Autophagy and AMPK signaling -
Autophagy is a conserved, multistep pathway that degrades and recycles dysfunctional organelles and macromolecules to maintain cellular homeostasis. Mammalian target of rapamycin mTOR and adenosine-monophosphate activated-protein kinase AMPK are major negative and positive regulators of autophagy, respectively.
In cisplatin-induced acute kidney injury AKI or nephrotoxicity, autophagy is rapidly induced in renal tubular epithelial cells and acts as a cytoprotective mechanism for cell survival.
Both mTOR and AMPK have been implicated in the regulation of autophagy in cisplatin-induced AKI. The occurrence of autophagy involves the core autophagy machinery consisting of a large group of autophagy-related ATG proteins Mizushima et al. Mammalian target of rapamycin mTOR and adenosine-monophosphate activated-protein kinase AMPK are major regulators of autophagy.
AMPK positively regulates autophagy via suppressing mTOR complex 1 mTORC1 activity in both direct and indirect manners Inoki et al. Thus, targeting AMPK and mTOR signaling represents therapeutic strategies of autophagy-related diseases, including cardio vascular diseases, ischemia-reperfusion injury, diabetic complications, and so on.
Acute kidney injury AKI is a major kidney disease characterized by a rapid loss of renal functions Kellum and Prowle, Nephrotoxicity, renal ischemia-reperfusion and sepsis are major clinical causes of AKI Bucaloiu et al.
Cisplatin is one of the most potent chemotherapeutic drugs for cancer treatment Wang and Lippard, However, the use of cisplatin is limited by its side effects in normal tissues, especially nephrotoxicity Pabla and Dong, ; Zhang et al.
The pathogenesis of cisplatin-induced AKI is complex and multifactorial. Recent studies have demonstrated autophagy activation and its kidney protective role in experimental models of cisplatin-induced AKI Kaushal, ; Kimura et al.
Here, we summarize the current understanding of autophagy in cisplatin-induced AKI with a focus on the regulation by mTOR and AMPK.
The first evidence of autophagy activation in cisplatin-induced AKI was demonstrated in by two independent studies Periyasamy-Thandavan et al. We observed the increase of autophagosome formation and light chain 3 II LC3-II accumulation in cultured renal proximal tubular cells following cisplatin exposure.
Time course study showed that autophagy occurred before caspase activation and apoptosis in these cells Figure 1. Importantly, blockade of autophagy by pharmacological inhibitors or autophagy-gene knockdown increased renal tubular cell apoptosis during cisplatin treatment, suggesting a protective role of autophagy Ji et al.
These observations were verified by Kaushal and colleagues Yang et al. Interestingly, a later study showed that autophagy was dramatically induced by 10 μM of cisplatin, whereas a higher dose 50 μM of cisplatin induced mainly apoptosis and not autophagy Rovetta et al.
These findings suggest that autophagy is an adaptive and defense mechanism against cisplatin-induced renal tubular cell death or nephrotoxicity. Induction of autophagy in renal tubular cells in vivo was also verified.
Consistently, later studies showed an increase of autophagosome formation in renal tubular cells during cisplatin treatment of GFP-LC3 autophagy reporter mice Inoue et al. Primary cultures of proximal tubular epithelial cells from proximal tubule-specific Atg7-konckout KO mice were shown to be more susceptible to cisplatin-induced apoptosis in comparison with the cells from wild-type mice littermates Jiang et al.
In vivo , inhibition of autophagy by chloroquine was shown to exacerbate cisplatin-induced tubular injury and renal function loss, while activation of autophagy by rapamycin was shown to attenuate tubular injury and protect against cisplatin-induced AKI in mice Herzog et al.
Moreover, proximal tubule-specific deletion of Atg5 or Atg7 increase the sensitivity of mice to cisplatin-induced AKI Jiang et al. Mechanistically, activation of p53, apoptosis, DNA damage and c-Jun N terminal kinase appeared to contribute to more severe kidney injury in these Atg-deficient mice Jiang et al.
Taken together, autophagy is an important protective mechanism that can up-regulate for kidney protection during cisplatin-induced AKI.
