Griffiths LR. Chromosome 17 and the inducible nitric oxide synthase gene in human essential hypertension. Hum Genet. Perreault M, Marette A. Targeted disruption of inducible nitric oxide synthase protects against obesity-linked insulin resistance in muscle. Nat Med. Luft FC. Angiotensin, inflammation, hypertension, and cardiovascular disease. Curr Hypertens Rep. Nyui N, Tamura K, Yamaguchi S, Nakamaru M, Ishigami T, Yabana M, Kihara M, Ochiai H, Miyazaki N, Umemura S, Ishii M. Tissue angiotensinogen gene expression induced by lipopolysaccharide in hypertensive rats. Hotamisligil GS, Peraldi P, Budavari A, Ellis R, White MF, Spiegelman BM. Irsmediated inhibition of insulin receptor tyrosine kinase activity in tnf-alpha- and obesity-induced insulin resistance. Morisco C, Lembo G, Trimarco B. Insulin resistance and cardiovascular risk: new insights from molecular and cellular biology. Trends Cardiovasc Med. Pausova Z, Deslauriers B, Gaudet D, Tremblay J, Kotchen TA, Larochelle P, Cowley AW, Hamet P. Role of tumor necrosis factor-alpha gene locus in obesity and obesity-associated hypertension in frenchcanadians. Zinman B, Hanley AJ, Harris SB, Kwan J, Fantus IG. Circulating tumor necrosis factor-alpha concentrations in a native canadian population with high rates of type 2 diabetes mellitus. J Clin Endocrinol Metab. Dorffel Y, Latsch C, Stuhlmuller B, Schreiber S, Scholze S, Burmester GR, Scholze J. Preactivated peripheral blood monocytes in patients with essential hypertension. Winkler G, Lakatos P, Salamon F, Nagy Z, Speer G, Kovacs M, Harmos G, Dworak O, Cseh K. Elevated serum tnf-alpha level as a link between endothelial dysfunction and insulin resistance in normotensive obese patients. Diabet Med. Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Ofei F, Hurel S, Newkirk J, Sopwith M, Taylor R. Effects of an engineered human anti-tnf-alpha antibody cdp on insulin sensitivity and glycemic control in patients with niddm. Fernandez-Real JM, Vayreda M, Richart C, Gutierrez C, Broch M, Vendrell J, Ricart W. Circulating interleukin 6 levels, blood pressure, and insulin sensitivity in apparently healthy men and women. Humphries SE, Luong LA, Ogg MS, Hawe E, Miller GJ. Perrotta M, Lembo G, Carnevale D. The interactions of the immune system and the brain in hypertension. CurrHypertens Rep. Gonzalez W, Fontaine V, Pueyo ME, Laquay N, Messika-Zeitoun D, Philippe M, Arnal JF, Jacob MP, Michel JB. Molecular plasticity of vascular wall during n g -nitro- l -arginine methyl ester-induced hypertension: modulation of proinflammatory signals. Haynes WG, Morgan DA, Walsh SA, Mark AL, Sivitz WI. Receptor-mediated regional sympathetic nerve activation by leptin. Shek EW, Brands MW, Hall JE. Chronic leptin infusion increases arterial pressure. Aizawa-Abe M, Ogawa Y, Masuzaki H, Ebihara K, Satoh N, Iwai H, Matsuoka N, Hayashi T, Hosoda K, Inoue G, Yoshimasa Y, Nakao K. Pathophysiological role of leptin in obesity-related hypertension. Sowers JR, Epstein M, Frohlich ED. Diabetes, hypertension, and cardiovascular disease: an update. Zhang R, Reisin E. Obesity-hypertension: the effects on cardiovascular and renal systems. Henegar JR, Bigler SA, Henegar LK, Tyagi SC, Hall JE. Functional and structural changes in the kidney in the early stages of obesity. J Am SocNephrol. Palaniappan L, Carnethon M, Fortmann SP. Association between microalbuminuria and the metabolic syndrome: NHANES III. Castro JP, El-Atat FA, McFarlane SI, Aneja A, Sowers JR. Cardiometabolic syndrome: pathophysiology and treatment. Volpe M, Battistoni A, Gallo G, et al. Executive summary of the joint consensus document on cardiovascular disease prevention in Italy. High Blood Press Cardiovasc Prev. Carrizzo A, Izzo C, Oliveti M, et al. The main determinants of diabetes mellitus vascular complications: endothelial dysfunction and platelet hyperaggregation. PubMed Central Google Scholar. Iaccarino G, Franco D, Sorriento D, Strisciuglio T, Barbato E, Morisco C. Modulation of insulin sensitivity by exercise training: implications for cardiovascular prevention [published online ahead of print, Jul 31]. J Cardiovasc Transl Res. Borghi C, SIIA Task Force, Rossi F, SIF Task Force. Role of the renin-angiotensin-aldosterone system and its pharmacological inhibitors in cardiovascular diseases: complex and critical issues. Brown JM, Everett BM. Cardioprotective diabetes drugs: what cardiologists need to know. Cardiovasc Endocrinol Metab. Patel KV, Sarraju A, Needland IJ, McGuire DK. Cardiovascular Effects of Dipeptidyl Peptidase-4 Inhibitors and Glucagon-Like Peptide-1 Receptor Agonists : a Review for the General Cardiologist. Curr Cardiol Rep. Download references. Department of Advanced Biomedical Sciences, Federico II University of Naples, Via S. Pansini n. You can also search for this author in PubMed Google Scholar. Correspondence to Carmine Morisco. Open access funding provided by Università degli Studi di Napoli Federico II within the CRUI-CARE Agreement. Giuseppe Di Gioia received a grant from the Cardiopath PhD program. CM and CM had the idea for this article, RI, MAL, and GDG, performed the literature search, CM and CM drafted, and EB critically revised the work. Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. Reprints and permissions. Mancusi, C. et al. Insulin Resistance the Hinge Between Hypertension and Type 2 Diabetes. High Blood Press Cardiovasc Prev 27 , — Download citation. Received : 25 July Accepted : 05 September Published : 22 September Issue Date : December Anyone you share the following link with will be able to read this content:. Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative. Download PDF. Abstract Epidemiological studies have documented a high incidence of diabetes in hypertensive patients. Management of hyperglycaemia in type 2 diabetes, A consensus report by the American Diabetes Association ADA and the European Association for the Study of Diabetes EASD Article 24 September Recent Developments in Biomarkers for Diagnosis and Screening of Type 2 Diabetes Mellitus Article 10 March Metformin: clinical use in type 2 diabetes Article 02 August Use our pre-submission checklist Avoid common mistakes on your manuscript. Full size image. References Conen D, Ridker PM, Mora S, Buring JE, Glynn RJ. PubMed Google Scholar Meisinger C, Döring A, Heier M. CAS PubMed Google Scholar Kramer CK, von Mühlen D, Barrett-Connor E. CAS PubMed Google Scholar Stahl CH, Novak M, Lappas G, Wilhelmsen L, Björck L, Hansson P-O, Rosengren A. Google Scholar Derakhshan A, Bagherzadeh-Khiabani F, Arshi B, Ramezankhani A, Azizi F, Hadaegh F. PubMed PubMed Central Google Scholar Izzo R, de Simone G, Chinali M, Iaccarino G, Trimarco V, Rozza F, et al. PubMed PubMed Central Google Scholar Izzo R, de Simone G, Trimarco V, Gerdts E, Giudice R, Vaccaro O, De Luca N, Trimarco B. PubMed PubMed Central Google Scholar de Simone G, Wang W, Best LG, Yeh F, Izzo R, Mancusi C, Roman MJ, Lee ET, Howard BV, Devereux RB. PubMed PubMed Central Google Scholar Olefsky J, Farquhar JW, Reaven G. CAS PubMed Google Scholar Scherrer U, Randin D, Vollenweider P, Vollenweider L, Nicod P. CAS PubMed PubMed Central Google Scholar Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G, Baron AD. CAS PubMed PubMed Central Google Scholar Fukuda N, Satoh C, Hu WY, Nakayama M, Kishioka H, Kanmatsuse K. CAS PubMed Google Scholar Lembo G, Napoli R, Capaldo B, Rendina V, Iaccarino G, Volpe M, Trimarco B, Sacca L. CAS PubMed PubMed Central Google Scholar Sowers JR. PubMed Google Scholar Tabit CE, Chung WB, Hamburg NM, Vita JA. CAS PubMed Google Scholar Mancusi C, Losi MA, Izzo R, Canciello G, Manzi MV, Sforza A, De Luca N, Trimarco B, de Simone G. CAS PubMed Google Scholar Reaven GM, Lithell H, Landsberg L. CAS PubMed Google Scholar Grunfeld B, Balzareti M, Romo M, Gimenez M, Gutman R. Google Scholar Cheng LS, Davis RC, Raffel LJ, Xiang AH, Wang N, Quinones M, Wen PZ, Toscano E, Diaz J, Pressman S, Henderson PC, Azen SP, Hsueh WA, Buchanan TA, Rotter JI. CAS PubMed Google Scholar Miyaoka K, Kuwasako T, Hirano K, Nozaki S, Yamashita S, Matsuzawa Y. CAS PubMed Google Scholar Dos Santos JM, Moreli ML, Tewari S, Benite-Ribeiro SA. PubMed Google Scholar Lembo G, Iaccarino G, Vecchione C, Rendina V, Trimarco B. CAS PubMed PubMed Central Google Scholar Lee J, Pilch PF. CAS PubMed Google Scholar Cong LN, Chen H, Li Y, Zhou L, McGibbon MA, Taylor SI, Quon MJ. CAS PubMed Google Scholar Hansen L, Ikeda Y, Olsen GS, Busch AK, Mosthaf L. |
| Background | Insuli Supercharge your metabolism Clinical Investigation —, Article Google Scholar Pressude Legge V, Prwssure L, Energy recovery systems AR, Ciccarone A, Lenzi M, et al. Effects resietance Supercharge your metabolism peroxisomal proliferator-activated receptor-γ agonist pioglitazone on renal and hormonal responses to salt in healthy men. Meigs JB Invited commentary: insulin resistance syndrome? Informed consent was obtained from all subjects. Fujisaka S, Usui I, Ikutani M, Aminuddin A, Takikawa A, Tsuneyama K, et al. Zinn, A. Klein J, Fasshauer M, Ito M, Lowell BB, Benito M, Kahn CR. |
| Frontiers | Salt-Sensitivity of Blood Pressure and Insulin Resistance | Xnd ancestors were caloric restriction and lifespan faced prsesure survival resixtance, including famine, infection, Supercharge your metabolism bloo physical stress. In Insulin resistance and high blood pressure second study 31the participants an in the Copenhagen Male Study were divided into three groups on the basis of their fasting plasma TG and HDL-C concentrations. Prehypertension was defined as SBP values of — mmHg or DBP values of 80—89 mmHg on the second visit, in individuals without antihypertensive medication use. Hypertension, angiotensin ii, and oxidative stress. Baron AD, Brechtel G, Johnson A, Henry D. γδ T cells mediate angiotensin II-induced hypertension and vascular injury. |

Welche gute Frage
ist mit der vorhergehenden Mitteilung gar nicht einverstanden