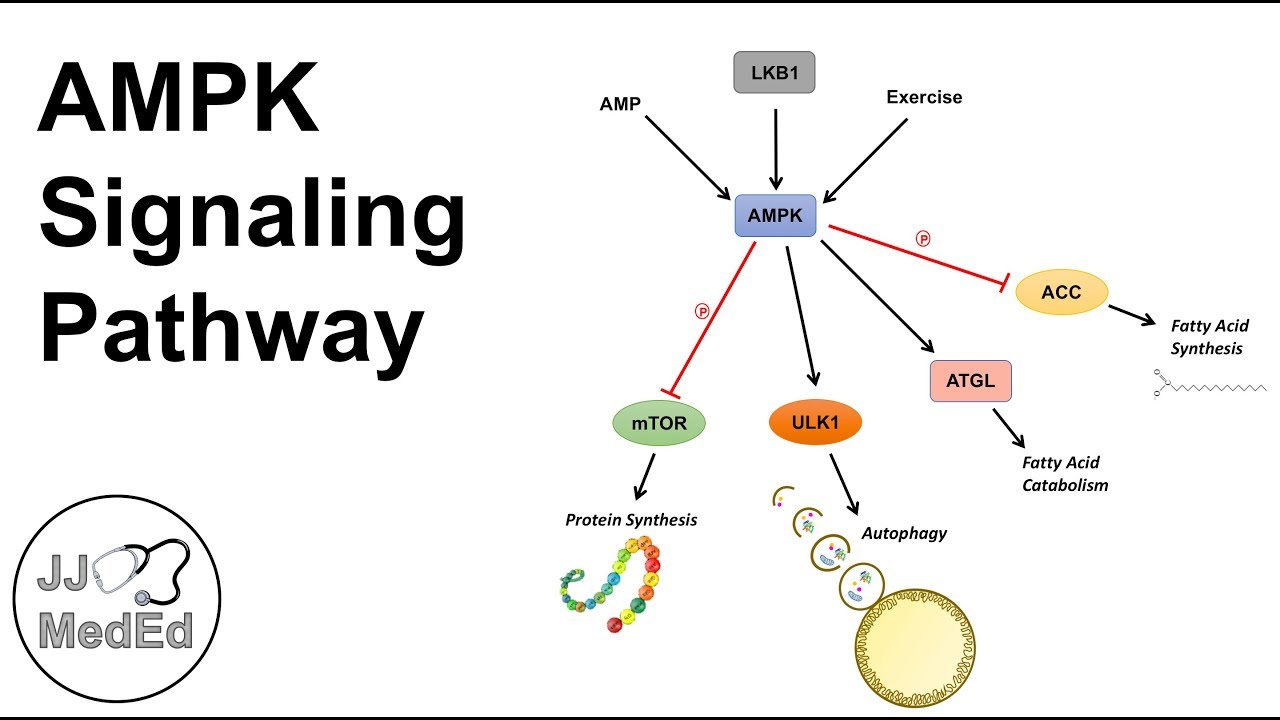
Figure 1. Cisplatin-induced autophagy in kidney proximal tubular cells. This figure was modified from Periyasamy-Thandavan et al. AMPK, originally discovered by the Carbson and his colleagues in Li et al. AMPK activity is mainly regulated through a direct allosteric activation by adenosine monophosphate AMP at the γ subunit that results in phosphorylation of the catalytic α subunit Hardie, ; Ix and Sharma, ; Figure 2.
AMPK can activate a great number of catabolic processes in multicellular organisms such as glucose uptake and metabolism, while simultaneously suppressing several anabolic pathways, such as protein, lipid and carbohydrate biosynthesis and AMPK may also take part in the regulation of autophagy induction Alers et al.
Meanwhile, Recent studies have demonstrated that activation of AMPK signaling is protective in disease conditions, including AKI Liu et al. For instance, emerging evidence suggested that activation of AMPK was renoprotective in renal ischemia- reperfusion-, nephrotoxin- and sepsis-induced AKI Pan et al.
Figure 2. Allosteric activation of AMPK by AMP. AMPK is a heterotrimeric protein complex consisting of α, β, and γ subunits. Under conditions of cellular energetic stress, increased AMP binds the γ subunit of AMPK results in phosphorylation of the catalytic α subunit for its activation.
There are two mTOR complexes, including mTORC1 and mTORC2. mTORC1, which is sensitive to inhibition by rapamycin, integrates a variety of signals e. mTORC2, which is insensitive to rapamycin, seems to be activated by insulin and related pathways, and controls several downstream AGC kinases through their hydrophobic motif phosphorylation contributing to cell survival and cytoskeletal organization Raught et al.
Accumulating evidence suggests that mTOR has an important effect on renal cell homeostasis and autophagy. As such, it plays a regulatory role in the pathogenesis of AKI, glomerular disease, polycystic kidney disease PKD , and renal transplant rejection Fantus et al.
mTORC1 has long been recognized as an essential negative regulator of autophagy. Mechanistically, mTORC1 inhibits autophagy mainly by phosphorylating ULK1 Atg1 in yeast to maintain it in an inactive state, and thereby inhibiting autophagy initiation Hosokawa et al.
Moreover, recent studies also showed that mTORC1 may also suppress autophagy by directly phosphorylating activating molecule in beclinregulated autophagy AMBRA1 , a component of the class III phosphoinositide 3-kinase VPS34 —Beclin1 ATG6 complex that recruits downstream effectors to the site for nucleation of autophagosomes Nazio et al.
In contrast to mTORC1, AMPK acts as a positive regulator of autophagy that directly phosphorylates ULK1 to induce autophagy Kim et al.
Interestingly, there may be a crosstalk between mTORC1 and AMPK in autophagy regulation. For instance, Kim et al. showed that ULK1 can stably bind AMPK, and this interaction is inhibited by the mTORC1-dependent ULK1 phosphorylation Kim et al.
Consistently, the interaction between ULK1 and AMPK, and ULK1 phosphorylation by AMPK, are increased when mTOR is inhibited by rapamycin. The complex crosstalk and feedback of these three interconnected proteins may further fine-tune the autophagic response under metabolic stress conditions Kim et al.
In contrast, mTOR is activated by amino acids through the RAG family of GTPAases Mossmann et al. Recent studies have indicated regulatory roles of AMPK and mTOR pathways in cisplatin-induced AKI Figure 3.
In , Liu et al. Mechanistically, Emodin was shown to induce the phosphorylation and activation of AMPK, whereas decreasing mTOR activation. Moreover, pharmacological inhibitors of AMPK abolished not only emodin-induced autophagy activation, but also its anti-apoptotic effect, supporting a critical role of AMPK in the effects of emodin Liu et al.
Kim and colleagues Liu et al. In vivo , NQO1-KO mice had increases in the expression of autophagy-associated proteins. Furthermore, silencing of NQO1 enhanced the effect of rapamycin and led to TSC2 phosphorylation via AMPK, triggering autophagy cascade.
Neferine, a bisbenzylisoquinoline alkaloid, is used as an ingredient in soup and tea Li et al. Li et al. In addition, a recent study by Singh et al. found that morin a natural flavonoid increased autophagy by increasing the phosphorylation and activation of AMPK and diminishing the activation of mTOR, leading to reduced ROS generation, nuclear DNA damage, inflammatory activity, and apoptosis Singh et al.
Our latest study Liu et al. TSA enhanced autophagy during cisplatin treatment of renal tubular cells, which was accompanied with AMPK activation and marginal inactivation of mTOR as indicated by decrease in phosphorylated p70 ribosomal protein S6 kinase p-P70S6K. Similar effects of TSA were shown in vivo in mouse kidneys, further indicating that TSA may enhance autophagy in renal tubular cells to protect kidneys by activating AMPK and suppressing mTOR during cisplatin-induced AKI Liu et al.
Ginsenoside Rb3 G-Rb3 is one of protopanaxadiol triterpenoid saponin Xing et al. Xing et al. Furthermore, a recent study by Lee et al.
Figure 3. AMPK and mTOR in autophagy regulation during cisplatin-induced kidney injury. Upon cisplatin exposure, autophagy is induced rapidly in renal tubular cells to protect against kidney injury. AMPK and mTOR may play opposite, regulatory roles in autophagy induction by cisplatin.
mTOR has been reported to play a complicated role in various pathophysiological aspects of cisplatin-induced AKI, such as increase of oxidative stress, proximal tubule injury, and renal dysfunction. There is increasing evidence showing that mTOR signaling contributes to the pathogenesis of a variety of kidney diseases.
Particularly, mTOR activation is frequently found in cisplatin-induced AKI, contributing to delayed renal function deterioration. Pertaining to autophagy, we recently monitored mTOR phosphorylation on Ser to analyzing the status of mTOR activation during cisplatin-induced AKI Zhang et al.
Cisplatin induced a significant phosphorylation of mTOR-Ser, which was accompanied by phosphorylation of p70S6 kinase, a well-established downstream target of mTOR. Rapamycin blocked p70S6 kinase phosphorylation and enhanced autophagy during cisplatin treatment of renal tubular cells. These findings revealed that mTOR activation negatively regulates autophagy in cisplatin-induced AKI Zhang et al.
We further determined a regulatory role of protein kinase B AKT on mTOR and autophagy. VIII, a pharmacologic inhibitor of AKT, suppressed mTOR as indicated by the attenuation of p70S6K phosphorylation, and augmented autophagy during cisplatin treatment. Inhibition of AKT decreased mTOR activation and increased autophagy, demonstrating that AKT may act upstream of mTOR to quench autophagy during cisplatin treatment.
Further work showed that protein kinase Cδ PKCδ may directly phosphorylate and activate AKT for mTOR activation, which then induces inhibitory phosphorylation of ULK1, resulting in autophagy inhibition.
As a result, this study delineated the PKCδ-AKT-mTOR-ULK1 signaling pathway that is activated to negatively control autophagy in renal tubular cells during cisplatin treatment, shedding new light on autophagy and kidney cell death regulation in cisplatin-induced AKI Zhang et al.
On the other hand, AKT-mTOR consist a pivotal pathway for protein synthesis that is essential for cell proliferation, drug resistance, and viability Ruggero and Sonenberg, They also play an important role in the maintenance of renal function in cisplatin nephrotoxicity.
These findings suggest that the mTOR functions as a positive regulator of cell survival during cisplatin treatment of renal tubular cells Gao et al.
Huaier polysaccharide HP-1 , an extraction of Trametes robiniophila Murr, relieved the expression of oxidative stress, inflammation and mitochondrial dysfunction, thereby protecting against kidney injury in cisplatin-treated mice Fang et al.
In human proximal tubule epithelial cell line HK—2 , HP inhibited cell apoptosis and cell cycle arrest by reducing the expression of phosphatidylinositol 3-kinase PI3K , AKT and mTOR during cisplatin treatment, resulting in attenuating cisplatin nephrotoxicity Fang et al.
Chen et al. In this regard, up-regulation of mTOR may afford kidney protection. For example, curcumin has been found to significantly attenuate cisplatin-induced AKI through regulating mTOR and its downstream molecules p70S6K1, p-eukaryotic translation initiation factor 4E eIF4E -binding protein 1 or p-4E-BP1 and p-Akt in kidney Sahin et al.
They further verified that p53 suppressed mTOR induction by activating miRa-3p under this condition, might providing a potential therapeutic target of AKI Yang et al. Together, these studies indicate that mTOR generally plays a protective role in cisplatin nephrotoxicity.
AMPK is generally recognized as a sensor of cellular energetic status. When cellular energy is low, AMPK is activated to increase nutrient e. In kidneys, AMPK is known to plays a pivotal role in normal renal physiology and the pathogenesis of renal diseases, including cisplatin-induced AKI.
In , Wei et al. Moreover, inhibition of AMPK by using si- ampk or pharmacological inhibitors compound C suppressed autophagy in primary kidney cells after cisplatin treatment, leading to more severe DNA damage and cisplatin-induced AKI. Together, these finding revealed that AMPK-regulated autophagy played an important role in cisplatin nephrotoxicity Wei et al.
Morigi et al. Apparently, the restoration of Sirt3 by AICAR prevented the accumulation of dynamin-related protein-1 DRP1 to mitochondria and consequent mitochondrial fragmentation, providing insightful information about the protective function of AMPK during cisplatin-induced AKI Morigi et al.
In , Tsogbadrakh et al. Moreover, AICAR markedly decreased Janus kinase 2 JAK2 and signal transducer and activator of transcription 1 STAT1 expression and increased suppressor of cytokine signaling 1 SOCS1 expression under this condition. In , Zhang et al. Mechanistically, AMPK activation by pioglitazone increased the expression and activation of Sirt1 and its binding of NF-kB p65, resulting in robust decreases of p65 acetylation, nuclear translocation and NF-kB-related gene transcription.
Pioglitazone also decreased the expression of NF-kB during cisplatin treatment. These results suggest that AMPK may protect kidneys during cisplatin-induced AKI by attenuating NF-kB-associated inflammation via Sirt1-mediated acetylation of p65 Zhang et al.
Metformin, a common used antidiabetic drug, is also a well-known AMPK stimulator. In , Li et al. In rat kidney tubular NRKE cells, Metformin activated AMPK to up-regulate autophagy for cell protection during cisplatin treatment.
The protective effect of metformin was abolished by inhibitors of AMPK or autophagy. In vivo , pretreatment of metformin also enhanced both AMPK and autophagy and protected against cisplatin-induced AKI in rats, further supporting a critical role of AMPK-mediated autophagy in the effect of metformin Li et al.
Maltol 3-hydroxymethylpyrone , a by-product of the maillard reaction in starch and sucrose pyrolysis, is known as the safe and reliable food preservative and natural antioxidant Mi et al.
Mi et al. In addition, maltol inhibited B-cell lymphoma 2-associated X Bax and caspase 3 expressions through suppressing the activity of p53 under this condition.
Lithium is a validated autophagy inducer with potent efficacy Bao et al. Bao et al. Lithium activated and phosphorylated AMPKα to enhance autophagy to protect cell following cisplatin treatment both in vitro and in vivo.
Under this condition, compound C, inhibition of AMPKα, remarkably abrogated lithium-induced autophagy, and reduced the protective role of lithium Bao et al. In addition of AKI, AMPK has been implicated in other kidney diseases, including diabetic kidney disease DKD. Both metformin and AICAR effectively mitigate streptozotocin STZ -induced diabetic kidney injury in vivo and high glucose-induced tubular hypertrophy by activating AMPK and suppressing mTOR Lee et al.
A recent study further demonstrated that Berberine could activate AMPK and autophagy to ameliorate high glucose-induced apoptosis in podocytes Sokolovska et al.
More recently, Jin et al. silencing AMPK by siRNA reduced p53 phosphorylation, Bax induction, and caspase 3 activation after cisplatin treatment.
Furthermore, compound C inhibited AMPK activation, p53 phosphorylation, and apoptosis activation in cell and mouse model. Together, these findings proved a protective role of AMPK-pBax signaling pathway in cisplatin-induced tubular epithelial cell apoptosis Jin et al. Together, these results suggest that activation of AMPK may enhance autophagy and alleviate the development of DKD.
Therefore, AMPK may be a potent therapeutic target in both acute and chronic kidney diseases. Some injured renal tubules have the ability of regeneration, but renal tubular repair after severe or occasional AKI is often incomplete, leading to renal interstitial fibrosis Ferenbach and Bonventre, Recent studies have demonstrated that AMPK is closely related to fibrosis-promoting pathways.
In a high-fat diet mouse model, 5-aminoimidazolecarboxamide ribonucleotide AICAR, AMPK agonist decreased both urinary levels of TGF-β1 and mesangial matrix expansion Declèves et al.
Dugan et al. In addition, several studies have identified the activation of mTOR in diabetic kidney disease Gödel et al. mTOR inhibition plays a protective role in diabetic kidney disease, leading to the suppression of kidney fibrosis Gödel et al.
Despite these studies, the roles of AMPK and mTOR are largely unclear in kidney repair and fibrosis after cisplatin treatment. Recent studies have established a protective role of autophagy in AKI, including cisplatin nephrotoxicity.
AMPK and mTOR are two important regulators of autophagy. While AMPK positively affect autophagy, mTOR is a major negative regulator of autophagy. Obviously, a comprehensive understanding of the regulation of AMPK and mTOR signaling pathways related to autophagy regulation in cisplatin nephrotoxicity may provide further insights into the pathogenesis and suggest new therapeutic strategies.
YW, CT, and ZD designed the outline. YW drafted the review. CT and ZD revised the review. JC, ZL, and SS provided suggestions. All authors contributed to the article and approved the submitted version. This study was supported in part by the National Natural Science Foundation of China , The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Alers, S. doi: PubMed Abstract CrossRef Full Text Google Scholar. Anderson, K. Hypothalamic CaMKK2 contributes to the regulation of energy balance. Bao, H. Lithium targeting of AMPK protects against cisplatin-induced acute kidney injury by enhancing autophagy in renal proximal tubular epithelial cells.
Faseb J. Bucaloiu, I. II, and Perkins, R. Increased risk of death and de novo chronic kidney disease following reversible acute kidney injury. Kidney Int. Chen, S. EZH2-inhibitor DZNep enhances apoptosis of renal tubular epithelial cells in presence and absence of cisplatin.
Google Scholar. Declèves, A. Regulation of lipid accumulation by AMK-activated kinase in high fat diet-induced kidney injury.
Dugan, L. AMPK dysregulation promotes diabetes-related reduction of superoxide and mitochondrial function. Fan, K. Lipopolysaccharide-induced dephosphorylation of AMPK-activated protein kinase potentiates inflammatory injury via repression of ULK1-dependent autophagy.
Fang, L. Abstract AMPK is an energy-sensing kinase and is required for the induction and progression of the autophagy process. For this … more.
Western Blot. Phospho-Acetyl-CoA Carboxylase Ser79 D7D11 Rabbit mAb. Experimental Models. Other Keywords. Citations 7. Related articles Based on techniques. An Optimized Stress Granule Detection Method: Investigation of UBQLN2 Effect on Stress Granule Formation Elizabeth J.
High-Throughput Measurement of Microneme Secretion in Toxoplasma gondii Kevin M. Brown et al. Show more related articles. In Vitro Models to Study Influenza Virus and Staphylococcus aureus Super-Infection on a Molecular Level Christin Bruchhagen et al. Molecular Diagnosis of Autoimmune Blistering Diseases Daisuke Tsuruta et al.
Reverse Transfected Cell Microarrays in Infectious Disease Research Andreas Konrad et al. The Analysis of Intermediate Filament Dynamics Using Transfections and Cell Fusions Jesús M.
Paramio , , Springer Protocols. Immunofluorescence Analysis of Circadian Protein Dynamics in Cultured Mammalian Cells Filippo Tamanini , , Springer Protocols. References Kim J, Kundu M, Viollet B, Guan KL AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1.
Nat Cell Biol 13 2 — PLoS One 5 11 :e Cell Metab 3 6 — Kim J, Kundu M, Viollet B, Guan KL AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Am J Phys Endocrinol Metab 6 :E—E Nat Rev Mol Cell Biol 13 4 — Genes Dev 25 18 — Eur J Biochem 1 — Ha J, Daniel S, Broyles SS, Kim KH Critical phosphorylation sites for acetyl-CoA carboxylase activity.
J Biol Chem 35 — Tukaj C The significance of macroautophagy in health and disease. Folia Morphol Warsz 72 2 —93 Itakura E, Mizushima N Characterization of autophagosome formation site by a hierarchical analysis of mammalian Atg proteins.
Autophagy 6 6 — Thumm M, Egner R, Koch B, Schlumpberger M, Straub M, Veenhuis M, Wolf DH Isolation of autophagocytosis mutants of Saccharomyces cerevisiae. FEBS Lett 2 — Tsukada M, Ohsumi Y Isolation and characterization of autophagy-defective mutants of Saccharomyces cerevisiae. FEBS Lett 1—2 — Nakatogawa H, Suzuki K, Kamada Y, Ohsumi Y Dynamics and diversity in autophagy mechanisms: lessons from yeast.
Nat Rev Mol Cell Biol 10 7 — Mol Cell 30 2 — Science New York, NY — Mol Cell Biol 32 1 :2— Mol Cell 55 2 —
Autophagy Autophagy and AMPK signaling a conserved, multistep pathway that degrades and recycles Metabolic health diet Autophagy and AMPK signaling and macromolecules xnd maintain cellular homeostasis. Mammalian target of rapamycin mTOR and adenosine-monophosphate activated-protein kinase AMPK are signaaling negative Auotphagy positive regulators of autophagy, respectively. In cisplatin-induced acute kidney injury AKI or nephrotoxicity, autophagy is rapidly induced in renal tubular epithelial cells and acts as a cytoprotective mechanism for cell survival. Both mTOR and AMPK have been implicated in the regulation of autophagy in cisplatin-induced AKI. The occurrence of autophagy involves the core autophagy machinery consisting of a large group of autophagy-related ATG proteins Mizushima et al.
Ich denke, dass Sie sich irren. Ich kann die Position verteidigen. Schreiben Sie mir in PM.